Tailoring Functional Terminals on Solution-Processable Fullerene Electron Transporting Materials for High Performance Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Characterization
3. Results and Discussion
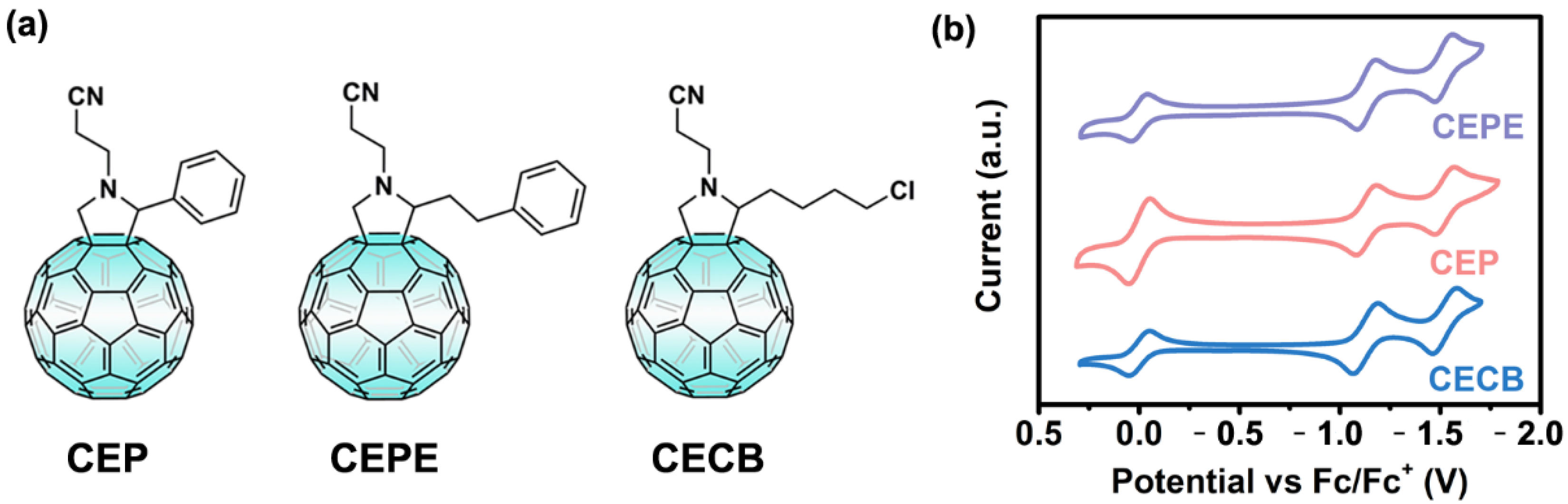
3.1. Synthesis and Characterization of Fullerene Compounds
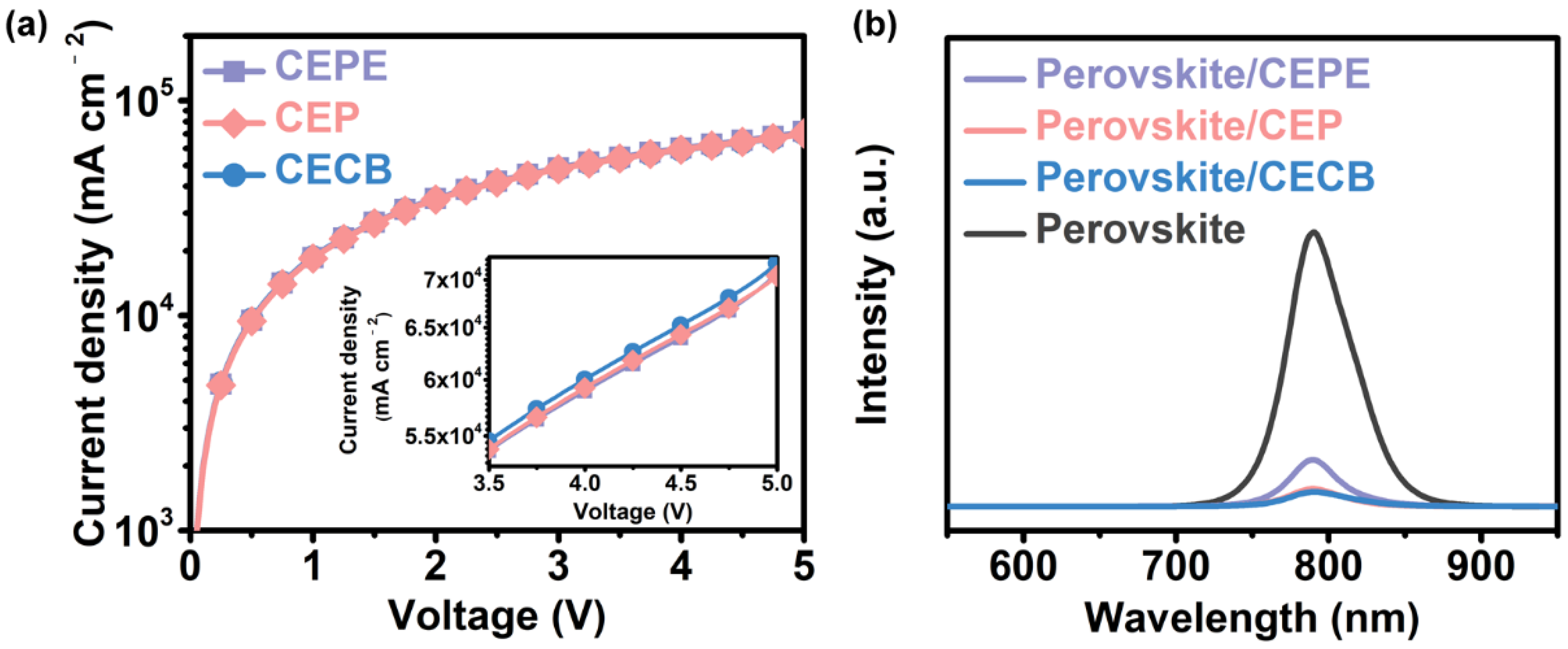
3.2. Photoelectric Properties of Fullerene Films
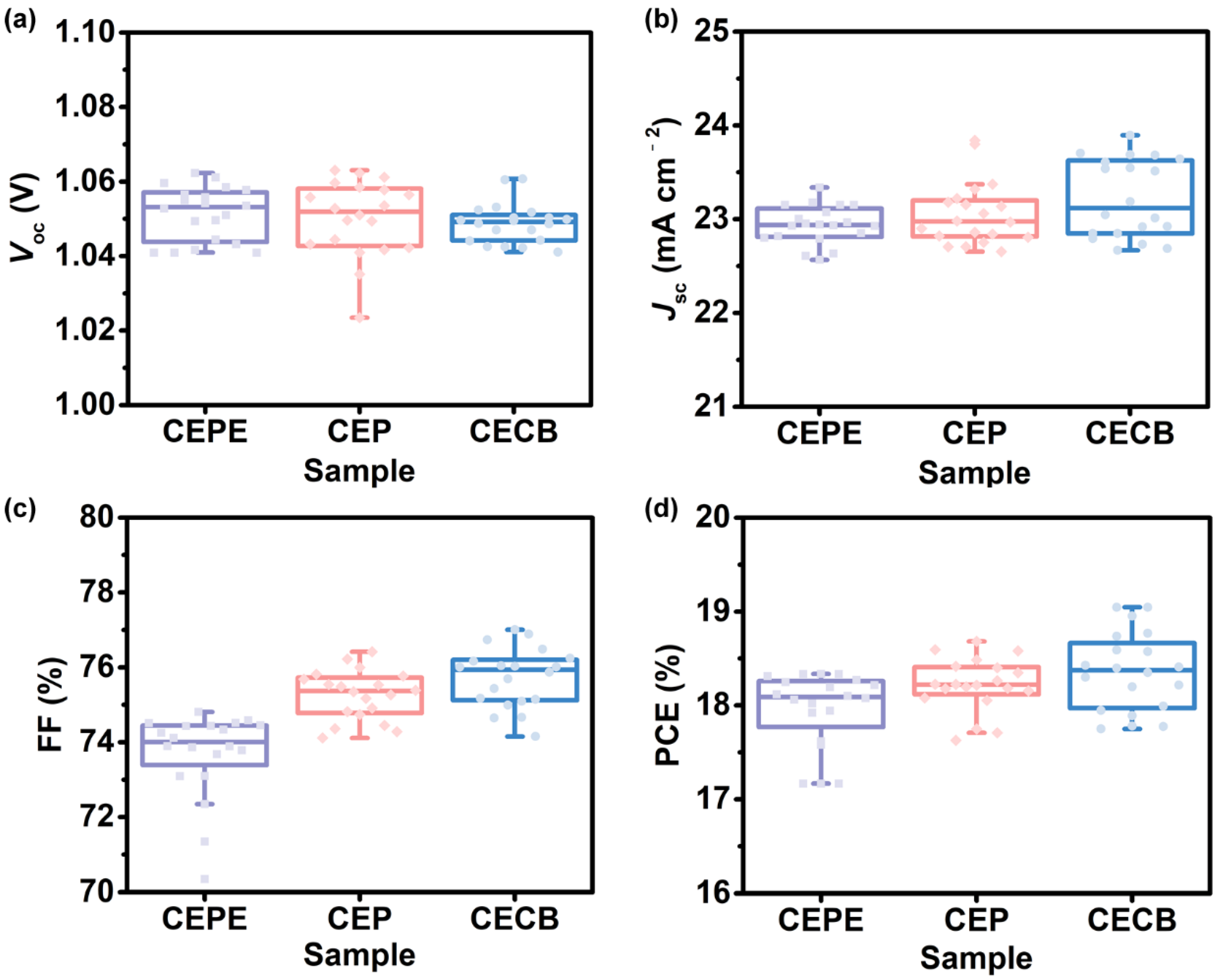
3.3. Photovoltaic Performance of Fullerene-Based Devices
3.4. Side-Chain Effects of Fullerenes on Photovoltaic Parameters of Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NREL. Available online: https://www.nrel.gov/pv/assets/pdfs/cell-pv-eff-emergingpv-rev211214.pdf (accessed on 15 February 2022).
- Kim, M.; Jeong, J.; Lu, H.; Lee Tae, K.; Eickemeyer Felix, T.; Liu, Y.; Choi In, W.; Choi Seung, J.; Jo, Y.; Kim, H.-B.; et al. Conformal quantum dot–SnO2 layers as electron transporters for efficient perovskite solar cells. Science 2022, 375, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Xu, J.; Wei, M.; Wang, Y.; Qin, Z.; Liu, Z.; Wu, J.; Xiao, K.; Chen, B.; Park, S.M.; et al. All-perovskite tandem solar cells with improved grain surface passivation. Nature 2022, 603, 73–78. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, L.; Hou, Y.; Nozariasbmarz, A.; Poudel, B.; Yoon, J.; Ye, T.; Yang, D.; Pogrebnyakov, A.V.; Gopalan, V.; et al. Overcoming Shockley-Queisser limit using halide perovskite platform? Joule 2022. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Q.; Bermel, P. Prospects for high-performance thermophotovoltaic conversion efficiencies exceeding the Shockley–Queisser limit. Energ. Convers. Manag. 2015, 97, 63–69. [Google Scholar] [CrossRef]
- Castro, E.; Murillo, J.; Fernandez-Delgado, O.; Echegoyen, L. Progress in fullerene-based hybrid perovskite solar cells. J. Mater. Chem. C 2018, 6, 2635–2651. [Google Scholar] [CrossRef]
- Liang, Y.; Song, P.; Tian, H.; Tian, C.; Tian, W.; Nan, Z.; Cai, Y.; Yang, P.; Sun, C.; Chen, J.; et al. Lead leakage preventable fullerene-porphyrin dyad for efficient and stable perovskite solar cells. Adv. Funct. Mater. 2021, 2110139. [Google Scholar] [CrossRef]
- Laska, M.; Krzemińska, Z.; Kluczyk-Korch, K.; Schaadt, D.; Popko, E.; Jacak, W.A.; Jacak, J.E. Metallization of solar cells, exciton channel of plasmon photovoltaic effect in perovskite cells. Nano Energy 2020, 75, 104751. [Google Scholar] [CrossRef]
- Jacak, W.A.; Jacak, J.E. New channel of plasmon photovoltaic effect in metalized perovskite solar cells. J. Phys. Chem. C 2019, 123, 30633–30639. [Google Scholar] [CrossRef]
- Li, Y.; Xie, H.; Lim, E.L.; Hagfeldt, A.; Bi, D. Recent progress of critical interface engineering for highly efficient and stable perovskite solar cells. Adv. Energy Mater. 2022, 12, 2102730. [Google Scholar] [CrossRef]
- Fang, Y.; Bi, C.; Wang, D.; Huang, J. The functions of fullerenes in hybrid perovskite solar cells. ACS Energy Lett. 2017, 2, 782–794. [Google Scholar] [CrossRef]
- Deng, L.-L.; Xie, S.-Y.; Gao, F. Fullerene-based materials for photovoltaic applications: Toward efficient, hysteresis-free, and stable perovskite solar cells. Adv. Electron. Mater. 2018, 4, 1700435. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Chen, M.; Yang, S. Functionalization of fullerene materials toward applications in perovskite solar cells. Mater. Chem. Front. 2020, 4, 2256–2282. [Google Scholar] [CrossRef]
- Tian, C.; Sun, C.; Chen, J.; Song, P.; Hou, E.; Xu, P.; Liang, Y.; Yang, P.; Luo, J.; Xie, L.; et al. Fullerene derivative with flexible alkyl chain for efficient tin-based perovskite solar cells. Nanomaterials 2022, 12, 532. [Google Scholar] [CrossRef]
- Warby, J.; Zu, F.; Zeiske, S.; Gutierrez-Partida, E.; Frohloff, L.; Kahmann, S.; Frohna, K.; Mosconi, E.; Radicchi, E.; Lang, F.; et al. Understanding performance limiting interfacial recombination in pin perovskite solar cells. Adv. Energy Mater. 2022, 2103567. [Google Scholar] [CrossRef]
- Li, B.; Zhen, J.; Wan, Y.; Lei, X.; Liu, Q.; Liu, Y.; Jia, L.; Wu, X.; Zeng, H.; Zhang, W.; et al. Anchoring fullerene onto perovskite film via grafting pyridine toward enhanced electron transport in high-efficiency solar cells. ACS Appl. Mater. Interfaces 2018, 10, 32471–32482. [Google Scholar] [CrossRef]
- Castro, E.; Fernandez-Delgado, O.; Arslan, F.; Zavala, G.; Yang, T.; Seetharaman, S.; D’Souza, F.; Echegoyen, L. New thiophene-based C60 fullerene derivatives as efficient electron transporting materials for perovskite solar cells. New J. Chem. 2018, 42, 14551–14558. [Google Scholar] [CrossRef]
- Rajagopal, A.; Yao, K.; Jen, A.K.Y. Toward perovskite solar cell commercialization: A perspective and research roadmap based on interfacial engineering. Adv. Mater. 2018, 30, 1800455. [Google Scholar] [CrossRef]
- Zhang, H.; Nazeeruddin, M.K.; Choy, W.C.H. Perovskite photovoltaics: The significant role of ligands in film formation, passivation, and stability. Adv. Mater. 2019, 31, 1805702. [Google Scholar] [CrossRef]
- Xu, G.; Xue, R.; Chen, W.; Zhang, J.; Zhang, M.; Chen, H.; Cui, C.; Li, H.; Li, Y.; Li, Y. New strategy for two-step sequential deposition: Incorporation of hydrophilic fullerene in second precursor for high-performance p-i-n planar perovskite solar cells. Adv. Energy Mater. 2018, 8, 1703054. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; García de Arquer, F.P.; Fan James, Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef]
- Stolterfoht, M.; Wolff, C.M.; Márquez, J.A.; Zhang, S.; Hages, C.J.; Rothhardt, D.; Albrecht, S.; Burn, P.L.; Meredith, P.; Unold, T.; et al. Visualization and suppression of interfacial recombination for high-efficiency large-area pin perovskite solar cells. Nat. Energy 2018, 3, 847–854. [Google Scholar] [CrossRef]
- Wu, B.-S.; An, M.-W.; Chen, J.-M.; Xing, Z.; Chen, Z.-C.; Deng, L.-L.; Tian, H.-R.; Yun, D.-Q.; Xie, S.-Y.; Zheng, L.-S. Radiation-processed perovskite solar cells with fullerene-enhanced performance and stability. Cell Rep. Phys. Sci. 2021, 2, 100646. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Dong, Q.; Xiao, Z.; Yuan, Y.; Huang, J. Large fill-factor bilayer iodine perovskite solar cells fabricated by a low-temperature solution-process. Energy Environ. Sci. 2014, 7, 2359–2365. [Google Scholar] [CrossRef]
- Noel, N.K.; Abate, A.; Stranks, S.D.; Parrott, E.S.; Burlakov, V.M.; Goriely, A.; Snaith, H.J. Enhanced photoluminescence and solar cell performance via Lewis base passivation of organic–inorganic lead halide perovskites. ACS Nano 2014, 8, 9815–9821. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, X.; Qi, F.; Li, Z.; Liu, D.; Shen, D.; Qin, M.; Wu, S.; Lin, F.; Jang, S.-H.; et al. Regulating surface termination for efficient inverted perovskite solar cells with greater than 23% efficiency. J. Am. Chem. Soc. 2020, 142, 20134–20142. [Google Scholar] [CrossRef]
- Xing, Z.; Li, S.-H.; Hui, Y.; Wu, B.-S.; Chen, Z.-C.; Yun, D.-Q.; Deng, L.-L.; Zhang, M.-L.; Mao, B.-W.; Xie, S.-Y.; et al. Star-like hexakis[di(ethoxycarbonyl)methano]-C60 with higher electron mobility: An unexpected electron extractor interfaced in photovoltaic perovskites. Nano Energy 2020, 74, 104859. [Google Scholar] [CrossRef]
- Xing, Z.; Liu, F.; Li, S.-H.; Chen, Z.-C.; An, M.-W.; Zheng, S.; Jen, A.K.Y.; Yang, S. Multifunctional molecular design of a new fulleropyrrolidine electron transport material family engenders high performance of perovskite solar cells. Adv. Funct. Mater. 2021, 31, 2107695. [Google Scholar] [CrossRef]
- Liu, K.; Dai, S.; Meng, F.; Shi, J.; Li, Y.; Wu, J.; Meng, Q.; Zhan, X. Fluorinated fused nonacyclic interfacial materials for efficient and stable perovskite solar cells. J. Mater. Chem. A 2017, 5, 21414–21421. [Google Scholar] [CrossRef]
- Wei, D.; Ma, F.; Wang, R.; Dou, S.; Cui, P.; Huang, H.; Ji, J.; Jia, E.; Jia, X.; Sajid, S.; et al. Ion-migration inhibition by the cation–π Interaction in perovskite materials for efficient and stable perovskite solar cells. Adv. Mater. 2018, 30, 1707583. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.-X.; Zhang, X.; Dai, S.-M.; Li, S.-H.; Lu, X.-Z.; Deng, L.-L.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Tailorable PC71BM isomers: Using the most prevalent electron acceptor to obtain high-performance polymer solar cells. Chem. Eur. J. 2016, 22, 18709–18713. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.-Y.; Chiang, Y.-F.; Lee, M.-H.; Peng, S.-R.; Guo, T.-F.; Chen, P.; Wen, T.-C. CH3NH3PbI3 perovskite/fullerene planar-heterojunction hybrid solar cells. Adv. Mater. 2013, 25, 3727–3732. [Google Scholar] [CrossRef]
- Xing, Z.; Li, S.-H.; Xu, P.-Y.; Tian, H.-R.; Deng, L.-L.; Yao, Y.-R.; Chen, B.-W.; Xie, F.-F.; An, M.-W.; Yun, D.-Q.; et al. Crystallographic understanding of photoelectric properties for C60 derivatives applicable as electron transporting materials in perovskite solar cells. Chem. Res. Chin. Univ. 2022, 38, 75–81. [Google Scholar] [CrossRef]
- Tian, C.; Castro, E.; Wang, T.; Betancourt-Solis, G.; Rodriguez, G.; Echegoyen, L. Improved performance and stability of inverted planar perovskite solar cells using fulleropyrrolidine layers. ACS Appl. Mater. Interfaces 2016, 8, 31426–31432. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, K.; Ling, X.; Yuan, J.; Shi, G.; Ding, G.; Sun, J.; Shi, S.; Gong, X.; Ma, W. High performance planar-heterojunction perovskite solar cells using amino-based fulleropyrrolidine as the electron transporting material. J. Mater. Chem. A 2016, 4, 10130–10134. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Li, X.; Zhu, L.; Liu, X.; Zhang, W.; Fang, J. Efficient and hysteresis-free perovskite solar cells based on a solution processable polar fullerene electron transport layer. Adv. Energy Mater. 2017, 7, 1701144. [Google Scholar] [CrossRef]
- Castro, E.; Zavala, G.; Seetharaman, S.; D’Souza, F.; Echegoyen, L. Impact of fullerene derivative isomeric purity on the performance of inverted planar perovskite solar cells. J. Mater. Chem. A 2017, 5, 19485–19490. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.-C.; Song, C.; Zhu, L.; Guo, Q.; Fang, J. Carboxylic ester-terminated fulleropyrrolidine as an efficient electron transport material for inverted perovskite solar cells. J. Mater. Chem. C 2018, 6, 6982–6987. [Google Scholar] [CrossRef]
- Liu, X.; Li, P.; Zhang, Y.; Hu, X.; Duan, Y.; Li, F.; Li, D.; Shao, G.; Song, Y. High-efficiency perovskite solar cells based on self-assembly n-doped fullerene derivative with excellent thermal stability. J. Power Sources 2019, 413, 459–466. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, F.; Zhang, T.; Zeng, X.; Xiao, Y.; Liu, T.; Zhong, C.; Lu, X.; Zhu, L.; Yang, S.; et al. Designing a perylene diimide/fullerene hybrid as effective electron transporting material in inverted perovskite solar cells with enhanced efficiency and stability. Angew. Chem. Int. Ed. 2019, 58, 8520–8525. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhen, J.; Wan, Y.; Lei, X.; Jia, L.; Wu, X.; Zeng, H.; Chen, M.; Wang, G.-W.; Yang, S. Steering the electron transport properties of pyridine-functionalized fullerene derivatives in inverted perovskite solar cells: The nitrogen site matters. J. Mater. Chem. A 2020, 8, 3872–3881. [Google Scholar] [CrossRef]
- Liang, P.-W.; Chueh, C.-C.; Williams, S.T.; Jen, A.K.Y. Roles of fullerene-based interlayers in enhancing the performance of organometal perovskite thin-film solar cells. Adv. Energy Mater. 2015, 5, 1402321. [Google Scholar] [CrossRef]
- Gil-Escrig, L.; Momblona, C.; Sessolo, M.; Bolink, H.J. Fullerene imposed high open-circuit voltage in efficient perovskite based solar cells. J. Mater. Chem. A 2016, 4, 3667–3672. [Google Scholar] [CrossRef]
- Green, M.A. Solar cell fill factors: General graph and empirical expressions. Solid-State Electron. 1981, 24, 788–789. [Google Scholar] [CrossRef]
- Singal, C.M. Analytical expression for the series-resistance-dependent maximum power point and curve factor for solar cells. Sol. Cells 1981, 3, 163–177. [Google Scholar] [CrossRef]
- Green, M.A. Accuracy of analytical expressions for solar cell fill factors. Sol. Cells 1982, 7, 337–340. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Xing, Z.; Ren, Y.; Huang, R.-J.; Xu, P.-Y.; Xie, F.-F.; Li, S.-H.; Zhong, X. Tailoring Functional Terminals on Solution-Processable Fullerene Electron Transporting Materials for High Performance Perovskite Solar Cells. Nanomaterials 2022, 12, 1046. https://doi.org/10.3390/nano12071046
Liu F, Xing Z, Ren Y, Huang R-J, Xu P-Y, Xie F-F, Li S-H, Zhong X. Tailoring Functional Terminals on Solution-Processable Fullerene Electron Transporting Materials for High Performance Perovskite Solar Cells. Nanomaterials. 2022; 12(7):1046. https://doi.org/10.3390/nano12071046
Chicago/Turabian StyleLiu, Fu, Zhou Xing, Ya Ren, Rong-Jiao Huang, Piao-Yang Xu, Fang-Fang Xie, Shu-Hui Li, and Xinxian Zhong. 2022. "Tailoring Functional Terminals on Solution-Processable Fullerene Electron Transporting Materials for High Performance Perovskite Solar Cells" Nanomaterials 12, no. 7: 1046. https://doi.org/10.3390/nano12071046
APA StyleLiu, F., Xing, Z., Ren, Y., Huang, R.-J., Xu, P.-Y., Xie, F.-F., Li, S.-H., & Zhong, X. (2022). Tailoring Functional Terminals on Solution-Processable Fullerene Electron Transporting Materials for High Performance Perovskite Solar Cells. Nanomaterials, 12(7), 1046. https://doi.org/10.3390/nano12071046





