Synthesis of Superionic Conductive Li1+x+yAlxSiyTi2−xP3−yO12 Solid Electrolytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. LASTP Powder Synthesis
2.2. Fabrication of the LASTP Pellet
2.3. Material Characterizations
2.4. Electrochemical Test
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Diouf, B.; Pode, R. Potential of lithium-ion batteries in renewable energy. Renew. Energy 2015, 76, 375–380. [Google Scholar] [CrossRef]
- Yang, C.; Ji, X.; Fan, X.; Gao, T.; Suo, L.; Wang, F.; Sun, W.; Chen, J.; Chen, L.; Han, F.; et al. Flexible Aqueous Li-Ion Battery with High Energy and Power Densities. Adv. Mater. 2017, 29, 1701972. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bo, Y.; Li, D.; Liang, Y.; Zhang, J.; Cao, M.; Guo, R.; Zhu, Z.; Ci, L.; Li, M.; et al. Safe and Stable Lithium Metal Batteries Enabled by an Amide-Based Electrolyte. Nano-Micro Lett. 2022, 14, 44. [Google Scholar] [CrossRef]
- Ogawa, H.; Mori, H. Lithium salt/amide-based deep eutectic electrolytes for lithium-ion batteries: Electrochemical, thermal and computational study. Phys. Chem. Chem. Phys. 2020, 22, 8853–8863. [Google Scholar] [CrossRef]
- Pham, H.Q.; Lee, H.-Y.; Hwang, E.-H.; Kwon, Y.-G.; Song, S.-W. Non-flammable organic liquid electrolyte for high-safety and high-energy density Li-ion batteries. J. Power Sources 2018, 404, 13–19. [Google Scholar] [CrossRef]
- Tu, Z.; Nath, P.; Lu, Y.; Tikekar, M.D.; Archer, L.A. Nanostructured Electrolytes for Stable Lithium Electrodeposition in Secondary Batteries. Acc. Chem. Res. 2015, 48, 2947–2956. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mendez, R.; Smith, J.G.; Neuefeind, J.C.; Siegel, D.J.; Sakamoto, J. Correlating Macro and Atomic Structure with Elastic Properties and Ionic Transport of Glassy Li2S-P2S5 (LPS) Solid Electrolyte for Solid-State Li Metal Batteries. Adv. Energy Mater. 2020, 10, 2000335. [Google Scholar] [CrossRef]
- Kim, K.J.; Balaish, M.; Wadaguchi, M.; Kong, L.; Rupp, J.L.M. Solid-State Li–Metal Batteries: Challenges and Horizons of Oxide and Sulfide Solid Electrolytes and Their Interfaces. Adv. Energy Mater. 2021, 11, 2002689. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, Y.; Zhou, Y.; Hu, Z.; Zhu, X. Influence of Liquid Solutions on the Ionic Conductivity of Li1.3Al0.3Ti1.7(PO4)3 Solid Electrolytes. ChemElectroChem 2019, 6, 6016–6026. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Subramanian, R.; Clearfield, A. Lithium ion conductors in the system AB(IV)2(PO4)3 (B = Ti, Zr and Hf). Solid State Ion. 1986, 18–19, 562–569. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Sahami, S.; Shea, S.W.; Zhang, Z. Preparation and conductivity measurements of SiS2 Li2S glasses doped with LiBr and LiCl. Solid State Ion. 1986, 18–19, 368–371. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, K.; Yu, C.; Chen, X.; Zhang, C.; Yao, Y.; Jiang, B.; Long, H. Recent progress of composite solid polymer electrolytes for all-solid-state lithium metal batteries. Chin. Chem. Lett. 2021, 32, 2659–2678. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, W.; Zhang, W.; Zheng, X.; Wu, M.-B.; Wei, W.; Tao, Y.; Li, Z.; Yang, Q.-H. Reduction of Graphene Oxide by Hydrogen Sulfide: A Promising Strategy for Pollutant Control and as an Electrode for Li-S Batteries. Adv. Energy Mater. 2014, 4, 1301565. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic solid Li ion conductors: An overview. Solid State Ion. 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.-P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Jian, Z.; Hu, Y.-S.; Ji, X.; Chen, W. NASICON-Structured Materials for Energy Storage. Adv. Mater. 2017, 29, 1601925. [Google Scholar] [CrossRef] [PubMed]
- Anantharamulu, N.; Rao, K.K.; Rambabu, G.; Kumar, B.V.; Radha, V.; Vithal, M. A wide-ranging review on Nasicon type materials. J. Mater. Sci. 2011, 46, 2821–2837. [Google Scholar] [CrossRef]
- Arjmandi, H.R.; Grieshammer, S. Defect formation and migration in Nasicon Li1+xAlxTi2−x(PO4)3. Phys. Chem. Chem. Phys. 2019, 21, 24232–24238. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Gautam, G.S.; Kolli, S.K.; Chotard, J.-N.; Cheetham, A.K.; Masquelier, C.; Canepa, P. Phase Behavior in Rhombohedral NaSiCON Electrolytes and Electrodes. Chem. Mater. 2020, 32, 7908–7920. [Google Scholar] [CrossRef]
- Pfalzgraf, D.; Mutter, D.; Urban, D.F. Atomistic analysis of Li migration in Li1+xAlxTi2−x(PO4)3 (LATP) solid electrolytes. Solid State Ion. 2021, 359, 115521. [Google Scholar] [CrossRef]
- Martínez-Juárez, A.; Pecharromán, C.; Iglesias, J.E.; Rojo, J.M. Relationship between Activation Energy and Bottleneck Size for Li+ Ion Conduction in NASICON Materials of Composition LiMM‘(PO4)3; M, M’ = Ge, Ti, Sn, Hf. J. Phys. Chem. B 1998, 102, 372–375. [Google Scholar] [CrossRef]
- Schmidt, W.; Bottke, P.; Sternad, M.; Gollob, P.; Hennige, V.; Wilkening, M. Small Change—Great Effect: Steep Increase of Li Ion Dynamics in Li4Ti5O12 at the Early Stages of Chemical Li Insertion. Chem. Mater. 2015, 27, 1740–1750. [Google Scholar] [CrossRef]
- Jackman, S.D.; Cutler, R.A. Effect of microcracking on ionic conductivity in LATP. J. Power Sources 2012, 218, 65–72. [Google Scholar] [CrossRef]
- Fu, J. Fast Li+ Ion Conduction in Li2O-Al2O3-TiO2-SiO2-P2O2 Glass-Ceramics. J. Am. Ceram. Soc. 1997, 80, 1901–1903. [Google Scholar] [CrossRef]
- Banerjee, S.; Devi, P.S.; Topwal, D.; Mandal, S.; Menon, K. Enhanced Ionic Conductivity in Ce0.8Sm0.2O1.9: Unique Effect of Calcium Co-doping. Adv. Funct. Mater. 2007, 17, 2847–2854. [Google Scholar] [CrossRef]
- Chung, H.; Kang, B. Increase in grain boundary ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 by adding excess lithium. Solid State Ion. 2014, 263, 125–130. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Lu, L. Influence of crystallization temperature on ionic conductivity of lithium aluminum germanium phosphate glass-ceramic. J. Power Sources 2015, 290, 123–129. [Google Scholar] [CrossRef]
- Davis, C., III; Nino, J.C. Microwave Processing for Improved Ionic Conductivity in Li2O–Al2O3–TiO2–P2O5 Glass-Ceramics. J. Am. Ceram. Soc. 2015, 98, 2422–2427. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Z.; Huang, B.; Zhou, J.; Xu, X. Influence of phosphorus sources on lithium ion conducting performance in the system of Li2O–Al2O3–GeO2–P2O5 glass–ceramics. Solid State Ion. 2015, 270, 61–65. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, H.; Lee, Y.-G.; Matsui, M.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Tape-Cast Water-Stable NASICON-Type High Lithium Ion Conducting Solid Electrolyte Films for Aqueous Lithium-Air Batteries. J. Electrochem. Soc. 2015, 162, A1265–A1271. [Google Scholar] [CrossRef]
- Chang, C.-M.; Lee, Y.I.; Hong, S.-H.; Park, H.-M. Spark Plasma Sintering of LiTi2(PO4)3-Based Solid Electrolytes. J. Am. Ceram. Soc. 2005, 88, 1803–1807. [Google Scholar] [CrossRef]
- El-Shinawi, H.; Regoutz, A.; Payne, D.J.; Cussen, E.J.; Corr, S.A. NASICON LiM2(PO4)3 electrolyte (M = Zr) and electrode (M = Ti) materials for all solid-state Li-ion batteries with high total conductivity and low interfacial resistance. J. Mater. Chem. A 2018, 6, 5296–5303. [Google Scholar] [CrossRef] [Green Version]
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G. Ionic conductivity and sinterability of lithium titanium phosphate system. Solid State Ion. 1990, 40–41, 38–42. [Google Scholar] [CrossRef]
- Rusdi, H.; Mohamed, N.S.; Subban, R.H.Y. Effects of temperatures to the electrical properties of Li1.6Al0.6Sn1.4P3O12 NASICON type solid electrolytes. AIP Conf. Proc. 2017, 1877, 50005. [Google Scholar] [CrossRef] [Green Version]

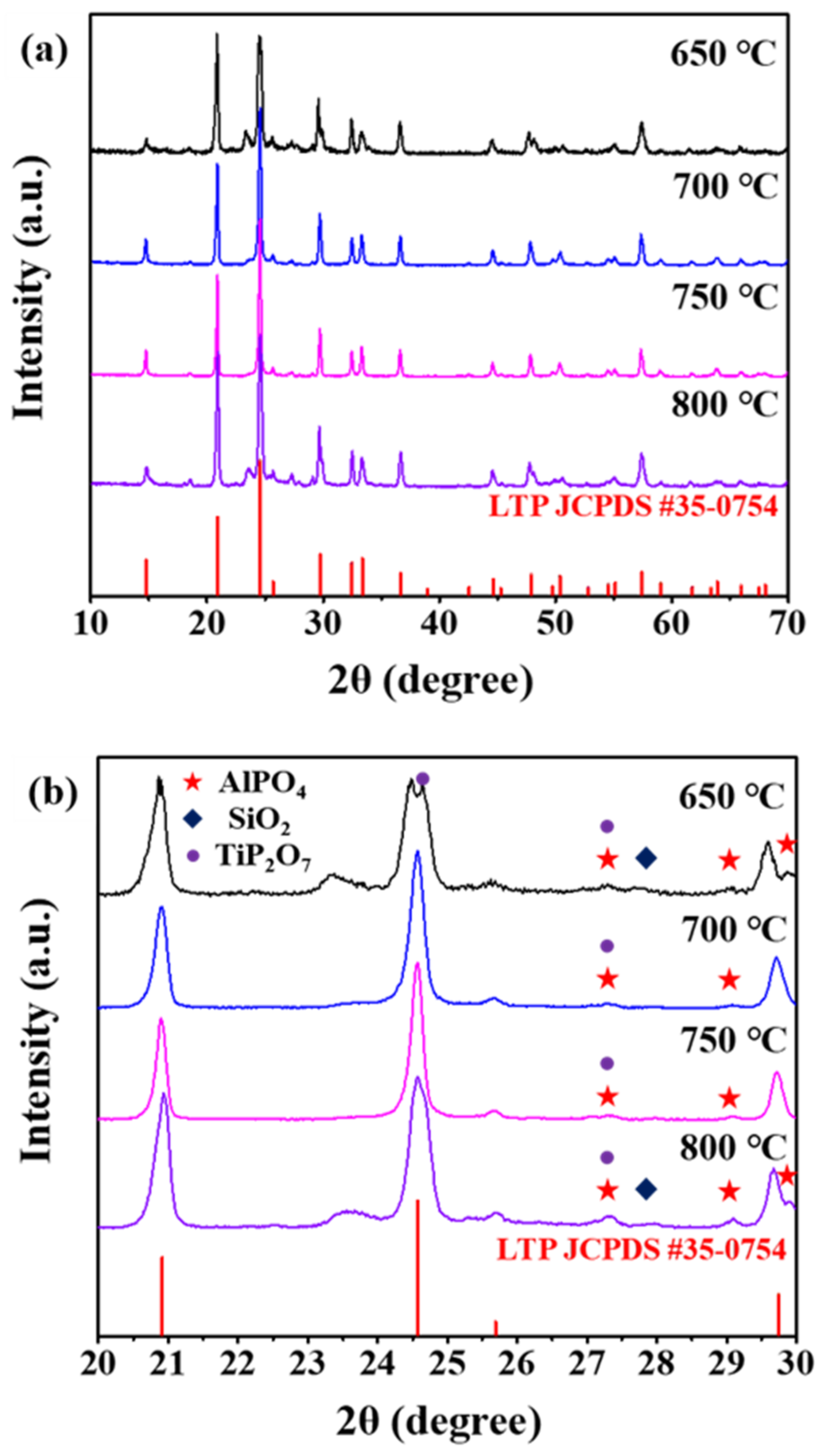
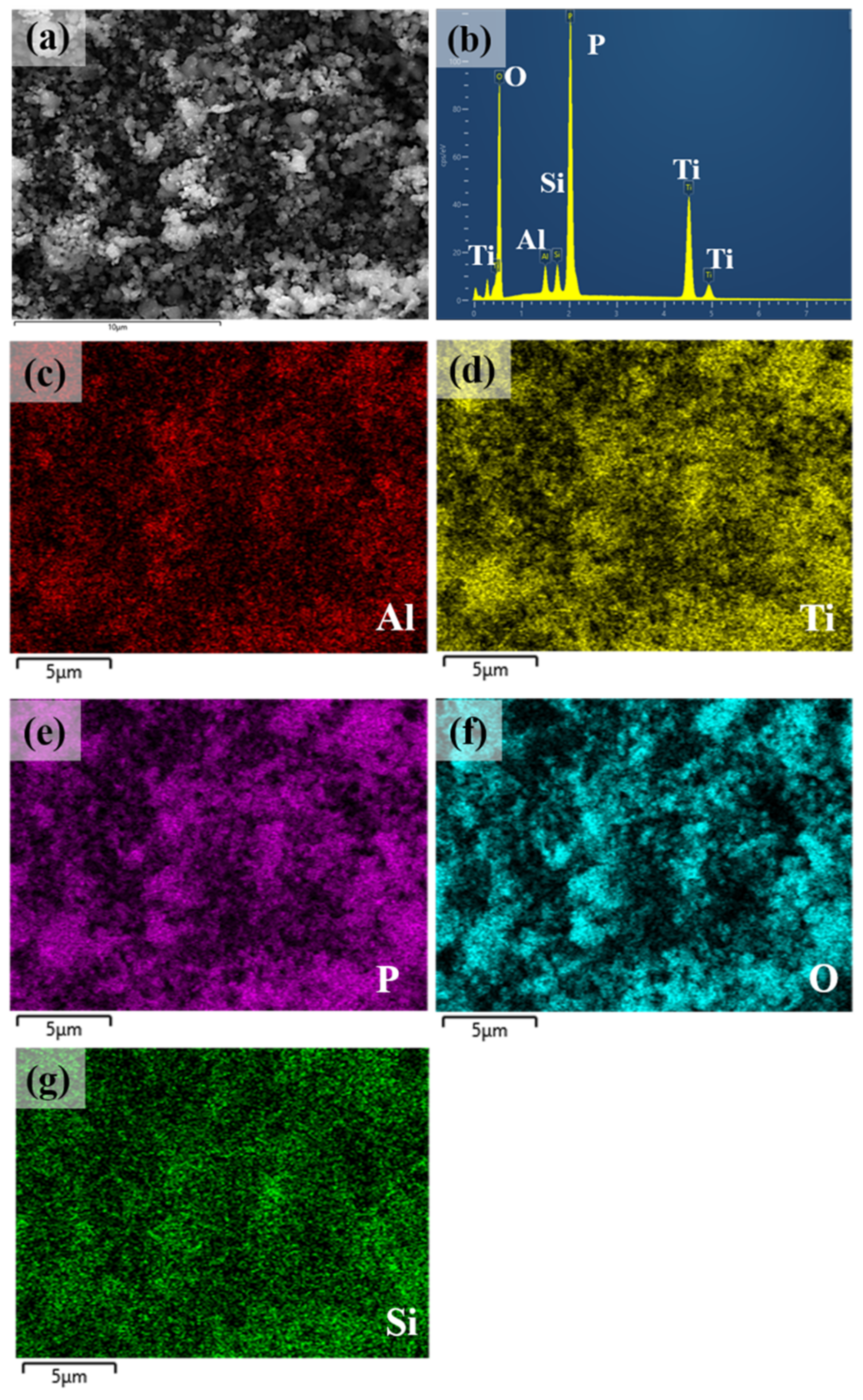
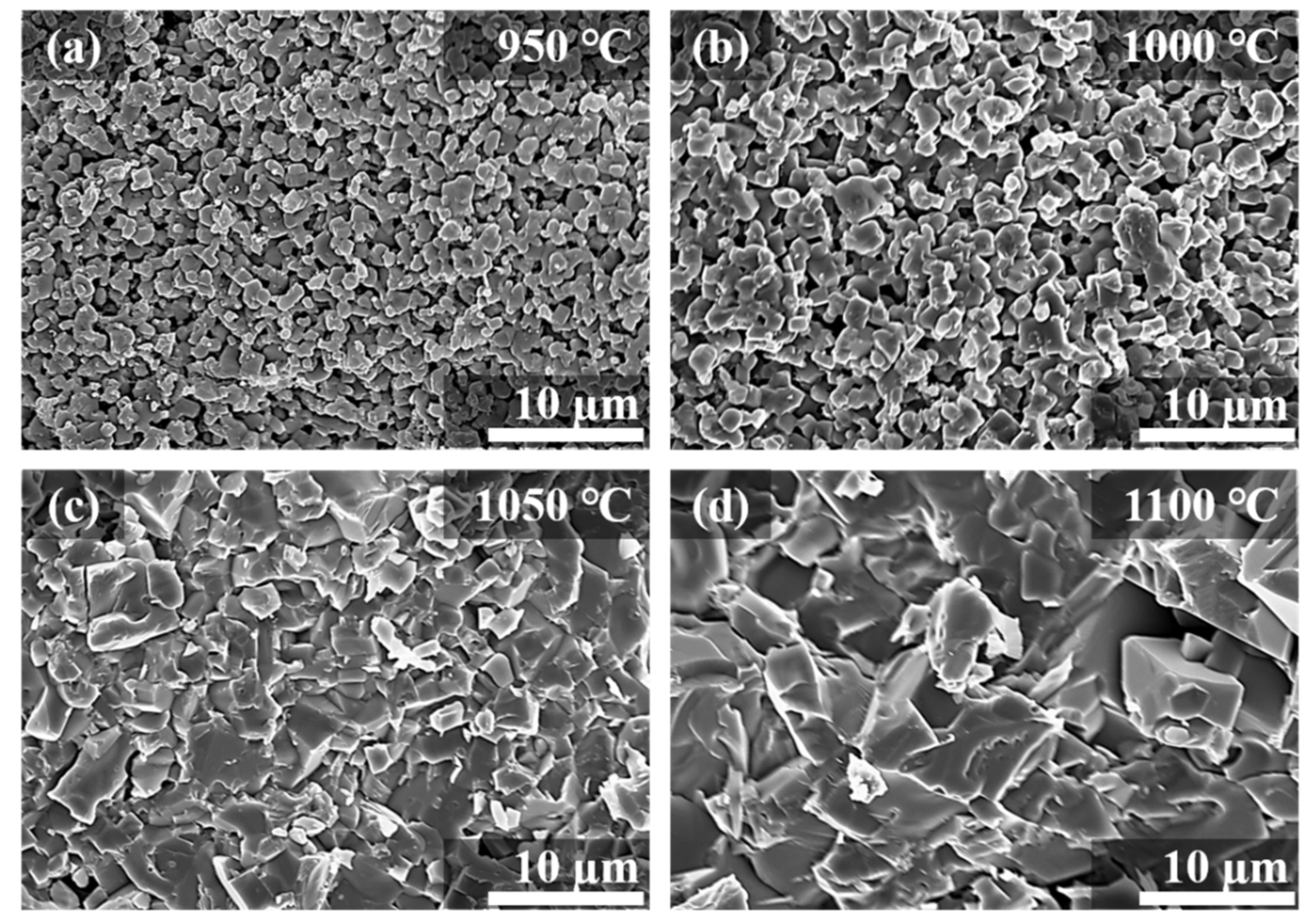
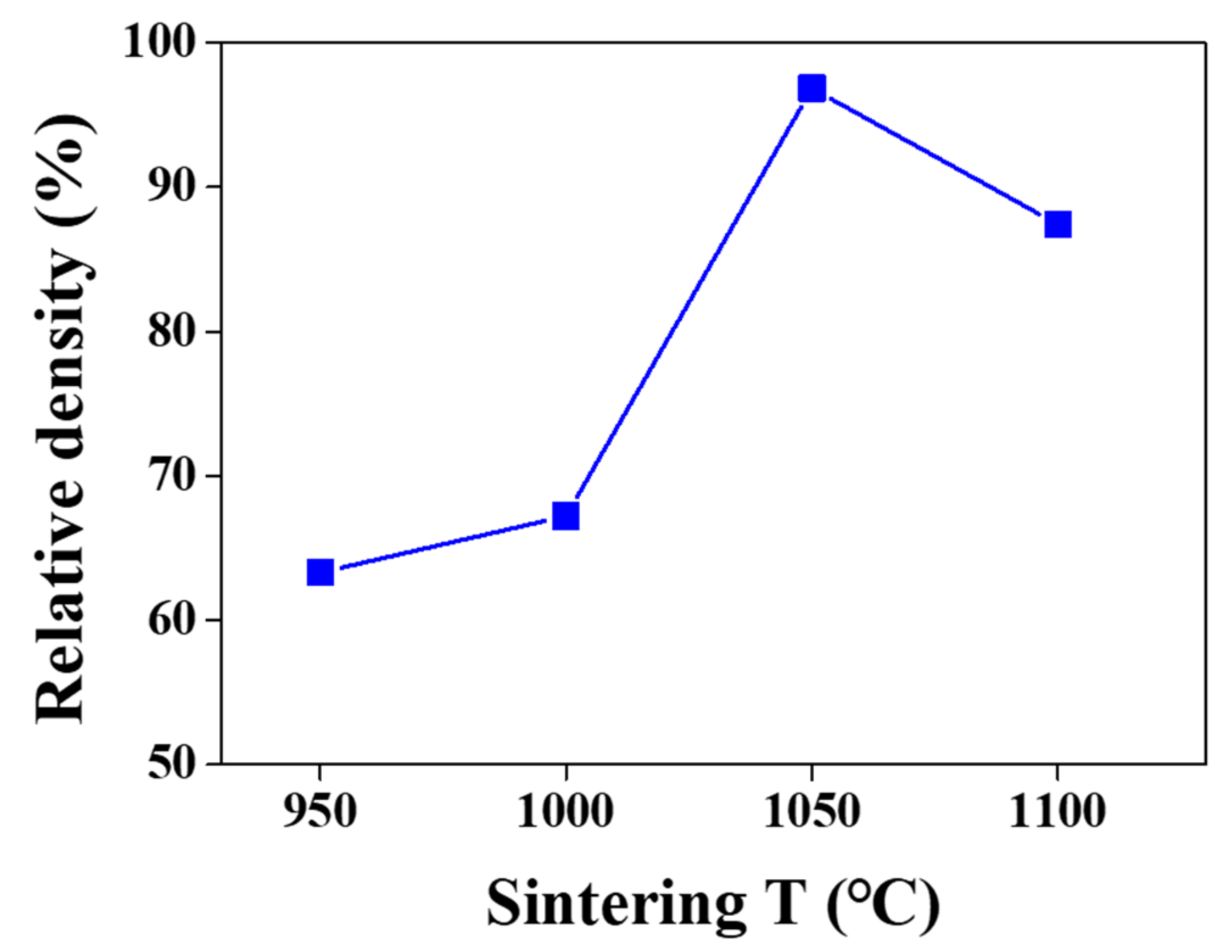
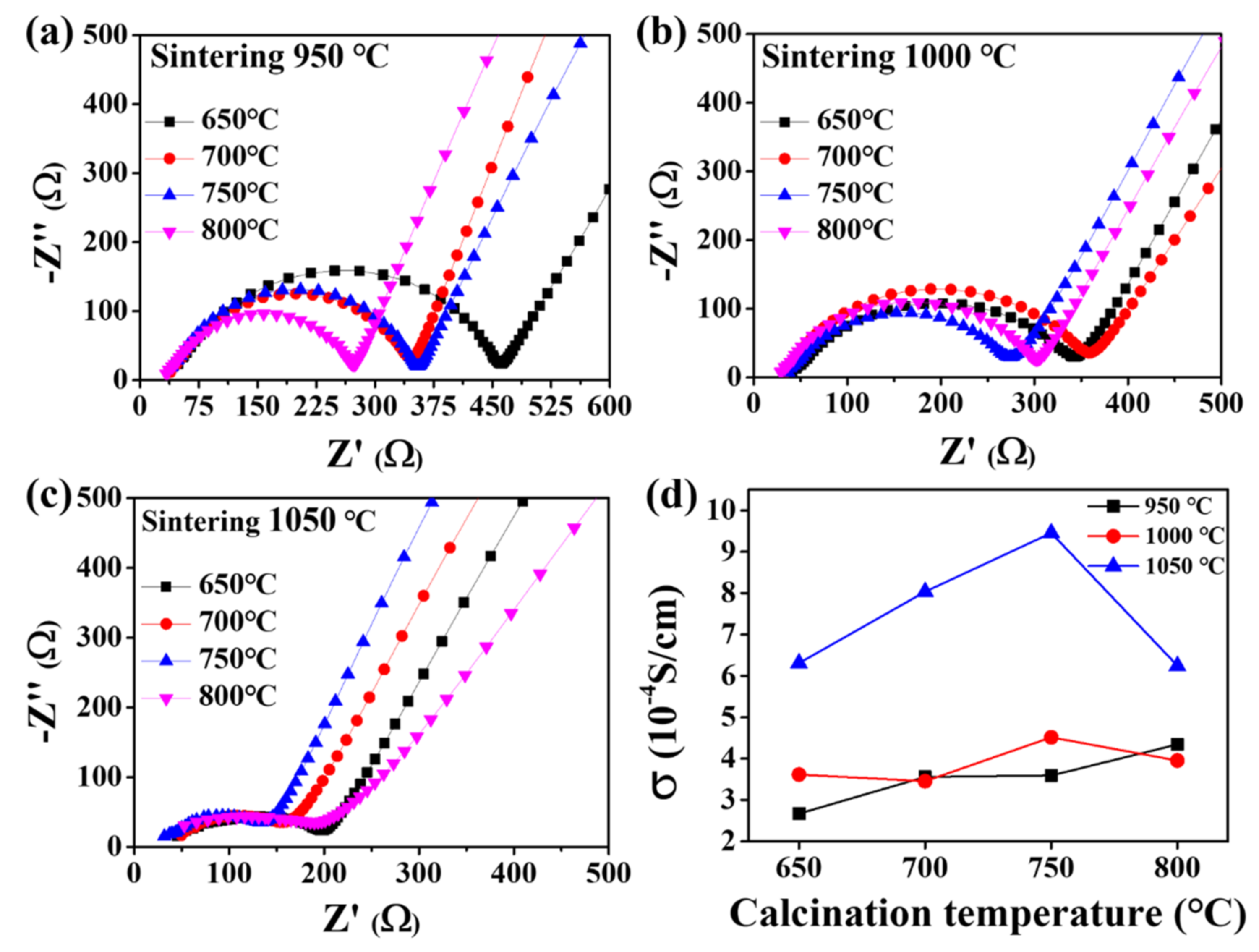
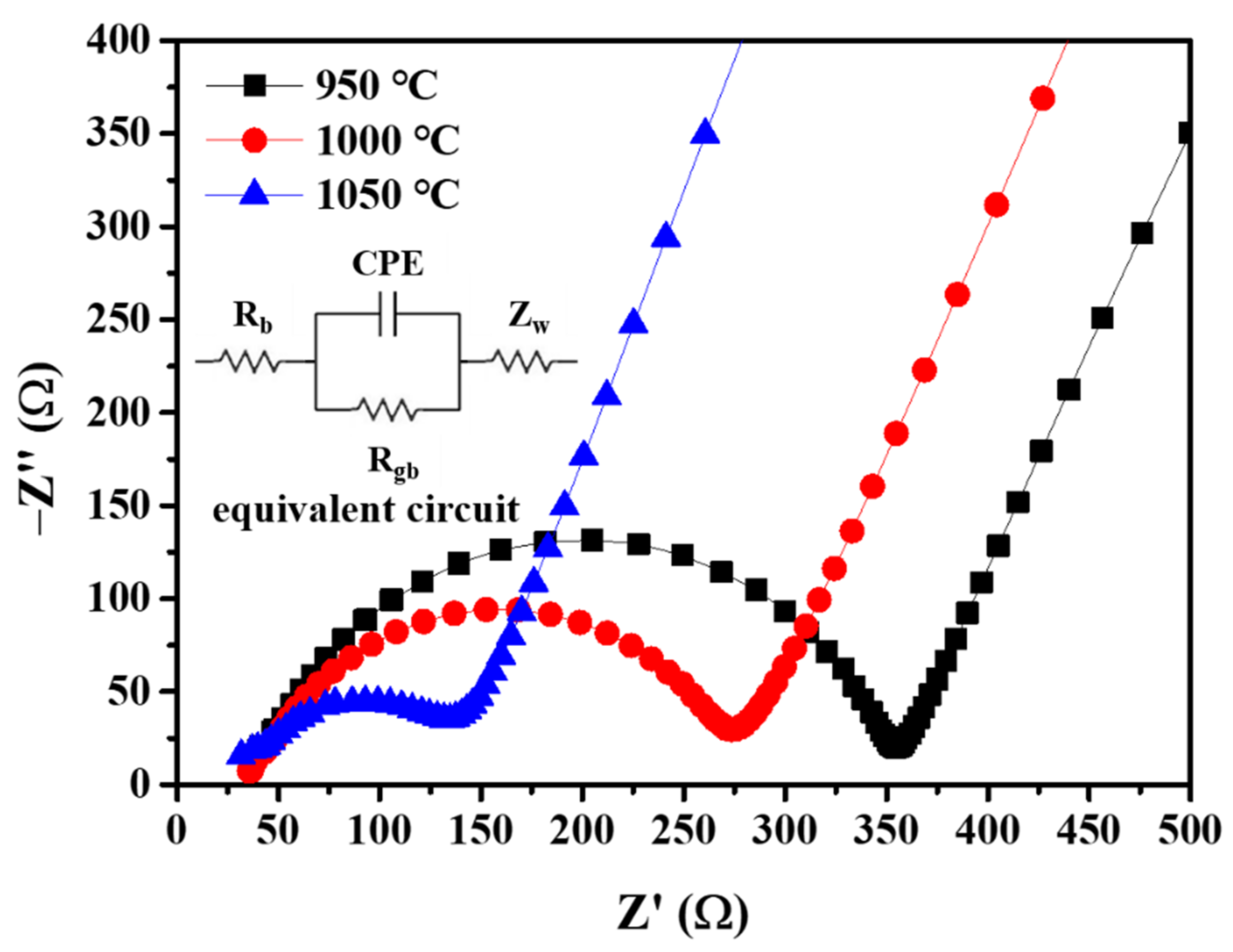
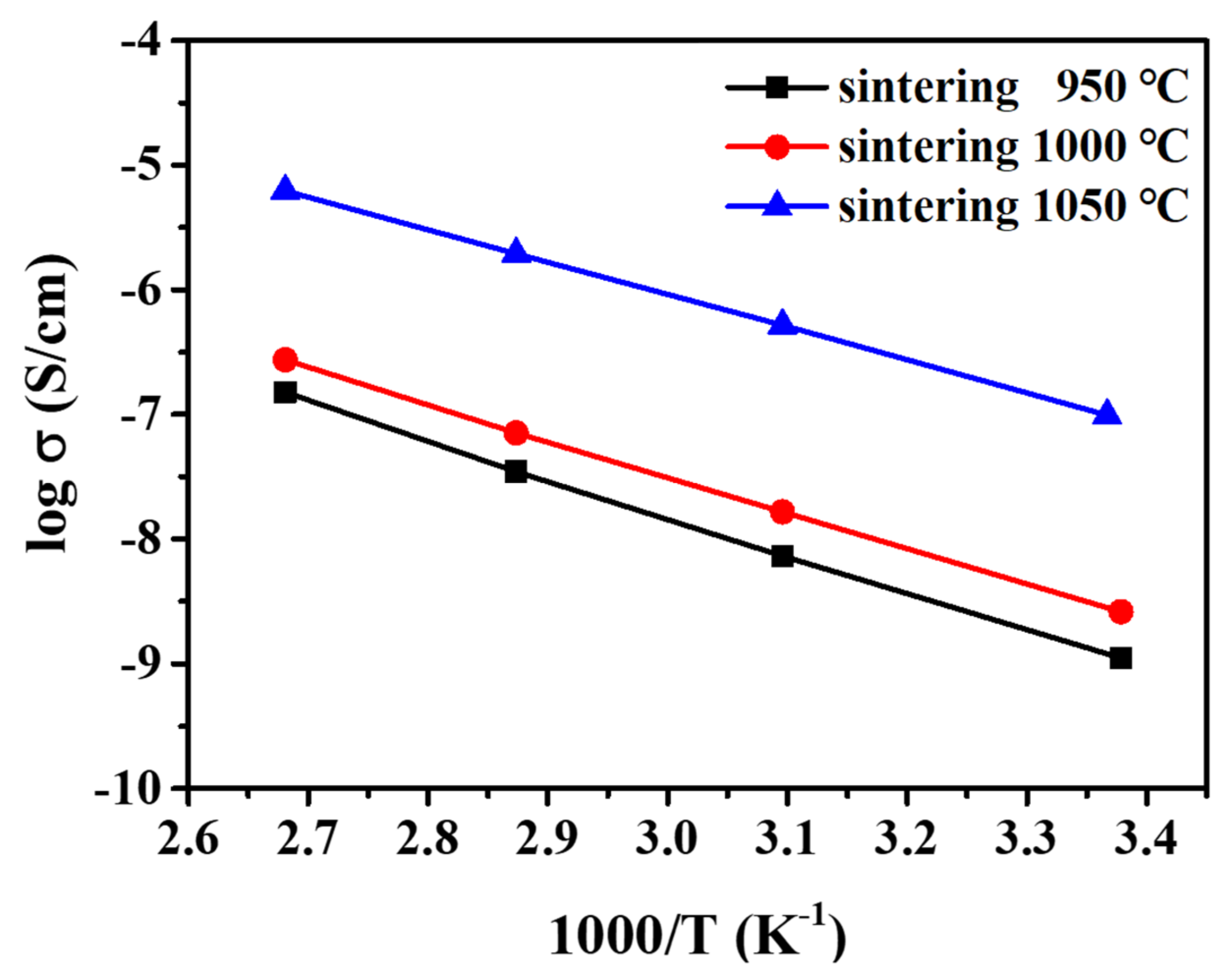
| Sintering T (°C) | Relative Density (%) |
|---|---|
| 950 | 63.30 |
| 1000 | 67.23 |
| 1050 | 96.84 |
| 1100 | 87.43 (fracture) |
| Sintering | 950 °C | 1000 °C | 1050 °C | ||||
|---|---|---|---|---|---|---|---|
| Calcination | t (cm) | σ (10−4 S cm−1) | t (cm) | σ (10−4 S cm−1) | t (cm) | σ (10−4 S cm−1) | |
| 650 °C | 0.097 | 2.670 | 0.097 | 3.615 | 0.098 | 6.390 | |
| 700 °C | 0.096 | 3.558 | 0.097 | 3.453 | 0.097 | 8.036 | |
| 750 °C | 0.098 | 3.591 | 0.100 | 4.514 | 0.100 | 9.455 | |
| 800 °C | 0.094 | 4.344 | 0.093 | 3.952 | 0.093 | 6.244 | |
| Sintering T (°C) | σb (mS cm−1) | σgb (mS cm−1) |
|---|---|---|
| 950 | 2.8967 | 0.3966 |
| 1000 | 3.2524 | 0.5350 |
| 1050 | 3.6651 | 1.2355 |
| Sintering T (°C) | Ea (σT) (eV) | σT (10−4 S cm-1) |
|---|---|---|
| 950 | 0.263 | 3.591 |
| 1000 | 0.249 | 4.514 |
| 1050 | 0.226 | 9.455 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.; Na, D.; Baek, J.; Kim, S.; Mamidi, S.; Lee, C.-R.; Seo, H.-K.; Seo, I. Synthesis of Superionic Conductive Li1+x+yAlxSiyTi2−xP3−yO12 Solid Electrolytes. Nanomaterials 2022, 12, 1158. https://doi.org/10.3390/nano12071158
Jeong H, Na D, Baek J, Kim S, Mamidi S, Lee C-R, Seo H-K, Seo I. Synthesis of Superionic Conductive Li1+x+yAlxSiyTi2−xP3−yO12 Solid Electrolytes. Nanomaterials. 2022; 12(7):1158. https://doi.org/10.3390/nano12071158
Chicago/Turabian StyleJeong, Hyeonwoo, Dan Na, Jiyeon Baek, Sanggil Kim, Suresh Mamidi, Cheul-Ro Lee, Hyung-Kee Seo, and Inseok Seo. 2022. "Synthesis of Superionic Conductive Li1+x+yAlxSiyTi2−xP3−yO12 Solid Electrolytes" Nanomaterials 12, no. 7: 1158. https://doi.org/10.3390/nano12071158
APA StyleJeong, H., Na, D., Baek, J., Kim, S., Mamidi, S., Lee, C.-R., Seo, H.-K., & Seo, I. (2022). Synthesis of Superionic Conductive Li1+x+yAlxSiyTi2−xP3−yO12 Solid Electrolytes. Nanomaterials, 12(7), 1158. https://doi.org/10.3390/nano12071158








