Improving the Stability of Ball-Milled Lead Halide Perovskites via Ethanol/Water-Induced Phase Transition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Cs4PbBr6 Crystal
2.2.2. Mechanochemical Synthesis of Cs4PbBr6 Microcrystals (MCs)
2.2.3. Synthesis of Ethanol/Water-Induced CsPbBr3 MCs
2.2.4. Synthesis of CsPbBr3 MCs through the Hot-Injection Method
2.3. Structural and Optical Characterization
3. Results and Discussion
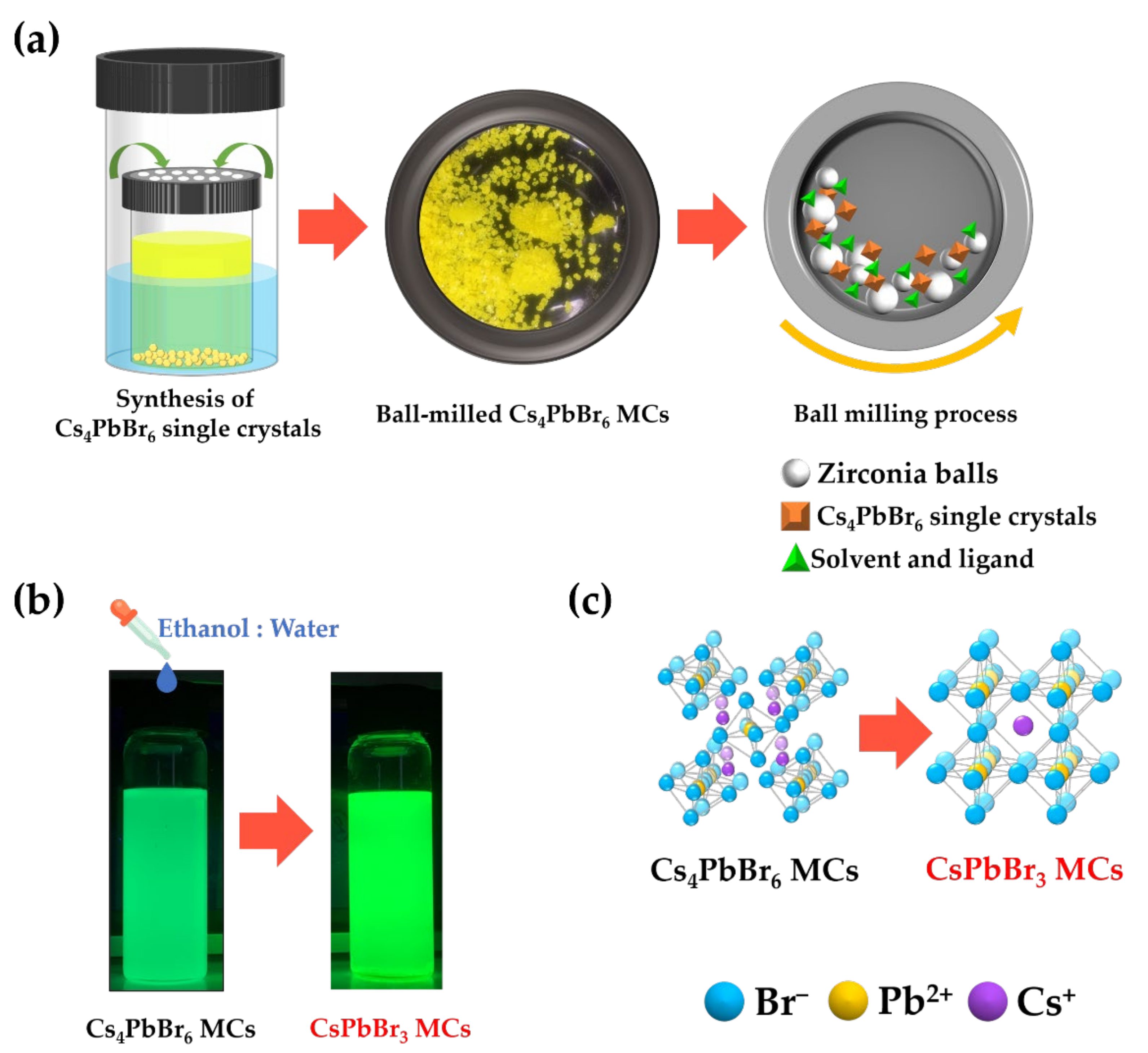
3.1. Synthesis of CsPbBr3 MCs
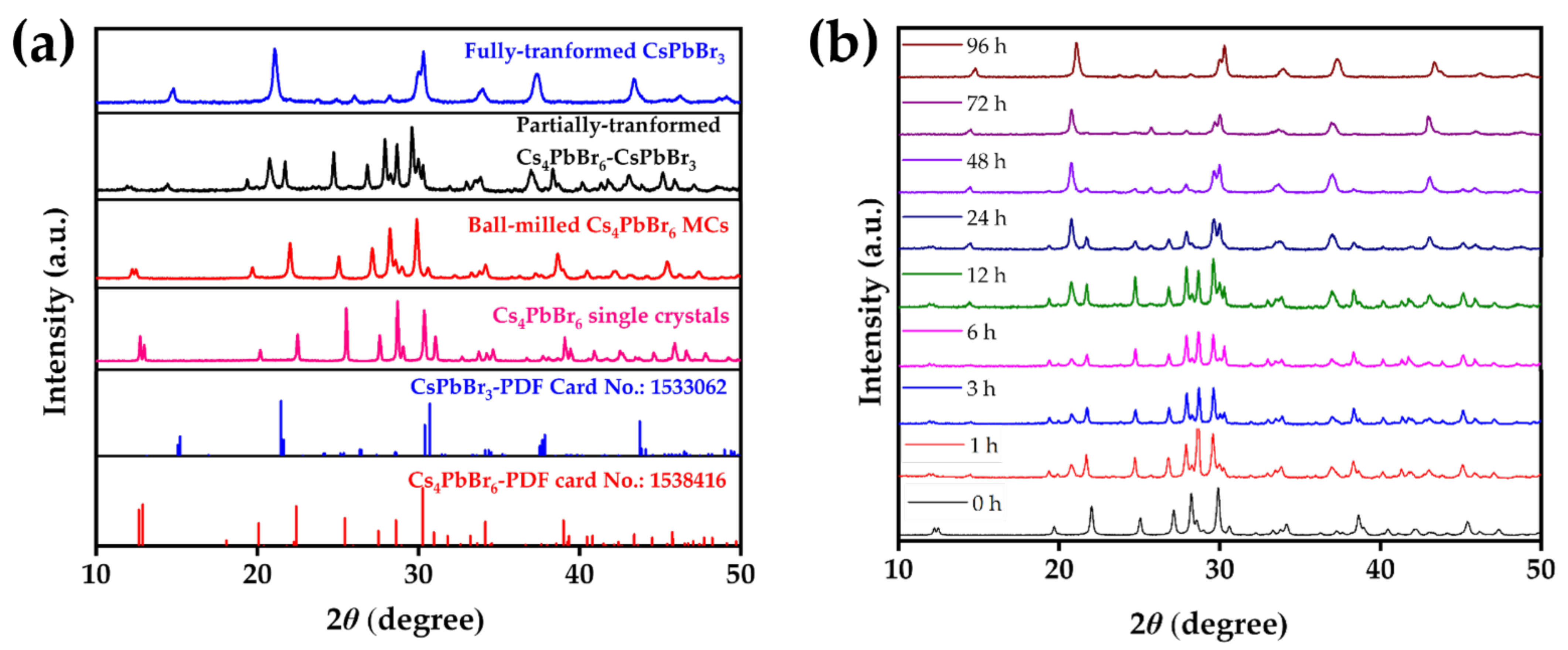
3.2. XRD Analysis
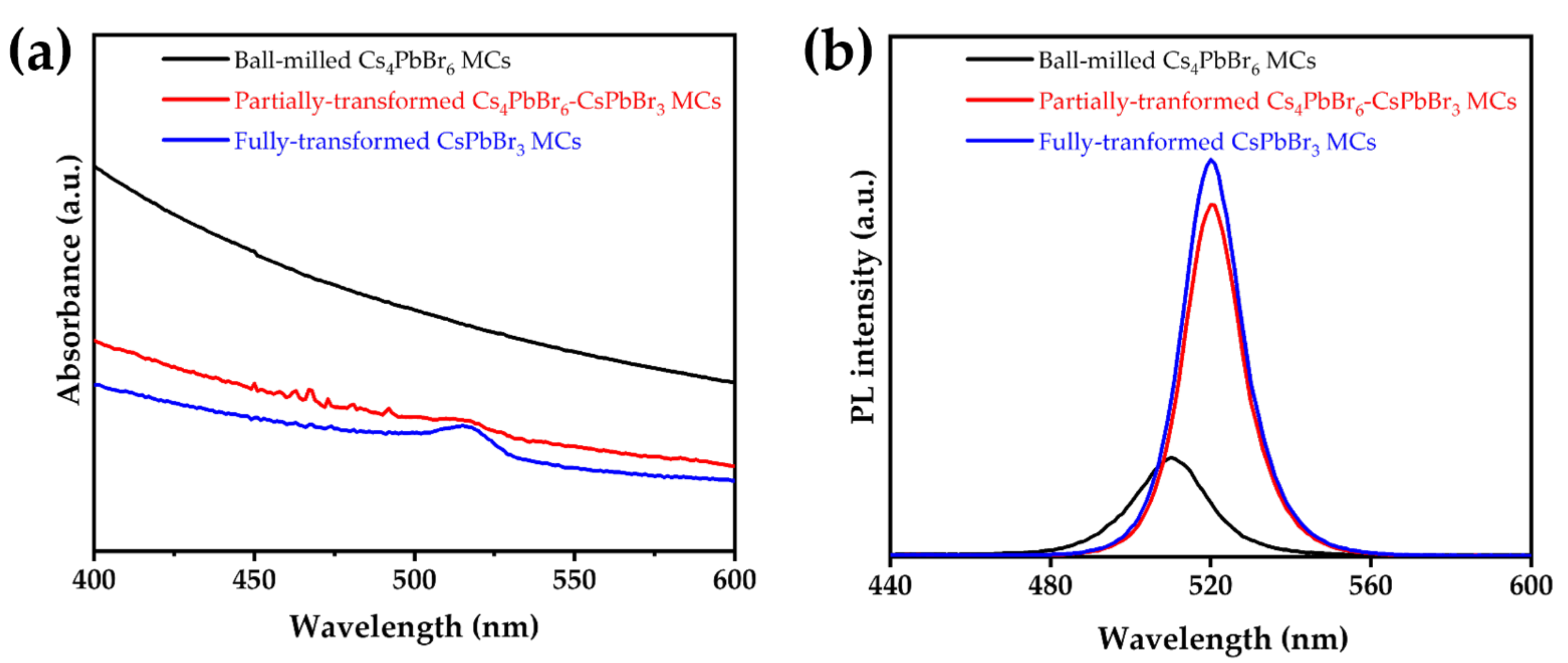
3.3. Absorption and Emission Spectroscopy
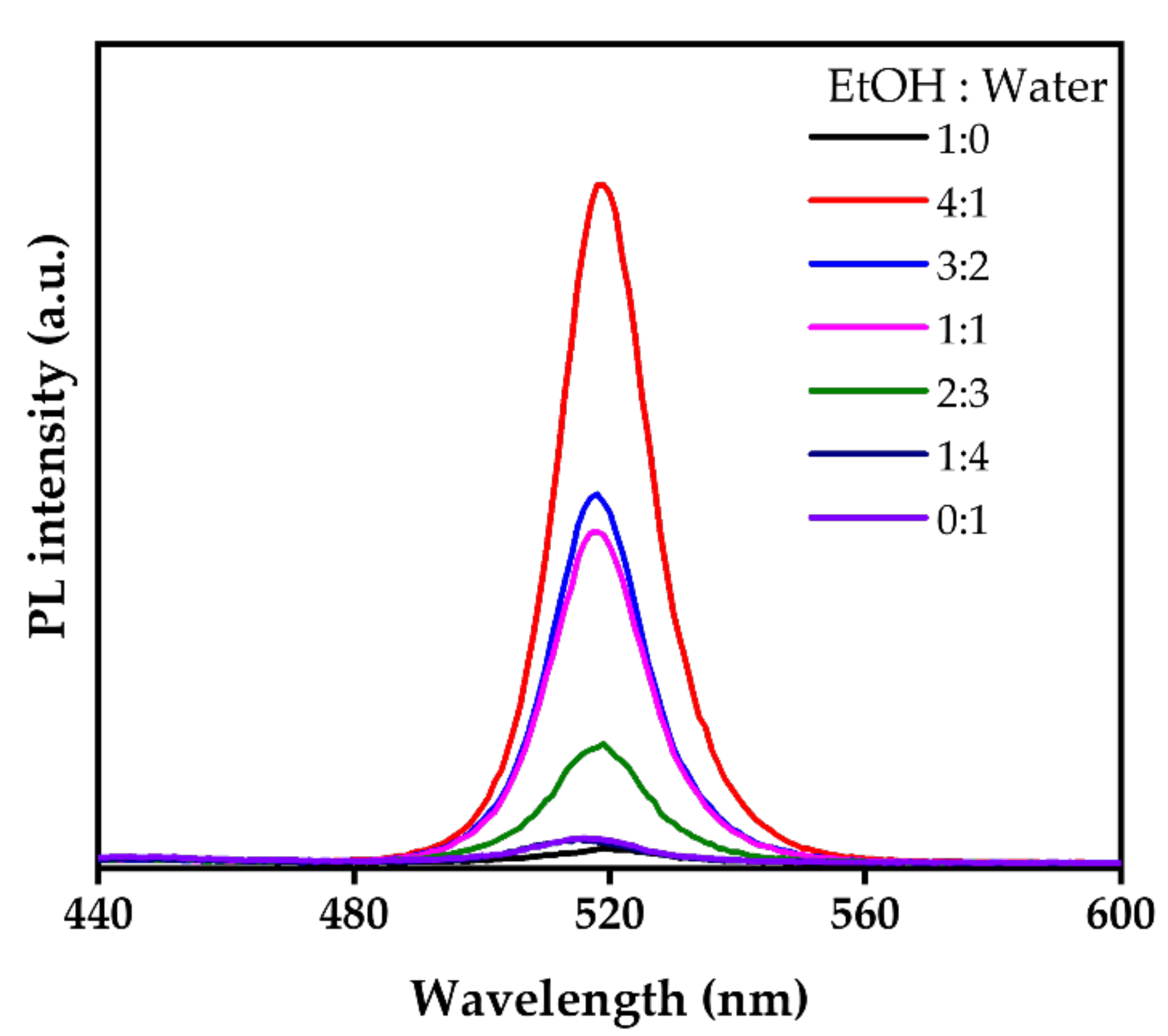
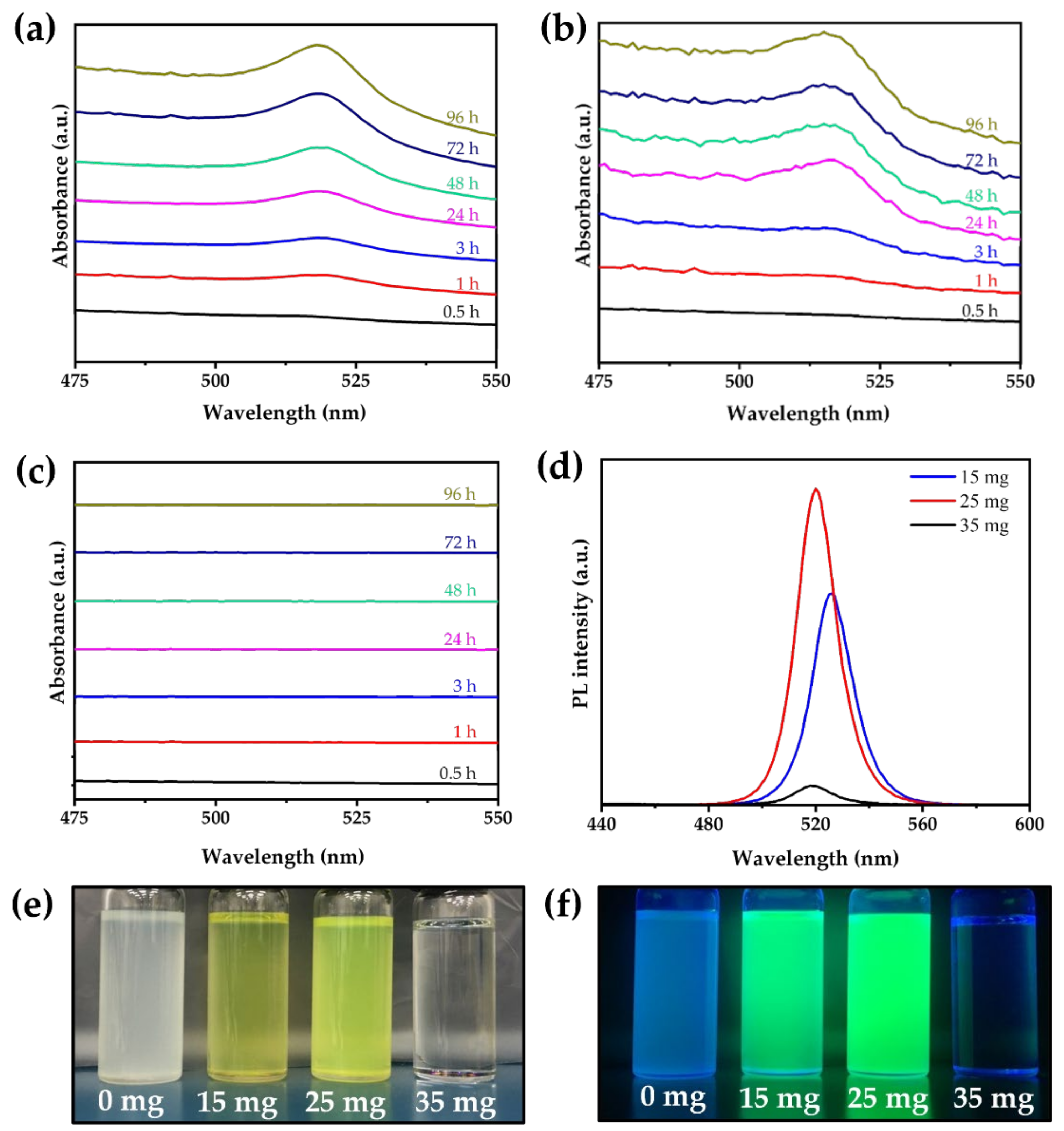
3.4. Phase Transformation Conditions and Mechanisms
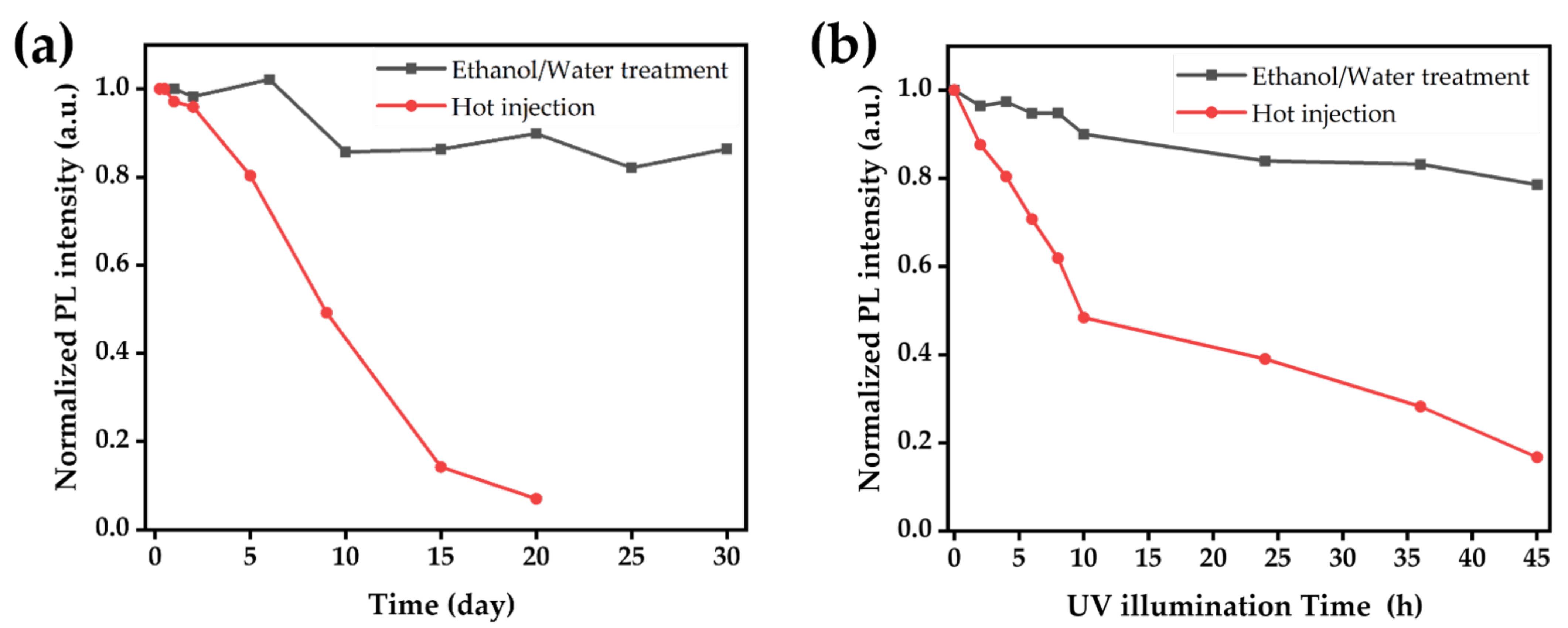
3.5. Stability of Ethanol/Water-Induced CsPbBr3 MCs
3.6. Thermal Stability of the CsPbBr3 MC Thin Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, N.; Li, F.; Turedi, B.; Sinatra, L.; Sarmah, S.P.; Parida, M.R.; Saidaminov, M.I.; Murali, B.; Burlakov, V.M.; Goriely, A. Pure crystal orientation and anisotropic charge transport in large-area hybrid perovskite films. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J. Highly efficient perovskite-quantum-dot light-emitting diodes by surface engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef]
- Adhikari, G.C.; Thapa, S.; Zhu, H.; Zhu, P. Mg2+-alloyed all-inorganic halide perovskites for white light-emitting diodes by 3D-printing method. Adv. Opt. Mater. 2019, 7, 1900916. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Yoon, H.C.; Kang, H.; Lee, S.; Oh, J.H.; Yang, H.; Do, Y.R. Study of perovskite QD down-converted LEDs and six-color white LEDs for future displays with excellent color performance. ACS Appl. Mater. Interfaces 2016, 8, 18189–18200. [Google Scholar] [CrossRef]
- Zhai, W.; Lin, J.; Li, C.; Hu, S.; Huang, Y.; Yu, C.; Wen, Z.; Liu, Z.; Fang, Y.; Tang, C. Solvothermal synthesis of cesium lead halide perovskite nanowires with ultra-high aspect ratios for high-performance photodetectors. Nanoscale 2018, 10, 21451–21458. [Google Scholar] [CrossRef]
- Ding, J.; Du, S.; Zuo, Z.; Zhao, Y.; Cui, H.; Zhan, X. High detectivity and rapid response in perovskite CsPbBr3 single-crystal photodetector. J. Phys. Chem. C 2017, 121, 4917–4923. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Adinolfi, V.; Comin, R.; Abdelhady, A.L.; Peng, W.; Dursun, I.; Yuan, M.; Hoogland, S.; Sargent, E.H.; Bakr, O.M. Planar-integrated single-crystalline perovskite photodetectors. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Nafradi, B.; Nafradi, G.; Forro, L.; Horvath, E. X-ray imaging: Perovskites target X-ray detection. Nat. Photonics 2016, 10, 288. [Google Scholar]
- Heo, J.H.; Shin, D.H.; Park, J.K.; Kim, D.H.; Lee, S.J.; Im, S.H. High-performance next-generation perovskite nanocrystal scintillator for nondestructive X-ray imaging. Adv. Mater. 2018, 30, 1801743. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fu, K.; Sun, R.; Lian, H.; Hu, X.; Zhang, Y. Ultra-stable CsPbBr3 perovskite nanosheets for X-ray imaging screen. Nano-Micro Lett. 2019, 11, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Chen, J.; Bakr, O.M.; Mohammed, O.F. Metal halide perovskites for X-ray imaging scintillators and detectors. ACS Energy Lett. 2021, 6, 739–768. [Google Scholar] [CrossRef]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; De Luca, G.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 2015, 6, 1–9. [Google Scholar]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Saidaminov, M.I.; Dursun, I.; Yang, H.; Murali, B.; Alarousu, E.; Yengel, E.; Alshankiti, B.A.; Bakr, O.M.; Mohammed, O.F. Zero-dimensional Cs4PbBr6 perovskite nanocrystals. J. Phys. Chem. Lett. 2017, 8, 961–965. [Google Scholar] [CrossRef]
- Krieg, F.; Ochsenbein, S.T.; Yakunin, S.; Ten Brinck, S.; Aellen, P.; Suess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y. Colloidal CsPbX3 (X = Cl, Br, I) nanocrystals 2.0: Zwitterionic capping ligands for improved durability and stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Pan, J.; Shang, Y.; Yin, J.; De Bastiani, M.; Peng, W.; Dursun, I.; Sinatra, L.; El-Zohry, A.M.; Hedhili, M.N.; Emwas, A.-H. Bidentate ligand-passivated CsPbI3 perovskite nanocrystals for stable near-unity photoluminescence quantum yield and efficient red light-emitting diodes. J. Am. Chem. Soc. 2017, 140, 562–565. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chesman, A.S.; Jasieniak, J.J. Stabilizing the cubic perovskite phase of CsPbI3 nanocrystals by using an alkyl phosphinic acid. Chem. Commun. 2017, 53, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T. Highly luminescent phase-stable CsPbI3 perovskite quantum dots achieving near 100% absolute photoluminescence quantum yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Guhrenz, C.; Kirch, A.; Sonntag, L.; Bauer, C.; Fan, X.; Wang, J.; Reineke, S.; Gaponik, N.; Eychmuller, A. Highly luminescent and water-resistant CsPbBr3–CsPb2Br5 perovskite nanocrystals coordinated with partially hydrolyzed poly (methyl methacrylate) and polyethylenimine. ACS Nano 2019, 13, 10386–10396. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, S.; Cao, F.; Zhou, J.; Wu, Q.; Wang, H.; Li, X.; Yin, L.; Yang, X. Ultrastable inorganic perovskite nanocrystals coated with a thick long-chain polymer for efficient white light-emitting diodes. Chem. Mater. 2019, 31, 1936–1940. [Google Scholar] [CrossRef]
- Imran, M.; Ijaz, P.; Goldoni, L.; Maggioni, D.; Petralanda, U.; Prato, M.; Almeida, G.; Infante, I.; Manna, L. Simultaneous cationic and anionic ligand exchange for colloidally stable CsPbBr3 nanocrystals. ACS Energy Lett. 2019, 4, 819–824. [Google Scholar] [CrossRef]
- Ashner, M.N.; Shulenberger, K.E.; Krieg, F.; Powers, E.R.; Kovalenko, M.V.; Bawendi, M.G.; Tisdale, W.A. Size-dependent biexciton spectrum in CsPbBr3 perovskite nanocrystals. ACS Energy Lett. 2019, 4, 2639–2645. [Google Scholar] [CrossRef]
- Wei, S.; Yang, Y.; Kang, X.; Wang, L.; Huang, L.; Pan, D. Room-temperature and gram-scale synthesis of CsPbX3 (X = Cl, Br, I) perovskite nanocrystals with 50–85% photoluminescence quantum yields. Chem. Commun. 2016, 52, 7265–7268. [Google Scholar] [CrossRef]
- Li, X.; Yu, D.; Cao, F.; Gu, Y.; Wei, Y.; Wu, Y.; Song, J.; Zeng, H. Healing all-inorganic perovskite films via recyclable dissolution–recyrstallization for compact and smooth carrier channels of optoelectronic devices with high stability. Adv. Funct. Mater. 2016, 26, 5903–5912. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- El Ajjouri, Y.; Palazon, F.; Sessolo, M.; Bolink, H.J. Single-source vacuum deposition of mechanosynthesized inorganic halide perovskites. Chem. Mater. 2018, 30, 7423–7427. [Google Scholar] [CrossRef] [Green Version]
- Manukyan, K.; Yeghishyan, A.; Moskovskikh, D.; Kapaldo, J.; Mintairov, A.; Mukasyan, A. Mechanochemical synthesis of methylammonium lead iodide perovskite. J. Mater. Sci. 2016, 51, 9123–9130. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Nazarenko, O.; Dirin, D.N.; Kovalenko, M.V. Low-cost synthesis of highly luminescent colloidal lead halide perovskite nanocrystals by wet ball milling. ACS Appl. Nano Mater. 2018, 1, 1300–1308. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Hu, H.; Xu, Y.; Jiang, S.; Chen, M.; Zhong, Q.; Yang, D.; Liu, Q.; Zhao, Y.; Sun, B. From nonluminescent Cs4PbX6 (X = Cl, Br, I) nanocrystals to highly luminescent CsPbX3 nanocrystals: Water-triggered transformation through a CsX-stripping mechanism. Nano Lett. 2017, 17, 5799–5804. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-H.; Han, J.H.; Yin, W.; Park, C.; Park, Y.; Ahn, T.K.; Cho, J.H.; Jung, D.-Y. Photoresponse of CsPbBr3 and Cs4PbBr6 perovskite single crystals. J. Phys. Chem. Lett. 2017, 8, 565–570. [Google Scholar] [CrossRef]
- Turedi, B.; Lee, K.J.; Dursun, I.; Alamer, B.; Wu, Z.; Alarousu, E.; Mohammed, O.F.; Cho, N.; Bakr, O.M. Water-induced dimensionality reduction in metal-halide perovskites. J. Phys. Chem. C 2018, 122, 14128–14134. [Google Scholar] [CrossRef] [Green Version]
- Akkerman, Q.A.; Park, S.; Radicchi, E.; Nunzi, F.; Mosconi, E.; De Angelis, F.; Brescia, R.; Rastogi, P.; Prato, M.; Manna, L. Nearly monodisperse insulator Cs4PbX6 (X = Cl, Br, I) nanocrystals, their mixed halide compositions, and their transformation into CsPbX3 nanocrystals. Nano Lett. 2017, 17, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, J.; Luo, Z.; Sun, Z.; Pan, N.; Ding, H.; Wang, X. Unveiling solvent-related effect on phase transformations in CsBr–PbBr2 system: Coordination and ratio of precursors. Chem. Mater. 2018, 30, 5846–5852. [Google Scholar] [CrossRef]
- Yang, L.; Wang, T.; Min, Q.; Liu, B.; Liu, Z.; Fan, X.; Qiu, J.; Xu, X.; Yu, J.; Yu, X. High water resistance of monoclinic CsPbBr3 nanocrystals derived from zero-dimensional cesium lead halide perovskites. ACS Omega 2019, 4, 6084–6091. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Sichert, J.A.; Tong, Y.; Mutz, N.; Vollmer, M.; Fischer, S.; Milowska, K.Z.; García Cortadella, R.; Nickel, B.; Cardenas-Daw, C.; Stolarczyk, J.K. Quantum size effect in organometal halide perovskite nanoplatelets. Nano Lett. 2015, 15, 6521–6527. [Google Scholar] [CrossRef]
- Shang, Y.; Liao, Y.; Wei, Q.; Wang, Z.; Xiang, B.; Ke, Y.; Liu, W.; Ning, Z. Highly stable hybrid perovskite light-emitting diodes based on Dion-Jacobson structure. Sci. Adv. 2019, 5, eaaw8072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, G.; Erdem, O.; Hübner, R.; Georgi, M.; Wei, W.; Fan, X.; Wang, J.; Demir, H.V.; Gaponik, N. Mechanosynthesis of polymer-stabilized lead bromide perovskites: Insight into the formation and phase conversion of nanoparticles. Nano Res. 2021, 14, 1078–1086. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Du, K.; Gao, X.; Lu, Y.; Wen, D.; Yao, S.; Feng, J.; Zhang, H. One-step conversion of CsPbBr3 into Cs4PbBr6/CsPbBr3@ Ta2O5 core–shell microcrystals with enhanced stability and photoluminescence. J. Mater. Chem. C 2021, 9, 1228–1234. [Google Scholar] [CrossRef]
- Hu, H.; Wu, L.; Tan, Y.; Zhong, Q.; Chen, M.; Qiu, Y.; Yang, D.; Sun, B.; Zhang, Q.; Yin, Y. Interfacial synthesis of highly stable CsPbX3/oxide Janus nanoparticles. J. Am. Chem. Soc. 2018, 140, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, D.; Guo, C.; Jiang, X.; Li, M.; Xu, T.; Zhu, J.; Fan, B.; Liu, W.; Shao, G. CsPbBr3 nanocrystals prepared by high energy ball milling in one-step and structural transformation from CsPbBr3 to CsPb2Br5. Appl. Surf. Sci. 2021, 543, 148782. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Almutlaq, J.; Sarmah, S.; Dursun, I.; Zhumekenov, A.A.; Begum, R.; Pan, J.; Cho, N.; Mohammed, O.F.; Bakr, O.M. Pure Cs4PbBr6: Highly luminescent zero-dimensional perovskite solids. ACS Energy Lett. 2016, 1, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Sousa, J.; Vivas, M.; Ferrari, J.; Mendonca, C.; Schiavon, M. Determination of particle size distribution of water-soluble CdTe quantum dots by optical spectroscopy. RSC Adv. 2014, 4, 36024–36030. [Google Scholar] [CrossRef]
- Du, X.; Wu, G.; Cheng, J.; Dang, H.; Ma, K.; Zhang, Y.-W.; Tan, P.-F.; Chen, S. High-quality CsPbBr3 perovskite nanocrystals for quantum dot light-emitting diodes. RSC Adv. 2017, 7, 10391–10396. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Manh, N.T.; Thai, H.T.; Jeong, S.-K.; Lee, Y.-W.; Cho, Y.; Ahn, W.; Choi, Y.; Cho, N. Improving the Stability of Ball-Milled Lead Halide Perovskites via Ethanol/Water-Induced Phase Transition. Nanomaterials 2022, 12, 920. https://doi.org/10.3390/nano12060920
Kim J, Manh NT, Thai HT, Jeong S-K, Lee Y-W, Cho Y, Ahn W, Choi Y, Cho N. Improving the Stability of Ball-Milled Lead Halide Perovskites via Ethanol/Water-Induced Phase Transition. Nanomaterials. 2022; 12(6):920. https://doi.org/10.3390/nano12060920
Chicago/Turabian StyleKim, Jinyoung, Nguyen The Manh, Huynh Tan Thai, Soon-Ki Jeong, Young-Woo Lee, Younghyun Cho, Wook Ahn, Yura Choi, and Namchul Cho. 2022. "Improving the Stability of Ball-Milled Lead Halide Perovskites via Ethanol/Water-Induced Phase Transition" Nanomaterials 12, no. 6: 920. https://doi.org/10.3390/nano12060920
APA StyleKim, J., Manh, N. T., Thai, H. T., Jeong, S.-K., Lee, Y.-W., Cho, Y., Ahn, W., Choi, Y., & Cho, N. (2022). Improving the Stability of Ball-Milled Lead Halide Perovskites via Ethanol/Water-Induced Phase Transition. Nanomaterials, 12(6), 920. https://doi.org/10.3390/nano12060920






