Abstract
A porous ball-flower-like Co3O4/Fe2O3 heterostructural photocatalyst was synthesized via a facile metal–organic-framework-templated method, and showed an excellent degradation performance in the model molecule rhodamine B under visible light irradiation. This enhanced photocatalytic activity can be attributed to abundant photo-generated holes and hydroxyl radicals, and the combined effects involving a porous structure, strong visible-light absorption, and improved interfacial charge separation. It is notable that the ecotoxicity of the treated reaction solution was also evaluated, confirming that an as-synthesized Co3O4/Fe2O3 catalyst could afford the sunlight-driven long-term recyclable degradation of dye-contaminated wastewater into non-toxic and colorless wastewater.
1. Introduction
Various pollutants in water environments can directly cause serious harm to the lives and health of human beings, animals and plants. Organic dyes, for example rhodamine B (RhB), methylene blue and methyl orange, as one of the most common industrial pollution sources at present, have attracted tremendous attention because of their geno- and ecotoxicity [1,2,3,4,5,6]. Therefore, the development of water treatment technologies regarding dye degradation has become a top priority. Among various methods, photocatalysis is recognized as one green and efficient alternative for organic pollutant degradation, where its key issue lies in the facile preparation of highly active and stable photocatalysts [7,8,9,10].
As one of the most promising multi-functional materials, metal–organic frameworks (MOFs) are often considered to be novel photocatalysts due to their abundant and editable active sites and large surface area. However, some of their defects, such as poor light absorption and metal ion leaching due to an unstable structure, may seriously limit their practical applications [11,12,13]. In order to solve these problems, in this study, a flower-like cobalt 2,5-thiophenedicarboxylic coordination polymer (Co-TDC) was used as a template to synthesize a novel Co3O4/Fe2O3 heterostructural photocatalyst with improved light harvesting and photocatalytic performance. The facile preparation, structural versatility, and superior dye degradation performance of this Co3O4/Fe2O3 heterostructure provides new inspirations for the development of higher-performance photocatalysts towards water environment remediation.
2. Materials and Methods
2.1. Synthesis of Ball-Flower-like Porous Co3O4/Fe2O3 Heterostructure
Briefly, 0.1 g of Co-TDC (Sinopharm Group Co., Ltd., Shanghai, China) and 0.0482 g of FeCl3·6H2O (Sinopharm Group Co., Ltd., Shanghai, China) were added into 5 mL of deionized water. After 30 min of ultrasonic treatment, the mixture was dried at 60 °C for 12 h. Afterwards, the obtained powder was calcined at 550 °C for 2 h. The final Co3O4/Fe2O3 product is denoted as CF in this work for convenience.
2.2. Characterization
The chemical composition and phase structure of samples were analyzed by X-ray powder diffraction (XRD, SmartLab® by Rigaku, Tokyo, Japan). The morphology was recorded using field-emission scanning electron microscopy (SEM, JSM-7800F by JEOL, Japan) and transmission electron microscopy (TEM, JEM-2100F by JEOL, Japan). X-ray photoelectron spectroscopy (XPS, EscaLab 250Xi by Thermo Fisher Scientific, Waltham, MA, USA) was performed to investigate element distribution and valence states. The magnetism and optical properties of samples were studied using vibrating sample magnetometer (VSM, LakeShore7404 by Quantum Design, San Diego, CA, USA) and diffuse-reflection spectroscopy (DRS, Cary-5000 by Agilent, Santa Clara, CA, USA).
2.3. Photocatalysis Measurements
The adsorption and photocatalysis processes of as-prepared catalysts were evaluated by the degradation of RhB in an aqueous solution under visible light irradiation at room temperature (ca. 25 °C). A 500 W xenon lamp with a cut-off filter (λ > 420 nm) was used to generate visible light. Amounts of 0.1 g of catalyst powder and 50 mL RhB aqueous solution (initial solution pH ≈ 4) were added to a 100 mL quartz tube and continuously stirred during the degradation experiment. Before irradiation, the reaction solution was magnetically stirred in the dark for 30 min to reach complete adsorption/desorption equilibrium. During the photocatalytic experiment, 5 mL reaction solution was extracted every 10 min, and the concentration of residual RhB was determined by measuring its absorbance at 590 nm on a UV-visible spectrometer (UV-3600i Plus by Shimadzu, Kyoto, Japan). The 5 mL solution was added back into the reaction solution after measurement.
3. Results and Discussion
The chemical composition and crystal structure of CF were analyzed by XRD. As shown in Figure 1a, the characteristic diffraction peaks located at 19.1°, 31.2°, 36.8°, 44.7°, 59.1°, and 65.1° could be attributed to Co3O4 (PDF#42-1467), while the other peaks at 35.6° and 62.9° could be assigned to Fe2O3 (PDF#39-1346), indicating that the as-prepared sample was composed of Co3O4 and Fe2O3. The chemical states of the sample surface were further analyzed by XPS. Considering the sample preparation method, only cobalt element was studied emphatically. In Co 2p spectra (Figure 1b), the asymmetric peaks at around 780.7 eV and 796.8 eV, and shake-up type satellite peaks at 785.8 eV and 802.3 eV of Co-TDC, could be well-indexed to Co2+, implying that cobalt in Co-TDC was only in the form of Co (II). On the other hand, for CF, two new peaks could be identified at around 779.4 eV and 794.3 eV, which were both ascribed to Co3+ [14,15,16,17,18]. This revealed that Co2+ in Co-TDC was partially oxidized to Co3+ during calcination, and thus Co3O4 was obtained as a result. Meanwhile, Fe 2p spectrum of CF was also recorded, as shown in Figure S1. It was revealed that Fe3+ ions were still dominant, which corresponded to the Fe2O3 phase. However, the minor peak at around 732.2 eV suggests that a little Fe3+ was reduced to Fe2+ along with the oxidation of Co2+ to Co3+ [19,20,21,22,23].
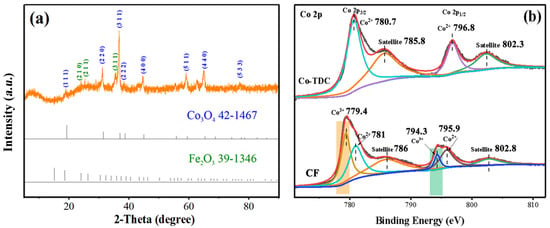
Figure 1.
XRD pattern (a) and Co 2p XPS spectra (b) of as-synthesized CF catalyst.
SEM images of the CF heterostructure are shown in Figure 2a–c. It can be observed from Figure 2a,b that CF has a regular ball-flower-like morphology with a spherical size ranged at 10–20 μm, which was retained from the Co-TDC template, as shown in Figure S2 of the Supplementary Materials. It is worth noting that the sheet-like fundamental units of Co3O4 in CF became much more porous after calcination, with large numbers of ~200 nm Fe2O3 nanoparticles (Figure S3) embedded within the pores, as indicated by the yellow arrows in Figure 2c, which facilitate the adsorption and degradation of dye molecules on the surface. Moreover, the elemental mapping profiles in Figure S4 also help to verify that the distribution of Fe2O3 within highly porous Co3O4 is uniform while it is random. In order to further determine the chemical composition of the synthesized catalyst, HRTEM image was also recorded, as shown in Figure 2d. The identified two lattice fringes with an interval of 0.25 and 0.20 nm could be indexed to the (311) facet of Fe2O3 and (400) facet of Co3O4, respectively, which is in good agreement with the XRD result.
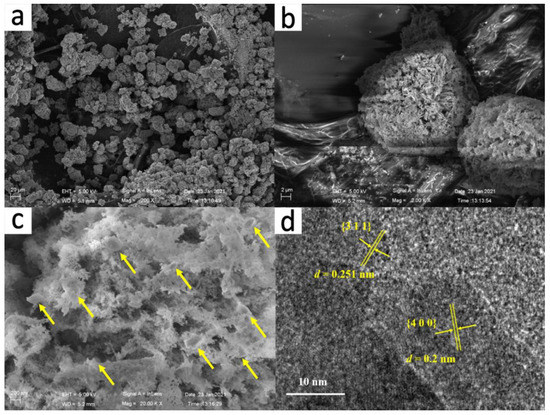
Figure 2.
Representative SEM images (a–c), and HRTEM image (d) of the CF catalyst.
The degradation efficiencies of different samples for RhB are displayed in Figure 3a. When the catalyst was not present in the solution, RhB could hardly undergo self-degradation under visible light (i.e., black plots). The reaction solution was first stirred in the dark for 30 min for the catalyst–RhB interface to reach the adsorption/desorption equilibrium. Typically, the contribution of RhB removal by adsorption is lower than 20%, which is in proportion to the surface area of the catalyst. In photocatalysis systems, CF demonstrated a superior performance than Co-TDC and Fe2O3, indicating that CF possesses the highest photocatalytic activity. This could be explained by the following aspects: (i) The highly porous structure of CF provided abundant active sites, as revealed in Figure 2b,c [24,25,26]; (ii) The p-n heterojunction that formed between Fe2O3 and Co3O4 could promote the separation of photo-generated electron and hole pairs [27,28,29,30]. The promoted charge separation, and thus the inhibited charge recombination, was witnessed by the significantly decreased photoluminescence (PL) intensity of CF composites compared to pristine Fe2O3 particles, as displayed in Figure S5 of the Supplementary Materials [31,32,33,34,35,36,37,38]. The variation in the RhB degradation efficiency of CF in different pH conditions is presented in Figure 3b, suggesting that the catalyst could maintain a superior photocatalytic degradation activity in the pH range of 4–10, despite the fact that the degradation rate decreased to a certain extent in a strong acid environment (pH ≤ 2). This may be due to the dissolving of oxides by strong acid, resulting in a loss of active material in the CF catalyst for the degradation of RhB. However, considering that the actual surface water or groundwater is mostly weakly acidic or weakly alkaline, the CF catalyst is still applicable to the oxidative degradation of organic pollutants in natural water bodies.
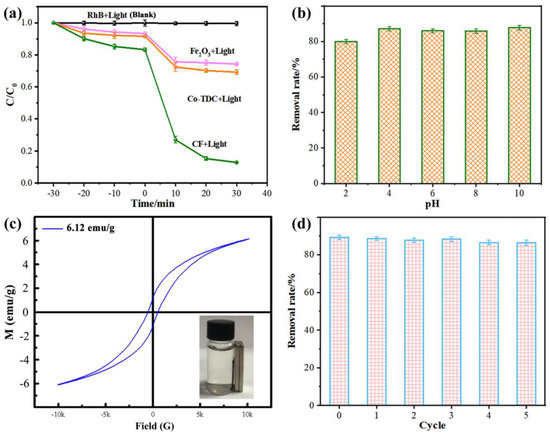
Figure 3.
(a) RhB degradation efficiencies of different samples; (b) the effect of pH of reaction solution; (c) VSM curve of CF; (d) recyclability of CF photocatalyst for RhB degradation.
The service life of a catalyst is an important technical indicator for evaluating its potential for practical usage. After the reaction, the catalyst in the solution could be easily and quickly separated due to its magnetism, as revealed in Figure 3c. Then, the recycled CF catalyst was rinsed with ethanol solution to remove the residual organics on the surface. Afterwards, it could be reused for RhB removal under the same conditions, as presented in Figure 3d. An excellent degradation efficiency of >86% was achieved after the CF catalyst was recycled and reused for five cycles, which maintained about 97% efficiency of the initial cycle (i.e., ~89.1%), confirming the recyclability of CF for long-term dye degradation in practical wastewater treatment.
Figure 4a displays the optical absorption of samples. It is observed that the absorption of Co-TDC is far lower than CF in the visible-light band. The CF catalyst maintains a superior absorption in the range of 550–750 nm, suggesting its capability for a visible-light-driven photocatalytic reaction. In addition, the threshold wavelengths of Co-TDC and CF are determined to be 619 nm and 685 nm, respectively. The corresponding bandgap and conduction band (CB)/valence band (VB) position can be calculated according to the following formulas [39,40,41]:
where Eg, λg, Ee, ECB, and EVB represent the bandgap, threshold wavelength, energy of free electrons on the hydrogen scale (~4.5 eV), and the CB and VB position, respectively. The values χ, n, and N represent the electronegativity of the constituent atom, number of species, and total number of atoms in the compound, respectively. The calculated Eg of Co-TDC and CF are 1.72 eV and 1.57 eV, indicating that the CF hybrid possesses a narrower bandgap, and thus requires less excitation energy. Thereby, Figure 4b depicts the photocatalytic mechanism of CF under visible light illumination. The photo-generated electrons in CB cannot reduce O2 to ·O2− because the ECB of Co3O4 and Fe2O3 are more positive than E(O2/·O2−) (−0.33 V vs. NHE), while the photo-generated holes are capable of oxidizing OH− to hydroxyl radicals (·OH) as the EVB of Co3O4 and Fe2O3 are more negative than E(·OH/OH−) (1.97 V vs. NHE) [42,43,44]. In order to further verify this perception, quenching experiments were carried out using tert-butyl alcohol (TBA), ammonium oxalate (AO) and L-ascorbic acid (L-AA) to quench the ·OH, photo-generated holes and ·O2−, respectively [45,46]. It can be observed from Figure S6 that the degradation efficiency of RhB clearly decreases in presence of TBA and AO. Therefore, it can be deduced that the main reactive species involved in the photocatalytic reaction are photo-generated holes and hydroxyl radicals (·OH), which consequently degrade RhB molecules to colorless small molecules.
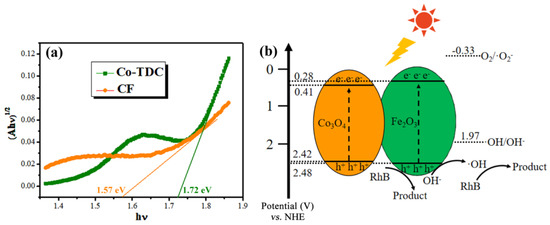
Figure 4.
(a) Tauc plots, i.e., plots of (αhν)0.5 vs. photon energy (hν), derived from diffuse-reflectance spectra of the Co-TDC and CF samples; (b) Band alignment and photocatalytic mechanism of the CF heterojunction under visible light illumination.
In order to evaluate the ecological toxicity of the RhB solution before and after treatment, Chlorella vulgaris (FACHB-8) was used as the model aquatic organism being tested, and the toxicity of the residual RhB after the photocatalytic reaction was assessed according to its growth inhibition rate to C. vulgaris. A detailed experimental method for algae density measurement is presented in the Supplementary Materials, which could be referred to as the standard GBT 21805-2008 [47]. As exhibited in Figure 5, the growth of C. vulgaris was significantly suppressed in the original RhB solution, and the inhibition rate doubled as time increases. In contrast, C. vulgaris could grow normally in the solution after reaction, and the remaining intermediate and final products showed a neglectable influence within 24 h. Even when the incubation time was extended to 96 h, the growth inhibition rate was still about 1%, which is only 15.6% of the original RhB solution. This demonstrates that the CF catalyst can effectively degrade and mineralize RhB molecules to nearly non-toxic products.
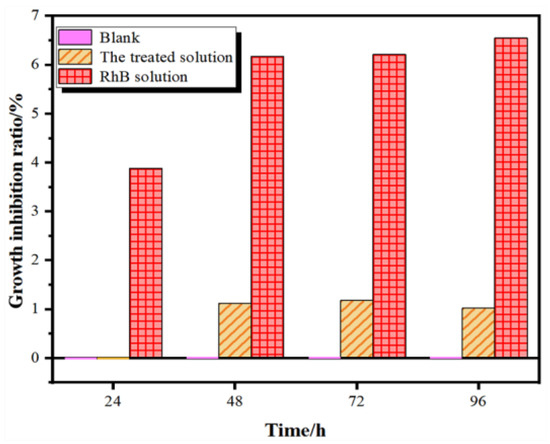
Figure 5.
Time-dependent growth inhibition rates of C. vulgaris in different solutions.
4. Conclusions
In summary, a highly active and stable Co3O4/Fe2O3 heterostructural photocatalyst was prepared by a facile MOF-templated method, with its structure, morphology and optical properties verified by XRD, XPS, SEM and UV-visible DRS methodology. The results indicate that the CF catalyst showed a strong visible-light absorption and high photocatalytic activity towards RhB degradation. By calculating the CB and VB position, it could be inferred that hydroxyl radicals and photo-generated holes were the dominant active species in the reaction. Furthermore, the 96 h growth inhibition rate of C. vulgaris by the treated RhB solution was 84.4% lower than the original solution, confirming the potential of the CF photocatalyst for the sunlight-driven long-term degradation of dye molecules into non-toxic and colorless ones.
Supplementary Materials
The followings are available online at https://www.mdpi.com/article/10.3390/nano12060904/s1, Figure S1: Fe 2p XPS spectrum of as-synthesized CF catalyst, Figure S2: SEM images of pure Co-TDC at different magnifications, Figure S3: SEM images of pure Fe2O3 at different magnifications, Figure S4: Elemental mapping profiles of as-synthesized CF catalyst: (a) O Kα1, (b) Co Kα1, (c) Fe Kα1, Figure S5: PL spectra of CF catalyst comparing with pure Fe2O3 at excitation wavelength of 355 nm, Figure S6: Photocatalytic degradation efficiency of RhB with and without quenching agent, Figure S7: Photocatalytic degradation efficiency of RhB by as-synthesized CF catalyst comparing with commercial Degussa/Evonik P25-TiO2 catalyst, Figure S8: Photocatalytic degradation efficiency of RhB by CF over a prolonged period of time; Experimental biota for toxicity test and algae density measurement.
Author Contributions
Conceptualization, Q.C. and Y.B.; Data curation, Q.L., J.Z., L.-W.S. and J.X.; Formal analysis, Q.L. and J.Z.; Funding acquisition, Q.C.; Investigation, Q.L., J.Z. and J.X.; Methodology, Q.C., Q.L., Z.P., J.Z. and L.-W.S.; Project administration, Q.C. and Y.C.; Resources, Q.C., Z.P., L.-W.S. and Y.C.; Supervision, Q.C., L.-W.S., J.C. and Y.B.; Validation, L.-W.S.; Writing—original draft, Q.L.; Writing—review and editing, Q.C., L.-W.S. and Y.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number: 52101213) and the Science and Technology Department of Jiangsu Province of China (grant number: BK20210261). The APC was funded by Southeast University of China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Qi Cao would like to thank the support from the “Zhi-Shan” Scholars Programme of Southeast University of China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, J.; Yang, D.; Hall, D.R.; Liu, J.; Sun, J.; Gu, W.; Tang, S.; Alharbi, H.A.; Jones, P.D.; Krause, H.M.; et al. Toxicokinetics of Brominated Azo Dyes in the Early Life Stages of Zebrafish (Danio rerio) Is Prone to Aromatic Substituent Changes. Environ. Sci. Technol. 2020, 54, 4421–4431. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jia, Y.; Li, H.; Wu, Z.; Huang, T.; Zhang, H. Enhanced pyrocatalysis of the pyroelectric BiFeO3/g-C3N4 heterostructure for dye decomposition driven by cold-hot temperature alternation. J. Adv. Ceram. 2021, 10, 338–346. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, X.; Yuan, K.; Yu, J.; Liu, Q.; Delaunay, J.-J.; Che, R. Gold nanoparticles decorated Ag(Cl,Br) micro-necklaces for efficient and stable SERS detection and visible-light photocatalytic degradation of Sudan, I. Appl. Catal. B Environ. 2017, 201, 607–616. [Google Scholar] [CrossRef]
- Cheng, Y.-F.; Cao, Q.; Zhang, J.; Wu, T.; Che, R. Efficient photodegradation of dye pollutants using a novel plasmonic AgCl microrods array and photo-optimized surface-enhanced Raman scattering. Appl. Catal. B Environ. 2017, 217, 37–47. [Google Scholar] [CrossRef]
- Cao, Q.; Che, R.; Chen, N. Scalable synthesis of Cu2S double-superlattice nanoparticle systems with enhanced UV/visible-light-driven photocatalytic activity. Appl. Catal. B Environ. 2015, 162, 187–195. [Google Scholar] [CrossRef]
- Cao, Q.; Che, R.; Chen, N. Facile and rapid growth of Ag2S microrod arrays as efficient substrates for both SERS detection and photocatalytic degradation of organic dyes. Chem. Commun. 2014, 50, 4931–4933. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Sadeghzadeh-Attar, A. Photocatalytic degradation evaluation of N–Fe codoped aligned TiO2 nanorods based on the effect of annealing temperature. J. Adv. Ceram. 2020, 9, 107–122. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Yuan, K.; Liu, Q.; Liang, C.; Wang, X.; Cheng, Y.-F.; Li, Q.; Wang, M.; Che, R. Porous Au–Ag Alloy Particles Inlaid AgCl Membranes As Versatile Plasmonic Catalytic Interfaces with Simultaneous, in Situ SERS Monitoring. ACS Appl. Mater. Interfaces 2015, 7, 18491–18500. [Google Scholar] [CrossRef]
- Cao, Q.; Che, R. Tailoring Au–Ag–S composite microstructures in one-pot for both SERS detection and photocatalytic degradation of plasticizers DEHA and DEHP. ACS Appl. Mater. Interfaces 2014, 6, 7020–7027. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, K.; Li, X.; Huang, L.; Liang, J.; Zheng, G.; Shan, G. Nickel-metal-organic framework nanobelt based composite membranes for efficient Sr2+ removal from aqueous solution. Environ. Sci. Ecotechnol. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Cheng, J.; Liang, J.; Dong, L.; Chai, J.; Zhao, N.; Ullah, S.; Wang, H.; Zhang, D.; Imtiaz, S.; Shan, G.; et al. Self-assembly of 2D-metal–organic framework/graphene oxide membranes as highly efficient adsorbents for the removal of Cs+ from aqueous solutions. RSC Adv. 2018, 8, 40813–40822. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhang, X.; Dong, X.; Ng, K.H.; Xie, Z.; Chen, I.-W.P.; Ng, Y.H.; Huang, J.; Lai, Y. Coupled porosity and heterojunction engineering: MOF-derived porous Co3O4 embedded on TiO2 nanotube arrays for water remediation. Chemosphere 2021, 274, 129799. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Hao, S.; Wu, Y.; Pei, K.; You, W.; Che, R. Interfacial charge redistribution in interconnected network of Ni2P–Co2P boosting electrocatalytic hydrogen evolution in both acidic and alkaline conditions. Chem. Eng. J. 2021, 424, 130444. [Google Scholar] [CrossRef]
- Li, R.; Fu, Q.; Zou, X.; Zheng, Z.; Luo, W.; Yan, L. Mn–Co–Ni–O thin films prepared by sputtering with alloy target. J. Adv. Ceram. 2020, 9, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, Y.; Tan, T.T.; Liu, Y.; Zheng, J.; Yang, Y.; Hou, X.; Feng, L.; Suo, G.; Ye, X.; et al. Thermoelectric performance enhancement by manipulation of Sr/Ti doping in two sublayers of Ca3Co4O9. J. Adv. Ceram. 2020, 9, 769–781. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Thayil, S.; Chang, A.; Sang, X.; Ma, X. Enhanced aging and thermal shock performance of Mn1.95−xCo0.21Ni0.84SrxO4 NTC ceramics. J. Adv. Ceram. 2021, 10, 258–270. [Google Scholar] [CrossRef]
- Liu, L.; Huang, X.; Wei, Z.; Duan, X.; Zhong, B.; Xia, L.; Zhang, T.; Wang, H.; Jia, D.; Zhou, Y.; et al. Solvents adjusted pure phase CoCO3 as anodes for high cycle stability. J. Adv. Ceram. 2021, 10, 509–519. [Google Scholar] [CrossRef]
- Song, B.; Yuan, K.; Wei, Y.; Chen, D.; Meng, F.; Cao, Q.; Song, M.; Liu, H. In-furnace control of arsenic vapor emissions using Fe2O3 microspheres with good sintering resistance. Environ. Sci. Technol. 2021, 55, 8613–8621. [Google Scholar] [CrossRef]
- Ye, F.; Dai, H.; Peng, K.; Li, T.; Chen, J.; Chen, Z.; Li, N. Effect of Mn doping on the microstructure and magnetic properties of CuFeO2 ceramics. J. Adv. Ceram. 2020, 9, 444–453. [Google Scholar] [CrossRef]
- Phor, L.; Chahal, S.; Kumar, V. Zn2+ substituted superparamagnetic MgFe2O4 spinel-ferrites: Investigations on structural and spin-interactions. J. Adv. Ceram. 2020, 9, 576–587. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Li, Z.; Liu, C.; Gong, W.; Tan, Q.; Han, B.; Yao, F.; Wang, K. Evolution of electromechanical properties in Fe-doped (Pb,Sr)(Zr,Ti)O3 piezoceramics. J. Adv. Ceram. 2021, 10, 587–595. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Liu, Q.; Jiang, Y.; Tang, Z. Resistive switching and optical properties of strontium ferrate titanate thin film prepared via chemical solution deposition. J. Adv. Ceram. 2021, 10, 1001–1010. [Google Scholar] [CrossRef]
- Hao, S.; Liu, J.; Cao, Q.; Zhao, Y.; Zhao, X.; Pei, K.; Zhang, J.; Chen, G.; Che, R. In-situ electrochemical pretreatment of hierarchical Ni3S2-based electrocatalyst towards promoted hydrogen evolution reaction with low overpotential. J. Colloid Interface Sci. 2020, 559, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Cao, Q.; Yang, L.; Che, R. Morphology-optimized interconnected Ni3S2 nanosheets coupled with Ni(OH)2 nanoparticles for enhanced hydrogen evolution reaction. J. Alloys Compd. 2020, 827, 154163. [Google Scholar] [CrossRef]
- Cao, Q.; Yu, J.; Yuan, K.; Zhong, M.; Delaunay, J.-J. Facile and Large-Area Preparation of Porous Ag3PO4 Photoanodes for Enhanced Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 19507–19512. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, C.-Y.; Zhu, L.-Y.; Cao, Q.; Yang, J.-H.; Li, X.-X.; Huang, W.; Wang, Y.-Y.; Lu, H.-L.; Zhang, D.W. Fabrication of a Micro-Electromechanical System-Based Acetone Gas Sensor Using CeO2 Nanodot-Decorated WO3 Nanowires. ACS Appl. Mater. Interfaces 2020, 12, 14095–14104. [Google Scholar] [CrossRef]
- Yuan, K.-P.; Zhu, L.-Y.; Cao, Q.; Ma, H.-P.; Tao, J.-J.; Huang, W.; Lu, H.-L. ALD-based hydrothermal facile synthesis of a dense WO3@TiO2–Fe2O3 nanodendrite array with enhanced photoelectrochemical properties. J. Mater. Chem. C 2020, 8, 6756–6762. [Google Scholar] [CrossRef]
- Yuan, K.; Cao, Q.; Lu, H.-L.; Zhong, M.; Zheng, X.; Chen, H.-Y.; Wang, T.; Delaunay, J.-J.; Luo, W.; Zhang, L.; et al. Oxygen-deficient WO3–x@TiO2–x core-shell nanosheets for efficient photoelectrochemical oxidation of neutral water solutions. J. Mater. Chem. A 2017, 5, 14697–14706. [Google Scholar] [CrossRef]
- Yuan, K.; Cao, Q.; Li, X.; Chen, H.-Y.; Deng, Y.; Wang, Y.-Y.; Luo, W.; Lu, H.-L.; Zhang, D.W. Synthesis of WO3@ZnWO4@ZnO–ZnO hierarchical nanocactus arrays for efficient photoelectrochemical water splitting. Nano Energy 2017, 41, 543–551. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, S.; Gadri, A. Synthesis, photoluminescence and magnetic properties of iron oxide (α-Fe2O3) nanoparticles through precipitation or hydrothermal methods. Phys. E Low Dimens. Syst. Nanostructures 2018, 101, 212–219. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, T.; Cheng, J.; Cao, Q.; Zheng, G.; Liang, S.; Wang, H.; Cao, M.S. Lightweight and high-performance microwave absorber based on 2D WS2–RGO heterostructures. Nano Micro Lett. 2019, 11, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Yu, J.; Cao, Y.; Delaunay, J.-J.; Che, R. Unusual effects of vacuum annealing on large-area Ag3PO4 microcrystalline film photoanode boosting cocatalyst- and scavenger-free water splitting. J. Mater. 2021, 7, 929–939. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, Y.-F.; Bi, H.; Zhao, X.; Yuan, K.; Liu, Q.; Li, Q.; Wang, M.; Che, R. Crystal defect-mediated band-gap engineering: A new strategy for tuning the optical properties of Ag2Se quantum dots toward enhanced hydrogen evolution performance. J. Mater. Chem. A 2015, 3, 20051–20055. [Google Scholar] [CrossRef]
- Lima, N.A.; Alencar, L.D.S.; Siu-Li, M.; Feitosa, C.A.C.; Mesquita, A.; M’peko, J.-C.; Bernardi, M.I.B. NiWO4 powders prepared via polymeric precursor method for application as ceramic luminescent pigments. J. Adv. Ceram. 2020, 9, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Luchechko, A.; Shpotyuk, Y.; Kravets, O.; Zaremba, O.; Szmuc, K.; Cebulski, J.; Ingram, A.; Golovchak, R.; Shpotyuk, O. Microstructure and luminescent properties of Eu3+-activated MgGa2O4:Mn2+ ceramic phosphors. J. Adv. Ceram. 2020, 9, 432–443. [Google Scholar] [CrossRef]
- Liu, N.; Mei, L.; Bin, J.; Zhang, Z.; Peng, Z. Effect of anionic group [SiO4]4−/[PO4]3− on the luminescence properties of Dy3+-doped tungstate structural compounds. J. Adv. Ceram. 2021, 10, 843–851. [Google Scholar] [CrossRef]
- Cao, Q.; Che, R. Synthesis of near-infrared fluorescent, elongated ring-like Ag2Se colloidal nanoassemblies. RSC Adv. 2014, 4, 16641–16646. [Google Scholar] [CrossRef]
- Li, C.; Cao, Q.; Wang, F.; Xiao, Y.; Li, Y.; Delaunay, J.-J.; Zhu, H. Engineering graphene and TMDs based van der Waals heterostructures for photovoltaic and photoelectrochemical solar energy conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef]
- Shao, Y.; Feng, K.; Guo, J.; Zhang, R.; He, S.; Wei, X.; Lin, Y.; Ye, Z.; Chen, K. Electronic structure and enhanced photoelectrocatalytic performance of RuxZn1−xO/Ti electrodes. J. Adv. Ceram. 2021, 10, 1025–1041. [Google Scholar] [CrossRef]
- Zhong, M.; Feng, Q.; Yuan, C.; Liu, X.; Zhu, B.; Meng, L.; Zhou, C.; Xu, J.; Wang, J.; Rao, G. Photocurrent density and electrical properties of Bi0.5Na0.5TiO3–BaNi0.5Nb0.5O3 ceramics. J. Adv. Ceram. 2021, 10, 1119–1128. [Google Scholar] [CrossRef]
- Siahroudi, M.G.; Daryakenari, A.A.; Molamahaleh, Y.B.; Cao, Q.; Daryakenari, M.A.; Delaunay, J.-J.; Siavoshi, H.; Molaei, F. Ethylene glycol assisted solvo-hydrothermal synthesis of NGr–Co3O4 nanostructures for ethanol electrooxidation. Int. J. Hydrogen Energy 2020, 45, 30357–30366. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Long, X.; Chen, L.; Cao, Q.; Wang, J.; Qiu, C.; Lim, J.; Yang, S. Formation of FeOOH Nanosheets Induces Substitutional Doping of CeO2−x with High-Valence Ni for Efficient Water Oxidation. Adv. Energy Mater. 2021, 11, 2002731. [Google Scholar] [CrossRef]
- Li, Z.; Dong, T.; Zhang, Y.; Wu, L.; Li, J.; Wang, X.; Fu, X. Studies on In(OH)ySz solid solutions: Syntheses, characterizations, electronic structure, and visible-light-driven photocatalytic activities. J. Phys. Chem. C 2007, 111, 4727–4733. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, M.; Cao, Q.; Sun, P.; Chen, Y.; Meng, F. The superoxide radicals’ production via persulfate activated with CuFe2O4@Biochar composites to promote the redox pairs cycling for efficient degradation of o-nitrochlorobenzene in soil. J. Hazard. Mater. 2020, 400, 122887. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Song, M.; Song, B.; Wei, Y.; Cao, Q.; Cao, Y. Enhanced degradation of Rhodamine B via α-Fe2O3 microspheres induced persulfate to generate reactive oxidizing species. Chemosphere 2020, 243, 125322. [Google Scholar] [CrossRef]
- Pei, Z.-T.; Xu, R.-R.; Liu, H.-Y.; Wang, W.-Q.; Zhang, M.; Zhang, L.-L.; Zhang, J.; Wang, W.-Q.; Yu, R.; Sun, L.-W. Development and application of a novel whole sediment toxicity test using immobilized sediment and Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2020, 189, 109979. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).