Enhancement of Gas Barrier Properties and Durability of Poly(butylene succinate-co-butylene adipate)-Based Nanocomposites for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Composites Preparation
2.2. Scanning Electron Microscopy (SEM)
2.3. Thermogravimetric Analysis (TGA)
2.4. Differential Scanning Calorimetry (DSC)
2.5. Tensile and Tear Tests
2.6. Rheology
2.7. Infrared Spectroscopy
2.8. Gas Barrier Properties
2.8.1. Water Vapor Transmission Rate
2.8.2. Oxygen Transmission Rate
2.9. UV–VIS Spectroscopy
2.10. Accelerated Photoaging
2.11. Natural Weathering
3. Results and Discussion
3.1. Initial Characterization of PBSA and PBSA-LDH Nanocomposite Films
3.1.1. Microscopy of Films
3.1.2. Thermal Properties
3.1.3. Infrared Spectroscopy
3.1.4. Mechanical Properties
3.1.5. Rheological Properties
3.1.6. Barrier Properties
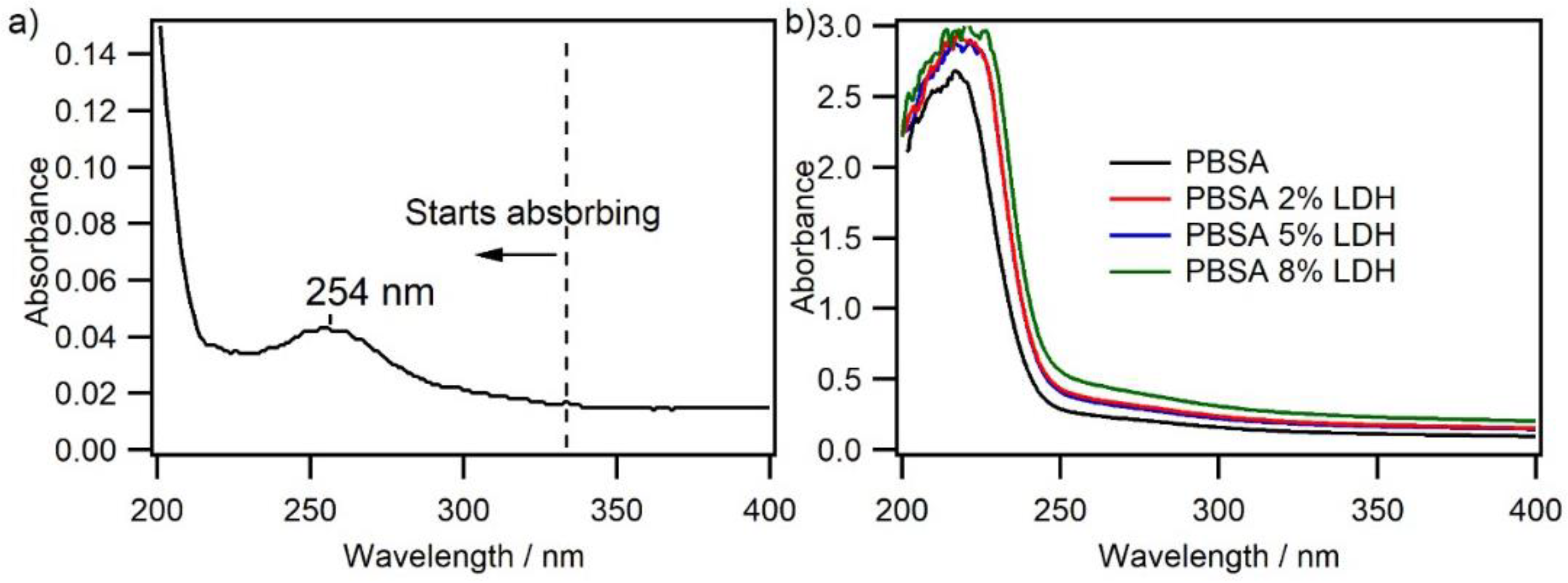
3.1.7. ®UV–VIS Spectroscopy
3.2. Photo-Durability of PBSA and PBSA Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raynaud, J. Valuing Plastics: The Business Case for Measuring, Managing and Disclosing Plastic Use in the Consumer Goods Industry; Richens, J., Russel, A., Eds.; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2014. [Google Scholar]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for Food Packaging Applications: An Overview. Nanomaterials 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D. A Review of the Biodegradability and Utility of Poly(caprolactone). J. Environ. Polym. Degrad. 1995, 3, 61–67. [Google Scholar] [CrossRef]
- Cosquer, R.; Pruvost, S.; Gouanvé, F. Improvement of Barrier Properties of Biodegradable Polybutylene Succinate/Graphene Nanoplatelets Nanocomposites Prepared by Melt Process. Membranes 2021, 11, 151. [Google Scholar] [CrossRef]
- Salomez, M.; George, M.; Fabre, P.; Touchaleaume, F.; Cesar, G.; Lajarrige, A.; Gastaldi, E. A Comparative Study of Degradation Mechanisms of PHBV and PBSA under Laboratory-Scale Composting Conditions. Polym. Degrad. Stab. 2019, 167, 102–113. [Google Scholar] [CrossRef]
- Commereuc, S.; Askanian, H.; Verney, V.; Celli, A.; Marchese, P. About Durability of Biodegradable Polymers: Structure/Degradability Relationships. Macromol. Symp. 2010, 296, 378–387. [Google Scholar] [CrossRef]
- Commereuc, S.; Askanian, H.; Verney, V.; Celli, A.; Marchese, P.; Berti, C. About the End Life of Novel Aliphatic and Aliphatic-Aromatic (Co)Polyesters after UV-Weathering: Structure/Degradability Relationships. Polym. Degrad. Stab. 2013, 98, 1321–1328. [Google Scholar] [CrossRef]
- Verney, V.; Ramoné, A.; Delor-Jestin, F.; Commereuc, S.; Koutny, M.; Perchet, G.; Troquet, J. Melt Viscoelastic Assessment of Poly(lactic acid) Composting: Influence of UV Ageing. Molecules. 2018, 23, 2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askanian, H.; Muranaka de Lima, D.; Commereuc, S.; Verney, V. Toward a Better Understanding of the Fused Deposition Modeling Process: Comparison with Injection Molding. 3D Print. Addit. Manuf. 2018, 5, 319–327. [Google Scholar] [CrossRef]
- Zaferani, S.H. Introduction of Polymer-Based Nanocomposites. In Polymer-based Nanocomposites for Energy and Environmental Applications; Jawaid, M., Khan, M.M., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Li, X.; Guo, M.; Bandyopadhyay, P.; Lan, Q.; Xie, H.; Liu, G.; Liu, X.; Cheng, X.; Kim, N.H.; Lee, J.H. Two-Dimensional Materials Modified Layered Double Hydroxides: A Series of Fillers for Improving Gas Barrier and Permselectivity of Poly(vinyl alcohol). Compos. B Eng. 2021, 207, 108568. [Google Scholar] [CrossRef]
- Yu, J.; Ruengkajorn, K.; Crivoi, D.-G.; Chen, C.; Buffet, J.-C.; O’Hare, D. High Gas Barrier Coating Using Non-Toxic Nanosheet Dispersions for Flexible Food Packaging Film. Nat. Commun. 2019, 10, 2398. [Google Scholar] [CrossRef]
- Bhatia, A.; Gupta, R.K.; Bhattacharya, S.N.; Choi, H.J. Effect of Clay on Thermal, Mechanical and Gas Barrier Properties of Biodegradable Poly(lactic acid)/Poly(butylene succinate) (PLA/PBS) Nanocomposites. Int. Polym. Process. 2010, 25, 5–14. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered Double Hydroxides: A Brief Review from Fundamentals to Application as Evolving Biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Clariant Produkte Deutschland GmbH. Product Sheet SORBACID-911; Clariant Produkte Deutschland GmbH: Moosburg, Germany, 2019. [Google Scholar]
- Siracusa, V.; Lotti, N.; Munari, A.; Rosa, M.D. Poly(butylene succinate) and Poly(butylene succinate-co-adipate) for Food Packaging Applications: Gas Barrier Properties after Stressed Treatments. Polym. Degrad. Stab. 2015, 119, 35–45. [Google Scholar] [CrossRef]
- Chiu, F.-C.; Hsieh, Y.-C.; Sung, Y.-C.; Liang, N.-Y. Poly(butylene succinate-co-adipate) Green Composites with Enhanced Rigidity: Influences of Dimension and Surface Modification of Kenaf Fiber Reinforcement. Ind. Eng. Chem. Res. 2015, 54, 12826–12835. [Google Scholar] [CrossRef]
- Wang, J.-M.; Wang, H.; Chen, E.-C.; Chen, Y.-J.; Wu, T.-M. Role of Organically-Modified Zn-Ti Layered Double Hydroxides in Poly(butylene succinate-co-adipate) Composites: Enhanced Material Properties and Photodegradation Protection. Polymers 2021, 13, 2181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-A.; Tsai, G.-S.; Chen, E.-C.; Wu, T.-M. Thermal Degradation Behaviors and Biodegradability of Novel Nanocomposites Based on Various Poly[(butylene succinate)-co-adipate] and Modified Layered Double Hydroxides. J. Taiwan Inst. Chem. Eng. 2017, 77, 263–270. [Google Scholar] [CrossRef]
- Eili, M.; Shameli, K.; Ibrahim, N.A.; Wan Yunus, W.M.Z. Degradability Enhancement of Poly(lactic acid) by Stearate-Zn3Al LDH Nanolayers. Int. J. Mol. Sci. 2012, 13, 7938–7951. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, K.; Camino, G. Polymer Nanocomposites Biodegradation. In Functional and Physical Properties of Polymer Nanocomposites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 57–91. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A Review on Polymer–Layered Silicate Nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Chaillot, D.; Bennici, S.; Brendlé, J. Layered Double Hydroxides and LDH-Derived Materials in Chosen Environmental Applications: A Review. Environ. Sci. Pollut. Res. 2021, 28, 24375–24405. [Google Scholar] [CrossRef] [PubMed]
- Seggiani, M.; Gigante, V.; Cinelli, P.; Coltelli, M.-B.; Sandroni, M.; Anguillesi, I.; Lazzeri, A. Processing and Mechanical Performances of Poly(butylene succinate-co-adipate) (PBSA) and Raw Hydrolyzed Collagen (HC) Thermoplastic Blends. Polym. Test. 2019, 77, 105900. [Google Scholar] [CrossRef]
- Lemaire, J.; Arnaud, R.; Lacoste, J. The Prediction of the Long-Term Photoageing of Solid Polymers. Acta Polym. 1988, 39, 27–32. [Google Scholar] [CrossRef]
- Messin, T.; Follain, N.; Guinault, A.; Sollogoub, C.; Gaucher, V.; Delpouve, N.; Marais, S. Structure and Barrier Properties of Multinanolayered Biodegradable PLA/PBSA Films: Confinement Effect via Forced Assembly Coextrusion. ACS Appl. Mater. Interfaces 2017, 9, 29101–29112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Guo, Y.; Marek, A.A.; Verney, V.; Leroux, F.; Tang, P.; Li, D.; Feng, Y. Design, Fabrication and Anti-Aging Behavior of a Multifunctional Inorganic–Organic Hybrid Stabilizer Derived from Co-Intercalated Layered Double Hydroxides for Polypropylene. Inorg. Chem. Front. 2019, 6, 2539–2549. [Google Scholar] [CrossRef]
- Sisti, L.; Totaro, G.; Fiorini, M.; Celli, A.; Coelho, C.; Hennous, M.; Verney, V.; Leroux, F. Poly(butylene succinate)/Layered Double Hydroxide Bionanocomposites: Relationships between Chemical Structure of LDH Anion, Delamination Strategy, and Final Properties. J. Appl. Polym. Sci. 2013, 130, 1931–1940. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Y.; Shi, Q.; Guo, S. Study of the Preparation and Properties of TPS/PBSA/PLA Biodegradable Composites. J. Compos. Sci. 2021, 5, 48. [Google Scholar] [CrossRef]
- Marek, A.A.; Verney, V.; Taviot-Gueho, C.; Totaro, G.; Sisti, L.; Celli, A.; Leroux, F. Outstanding Chain-Extension Effect and High UV Resistance of Polybutylene Succinate Containing Amino-Acid-Modified Layered Double Hydroxides. Beilstein J. Nanotechnol. 2019, 10, 684–695. [Google Scholar] [CrossRef]
- Nejati, K.; Rezvani, Z. Synthesis and Characterisation of Nanohybrids of Olsalazine-Intercalated Al–Mg Layered Double Hydroxide. J. Exp. Nanosci. 2012, 7, 412–425. [Google Scholar] [CrossRef]
- Tanaka, T.; Kameshima, Y.; Nishimoto, S.; Miyake, M. Determination of Carbonate Ion Contents in Layered Double Hydroxides by FTIR Spectrometry. Anal. Methods 2012, 4, 3925–3927. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Abdennouri, M.; Barka, N. Zn–Al Layered Double Hydroxides Intercalated with Carbonate, Nitrate, Chloride and Sulphate Ions: Synthesis, Characterisation and Dye Removal Properties. J. Taibah Univ. Sci. 2017, 11, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Kuan, C.-F.; Ma, C.-C.M.; Kuan, H.-C.; Wu, H.-L.; Liao, Y.-M. Preparation and Characterization of the Novel Water-Crosslinked Cellulose Reinforced Poly(butylene succinate) Composites. Compos. Sci. Technol. 2006, 66, 2231–2241. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M.; Okamoto, K. Structure and Properties of Nanocomposites Based on Poly(butylene succinate-co-adipate) and Organically Modified Montmorillonite. Macromol. Mater. Eng. 2005, 290, 759–768. [Google Scholar] [CrossRef]
- Han, S.-I.; Lim, J.S.; Kim, D.K.; Kim, M.N.; Im, S.S. In Situ Polymerized Poly(butylene succinate)/Silica Nanocomposites: Physical Properties and Biodegradation. Polym. Degrad. Stab. 2008, 93, 889–895. [Google Scholar] [CrossRef]
- Jin, C.; Christensen, P.A.; Egerton, T.A.; White, J.R. Effect of Anisotropy on Photo-Mechanical Oxidation of Polyethylene. Polymer 2003, 44, 5969–5981. [Google Scholar] [CrossRef]
- Jingwen, X. Biobased Nanocomposites for Packaging Applications—Synthesis Using Melt Extrusion of Poly(lactic acid), Poly(butylene succinate) and/or Starch Blended with Natural Nanofillers. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2015. [Google Scholar]
- Lange, J.; Wyser, Y. Recent Innovations in Barrier Technologies for Plastic Packaging—A Review. Packag. Technol. Sci. 2003, 16, 149–158. [Google Scholar] [CrossRef]
- Mhlabeni, T.; Kesavan Pillai, S.; Ray, S.S. Effect of Organically Modified Layered Double Hydroxides on the Properties of Poly(lactic acid)/Poly[(butylene succinate)-co-adipate] Immiscible Blends. J. Appl. Polym. Sci. 2020, 137, 48654. [Google Scholar] [CrossRef]
- Verney, V.; Michel, A. Representation of the Rheological Properties of Polymer Melts in Terms of Complex Fluidity. Rheol. Acta 1989, 28, 54–60. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustainable Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and Supramolecular Changes in Polybutylene Succinate (PBS) and Polybutylene Succinate Adipate (PBSA) Copolymer during Degradation in Various Environmental Conditions. Polymers 2018, 10, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alao, P.; Visnapuu, K.; Kallakas, H.; Poltimäe, T.; Kers, J. Natural Weathering of Bio-Based Façade Materials. Forests 2020, 11, 642. [Google Scholar] [CrossRef]
- Su, W.-F. Characterization of Polymer. In Principles of Polymer Design and Synthesis; Springer: Berlin/Heidelberg, Germany, 2013; pp. 89–110. [Google Scholar] [CrossRef] [Green Version]
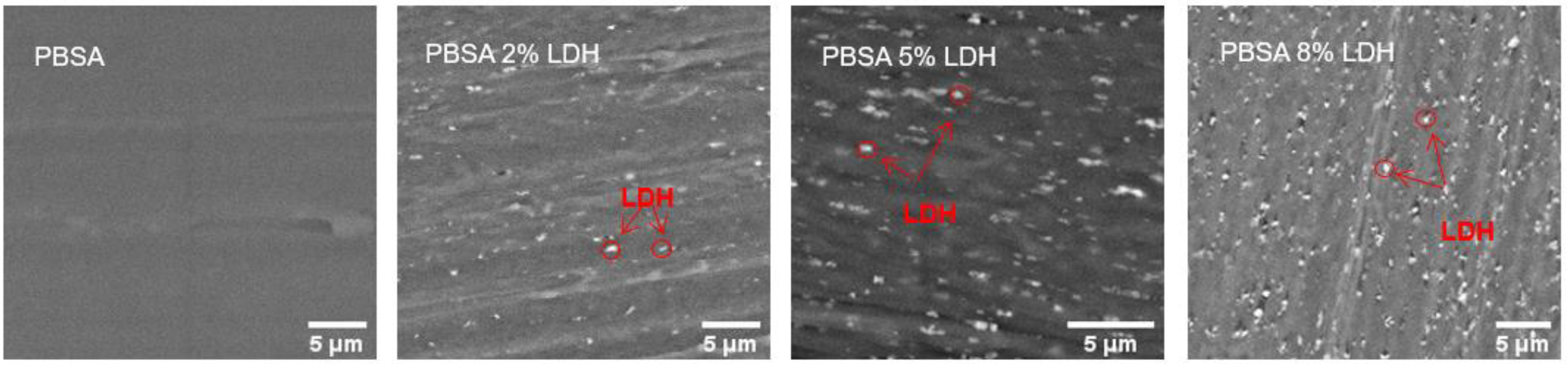

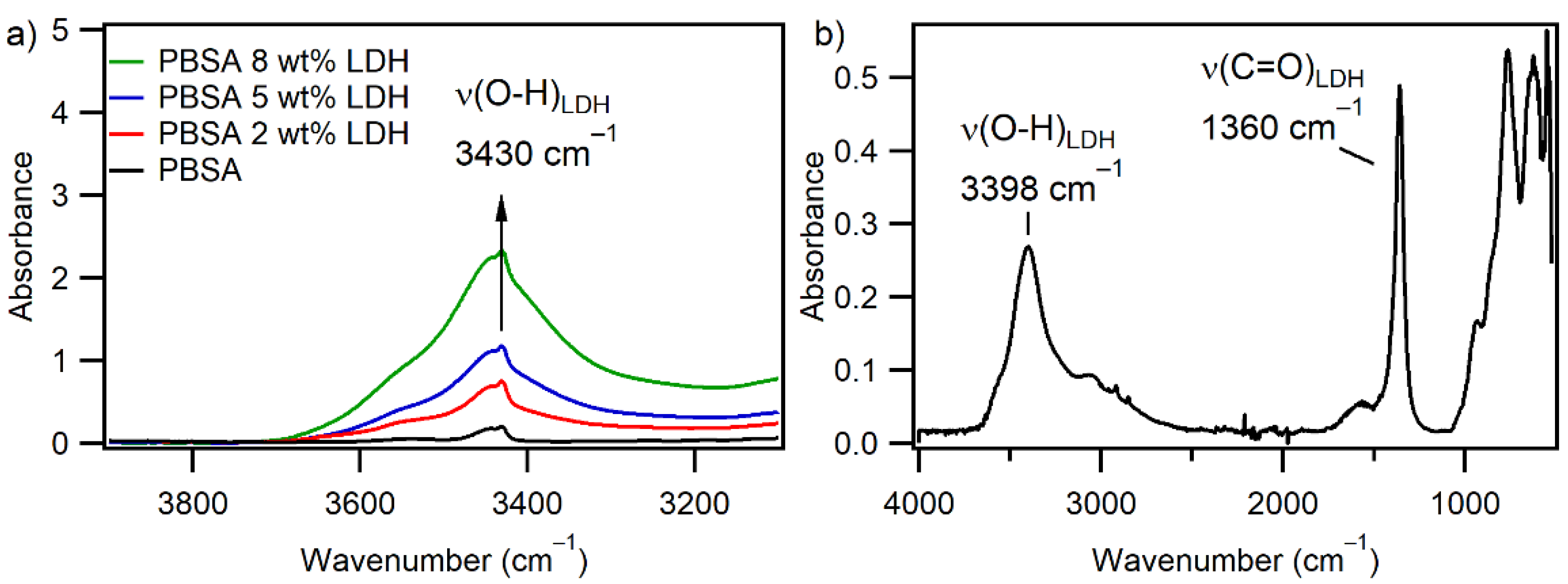
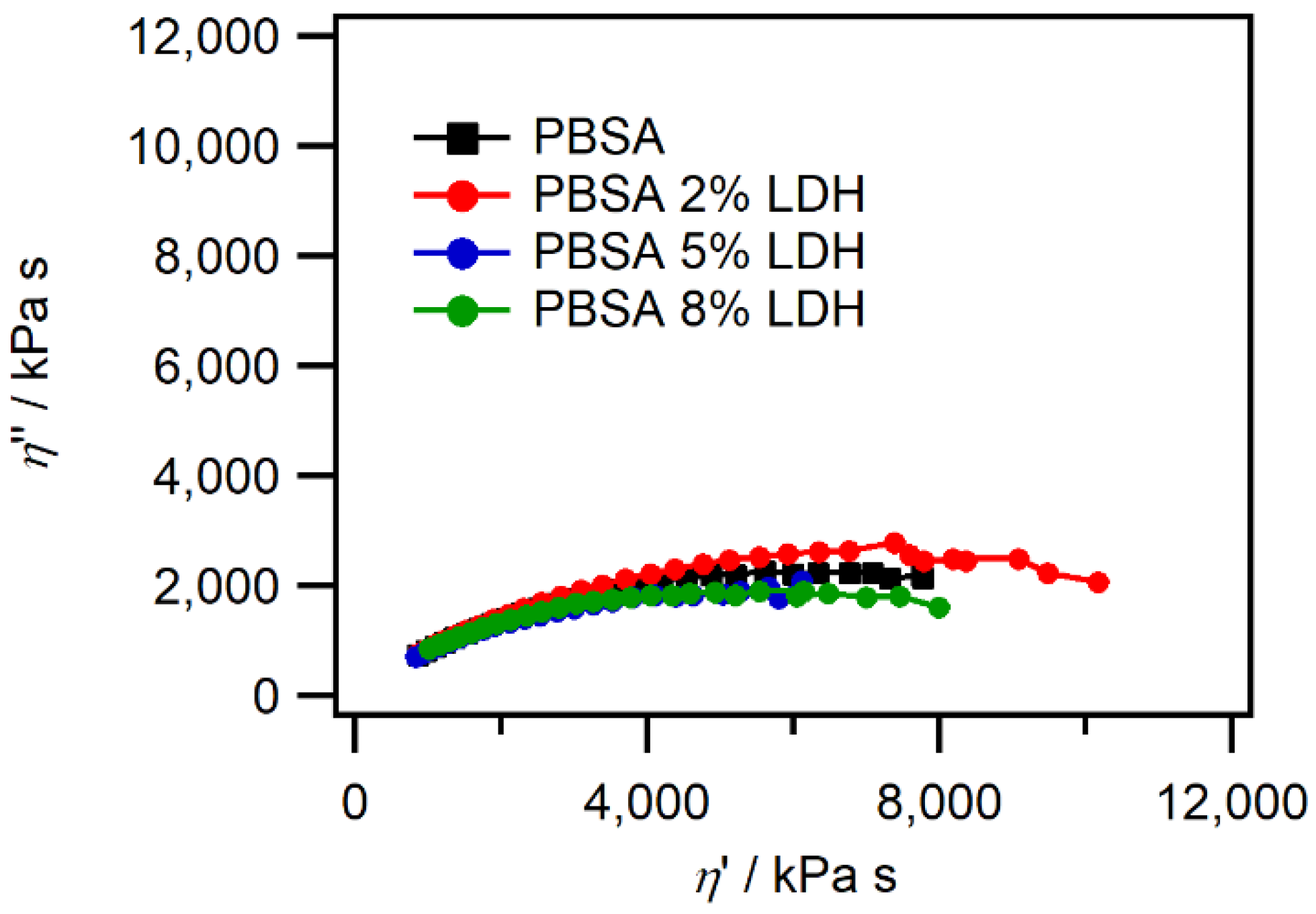
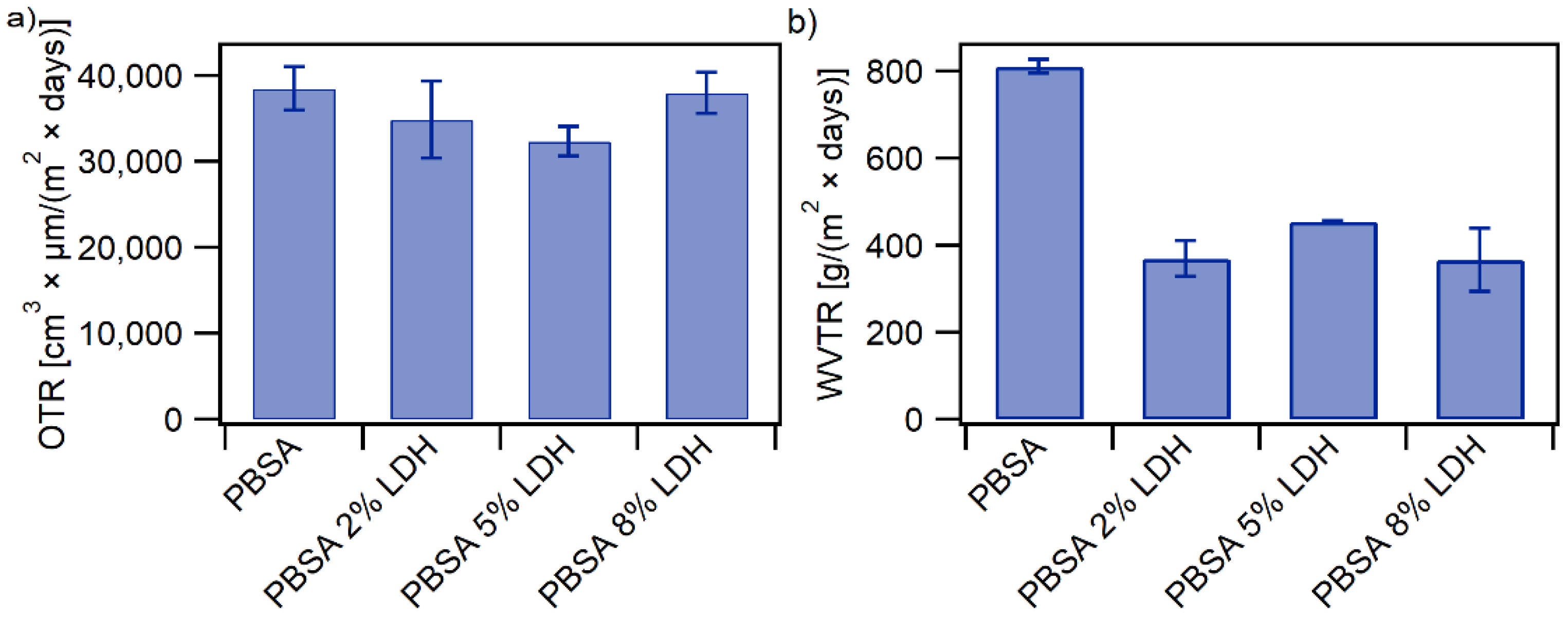


| Film | Tm, °C | ∆Hm, J g−1 | Tc, °C | Xc, % |
|---|---|---|---|---|
| PBSA | 89.1 | 42.4 | 56.0 | 36.3 |
| PBSA 2 wt% LDH | 88.9 | 41.3 | 55.1 | 36.1 |
| PBSA 5 wt% LDH | 88.1 | 37.3 | 50.8 | 33.6 |
| PBSA 8 wt% LDH | 87.7 | 34.9 | 49.8 | 32.5 |
| Film | Tensile Modulus, MPa | Stress at Break, MPa | Strain at Break, % | Tensile Modulus, MPa | Stress at Break, MPa | Strain at Break, % |
|---|---|---|---|---|---|---|
| Machine Direction | Transverse Direction | |||||
| PBSA | 172 ± 26 | 20.0 ± 0.8 | 520 ± 16 | 187 ± 54 | 21.9 ± 3.4 | 561 ± 60 |
| PBSA 2 wt% LDH | 255 ± 24 | 24.8 ± 2.5 | 19.4 ± 1.2 | 254 ± 7 | 24.1 ± 0.6 | 17.5 ± 1.0 |
| PBSA 5 wt% LDH | 335 ± 6 | 29.8 ± 0.5 | 18.4 ± 0.6 | 272 ± 8 | 24.4 ± 0.4 | 16.0 ± 1.6 |
| PBSA 8 wt% LDH | 212 ± 13 | 19.0 ± 1.0 | 15.6 ± 1.5 | 242 ± 9 | 19.6 ± 1.7 | 13.5 ± 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delorme, A.E.; Radusin, T.; Myllytie, P.; Verney, V.; Askanian, H. Enhancement of Gas Barrier Properties and Durability of Poly(butylene succinate-co-butylene adipate)-Based Nanocomposites for Food Packaging Applications. Nanomaterials 2022, 12, 978. https://doi.org/10.3390/nano12060978
Delorme AE, Radusin T, Myllytie P, Verney V, Askanian H. Enhancement of Gas Barrier Properties and Durability of Poly(butylene succinate-co-butylene adipate)-Based Nanocomposites for Food Packaging Applications. Nanomaterials. 2022; 12(6):978. https://doi.org/10.3390/nano12060978
Chicago/Turabian StyleDelorme, Astrid E., Tanja Radusin, Petri Myllytie, Vincent Verney, and Haroutioun Askanian. 2022. "Enhancement of Gas Barrier Properties and Durability of Poly(butylene succinate-co-butylene adipate)-Based Nanocomposites for Food Packaging Applications" Nanomaterials 12, no. 6: 978. https://doi.org/10.3390/nano12060978
APA StyleDelorme, A. E., Radusin, T., Myllytie, P., Verney, V., & Askanian, H. (2022). Enhancement of Gas Barrier Properties and Durability of Poly(butylene succinate-co-butylene adipate)-Based Nanocomposites for Food Packaging Applications. Nanomaterials, 12(6), 978. https://doi.org/10.3390/nano12060978








