Tailoring Nanoadsorbent Surfaces: Separation of Rare Earths and Late Transition Metals in Recycling of Magnet Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of SiO2 Nanoparticles
2.3. Surface Functionalization
2.4. Adsorption Isotherms and Kinetics with Late Transition Metals (LTMs) and REEs
2.5. Selectivity of Functionalized SiO2 Nanoparticles
2.6. Characterization
3. Results and Discussion
3.1. Characterization
3.2. Adsorption Equilibrium Isotherms
3.3. Selectivity and Desorption Tests with Functionalized SiO2 Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Mancheri, N.A.; Sprecher, B.; Bailey, G.; Ge, J.; Tukker, A. Effect of Chinese policies on rare earth supply chain resilience. Resour. Conserv. Recycl. 2019, 142, 101–112. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.M.; Van Gerven, T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Tripathy, P.K.; Mondal, K.; Khanolkar, A.R. One-step manufacturing process for neodymium-iron (magnet-grade) master alloy. Mater. Sci. Energy Technol. 2021, 4, 249–255. [Google Scholar] [CrossRef]
- Gergoric, M.; Ekberg, C.; Foreman, M.R.S.J.; Steenari, B.M.; Retegan, T. Characterization and Leaching of Neodymium Magnet Waste and Solvent Extraction of the Rare-Earth Elements Using TODGA. J. Sustain. Metall. 2017, 3, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.S.; Kim, C.J.; Chung, K.W.; Lee, S.J.; Joe, A.R.; Shin, Y.H.; Lee, S.I.; Yoo, S.J.; Kim, J.G. Leaching kinetics of neodymium in sulfuric acid from E-scrap of NdFeB permanent magnet. Korean J. Chem. Eng. 2014, 31, 706–711. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, Y.J.; Liao, C.H.; Popuri, S.R.; Tsai, S.L.; Hung, C.E. Selective leaching process for neodymium recovery from scrap Nd-Fe-B magnet. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 5825–5833. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Legaria, E.P.; Kessler, V. Separation of Rare Earth Elements from Other Elements. US Patent 10787722, 29 September 2020. [Google Scholar]
- Prodius, D.; Klocke, M.; Smetana, V.; Alammar, T.; Perez Garcia, M.; Windus, T.L.; Nlebedim, I.C.; Mudring, A.V. Rationally designed rare earth separation by selective oxalate solubilization. Chem. Commun. 2020, 56, 11386–11389. [Google Scholar] [CrossRef]
- Li, C.; Ramasamy, D.L.; Sillanpää, M.; Repo, E. Separation and concentration of rare earth elements from wastewater using electrodialysis technology. Sep. Purif. Technol. 2021, 254, 117442. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, C.; Li, F.; Dong, Y.; Sun, X. Process for the separation of thorium and rare earth elements from radioactive waste residues using Cyanex® 572 as a new extractant. Hydrometallurgy 2017, 169, 158–164. [Google Scholar] [CrossRef]
- García, A.C.; Latifi, M.; Amini, A.; Chaouki, J. Separation of radioactive elements from rare earth element-bearing minerals. Metals 2020, 10, 1524. [Google Scholar] [CrossRef]
- Hu, Y.; Florek, J.; Larivière, D.; Fontaine, F.G.; Kleitz, F. Recent advances in the separation of rare earth elements using mesoporous hybrid materials. Chem. Rec. 2018, 18, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Vardanyan, A.; Charnay, C.; Raehm, L.; Seisenbaeva, G.A.; Pleixats, R.; Durand, J.O. Synthesis of Cyclen-Functionalized Ethenylene-Based Periodic Mesoporous Organosilica Nanoparticles and Metal-Ion Adsorption Studies. ChemNanoMat 2020, 6, 1625–1634. [Google Scholar] [CrossRef]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Dudarko, O.; Kobylinska, N.; Mishra, B.; Kessler, V.G.; Tripathi, B.P.; Seisenbaeva, G.A. Facile strategies for synthesis of functionalized mesoporous silicas for the removal of rare-earth elements and heavy metals from aqueous systems. Microporous Mesoporous Mater. 2021, 315, 110919. [Google Scholar] [CrossRef]
- Shyam Sunder, G.S.; Rohanifar, A.; Alipourasiabi, N.; Lawrence, J.G.; Kirchhoff, J.R. Synthesis and Characterization of Poly(pyrrole-1-carboxylic acid) for Preconcentration and Determination of Rare Earth Elements and Heavy Metals in Water Matrices. ACS Appl. Mater. Interfaces 2021, 13, 34782–34792. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Ali, L.M.A.; Vardanyan, A.; Gary-Bobo, M.; Budnyak, T.M.; Kessler, V.G.; Durand, J.O. Mesoporous silica adsorbents modified with amino polycarboxylate ligands—Functional characteristics, health and environmental effects. J. Hazard. Mater. 2021, 406, 124698. [Google Scholar] [CrossRef] [PubMed]
- Ashour, R.M.; Samouhos, M.; Polido Legaria, E.; Svärd, M.; Högblom, J.; Forsberg, K.; Palmlöf, M.; Kessler, V.G.; Seisenbaeva, G.A.; Rasmuson, Å.C. DTPA-Functionalized Silica Nano- and Microparticles for Adsorption and Chromatographic Separation of Rare Earth Elements. ACS Sustain. Chem. Eng. 2018, 6, 6889–6900. [Google Scholar] [CrossRef] [Green Version]
- Florek, J.; Larivière, D.; Kählig, H.; Fiorilli, S.L.; Onida, B.; Fontaine, F.G.; Kleitz, F. Understanding Selectivity of Mesoporous Silica-Grafted Diglycolamide-Type Ligands in the Solid-Phase Extraction of Rare Earths. ACS Appl. Mater. Interfaces 2020, 12, 57003–57016. [Google Scholar] [CrossRef]
- Abdel-Magied, A.F.; Abdelhamid, H.N.; Ashour, R.M.; Zou, X.; Forsberg, K. Hierarchical porous zeolitic imidazolate frameworks nanoparticles for efficient adsorption of rare-earth elements. Microporous Mesoporous Mater. 2019, 278, 175–184. [Google Scholar] [CrossRef]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef] [PubMed]
- Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M. Extraction and Separation of Rare Earth Elements by Adsorption Approaches: Current Status and Future Trends. Sep. Purif. Rev. 2020, 50, 417–444. [Google Scholar] [CrossRef]
- Kobylinska, N.; Dudarko, O.; Kessler, V.; Seisenbaeva, G. Enhanced removal of Cr (III), Mn (II), Cd (II), Pb (II) and Cu (II) from aqueous solution by N-functionalized ordered silica. Chem. Afr. 2021, 4, 451–461. [Google Scholar] [CrossRef]
- Topel, S.D.; Legaria, E.P.; Tiseanu, C.; Rocha, J.; Nedelec, J.M.; Kessler, V.; Seisenbaeva, G.A. Hybrid silica nanoparticles for sequestration and luminescence detection of trivalent rare-earth ions (Dy3+ and Nd3+) in solution. J. Nanoparticle Res. 2014, 16, 2783. [Google Scholar] [CrossRef]
- Polido Legaria, E.; Samouhos, M.; Kessler, V.G.; Seisenbaeva, G.A. Toward Molecular Recognition of REEs: Comparative Analysis of Hybrid Nanoadsorbents with the Different Complexonate Ligands EDTA, DTPA, and TTHA. Inorg. Chem. 2017, 56, 13938–13948. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, D.L.; Khan, S.; Repo, E.; Sillanpää, M. Synthesis of mesoporous and microporous amine and non-amine functionalized silica gels for the application of rare earth elements (REE) recovery from the waste water-understanding the role of pH, temperature, calcination and mechanism in Light REE and Hea. Chem. Eng. J. 2017, 322, 56–65. [Google Scholar] [CrossRef]
- Noack, C.W.; Perkins, K.M.; Callura, J.C.; Washburn, N.R.; Dzombak, D.A.; Karamalidis, A.K. Effects of ligand chemistry and geometry on rare earth element partitioning from saline solutions to functionalized adsorbents. ACS Sustain. Chem. Eng. 2016, 4, 6115–6124. [Google Scholar] [CrossRef]
- Da’na, E.; Sayari, A. Adsorption of heavy metals on amine-functionalized SBA-15 prepared by co-condensation: Applications to real water samples. Desalination 2012, 285, 62–67. [Google Scholar] [CrossRef]
- Inoue, K.; Yoshizuka, K.; Ohto, K. Adsorptive separation of some metal ions by complexing agent types of chemically modified chitosan. Anal. Chim. Acta 1999, 388, 209–218. [Google Scholar] [CrossRef]
- Repo, E.; Kurniawan, T.A.; Warchol, J.K.; Sillanpää, M.E.T. Removal of Co(II) and Ni(II) ions from contaminated water using silica gel functionalized with EDTA and/or DTPA as chelating agents. J. Hazard. Mater. 2009, 171, 1071–1080. [Google Scholar] [CrossRef]
- Cegłowski, M.; Schroeder, G. Removal of heavy metal ions with the use of chelating polymers obtained by grafting pyridine-pyrazole ligands onto polymethylhydrosiloxane. Chem. Eng. J. 2015, 259, 885–893. [Google Scholar] [CrossRef]
- Yanovska, E.S.; Vretik, L.O.; Nikolaeva, O.A.; Polonska, Y.; Sternik, D.; Kichkiruk, O.Y. Synthesis and Adsorption Properties of 4-Vinylpyridine and Styrene Copolymer In Situ Immobilized on Silica Surface. Nanoscale Res. Lett. 2017, 12, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Matveichuk, Y.V.; Rakhman’Ko, E.M.; Yasinetskii, V.V. Thiocyanate complexes of d metals: Study of aqueous solutions by UV, visible, and IR spectrometry. Russ. J. Inorg. Chem. 2015, 60, 100–104. [Google Scholar] [CrossRef]
- Deng, Y.; Bai, W.; Zhang, X.; Chen, J.; Wang, S.; Lin, J.; Xu, Y. Effect of Silane on the Active Aging Resistance and Anticorrosive Behaviors of Natural Lacquer. ACS Omega 2018, 3, 4129–4140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Qi, Y.; Zhang, Z.; Yang, Q. Effect of N-(2-aminoethyl)-3-aminopropyltrimethoxysilane on the adhesion of themodified silicone tie-coating to epoxy primer. Coatings 2021, 11, 71. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Tzvetkova, P.; Nickolov, R. Modified and Unmodified Silica Gel used for Heavy Metal Ions Removal from Aqueous Solutions. J. Univ. Chem. Technol. Metall. 2012, 47, 498–504. [Google Scholar]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Barczak, M. Functionalization of mesoporous silica surface with carboxylic groups by Meldrum’s acid and its application for sorption of proteins. J. Porous Mater. 2019, 26, 291–300. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, T.F.; Morris, M.A. Hydroxylation methods for mesoporous silica and their impact on surface functionalisation. Microporous Mesoporous Mater. 2021, 317, 110989. [Google Scholar] [CrossRef]
- Tomina, V.V.; Mel’Nik, I.V.; Pogorilyi, R.P.; Kochkodan, V.M.; Zub, Y.L. Functionalization of the surface of ceramic membranes with 3-mercaptopropyl groups using the sol-gel method. Prot. Met. Phys. Chem. Surf. 2013, 49, 386–391. [Google Scholar] [CrossRef]
- Manousi, N.; Kabir, A.; Furton, K.G.; Zachariadis, G.A.; Anthemidis, A. Multi-Element Analysis Based on an Automated On-Line Microcolumn Separation/Preconcentration System Using a Novel Sol-Gel Thiocyanatopropyl-Functionalized Silica Sorbent Prior to ICP-AES for Environmental Water Samples. Molecules 2021, 26, 4461. [Google Scholar] [CrossRef]
- Bois, L.; Bonhommé, A.; Ribes, A.; Pais, B.; Raffin, G.; Tessier, F. Functionalized silica for heavy metal ions adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2003, 221, 221–230. [Google Scholar] [CrossRef]
- Lee, B.; Kim, Y.; Lee, H.; Yi, J. Synthesis of functionalized porous silicas via templating method as heavy metal ion adsorbents: The introduction of surface hydrophilicity onto the surface of adsorbents. Microporous Mesoporous Mater. 2001, 50, 77–90. [Google Scholar] [CrossRef]
- Liu, A.M.; Hidajat, K.; Kawi, S.; Zhao, D.Y. A new class of hybrid mesaporous materials with functionalized organic monolayers for selective adsorption of heavy metal ions. Chem. Commun. 2000, 15, 1145–1146. [Google Scholar] [CrossRef]
- Polido Legaria, E.; Topel, S.D.; Kessler, V.G.; Seisenbaeva, G.A. Molecular insights into the selective action of a magnetically removable complexone-grafted adsorbent. Dalton Trans. 2014, 44, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Tavlarides, L.L. Adsorption of neodymium(III) from aqueous solutions using a phosphorus functionalized adsorbent. Ind. Eng. Chem. Res. 2010, 49, 12567–12575. [Google Scholar] [CrossRef]
- Galhoum, A.A.; Mahfouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Atia, A.A.; Vincent, T.; Guibal, E. Diethylenetriamine-functionalized chitosan magnetic nano-based particles for the sorption of rare earth metal ions [Nd(III), Dy(III) and Yb(III)]. Cellulose 2015, 22, 2589–2605. [Google Scholar] [CrossRef]
- Aghayan, H.; Mahjoub, A.R.; Khanchi, A.R. Samarium and dysprosium removal using 11-molybdo-vanadophosphoric acid supported on Zr modified mesoporous silica SBA-15. Chem. Eng. J. 2013, 225, 509–519. [Google Scholar] [CrossRef]
- Ettehadi Gargari, J.; Sid Kalal, H.; Shakeri, A.; Khanchi, A. Synthesis and characterization of Silica/polyvinyl imidazole/H2PO4-core-shell nanoparticles as recyclable adsorbent for efficient scavenging of Sm(III) and Dy(III) from water. J. Colloid Interface Sci. 2017, 505, 745–755. [Google Scholar] [CrossRef]
- He, H.; Gan, Q.; Feng, C. Preparation and application of Ni(ii) ion-imprinted silica gel polymer for selective separation of Ni(ii) from aqueous solution. RSC Adv. 2017, 7, 15102–15111. [Google Scholar] [CrossRef] [Green Version]
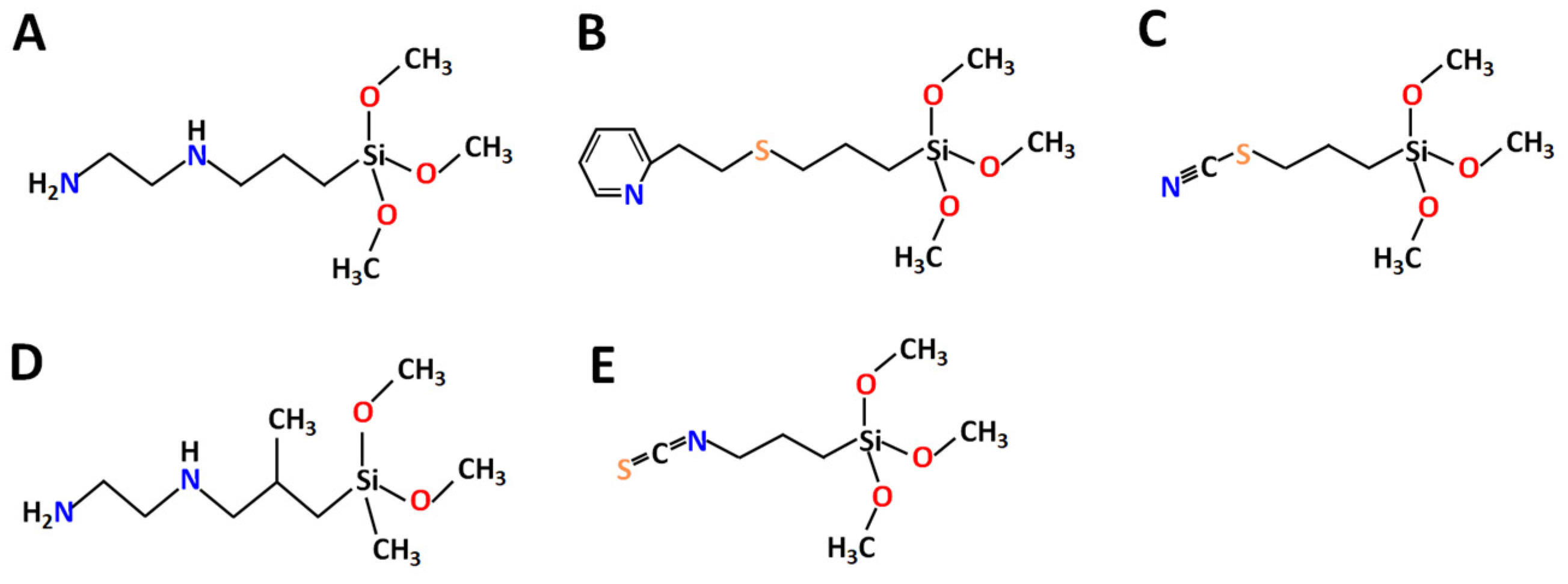


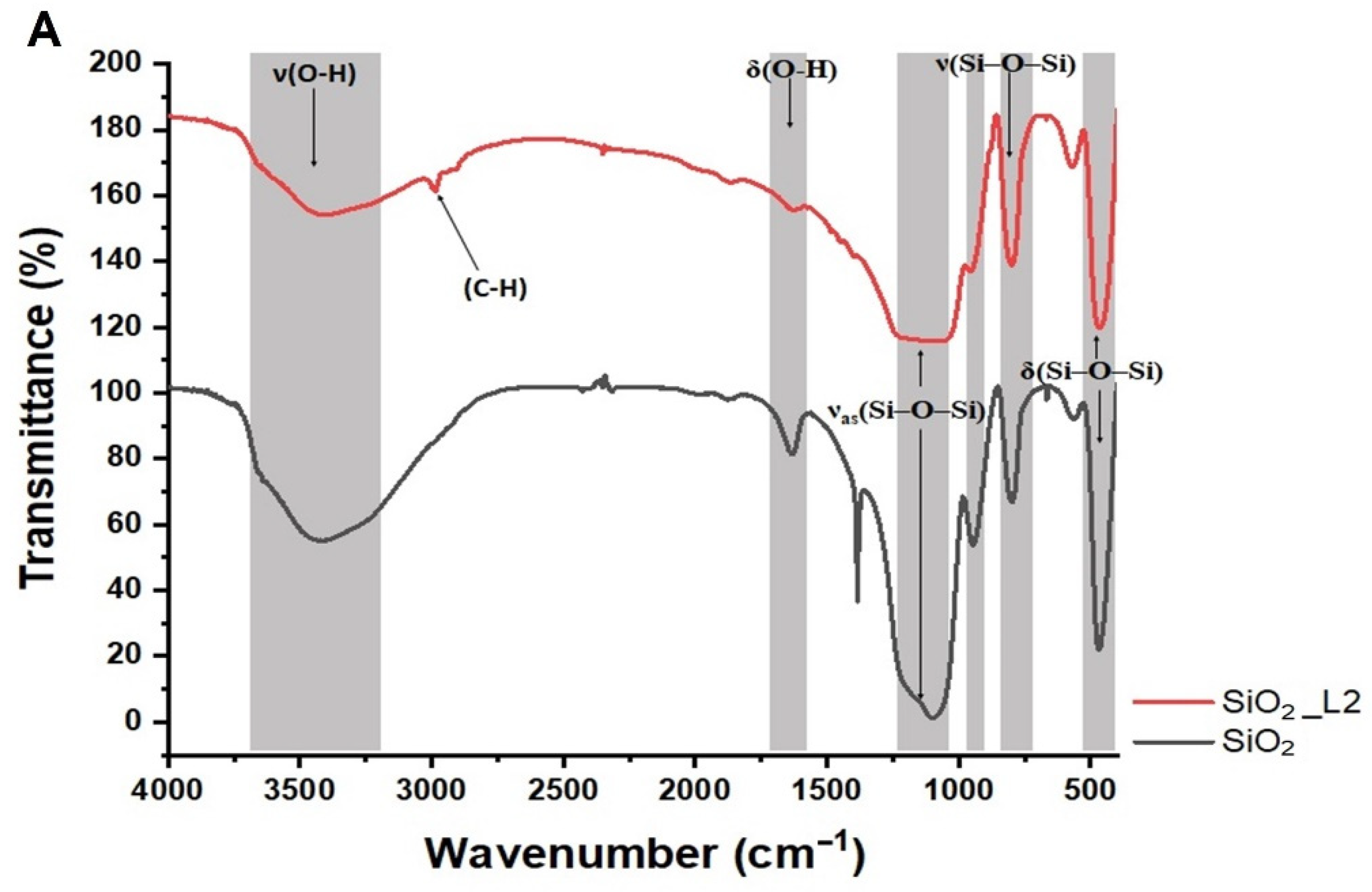
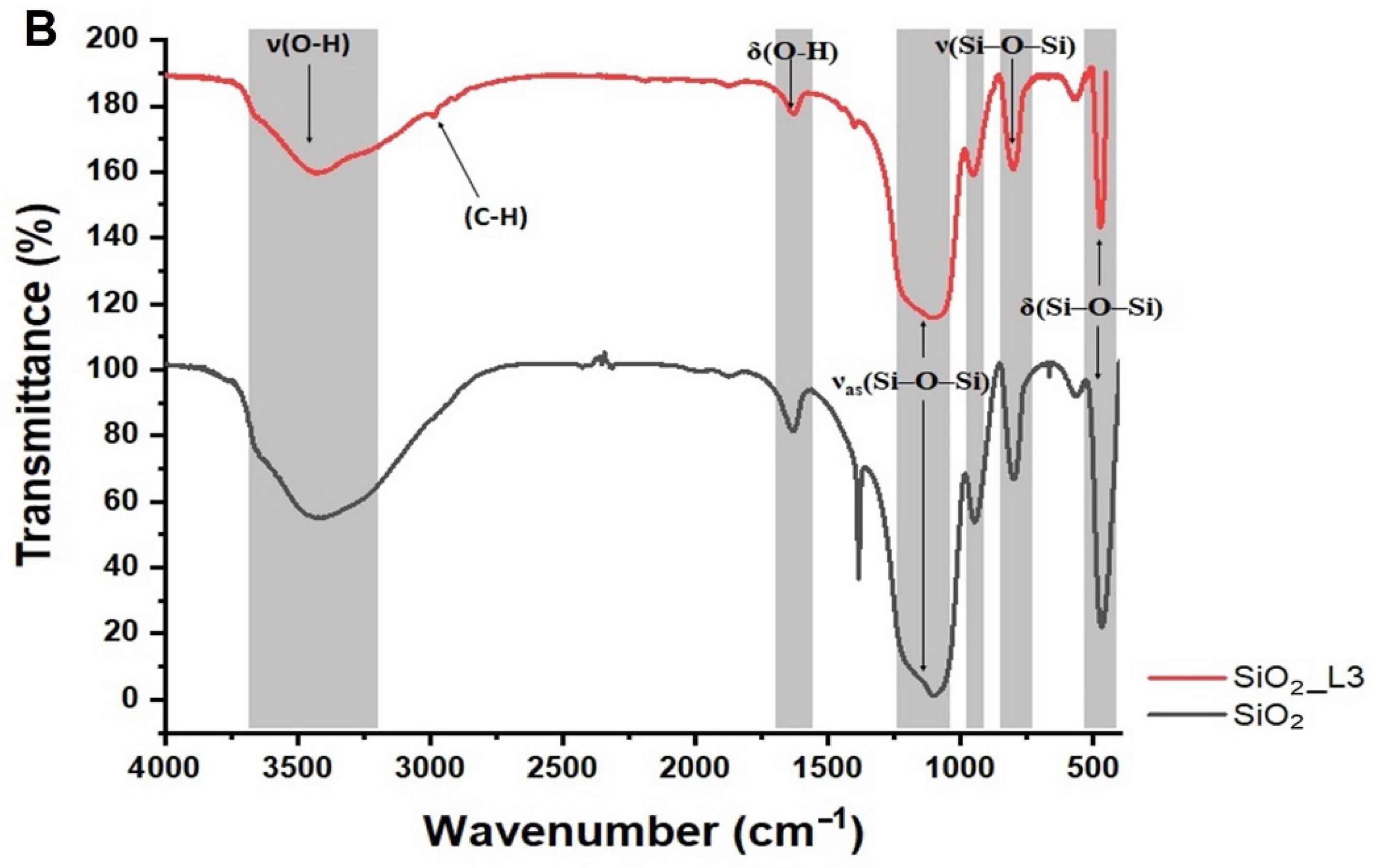
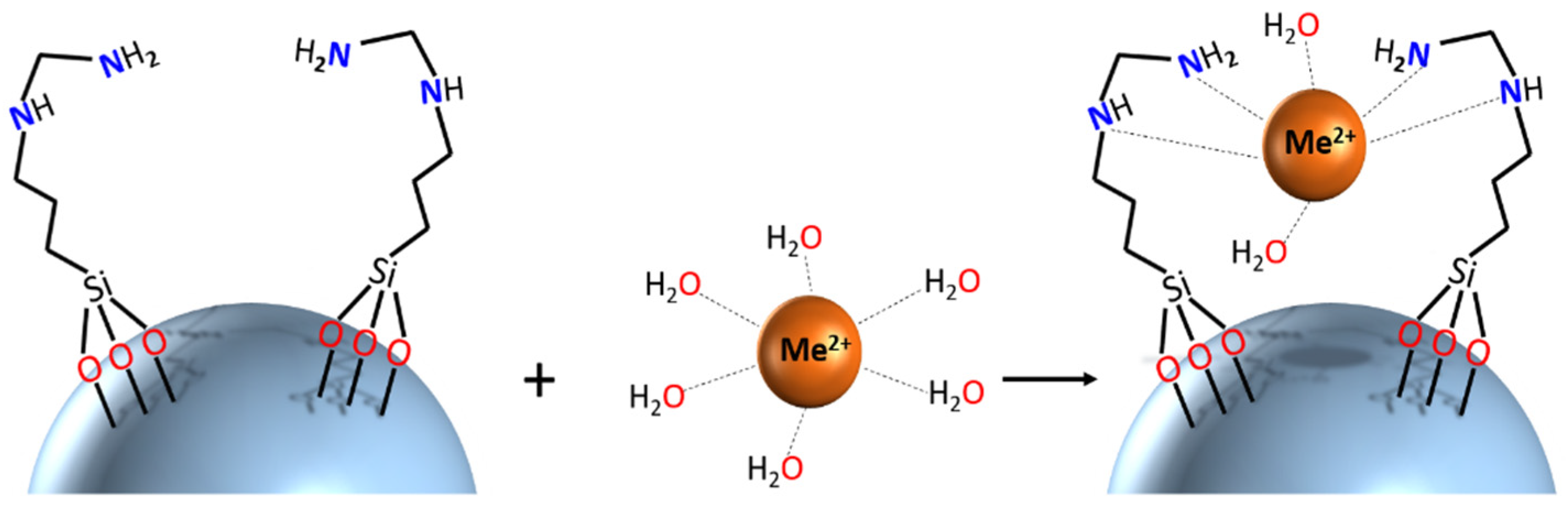


| Sample | Amount of Grafted Ligand (%) | Amount of Grafted Ligand (mmol/g) |
|---|---|---|
| SiO2_L1 | 23.45 | 1.61 |
| SiO2_L2 | 10.32 | 0.45 |
| SiO2_L3 | 9.28 | 0.63 |
| SiO2_L4 | 9.24 | 0.54 |
| SiO2_L5 | 9.62 | 0.65 |
| SiO2_L3_acid | 15.42 | 1.02 |
| SiO2_L4_acid | 9.23 | 0.54 |
| SiO2_L5_acid | 15.86 | 1.07 |
| Sample | Ni (mmol/g) | Co (mmol/g) | Nd (mmol/g) | Sm (mmol/g) |
|---|---|---|---|---|
| SiO2_L1 | 1.66 | 1.83 | 0.83 | 1.10 |
| SiO2_L2 | 0.55 | 0.66 | 0.50 | 0.56 |
| SiO2_L3 | 0.58 | 0.75 | 0.67 | 0.66 |
| SiO2_L4 | 0.57 | 0.67 | 0.44 | 0.77 |
| SiO2_L5 | 0.34 | 0.68 | 0.44 | 0.68 |
| SiO2_L3_acid | 1.00 | 1.33 | 0.75 | 0.83 |
| SiO2_L5_acid | 1.66 | 1.33 | 0.75 | 1.00 |
| Adsorbent | Metal | Uptake (mmol/g) | References |
|---|---|---|---|
| Phosphorus functionalized adsorbent | Nd(III) | 1.13 | [49] |
| DETA-functionalized chitosan magnetic nano-based particles | Nd(III) | 0.35 | [50] |
| Zr modified mesoporous silica SBA-15 | Sm(III) | 0.28 | [51] |
| Silica/polyvinyl imidazole/H2PO4-core-shell NPs | Sm(III) | 1.04 | [52] |
| Cyclen-functionalized ethenylene-based mesoporous organosilica NPs | Ni(II) | 3.94 | [14] |
| Cyclen-functionalized ethenylene-based mesoporous organosilica NPs | Co(II) | 3.84 | [14] |
| Ni(II) ion-imprinted silica gel polymer | Ni(II) | 0.35 | [53] |
| Sample | Ni/Nd | Co/Sm | Ni/Co |
|---|---|---|---|
| SiO2_L1 | 5.1:1 | 1:1.65 | 3.5:1 |
| SiO2_L2 | 1:1.78 | 1:12 | 1.6:1 |
| SiO2_L3 | 1:1 | 1:18 | 1:1 |
| SiO2_L4 | 1.33:1 | 1:18 | 1.85:1 |
| SiO2_L5 | 1:3 | 1:1 | 1.85:1 |
| SiO2_L3_acid | 1:2.5 | 6:1 | 1.3:1 |
| SiO2_L5_acid | 15:1 | 6:1 | 2.75:1 |
| Cycle 1 | Cycle 2 | Cycle 3 | ||||
|---|---|---|---|---|---|---|
| Sample | Adsorption (mmol/g) | Desorption (%) | Adsorption (mmol/g) | Desorption (%) | Adsorption (mmol/g) | Desorption (%) |
| SiO2_L1 | 1.66 | 100% | 1.5 | 100% | 1.33 | 90% |
| SiO2_L2 | 0.55 | 90% | 0.45 | 85% | 0.33 | 85% |
| SiO2_L3_acid | 1 | 100% | 0.88 | 95% | 0.83 | 90% |
| SiO2_L4 | 0.57 | 85% | 0.45 | 80% | 0.33 | 70% |
| SiO2_L5_acid | 1.66 | 95% | 1.5 | 95% | 1.4 | 90% |
| Cycle 1 | Cycle 2 | Cycle 3 | ||||
|---|---|---|---|---|---|---|
| Sample | Adsorption (mmol/g) | Desorption (%) | Adsorption (mmol/g) | Desorption (%) | Adsorption (mmol/g) | Desorption (%) |
| SiO2_L1 | 1.1 | 90% | 0.88 | 100% | 0.81 | 90% |
| SiO2_L2 | 0.56 | 85% | 0.45 | 85% | 0.33 | 70% |
| SiO2_L3_acid | 0.83 | 100% | 0.8 | 85% | 0.66 | 90% |
| SiO2_L4 | 0.77 | 80% | 0.55 | 80% | 0.43 | 70% |
| SiO2_L5_acid | 1 | 95% | 0.93 | 85% | 0.75 | 80% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vardanyan, A.; Guillon, A.; Budnyak, T.; Seisenbaeva, G.A. Tailoring Nanoadsorbent Surfaces: Separation of Rare Earths and Late Transition Metals in Recycling of Magnet Materials. Nanomaterials 2022, 12, 974. https://doi.org/10.3390/nano12060974
Vardanyan A, Guillon A, Budnyak T, Seisenbaeva GA. Tailoring Nanoadsorbent Surfaces: Separation of Rare Earths and Late Transition Metals in Recycling of Magnet Materials. Nanomaterials. 2022; 12(6):974. https://doi.org/10.3390/nano12060974
Chicago/Turabian StyleVardanyan, Ani, Anna Guillon, Tetyana Budnyak, and Gulaim A. Seisenbaeva. 2022. "Tailoring Nanoadsorbent Surfaces: Separation of Rare Earths and Late Transition Metals in Recycling of Magnet Materials" Nanomaterials 12, no. 6: 974. https://doi.org/10.3390/nano12060974
APA StyleVardanyan, A., Guillon, A., Budnyak, T., & Seisenbaeva, G. A. (2022). Tailoring Nanoadsorbent Surfaces: Separation of Rare Earths and Late Transition Metals in Recycling of Magnet Materials. Nanomaterials, 12(6), 974. https://doi.org/10.3390/nano12060974









