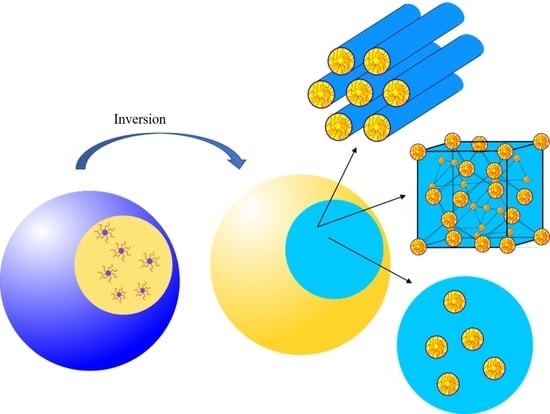
Inverse ISAsomes in Bio-Compatible Oils—Exploring Formulations in Squalane, Triolein and Olive Oil
Abstract
:1. Introduction
1.1. ISAsomes–Definition and Nomenclature
1.2. Stabilisation Schemes
1.3. Applications
1.4. Definition of Inverse ISAsomes and Research Goals
2. Materials and Methods
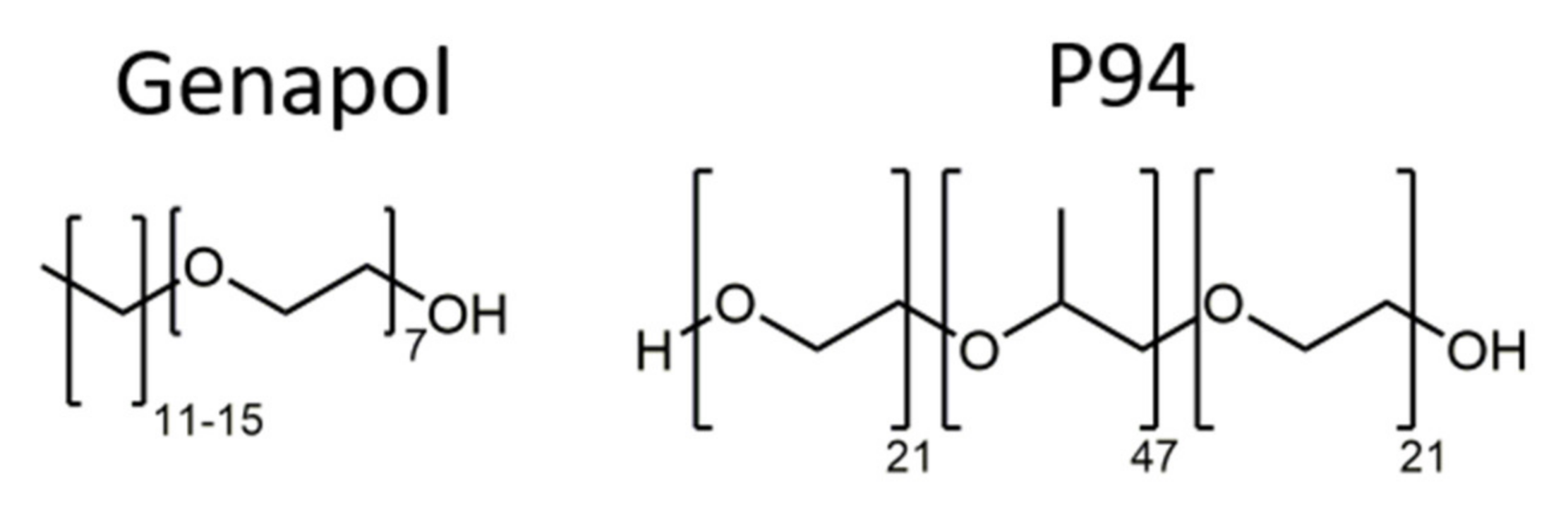
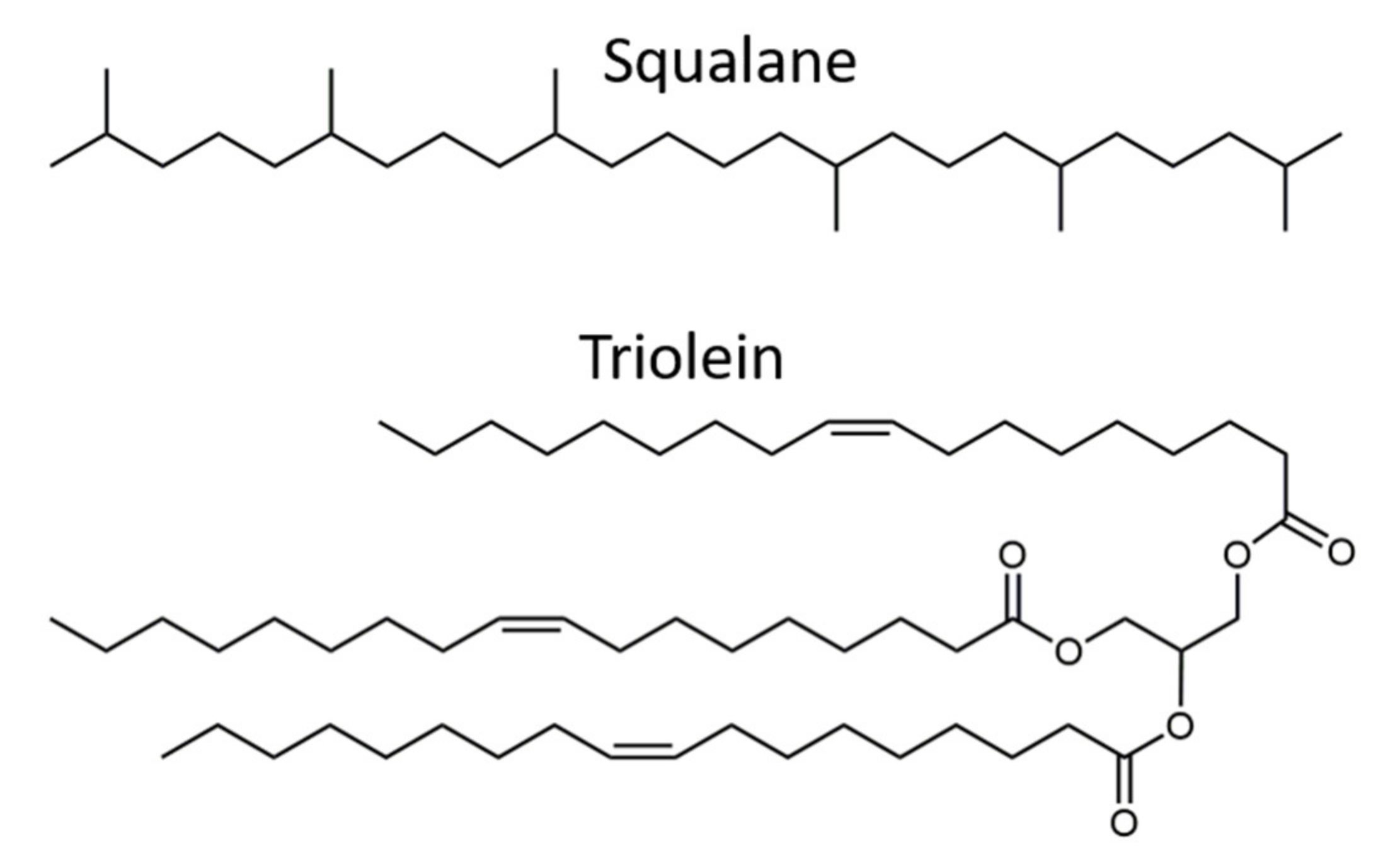
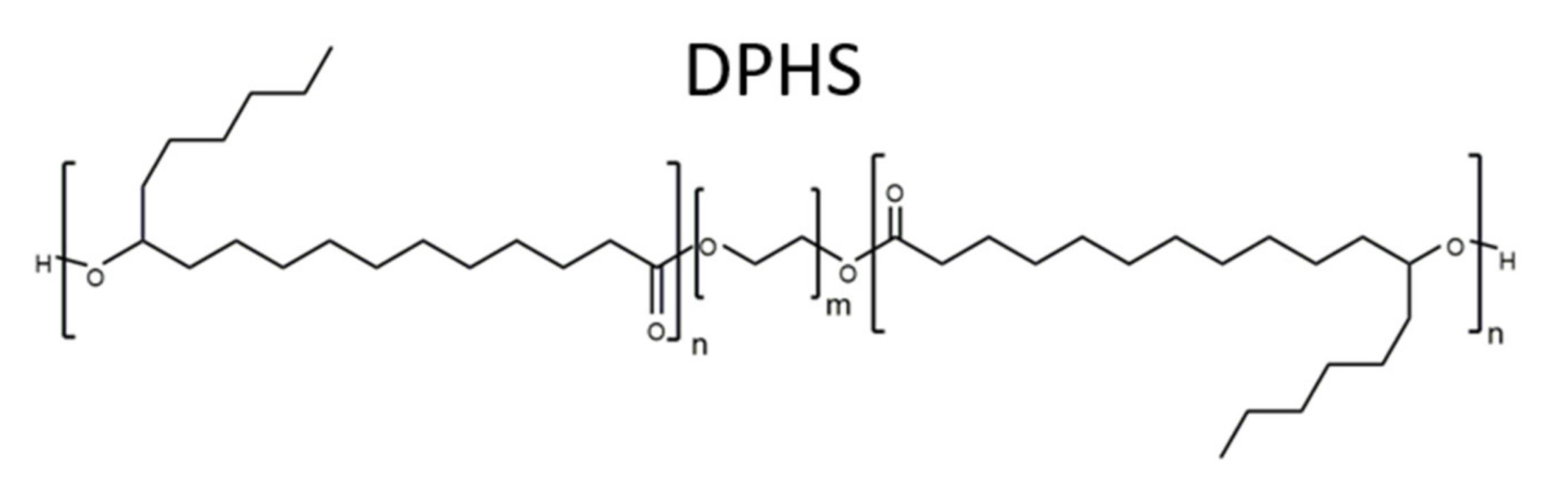
2.1. Materials
2.2. Preparation Methods
3. Results
3.1. Investigations on Bulk Samples
3.1.1. Genapol–Squalane System
3.1.2. P94/Triolein System
3.2. Dispersion and Stabilisation
3.2.1. Genapol/Squalane System
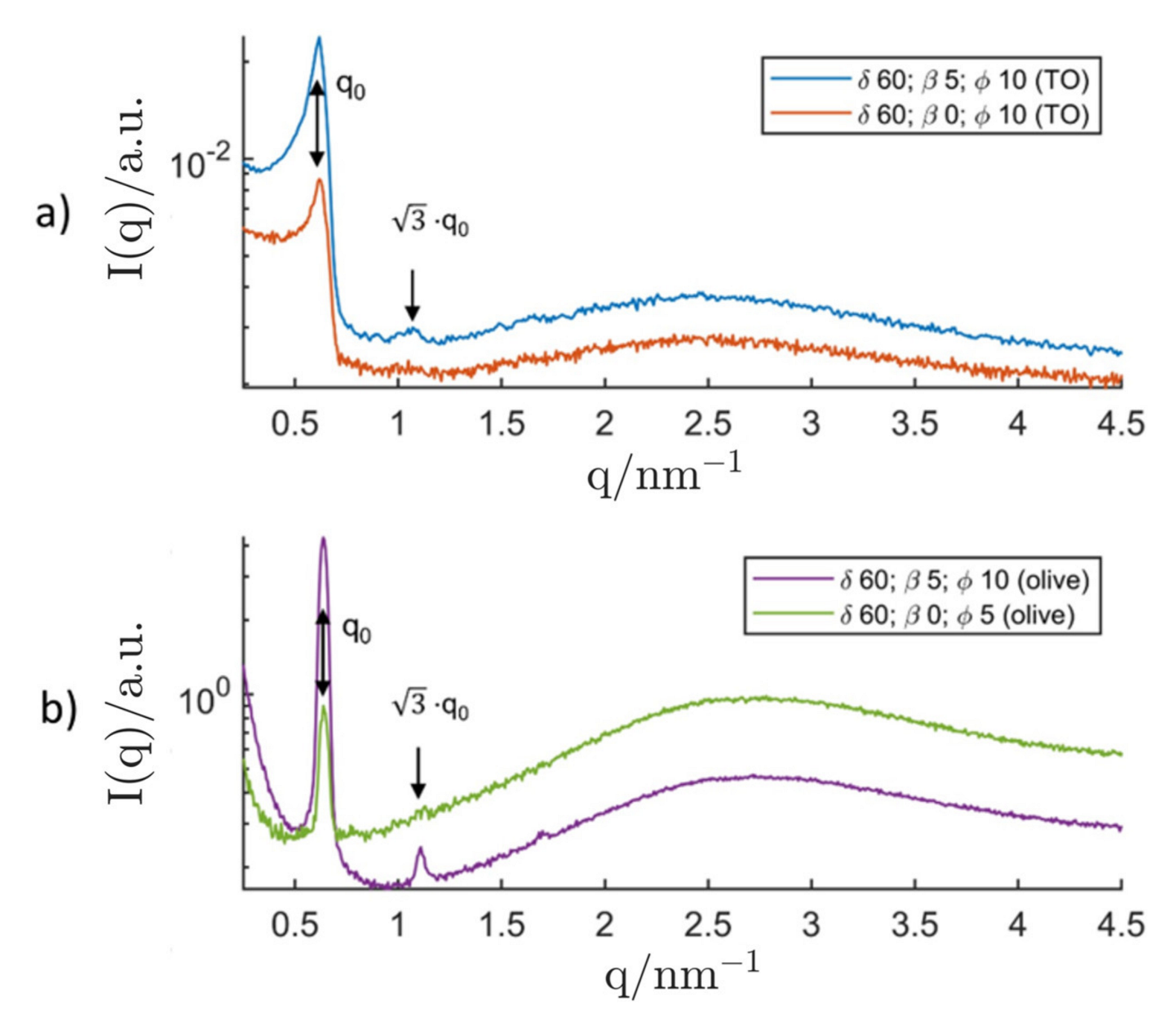
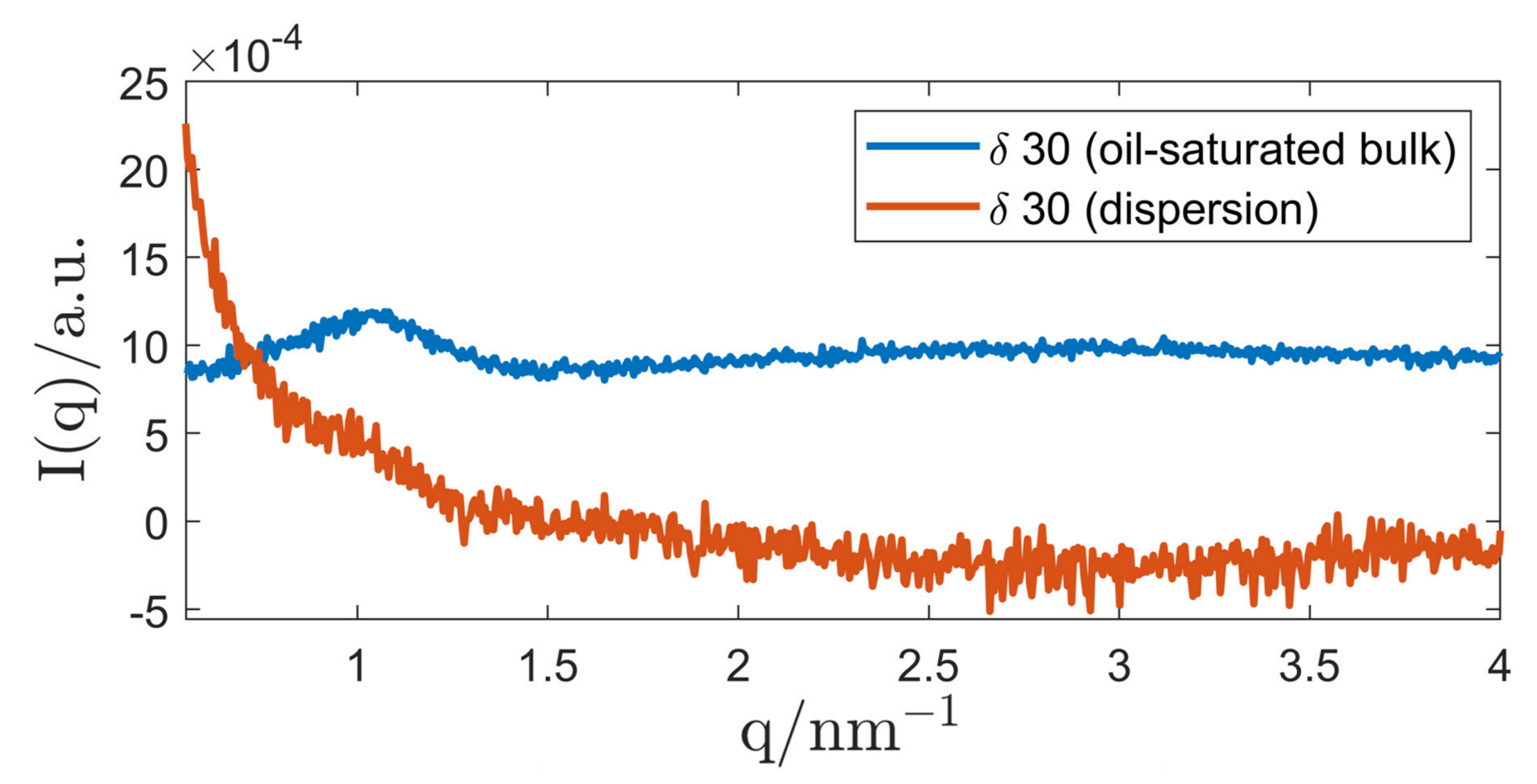
3.2.2. P94/Triolein System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huibers, P.D.T.; Lobanov, V.S.; Katritzky, A.R.; Shah, D.O.; Karelson, M. Prediction of Critical Micelle Concentration Using a Quantitative Structure–Property Relationship Approach. 1. Nonionic Surfactants. Langmuir 1996, 12, 1462–1470. [Google Scholar] [CrossRef]
- Swope, W.C.; Johnston, M.; Duff, A.I.; McDonagh, J.; Anderson, R.L.; Alva, G.; Tek, A.T.; Maschino, A.P. Challenge to Reconcile Experimental Micellar Properties of the CnEm Nonionic Surfactant Family. J. Phys. Chem. B 2019, 123, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakov, A.; Lee, M.-T.; Neimark, A.V. Prediction of the Critical Micelle Concentration of Nonionic Surfactants by Dissipative Particle Dynamics Simulations. J. Phys. Chem. Lett. 2013, 4, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.N. The energetics of micelle formation. Trans. Faraday Soc. 1955, 51, 561–569. [Google Scholar] [CrossRef]
- Larsson, K. On the structure of isotropic phases in lipid-water systems. Chem. Phys. Lipids 1972, 9, 181–195. [Google Scholar] [CrossRef]
- Dave, N.; Joshi, T. A Concise Review on Surfactants and Its Significance. Int. J. Appl. Chem. 2017, 13, 663–672. [Google Scholar]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchen, S.; Ahualli, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef]
- Shao, X.; Bor, G.; Al-Hosayni, S.; Salentinig, S.; Yaghmur, A. Structural characterization of self-assemblies of new omega-3 lipids: Docosahexaenoic acid and docosapentaenoic acid monoglycerides. Phys. Chem. Chem. Phys. 2018, 20, 23928–23941. [Google Scholar] [CrossRef]
- Pfrang, C.; Rastogi, K.; Cabrera-Martinez, E.R.; Seddon, A.; Dicko, C.; Labrador, A.; Plivelic, T.S.; Cowieson, N.; Squires, A.M. Complex three-dimensional self-assembly in proxies for atmospheric aerosols. Nat. Commun. 2017, 8, 1724. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Alexandridis, P.; Olsson, U.; Lindman, B. Self-Assembly of Amphiphilic Block Copolymers: The (EO)13(PO)30(EO)13-Water-p-Xylene System. Macromolecules 1995, 28, 7700–7710. [Google Scholar] [CrossRef]
- Khimani, M.; Patel, H.; Patel, V.I.; Parekh, P.; Vekariya, R.L. Self-assembly of stimuli-responsive block copolymers in aqueous solutions: An overview. Polym. Bull. 2020, 77, 5783–5810. [Google Scholar] [CrossRef]
- Coppola, L.; Oliviero, C.; Pogliani, L.; Ranieri, G.A.; Terenzi, M. A self-diffusion study in aqueous solution and lyotropic mesophases of amphiphilic block copolymers. Colloid Polym. Sci. 2000, 278, 434–442. [Google Scholar] [CrossRef]
- Wanka, G.; Hoffmann, H.; Ulbricht, W. Phase Diagrams and Aggregation Behavior of Poly(oxyethylene)-Poly(oxypropylene)-Poly(oxyethylene) Triblock Copolymers in Aqueous Solutions. Macromolecules 1994, 27, 4145–4159. [Google Scholar] [CrossRef]
- Zhai, J.; Fan, B.; Thang, S.; Drummond, C. Novel Amphiphilic Block Copolymers for the Formation of Stimuli-Responsive Non-Lamellar Lipid Nanoparticles. Molecules 2021, 26, 3648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Khan, A. Phase Behavior of Poly(Ethylene Oxide)-Poly (Propylene Oxide)-Poly (Ethylene Oxide) Triblock Copolymers in Water. Macromolecules 1995, 28, 3807–3812. [Google Scholar] [CrossRef]
- Tiwari, S.; Mall, C.; Prakash Solanki, P. Surfactant and Its Applications: A Review. Int. J. Eng. Res. Appl. 2018, 8, 61–66. [Google Scholar] [CrossRef]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Submicron Particles of Reversed Lipid Phases in Water Stabilized by a Nonionic Amphiphilic Polymer. Langmuir 1997, 13, 6964–6971. [Google Scholar] [CrossRef]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Cubic Lipid−Water Phase Dispersed into Submicron Particles. Langmuir 1996, 12, 4611–4613. [Google Scholar] [CrossRef]
- De Campo, L.; Yaghmur, A.; Sagalowicz, L.; Leser, M.E.; Watzke, H.; Glatter, O. Reversible Phase Transitions in Emulsified Nanostructured Lipid Systems. Langmuir 2004, 20, 5254–5261. [Google Scholar] [CrossRef]
- Larsson, K. Aqueous dispersions of cubic lipid–water phases. Curr. Opin. Colloid Interface Sci. 2000, 5, 64–69. [Google Scholar] [CrossRef]
- Abraham, T.; Hato, M.; Hirai, M. Glycolipid based cubic nanoparticles: Preparation and structural aspects. Colloids Surf. B Biointerfaces 2004, 35, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Mele, S.; Murgia, S.; Monduzzi, M. Monoolein based liquid crystals to form long-term stable emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2003, 228, 57–63. [Google Scholar] [CrossRef]
- Spicer, P.T.; Hayden, K.L.; Lynch, M.L.; Ofori-Boateng, A.; Burns, J.L. Novel Process for Producing Cubic Liquid Crystalline Nanoparticles (Cubosomes). Langmuir 2001, 17, 5748–5756. [Google Scholar] [CrossRef]
- Yaghmur, A.; de Campo, L.; Salentinig, S.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Oil-Loaded Monolinolein-Based Particles with Confined Inverse Discontinuous Cubic Structure (Fd3m). Langmuir 2005, 22, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Sureshkumar, A.; Badduri, N.; Jain, V. A Review on Lyotropic Liquid Crystals and Its Potential Applications. Nanosci. Nanotechnol. Asia 2021, 11, 50–63. [Google Scholar] [CrossRef]
- Monduzzi, M.; Ljusberg-Wahren, H.; Larsson, K. A 13C NMR Study of Aqueous Dispersions of Reversed Lipid Phases. Langmuir 2000, 16, 7355–7358. [Google Scholar] [CrossRef]
- Larsson, K. Cubic lipid-water phases: Structures and biomembrane aspects. J. Phys. Chem. 1989, 93, 7304–7314. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef] [Green Version]
- Yaghmur, A.; de Campo, L.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Emulsified Microemulsions and Oil-Containing Liquid Crystalline Phases. Langmuir 2004, 21, 569–577. [Google Scholar] [CrossRef]
- Kunieda, H.; Shigeta, K.; Nakamura, K.; Imae, T. Formation and structure of reverse vesicles. Trends Colloid Interface Sci. X 2007, 100, 1–5. [Google Scholar] [CrossRef]
- Weiss, T.M.; Narayanan, T.; Gradzielski, M. Dynamics of Spontaneous Vesicle Formation in Fluorocarbon and Hydrocarbon Surfactant Mixtures. Langmuir 2008, 24, 3759–3766. [Google Scholar] [CrossRef] [PubMed]
- Kunieda, H.; Akimaru, M.; Ushio, N.; Nakamura, K. Reverse Vesicles: Counter Structure of Biological Membranes. J. Colloid Interface Sci. 1993, 156, 446–453. [Google Scholar] [CrossRef]
- Fischer, S.; Exner, A.; Zielske, K.; Perlich, J.; Deloudi, S.; Steurer, W.; Lindner, P.; Förster, S. Colloidal quasicrystals with 12-fold and 18-fold diffraction symmetry. Proc. Natl. Acad. Sci. USA 2011, 108, 1810–1814. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, A.; Baez-Cotto, C.M.; Mann, T.J.; Mahanthappa, M.K. Dodecagonal quasicrystals of oil-swollen ionic surfactant micelles. Proc. Natl. Acad. Sci. USA 2021, 118, e2101598118. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.C. Calculation of HLB Values of Non-Ionic Surfactants. J. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- Griffin, W.C. Classification of Surface-Active Agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Guillot, S.; Salentinig, S.; Chemelli, A.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Influence of the Stabilizer Concentration on the Internal Liquid Crystalline Order and the Size of Oil-Loaded Monolinolein-Based Dispersions. Langmuir 2010, 26, 6222–6229. [Google Scholar] [CrossRef]
- Almgren, M.; Edwards, K.; Gustafsson, J. Cryotransmission electron microscopy of thin vitrified samples. Curr. Opin. Colloid Interface Sci. 1996, 1, 270–278. [Google Scholar] [CrossRef]
- Barauskas, J.; Johnsson, M.; Joabsson, F.; Tiberg, F. Cubic Phase Nanoparticles (Cubosome): Principles for Controlling Size, Structure, and Stability. Langmuir 2005, 21, 2569–2577. [Google Scholar] [CrossRef]
- Sagalowicz, L.; Michel, M.; Adrian, M.; Frossard, P.; Rouvet, M.; Watzke, H.J.; Yaghmur, A.; de Campo, L.; Glatter, O.; Leser, M.E. Crystallography of dispersed liquid crystalline phases studied by cryo-transmission electron microscopy. J. Microsc. 2006, 221, 110–121. [Google Scholar] [CrossRef]
- Chong, J.; Mulet, X.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. High-Throughput Discovery of Novel Steric Stabilizers for Cubic Lyotropic Liquid Crystal Nanoparticle Dispersions. Langmuir 2012, 28, 9223–9232. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.Y.T.; Mulet, X.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. Steric stabilisation of self-assembled cubic lyotropic liquid crystalline nanoparticles: High throughput evaluation of triblock polyethylene oxide-polypropylene oxide-polyethylene oxide copolymers. Soft Matter 2011, 7, 4768–4777. [Google Scholar] [CrossRef]
- Rodriguez, A.M.B.; Binks, B.P.; Sekine, T. Emulsion stabilisation by complexes of oppositely charged synthetic polyelectrolytes. Soft Matter 2018, 14, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Finkle, P.; Draper, H.D.; Hildebrand, J.H. The Theory of Emulsification1. J. Am. Chem. Soc. 1923, 45, 2780–2788. [Google Scholar] [CrossRef]
- Larson-Smith, K.; Jackson, A.; Pozzo, D.C. SANS and SAXS Analysis of Charged Nanoparticle Adsorption at Oil–Water Interfaces. Langmuir 2012, 28, 2493–2501. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.A.; Chevalier, Y. Effects of solid particle content on properties of o/w Pickering emulsions. J. Colloid Interface Sci. 2010, 351, 348–356. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Pickering Emulsions Stabilized by Monodisperse Latex Particles: Effects of Particle Size. Langmuir 2001, 17, 4540–4547. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Stability of oil-in-water emulsions stabilised by silica particles. Phys. Chem. Chem. Phys. 1999, 1, 3007–3016. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100, 503–546. [Google Scholar] [CrossRef]
- Ashby, N.P.; Binks, B.P. Pickering emulsions stabilised by Laponite clay particles. Phys. Chem. Chem. Phys. 2000, 2, 5640–5646. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Pirolt, F.; Iglesias, G.R.; Glatter, O. Lipid Transfer between Submicrometer Sized Pickering ISAsome Emulsions and the Influence of Added Hydrogel. Langmuir 2014, 30, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Sadeghpour, A.; Pirolt, F.; Glatter, O. Submicrometer-Sized Pickering Emulsions Stabilized by Silica Nanoparticles with Adsorbed Oleic Acid. Langmuir 2013, 29, 6004–6012. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Salonen, A.; Dulle, M.; Glatter, O. Salt-Induced Behavior of Internally Self-Assembled Nanodrops: Understanding Stabilization by Charged Colloids. In Trends in Colloid and Interface Science XXIV; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2011; pp. 27–31. [Google Scholar]
- Salonen, A.; Muller, F.; Glatter, O. Internally Self-Assembled Submicrometer Emulsions Stabilized by Spherical Nanocolloids: Finding the Free Nanoparticles in the Aqueous Continuous Phase. Langmuir 2010, 26, 7981–7987. [Google Scholar] [CrossRef]
- Muller, F.; Salonen, A.; Glatter, O. Phase behavior of Phytantriol/water bicontinuous cubic Pn3m cubosomes stabilized by Laponite disc-like particles. J. Colloid Interface Sci. 2010, 342, 392–398. [Google Scholar] [CrossRef]
- Muller, F.; Salonen, A.; Glatter, O. Monoglyceride-based cubosomes stabilized by Laponite: Separating the effects of stabilizer, pH and temperature. Colloids Surf. A Physicochem. Eng. Asp. 2010, 358, 50–56. [Google Scholar] [CrossRef]
- Salonen, A.; Muller, F.; Glatter, O. Dispersions of Internally Liquid Crystalline Systems Stabilized by Charged Disklike Particles as Pickering Emulsions: Basic Properties and Time-Resolved Behavior. Langmuir 2008, 24, 5306–5314. [Google Scholar] [CrossRef]
- Sarkar, A.; Dickinson, E. Sustainable food-grade Pickering emulsions stabilized by plant-based particles. Curr. Opin. Colloid Interface Sci. 2020, 49, 69–81. [Google Scholar] [CrossRef]
- Binks, B.P.; Muijlwijk, K.; Koman, H.; Poortinga, A.T. Food-grade Pickering stabilisation of foams by in situ hydrophobisation of calcium carbonate particles. Food Hydrocoll. 2017, 63, 585–592. [Google Scholar] [CrossRef]
- Dickinson, E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll. 2017, 68, 219–231. [Google Scholar] [CrossRef]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends Food Sci. Technol. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Linke, C.; Drusch, S. Pickering emulsions in foods—Opportunities and limitations. Crit. Rev. Food Sci. Nutr. 2018, 58, 1971–1985. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, Y.; Huang, Q. Recent advances on food-grade particles stabilized Pickering emulsions: Fabrication, characterization and research trends. Trends Food Sci. Technol. 2016, 55, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Food-grade particles for emulsion stabilization. Trends Food Sci. Technol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Jiao, J.; Burgess, D.J. Ostwald ripening of water-in-hydrocarbon emulsions. J. Colloid Interface Sci. 2003, 264, 509–516. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Hussein, H.; Masliyah, J.H. Water-in-Hydrocarbon Emulsions Stabilized by Asphaltenes at Low Concentrations. J. Colloid Interface Sci. 2000, 228, 52–63. [Google Scholar] [CrossRef]
- Neto, D.M.C.; Sad, C.C.C.; Silva, M.; Santos, F.D.; Pereira, L.B.; Corona, R.R.; Silva, S.R.C.; Bassane, J.F.P.; Castro, E.V.R.; Lacerda, V., Jr.; et al. Rheological study of the behaviour of water-in-oil emulsions of heavy oils. J. Pet. Sci. 2019, 173, 1323–1331. [Google Scholar] [CrossRef]
- Fingas, M.F. Water-in-Oil Emulsions: Formation and Prediction. J. Pet. Sci. Res. 2014, 3, 38. [Google Scholar] [CrossRef]
- Fingas, M.; Fieldhouse, B. Studies of the formation process of water-in-oil emulsions. Mar. Pollut. Bull. 2003, 47, 369–396. [Google Scholar] [CrossRef]
- Ushikubo, F.; Cunha, R. Stability mechanisms of liquid water-in-oil emulsions. Food Hydrocoll. 2014, 34, 145–153. [Google Scholar] [CrossRef]
- Flak, D.K.; Adamski, V.; Nowaczyk, G.; Szutkowski, K.; Synowitz, M.; Jurga, S.; Held-Feindt, J. AT101-Loaded Cubosomes as an Alternative for Improved Glioblastoma Therapy. Int. J. Nanomed. 2020, 15, 7415–7431. [Google Scholar] [CrossRef] [PubMed]
- Mahant, S.; Rao, R.; Souto, E.B.; Nanda, S. Analytical tools and evaluation strategies for nanostructured lipid carrier-based topical delivery systems. Expert Opin. Drug Deliv. 2020, 17, 963–992. [Google Scholar] [CrossRef]
- Faria, A.R.; Silvestre, O.; Maibohm, C.; Adão, R.; Silva, B.; Nieder, J.B. Cubosome nanoparticles for enhanced delivery of mitochondria anticancer drug elesclomol and therapeutic monitoring via sub-cellular NAD(P)H multi-photon fluorescence lifetime imaging. Nano Res. 2018, 12, 991–998. [Google Scholar] [CrossRef]
- Zhai, J.; Tan, F.H.; Luwor, R.B.; Srinivasa Reddy, T.; Ahmed, N.; Drummond, C.J.; Tran, N. In Vitro and In Vivo Toxicity and Biodistribution of Paclitaxel-Loaded Cubosomes as a Drug Delivery Nanocarrier: A Case Study Using an A431 Skin Cancer Xenograft Model. ACS Appl. Bio Mater. 2020, 3, 4198–4207. [Google Scholar] [CrossRef]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef]
- Bor, G.; Azmi, I.D.M.; Yaghmur, A. Nanomedicines for cancer therapy: Current status, challenges and future prospects. Ther. Deliv. 2019, 10, 113–132. [Google Scholar] [CrossRef]
- Prajapati, R.; Larsen, S.W.; Yaghmur, A. Citrem—Phosphatidylcholine nano-self-assemblies: Solubilization of bupivacaine and its role in triggering a colloidal transition from vesicles to cubosomes and hexosomes. Phys. Chem. Chem. Phys. 2019, 21, 15142–15150. [Google Scholar] [CrossRef]
- Azmi, I.D.M.; Østergaard, J.; Stürup, S.; Gammelgaard, B.; Urtti, A.; Moghimi, S.M.; Yaghmur, A. Cisplatin Encapsulation Generates Morphologically Different Multicompartments in the Internal Nanostructures of Nonlamellar Liquid-Crystalline Self-Assemblies. Langmuir 2018, 34, 6570–6581. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajik-Ahmadabad, B.; Chollet, L.; White, J.; Separovic, F.; Polyzos, A. Metallo-Cubosomes: Zinc-Functionalized Cubic Nanoparticles for Therapeutic Nucleotide Delivery. Mol. Pharm. 2019, 16, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Azmi, I.D.; Moghimi, S.M.; Yaghmur, A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [Green Version]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef] [Green Version]
- Zabara, M.; Senturk, B.; Gontsarik, M.; Ren, Q.; Rottmar, M.; Maniura, K.; Mezzenga, R.; Bolisetty, S.; Salentinig, S. Multifunctional Nano-Biointerfaces: Cytocompatible Antimicrobial Nanocarriers from Stabilizer-Free Cubosomes. Adv. Funct. Mater. 2019, 29, 1904007. [Google Scholar] [CrossRef]
- Chemelli, A.; Conde-Valentín, B.; Uhlig, F.; Glatter, O. Amino Acid Induced Modification of Self-Assembled Monoglyceride-Based Nanostructures. Langmuir 2015, 31, 10377–10381. [Google Scholar] [CrossRef]
- Chemelli, A.; Maurer, M.; Geier, R.; Glatter, O. Optimized Loading and Sustained Release of Hydrophilic Proteins from Internally Nanostructured Particles. Langmuir 2012, 28, 16788–16797. [Google Scholar] [CrossRef]
- Mehata, A.K.; Dehari, D.; Gupta, A.; Rabin, D.C.; Miya, A. Multifunctional Liquid Crystal Nanoparticles for Cancer Therapy. Curr. Nanomater. 2021, 6, 4–16. [Google Scholar] [CrossRef]
- Mohammad, Y.; Prentice, R.N.; Boyd, B.J.; Rizwan, S.B. Comparison of cubosomes and hexosomes for the delivery of phenytoin to the brain. J. Colloid Interface Sci. 2021, 605, 146–154. [Google Scholar] [CrossRef]
- Millart, E.; Lesieur, S.; Faivre, V. Superparamagnetic lipid-based hybrid nanosystems for drug delivery. Expert Opin. Drug Deliv. 2018, 15, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.D.; Maurya, A.K.; Fortunato, G.; Rottmar, M.; Zboray, R.; Erni, R.; Dommann, A.; Rossi, R.M.; Neels, A.; Sadeghpour, A. Responsive Nanofibers with Embedded Hierarchical Lipid Self-Assemblies. Langmuir 2020, 36, 11787–11797. [Google Scholar] [CrossRef] [PubMed]
- Gontsarik, M.; Yaghmur, A.; Ren, Q.; Maniura-Weber, K.; Salentinig, S. From Structure to Function: pH-Switchable Antimicrobial Nano-Self-Assemblies. ACS Appl. Mater. Interfaces 2019, 11, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Gontsarik, M.; Mohammadtaheri, M.; Yaghmur, A.; Salentinig, S. pH-Triggered nanostructural transformations in antimicrobial peptide/oleic acid self-assemblies. Biomater. Sci. 2018, 6, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A. pH Responsiveness of Hexosomes and Cubosomes for Combined Delivery of Brucea javanica Oil and Doxorubicin. Langmuir 2019, 35, 14532–14542. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.; Gontsarik, M.; Yaghmur, A.; Salentinig, S. pH-Responsive Nano-Self-Assemblies of the Anticancer Drug 2-Hydroxyoleic Acid. Langmuir 2019, 35, 7954–7961. [Google Scholar] [CrossRef] [Green Version]
- Mathews, P.D.; Mertins, O.; Angelov, B.; Angelova, A. Cubosomal lipid nanoassemblies with pH-sensitive shells created by biopolymer complexes: A synchrotron SAXS study. J. Colloid Interface Sci. 2021, 607, 440–450. [Google Scholar] [CrossRef]
- Mertins, O.; Mathews, P.D.; Angelova, A. Advances in the Design of pH-Sensitive Cubosome Liquid Crystalline Nanocarriers for Drug Delivery Applications. Nanomaterials 2020, 10, 963. [Google Scholar] [CrossRef]
- Boyd, B.J. Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int. J. Pharm. 2003, 260, 239–247. [Google Scholar] [CrossRef]
- Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers. Pharmaceutics 2020, 12, 587. [Google Scholar] [CrossRef]
- Tomšič, M.; Guillot, S.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Internally Self-Assembled Thermoreversible Gelling Emulsions: ISAsomes in Methylcellulose, κ-Carrageenan, and Mixed Hydrogels. Langmuir 2009, 25, 9525–9534. [Google Scholar] [CrossRef] [PubMed]
- Caselli, L.; Mendozza, M.; Muzzi, B.; Toti, A.; Montis, C.; Mello, T.; Mannelli, L.D.C.; Ghelardini, C.; Sangregorio, C.; Berti, D. Lipid Cubic Mesophases Combined with Superparamagnetic Iron Oxide Nanoparticles: A Hybrid Multifunctional Platform with Tunable Magnetic Properties for Nanomedical Applications. Int. J. Mol. Sci. 2021, 22, 9268. [Google Scholar] [CrossRef] [PubMed]
- Salentinig, S.; Sagalowicz, L.; Leser, M.E.; Tedeschi, C.; Glatter, O. Transitions in the internal structure of lipid droplets during fat digestion. Soft Matter 2010, 7, 650–661. [Google Scholar] [CrossRef]
- Salentinig, S.; Amenitsch, H.; Yaghmur, A. In Situ Monitoring of Nanostructure Formation during the Digestion of Mayonnaise. ACS Omega 2017, 2, 1441–1446. [Google Scholar] [CrossRef] [Green Version]
- Salentinig, S. Supramolecular structures in lipid digestion and implications for functional food delivery. Curr. Opin. Colloid Interface Sci. 2019, 39, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Salentinig, S.; Phan, S.; Hawley, A.; Boyd, B.J. Self-Assembly Structure Formation during the Digestion of Human Breast Milk. Angew. Chem. Int. Ed. 2015, 54, 1600–1603. [Google Scholar] [CrossRef]
- Mezzenga, R.; Schurtenberger, P.; Burbidge, A.; Michel, M. Understanding foods as soft materials. Nat. Mater. 2005, 4, 729–740. [Google Scholar] [CrossRef]
- Krog, N. Emulsifiers and Emulsions in Dairy Foods; Elsevier: Amsterdam, The Netherlands, 2002; pp. 891–900. [Google Scholar]
- Larsson, K. Lyotropic liquid crystals and their dispersions relevant in foods. Curr. Opin. Colloid Interface Sci. 2009, 14, 16–20. [Google Scholar] [CrossRef]
- Ubbink, J.; Mezzenga, R. Delivery of functionality in complex food systems: Introduction. Trends Food Sci. Technol. 2006, 17, 194–195. [Google Scholar] [CrossRef]
- Yaghmur, A. Nanoencapsulation of food ingredients by cubosomes and hexosomes. In Lipid-Based Nanostructures for Food Encapsulation Purposes; Academic Press: Cambridge, MA, USA, 2019; pp. 483–522. [Google Scholar] [CrossRef]
- Garti, N.; Clement, V.; Fanun, M.; Leser, M.E. Some Characteristics of Sugar Ester Nonionic Microemulsions in View of Possible Food Applications. J. Agric. Food Chem. 2000, 48, 3945–3956. [Google Scholar] [CrossRef]
- Fornasier, M.; Pireddu, R.; Del Giudice, A.; Sinico, C.; Nylander, T.; Schillén, K.; Galantini, L.; Murgia, S. Tuning lipid structure by bile salts: Hexosomes for topical administration of catechin. Colloids Surf. B Biointerfaces 2021, 199, 111564. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.; Espinosa, Y.G.; Norton, I.T. Encapsulation systems for the delivery of hydrophilic nutraceuticals: Food application. Biotechnol. Adv. 2017, 35, 450–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghpour, M.R.A. Lyotropic Liquid Crystalline Phases for the Formulation of Future Functional Foods. J. Nutr. Health Food Eng. 2016, 5, 1–5. [Google Scholar] [CrossRef]
- Cortesi, R.; Cappellozza, E.; Drechsler, M.; Contado, C.; Baldisserotto, A.; Mariani, P.; Carducci, F.; Pecorelli, A.; Esposito, E.; Valacchi, G. Monoolein aqueous dispersions as a delivery system for quercetin. Biomed. Microdevices 2017, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki, M.D.; Mitsou, E.; Yaghmur, A.; Xenakis, A.; Papadimitriou, V. Formulation and characterization of food-grade microemulsions as carriers of natural phenolic antioxidants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 130–136. [Google Scholar] [CrossRef]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of different nanocarriers for encapsulation of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 3468–3497. [Google Scholar] [CrossRef]
- Serieye, S.; Méducin, F.; Tidu, A.; Guillot, S. Incorporation of aromas in nanostructured monolinolein-based miniemulsions: A structural investigation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 802–808. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Beasley, D.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R. The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome. PLoS ONE 2015, 10, e0134116. [Google Scholar] [CrossRef]
- Glatter, O.; Glatter, I. Water-in-Oil Emulsions and Methods for Their Preparation. European Patent EP 2604253 2011, 9 November 2016. [Google Scholar]
- Kulkarni, C.V.; Mezzenga, R.; Glatter, O. Water-in-oil nanostructured emulsions: Towards the structural hierarchy of liquid crystalline materials. Soft Matter 2010, 6, 5615–5624. [Google Scholar] [CrossRef]
- Sreedharan, R.; Chidambaram, K. Drug/Vehicle Impacts and Formulation Centered Stratagems for Enhanced Transdermal Drug Permeation, Controlled Release and Safety: Unparalleled Past and Recent Innovations-An Overview. Curr. Drug Ther. 2019, 14, 192–209. [Google Scholar] [CrossRef]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Moitzi, C.; Guillot, S.; Fritz, G.; Salentinig, S.; Glatter, O. Phase Reorganization in Self-Assembled Systems through Interparticle Material Transfer. Adv. Mater. 2007, 19, 1352–1358. [Google Scholar] [CrossRef]
- Salonen, A.; Moitzi, C.; Salentinig, S.; Glatter, O. Material Transfer in Cubosome–Emulsion Mixtures: Effect of Alkane Chain Length. Langmuir 2010, 26, 10670–10676. [Google Scholar] [CrossRef] [PubMed]
- Pirolt, F.; Glatter, O.; Trimmel, G. Reverse Hexosome Dispersions in Alkanes—The Challenge of Inverting Structures. Langmuir 2018, 34, 8379–8387. [Google Scholar] [CrossRef]
- Hassan, T.; Mäder, K.; Maeder, K. Novel semisolid SNEDDS based on PEG-30-di-(polyhydroxystearate): Progesterone incorporation and in vitro digestion. Int. J. Pharm. 2015, 486, 77–87. [Google Scholar] [CrossRef]
- Milinkovic, J.; Petrovic, L.; Fraj, J.; Bučko, S.; Katona, J. Characteristics of W/O emulsions containing polymeric emulsifier PEG 30-dipolyhydroxystearate. Acta Period. Technol. 2016, 47, 219–230. [Google Scholar] [CrossRef]
- Koppel, D.E. Analysis of Macromolecular Polydispersity in Intensity Correlation Spectroscopy: The Method of Cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Alam, M.M.; Aramaki, K. Hexagonal Phase Based Gel-Emulsion (O/H1Gel-Emulsion): Formation and Rheology. Langmuir 2008, 24, 12253–12259. [Google Scholar] [CrossRef]
- Mariani, P.; Luzzati, V.; Delacroix, H. Cubic phases of lipid-containing systems: Structure analysis and biological implications. J. Mol. Biol. 1988, 204, 165–189. [Google Scholar] [CrossRef]
- Papadimitriou, V.; Dulle, M.; Wachter, W.; Sotiroudis, T.G.; Glatter, O.; Xenakis, A. Structure and Dynamics of Veiled Virgin Olive Oil: Influence of Production Conditions and Relation to its Antioxidant Capacity. Food Biophys. 2013, 8, 112–121. [Google Scholar] [CrossRef]
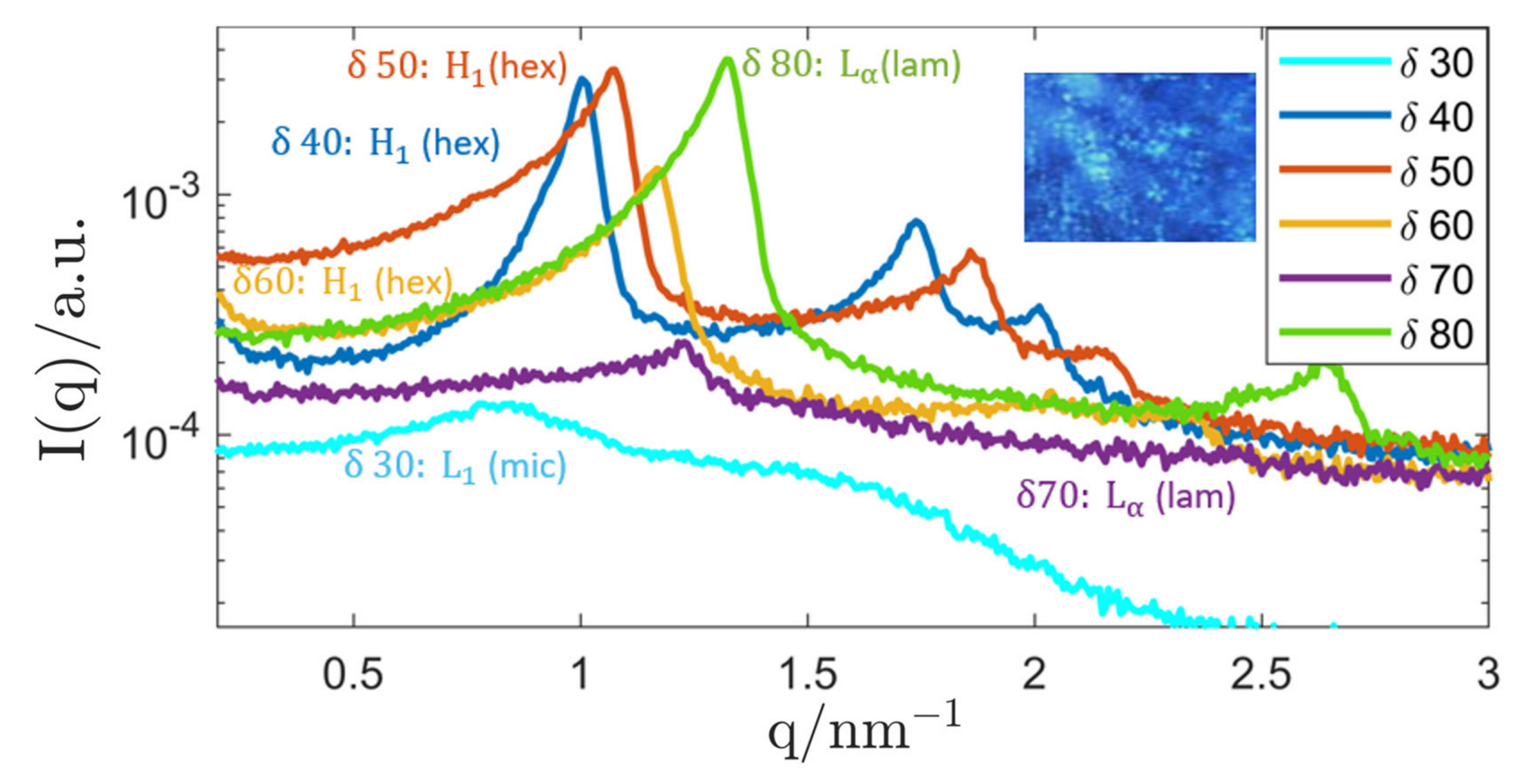
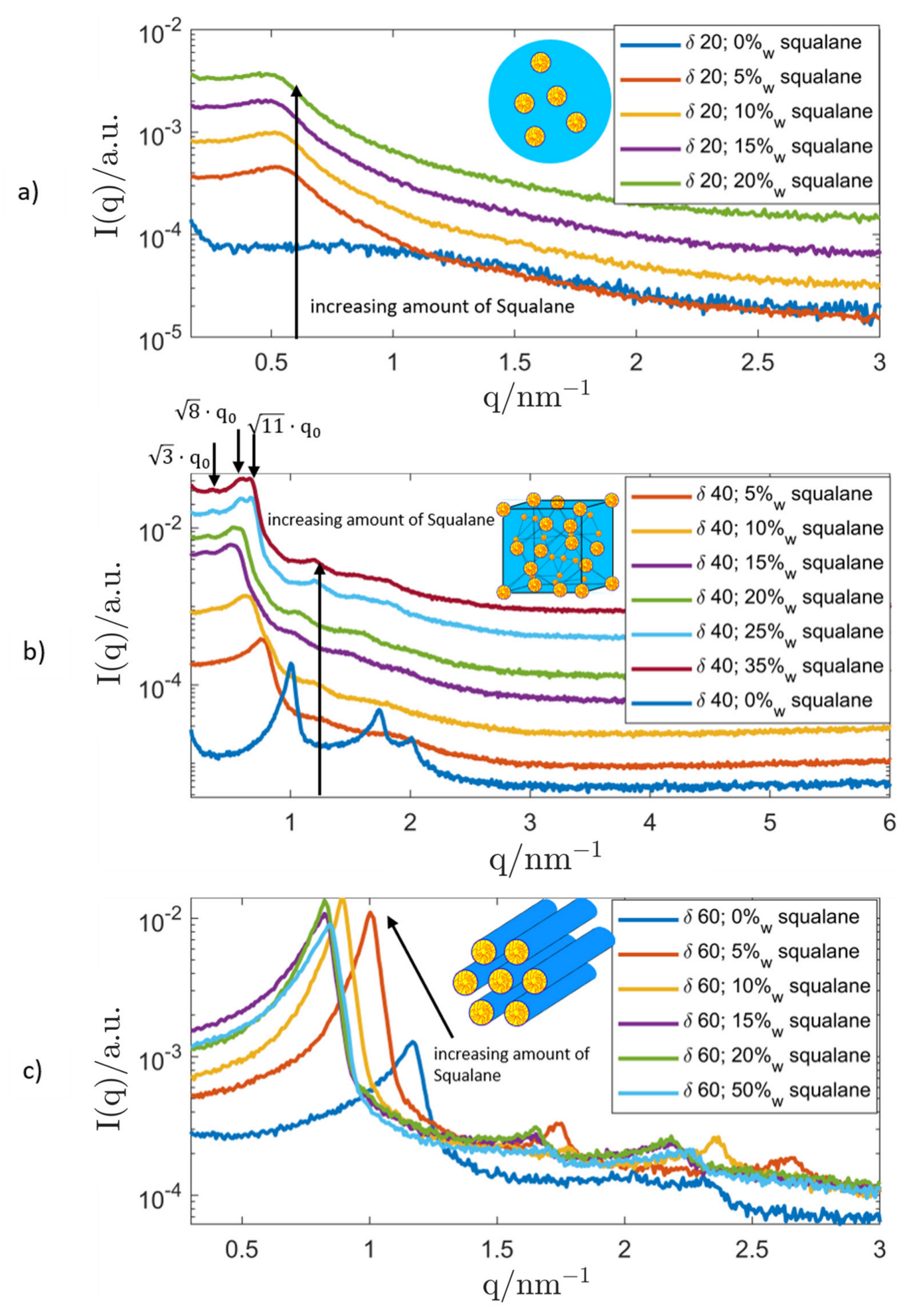
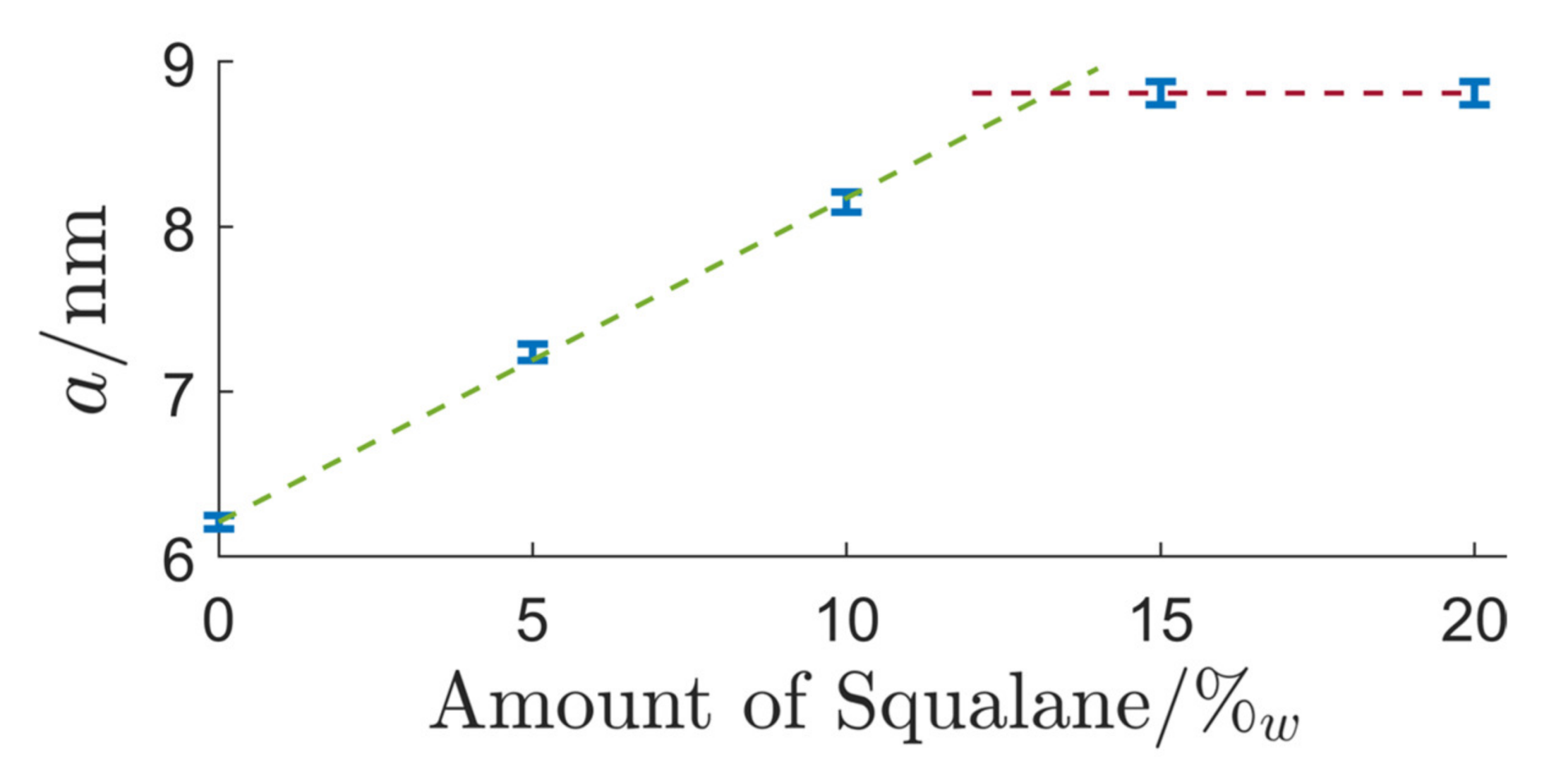
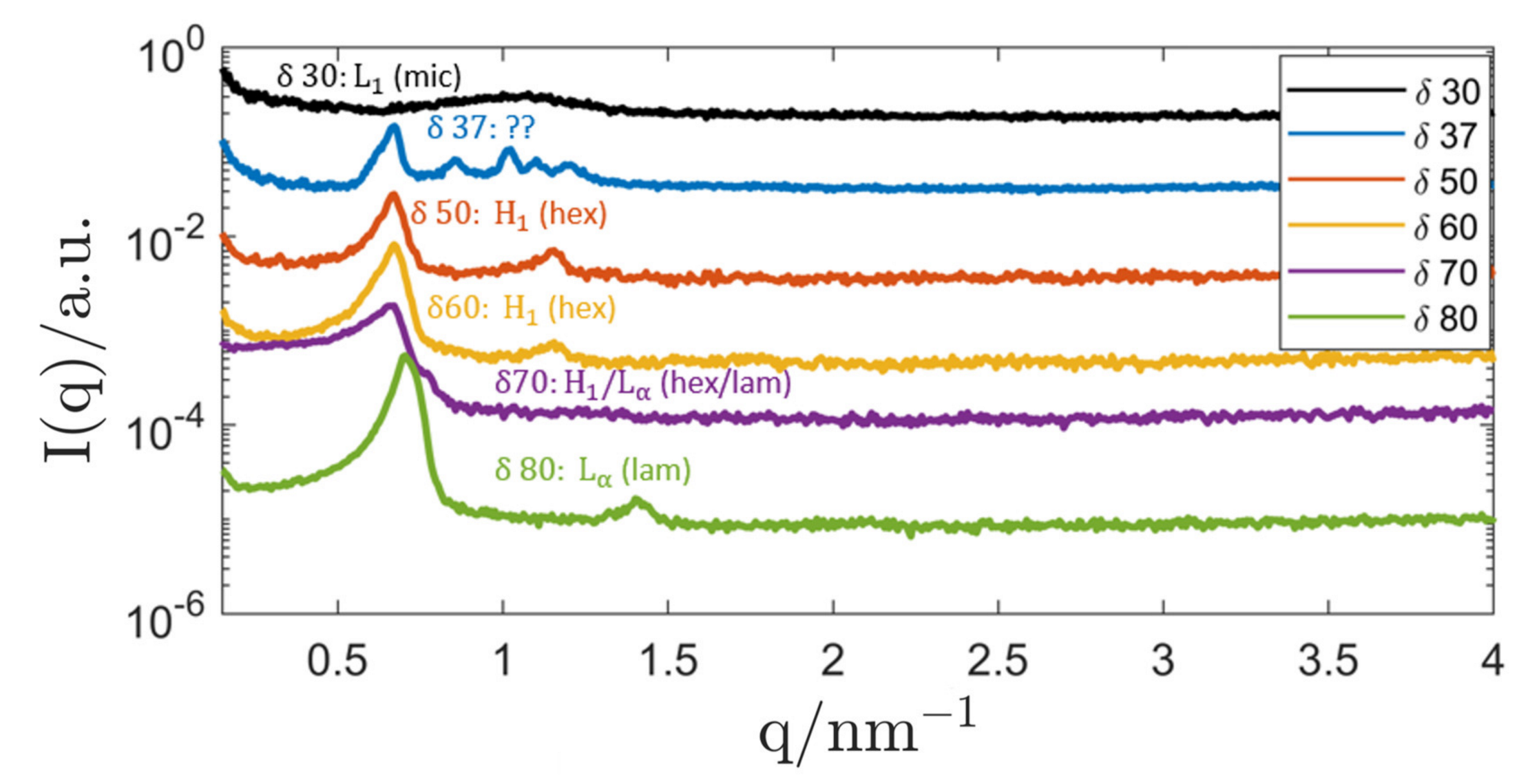
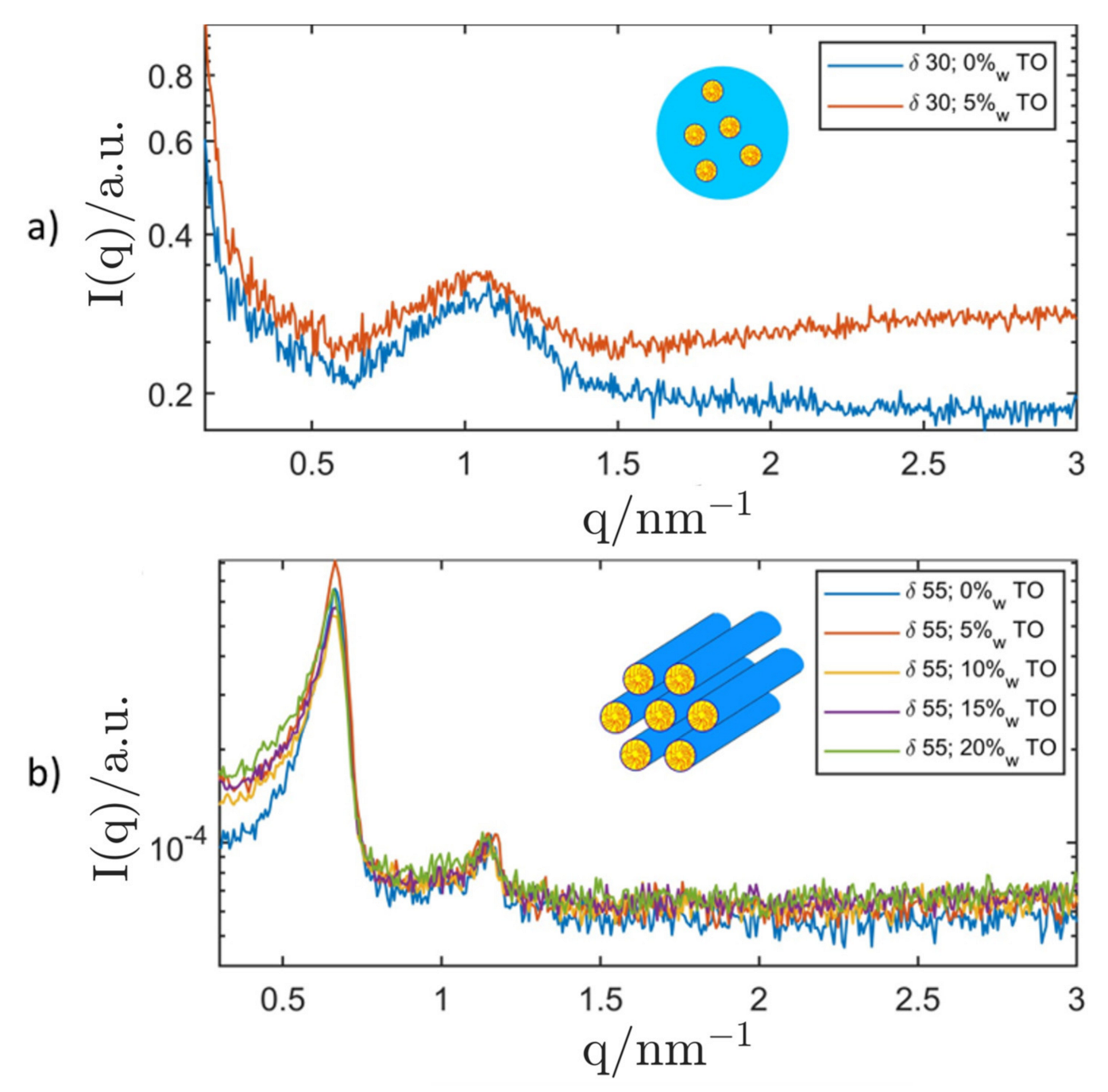
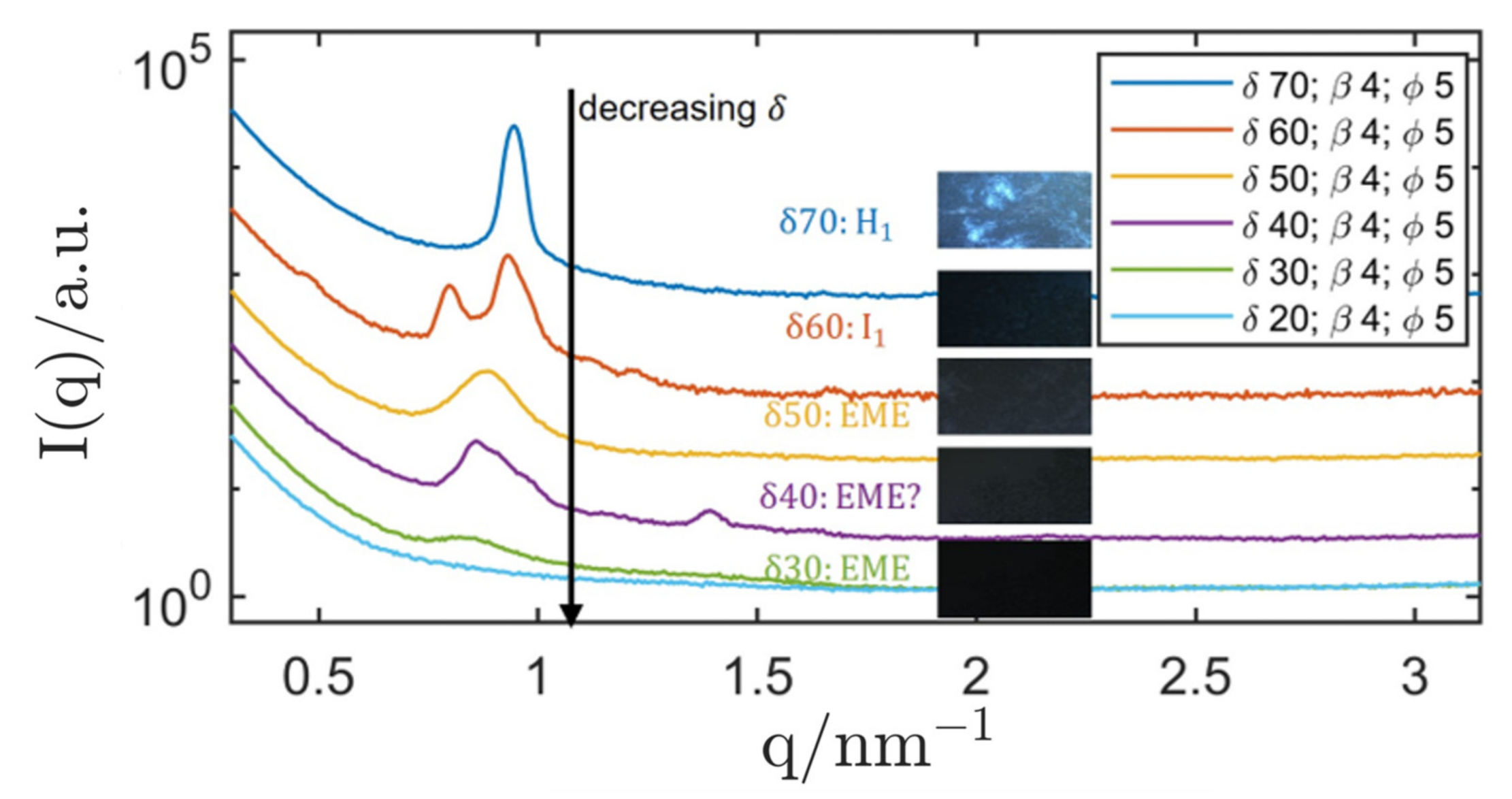
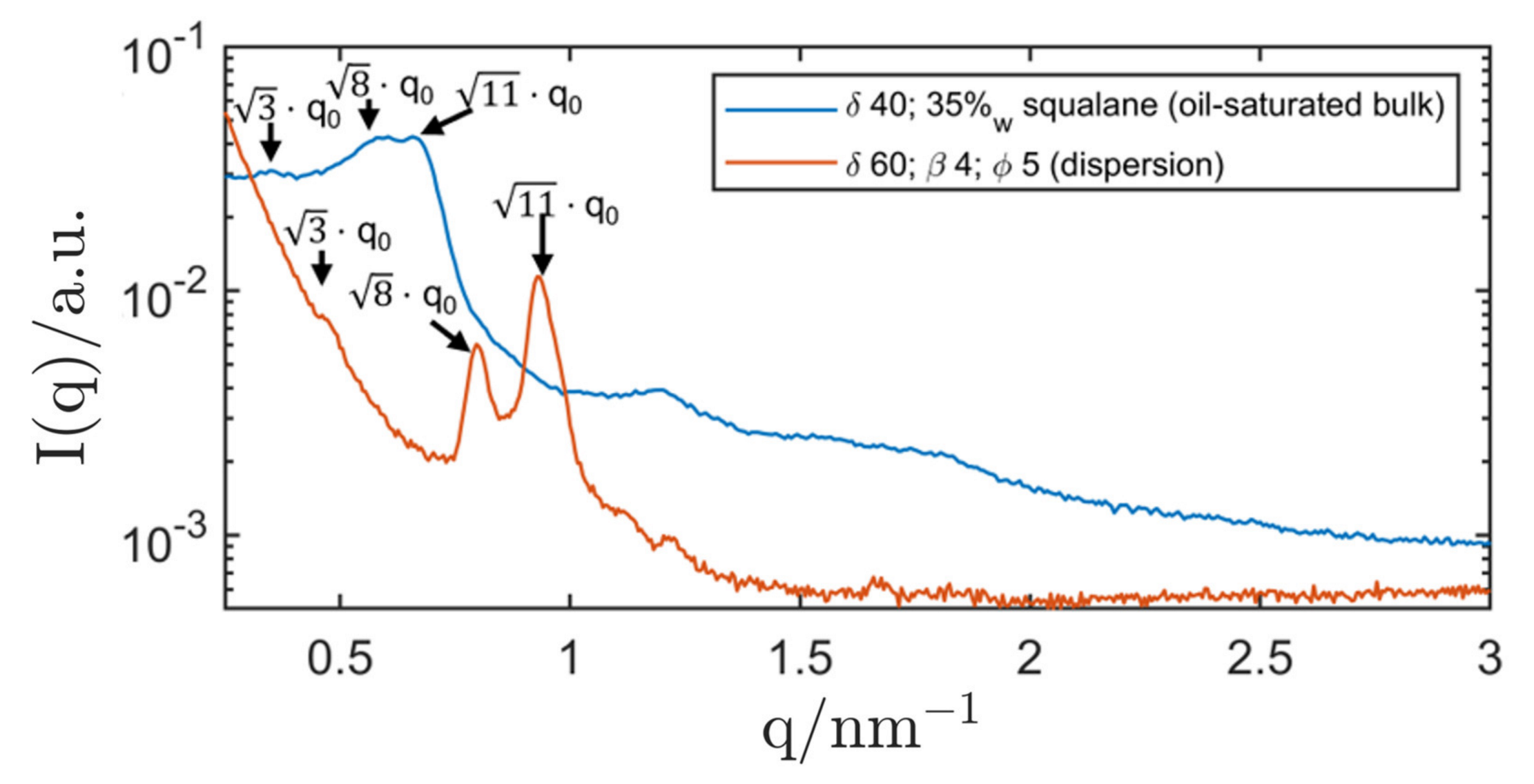
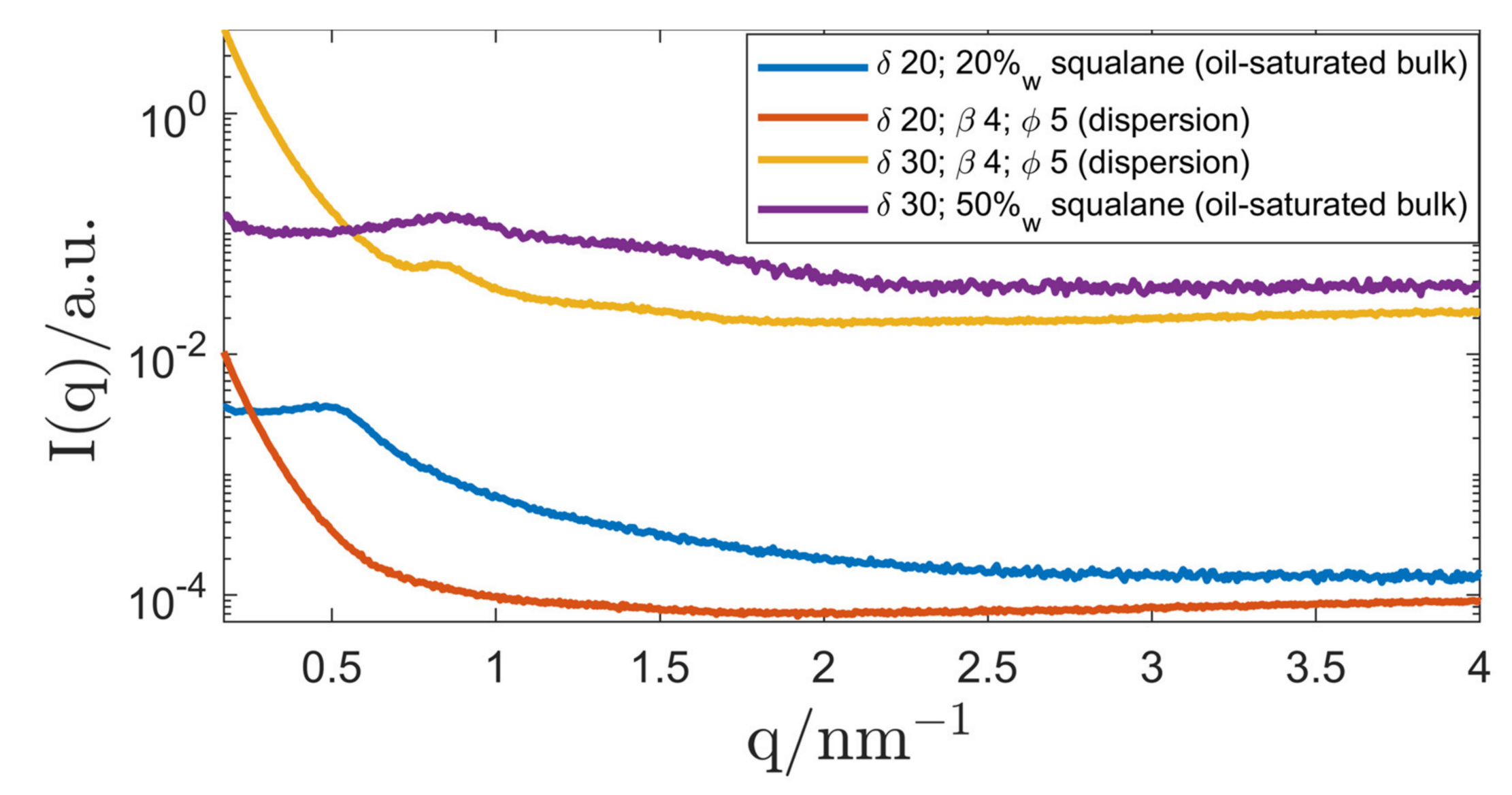
| System Composition | δ | LC-Phase | a/nm | |
|---|---|---|---|---|
| Gena | 20 |  | n.a. | |
| Gena+Squal20% | 20 | n.a. | ||
| Gena | 40 |  | 7.19 | |
| Gena+Squal25% | 40 | 31.1 | ||
| Gena | 60 |  | 6.21 | |
| Gena+Squal20% | 60 | 8.81 | ||
| System Composition | δ | LC-Phase | a/nm | |
|---|---|---|---|---|
| P94 | 30 |  | n.a. | |
| P94+Triolein5% | 30 | n.a. | ||
| P94 | 55 |  | 10.93 | |
| P94+Triolein20% | 55 | 10.93 | ||
| Oil Phase | δ | d/nm | PDI |
|---|---|---|---|
| Triolein | 30 | 216 | 23% |
| Triolein | 60 | 134 | 23% |
| Olive oil | 30 | 175 | 37% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trummer, F.; Glatter, O.; Chemelli, A. Inverse ISAsomes in Bio-Compatible Oils—Exploring Formulations in Squalane, Triolein and Olive Oil. Nanomaterials 2022, 12, 1133. https://doi.org/10.3390/nano12071133
Trummer F, Glatter O, Chemelli A. Inverse ISAsomes in Bio-Compatible Oils—Exploring Formulations in Squalane, Triolein and Olive Oil. Nanomaterials. 2022; 12(7):1133. https://doi.org/10.3390/nano12071133
Chicago/Turabian StyleTrummer, Florian, Otto Glatter, and Angela Chemelli. 2022. "Inverse ISAsomes in Bio-Compatible Oils—Exploring Formulations in Squalane, Triolein and Olive Oil" Nanomaterials 12, no. 7: 1133. https://doi.org/10.3390/nano12071133
APA StyleTrummer, F., Glatter, O., & Chemelli, A. (2022). Inverse ISAsomes in Bio-Compatible Oils—Exploring Formulations in Squalane, Triolein and Olive Oil. Nanomaterials, 12(7), 1133. https://doi.org/10.3390/nano12071133