Experimental Study on the Stability of a Novel Nanocomposite-Enhanced Viscoelastic Surfactant Solution as a Fracturing Fluid under Unconventional Reservoir Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Fe3O4@ZnO NCs
2.3. Preparation of the NC-VES
2.4. Characterization of Nanoparticles
2.5. Property Tests of the NC-VES
3. Results and Discussion
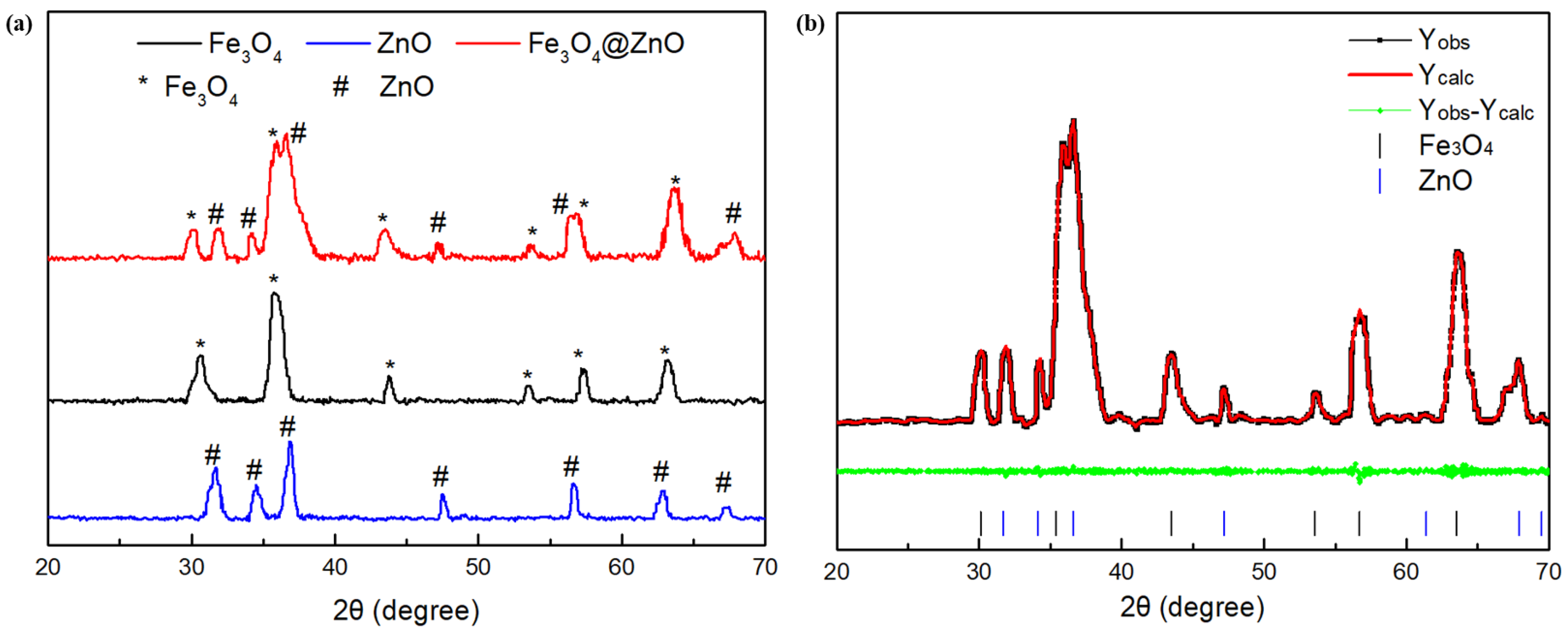
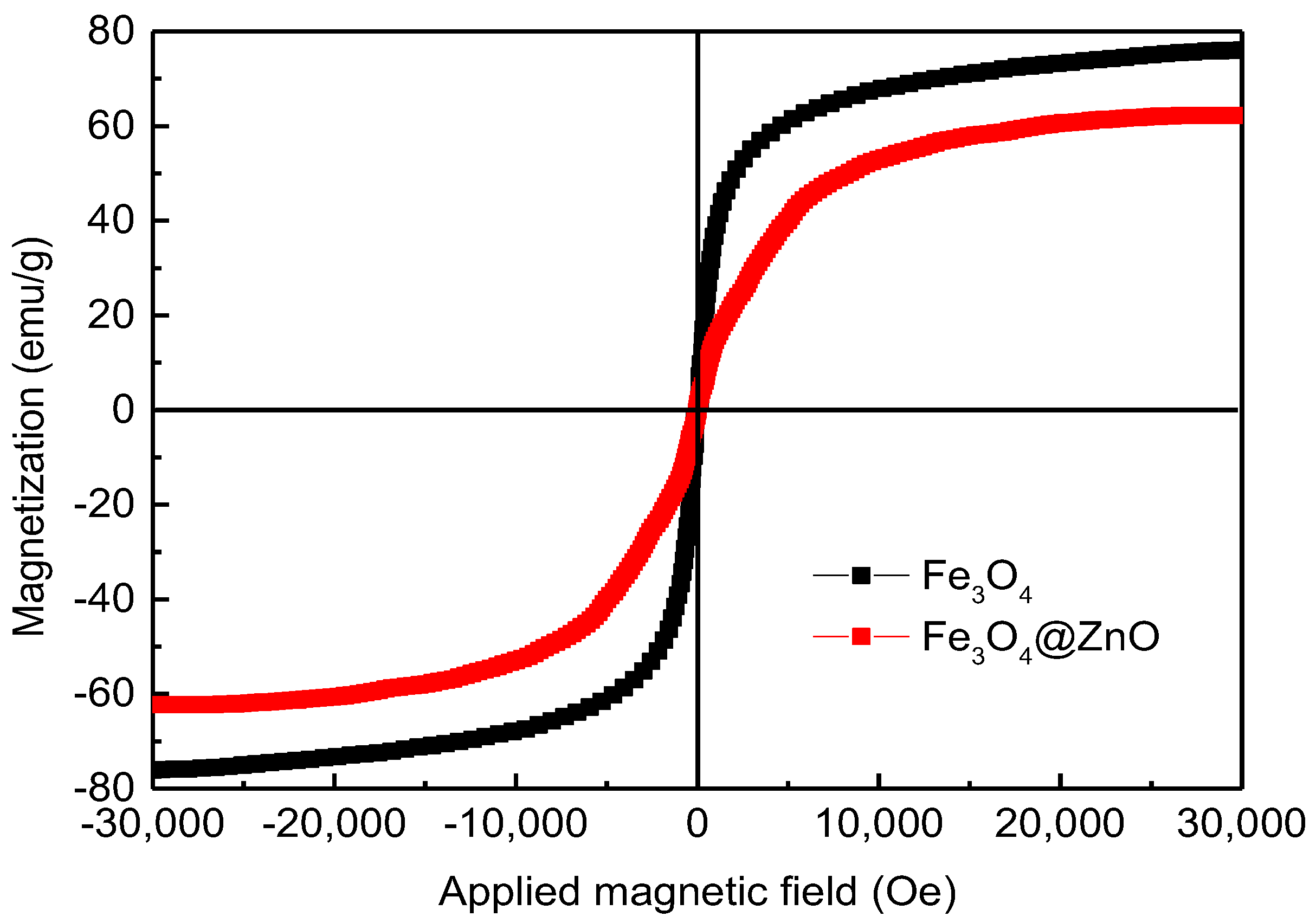
3.1. Characterization of Fe3O4@ZnO Nanocomposites
3.2. Stability of the NC-VES
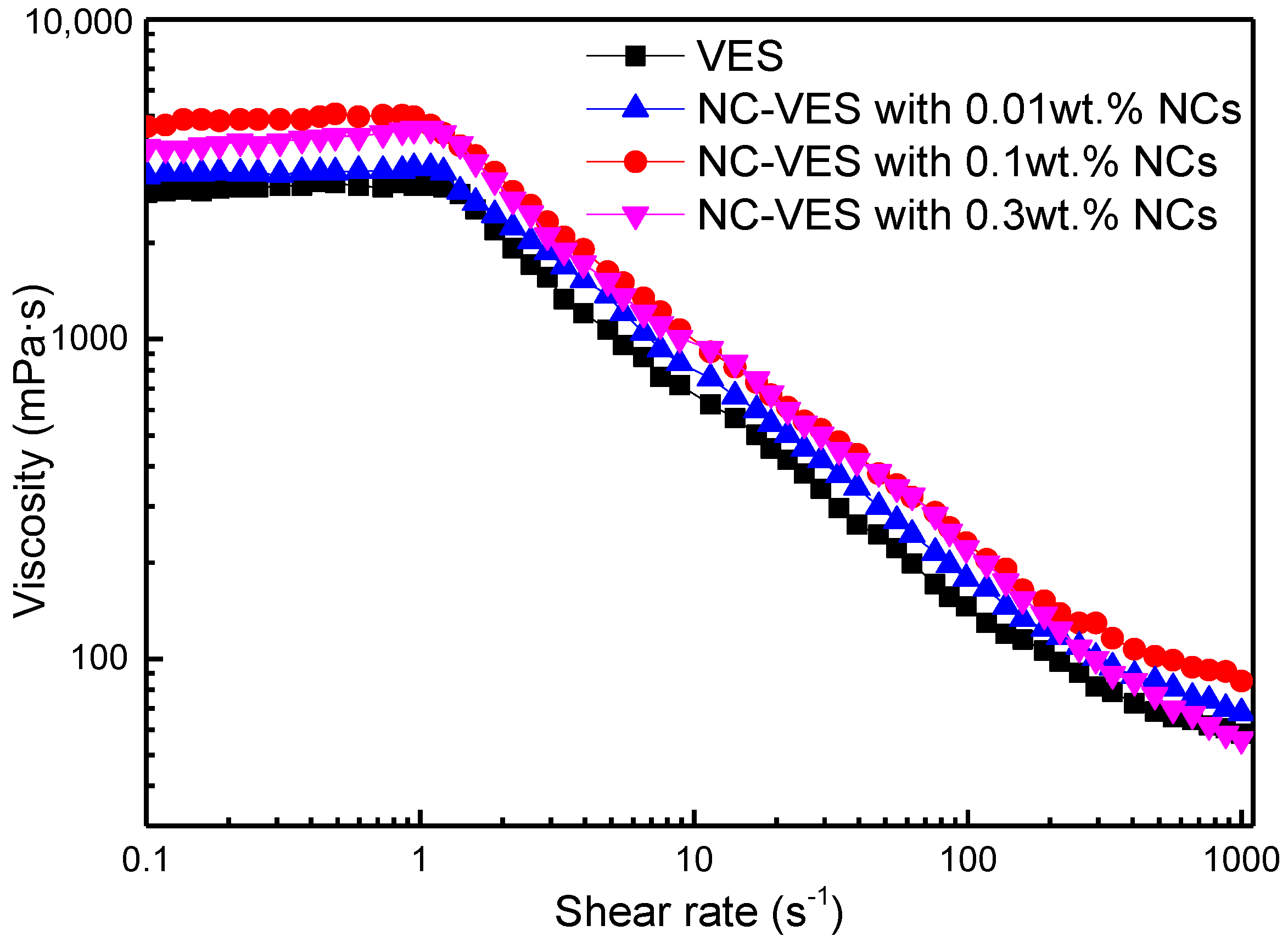
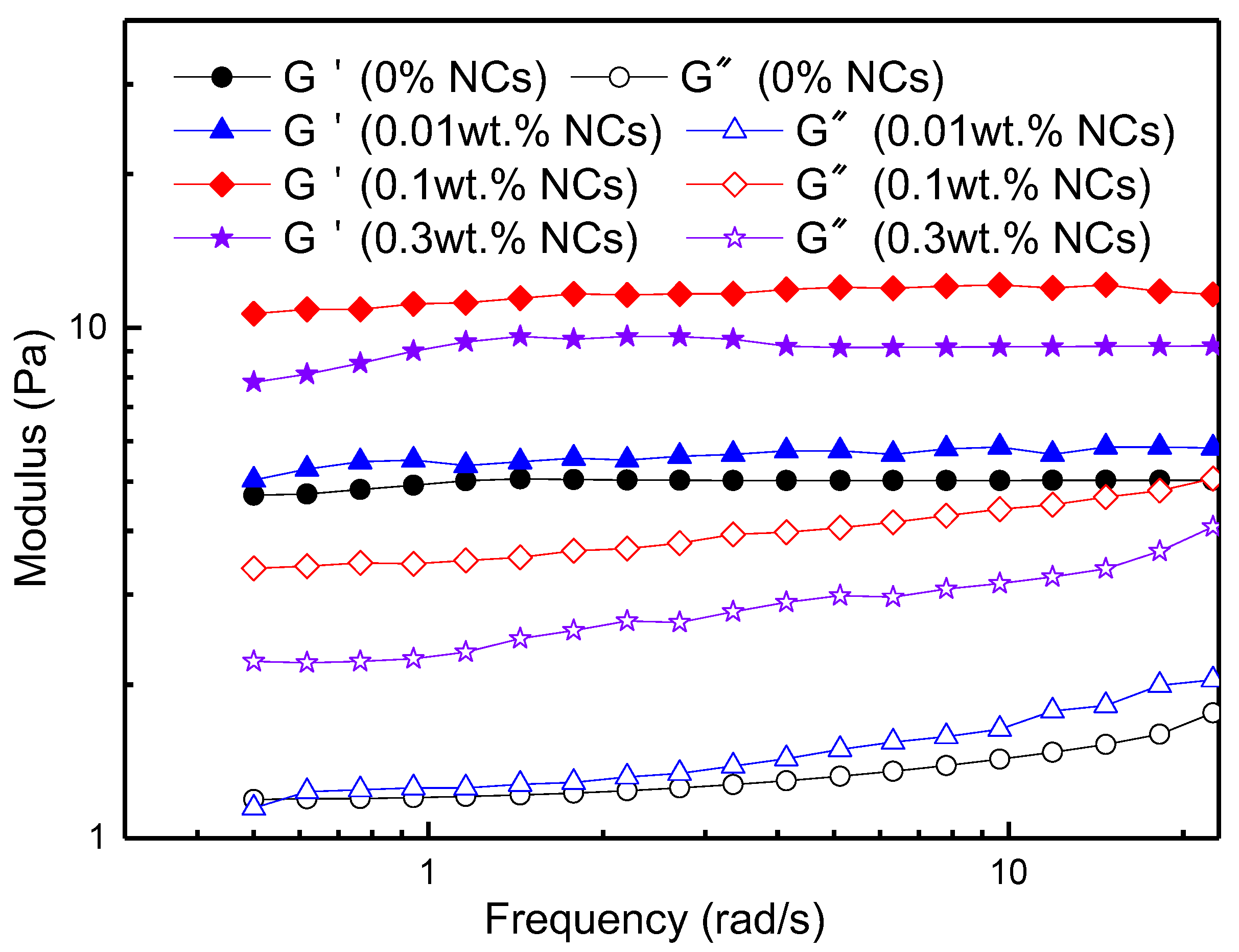
3.2.1. Effects of Shear Rate and NC Concentration
3.2.2. Effect of Temperature
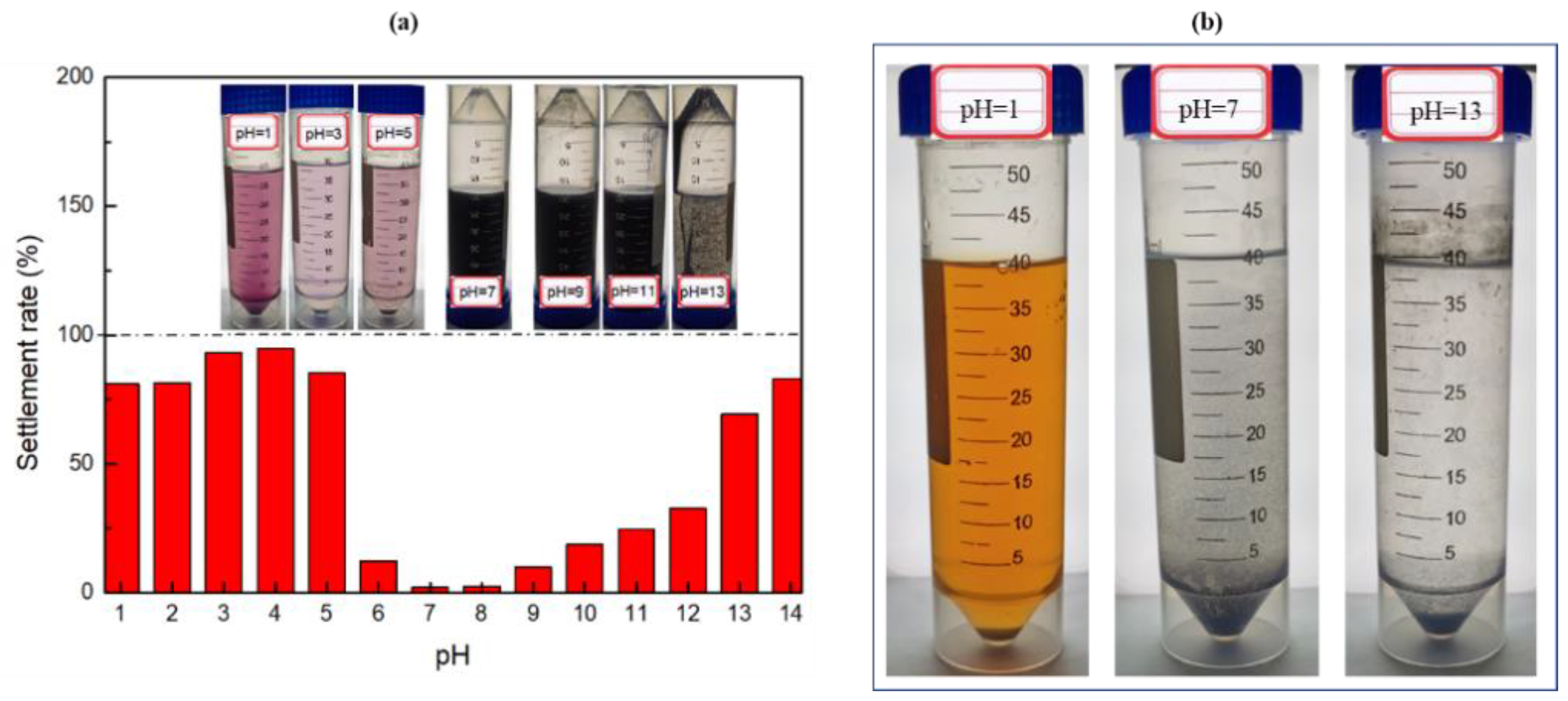
3.2.3. Effect of pH
3.3. Proppant-Transporting Performance and Gel-Breaking Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, M.L.; Si, X.D.; Zhang, Y.; Yuan, Z.H.; Yang, D.Y.; Gong, J. Performance evaluation of water control with nanoemulsion as pre-pad fluid in hydraulically fracturing tight gas formations. Energy Fuels 2017, 31, 3698–3707. [Google Scholar] [CrossRef]
- Andrés, J.C.; Omar, J.G.; Lazaros, G.P.; Gintaras, V.R. Disclosing water-energy-economics nexus in shale gas development. Appl. Energy 2018, 225, 710–731. [Google Scholar] [CrossRef]
- Tiffany, L.; Doug, D.; Shinji, M. Comparison of the degree of fouling at various flux rates and modes of operation using forward osmosis for remediation of produced water from unconventional oil and gas development. Sci. Total Environ. 2019, 675, 73–80. [Google Scholar] [CrossRef]
- Luo, M.L.; Jia, X.H.; Si, X.D.; Luo, S.; Zhan, Y.P. A novel polymer encapsulated silica nanoparticles for water control in development of fossil hydrogen energy-tight carbonate oil reservoir by acid fracturing. Int. J. Hydrogen Energy 2021, 46, 31191–31201. [Google Scholar] [CrossRef]
- Apergis, N.; Mustafa, G.; Dastidar, S.G. An analysis of the impact of unconventional oil and gas activities on public health: New evidence across Oklahoma counties. Energy Econ. 2021, 97, 105223. [Google Scholar] [CrossRef]
- Vishkai, M.; Gates, I. On multistage hydraulic fracturing in tight gas reservoirs: Montney Formation, Alberta, Canada. J. Pet. Sci. Eng. 2019, 174, 1127–1141. [Google Scholar] [CrossRef]
- Li, Y.Y.; Hu, W.; Zhang, Z.H.; Zhang, Z.B.; Shang, Y.J.; Han, L.L.; Wei, S.Y. Numerical simulation of hydraulic fracturing process in a naturally fractured reservoir based on a discrete fracture network model. J. Struct. Geol. 2021, 147, 104331. [Google Scholar] [CrossRef]
- Liu, J.R.; Sheng, J.J.; Emadibaladehi, H.; Tu, J.W. Experimental study of the stimulating mechanism of shut-in after hydraulic fracturing in unconventional oil reservoirs. Fuel 2021, 300, 120982. [Google Scholar] [CrossRef]
- Javadpour, F.; McClure, M.; Naraghi, M.E. Slip-corrected liquid permeability and its effect on hydraulic fracturing and fluid loss in shale. Fuel 2015, 160, 549–559. [Google Scholar] [CrossRef]
- Etoughe, P.; Siddhamshetty, P.; Cao, K.Y. Incorporation of sustainability in process control of hydraulic fracturing in unconventional reservoirs. Chem. Eng. Res. Des. 2018, 139, 62–76. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, J.C.; Peng, H.; Pan, R.; Aliu, A.O.; Lu, Q.L.; Yang, J. Synthesis and evaluation of a novel clean hydraulic fracturing fluid based on star-dendritic polymer. J. Nat. Gas Sci. Eng. 2017, 43, 179–189. [Google Scholar] [CrossRef]
- Xu, T.; Mao, J.C.; Zhang, Y.Z.; Yang, X.J.; Lin, C.; Du, A.Q.; Zhang, H. Application of gemini viscoelastic surfactant with high salt in brine-based fracturing fluid. Colloids Surf. A 2021, 611, 125838. [Google Scholar] [CrossRef]
- Baruah, A.; Pathak, A.K.; Ojha, K. Phase behaviour and thermodynamic properties of lamellar liquid crystal developed for viscoelastic surfactant based fracturing fluid. Chem. Eng. Sci. 2015, 131, 146–154. [Google Scholar] [CrossRef]
- Chauhan, G.; Verma, A.; Hazarika, A.; Ojha, K. Rheological, structural and morphological studies of Gum Tragacanth and its inorganic SiO2 nanocomposite for fracturing fluid application. J. Taiwan Inst. Chem. Eng. 2017, 80, 978–988. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, J.Z.; Mao, J.C.; Tan, H.Z.; Zhang, Y.; Song, Z.F. Review of friction reducers used in slickwater fracturing fluids for shale gas reservoirs. J. Nat. Gas Sci. Eng. 2019, 62, 302–313. [Google Scholar] [CrossRef]
- Wang, J.; Holditch, S.A.; McVay, D.A. Effect of gel damage on fracture fluid cleanup and long-term recovery in tight gas reservoirs. J. Nat. Gas Sci. Eng. 2012, 9, 108–118. [Google Scholar] [CrossRef]
- Liang, X.Y.; Zhou, F.J.; Liang, T.B.; Wang, C.Z.; Wang, J.; Yuan, S. Impacts of low harm fracturing fluid on fossil hydrogen energy production in tight reservoirs. Int. J. Hydrogen Energy 2020, 45, 21195–21204. [Google Scholar] [CrossRef]
- Yan, Z.H.; Dai, C.L.; Zhao, M.W.; Sun, Y.P.; Zhao, G. Development, formation mechanism and performance evaluation of a reusable viscoelastic surfactant solution as fracturing fluid. J. Ind. Eng. Chem. 2016, 37, 115–122. [Google Scholar] [CrossRef]
- Samuel, M.M.; Card, R.J.; Nelson, E.B.; Brown, J.E.; Vinod, P.S.; Temple, H.L.; Qu, Q.; Fu, D.K. Polymer-free fluid for fracturing applications. SPE Drill. Complet. 1999, 14, 240–246. [Google Scholar] [CrossRef]
- García, B.F.; Saraji, S. Mixed in-situ rheology of viscoelastic surfactant solutions using a hyperbolic geometry. J. Non-Newton. Fluid Mech. 2019, 270, 56–65. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Aleshina, A.L.; Arkharova, N.A.; Orekhov, A.S.; Kuklin, A.I.; Philippova, O.E. Disruption of cationic/anionic viscoelastic surfactant micellar networks by hydrocarbon as a basis of enhanced fracturing fluids clean-up. Nanomaterials 2020, 10, 2353. [Google Scholar] [CrossRef] [PubMed]
- Mushi, S.J.; Kang, W.L.; Yang, H.B.; Wang, P.X.; Hou, X.Y. Viscoelasticity and microstructural properties of zwitterionic surfactant induced by hydroxybenzoate salt for fracturing. J. Mol. Liq. 2020, 301, 112485. [Google Scholar] [CrossRef]
- Wu, X.P.; Song, Z.H.; Zhen, J.W.; Wang, H.B.; Yao, L.S.; Zhao, M.W.; Dai, C.L. A smart recyclable VES fluid for high temperature and high pressure fracturing. J. Pet. Sci. Eng. 2020, 190, 107097. [Google Scholar] [CrossRef]
- Wu, X.P.; Zhang, Y.; Sun, X.; Huang, Y.P.; Dai, C.L.; Zhao, M.W. A novel CO2 and pressure responsive viscoelastic surfactant fluid for fracturing. Fuel 2018, 229, 79–87. [Google Scholar] [CrossRef]
- Nettesheim, F.; Liberatore, M.W.; Hodgdon, T.K. Influence of nanoparticle addition on the properties of wormlike micellar solutions. Langmuir 2008, 24, 7718–7726. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, C.L.; Qian, Y.; Fan, X.Q.; Wu, Y.N.; Wu, X.P. Rheological properties and formation dynamic filtration damage evaluation of a novel nanoparticle-enhanced VES fracturing system constructed with wormlike micelles. Colloids Surf. A 2018, 533, 244–252. [Google Scholar] [CrossRef]
- Philippova, O.E.; Molchanov, V.S. Enhanced rheological properties and performance of viscoelastic surfactant fluids with embedded nanoparticles. Curr. Opin. Colloid Interface Sci. 2019, 43, 52–62. [Google Scholar] [CrossRef]
- García, B.F.; Saraji, S. Linear rheology of nanoparticle-enhanced viscoelastic surfactants. J. Mol. Liq. 2020, 300, 112215. [Google Scholar] [CrossRef]
- Luo, M.L.; Jia, Z.L.; Sun, H. Rheological behavior and microstructure of an anionic surfactant micelle solution with pyroelectric nanoparticle. Colloids Surf. A 2012, 395, 267–275. [Google Scholar] [CrossRef]
- Baruah, A.; Khilendra, K.; Pathak, A.K.; Ojha, K. Study on rheology and thermal stability of mixed (nonionic–anionic) surfactant based fracturing fluids. AICHE J. 2016, 62, 2177–2187. [Google Scholar] [CrossRef]
- Díez, R.; Medina, O.E.; Giraldo, L.J.; Cortés, F.B.; Franco, C.A. Development of nanofluids for the inhibition of formation damage caused by fines migration: Effect of the interaction of quaternary amine (CTAB) and MgO nanoparticle. Nanomaterials 2020, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.A.; RichardZabala, R.; Cortés, F.B. Nanotechnology applied to the enhancement of oil and gas productivity and recovery of Colombian fields. J. Pet. Sci. Eng. 2017, 157, 39–55. [Google Scholar] [CrossRef]
- Giraldo, L.J.; Diez, R.; Acevedo, S.; Cortés, F.B.; Franco, C.A. The effects of chemical composition of fines and nanoparticles on inhibition of formation damage caused by fines migration: Insights through a simplex-centroid mixture design of experiments. J. Pet. Sci. Eng. 2021, 203, 108494. [Google Scholar] [CrossRef]
- Dong, P.; Chen, X.; Guo, M.T.; Wu, Z.Y. Heterogeneous electro-Fenton catalysis with self-supporting CFP@MnO2-Fe3O4/C cathode for shale gas fracturing flowback wastewater. J. Hazard. Mater. 2021, 412, 125208. [Google Scholar] [CrossRef]
- Sengupta, S. An innovative approach to image fracture dimensions by injecting ferrofluids. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, United Arab Emirates, 11 November 2012. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Bryant, S.L.; Huh, C. Crosswell magnetic sensing of superparamagnetic nanoparticles for subsurface applica-tions. SPE J. 2015, 20, 1067–1082. [Google Scholar] [CrossRef]
- Luo, M.L.; Si, X.D.; Li, M.Z.; Jia, X.H.; Yang, Y.L.; Zhan, Y.P. Experimental study on the drag reduction performance of clear fracturing fluid using wormlike surfactant micelles and magnetic nanoparticles under a magnetic field. Nanomaterials 2021, 11, 885. [Google Scholar] [CrossRef]
- Ali, A.M.; Yahya, N.; Qureshi, S. Interactions of ferro-nanoparticles (hematite and magnetite) with reservoir sandstone: Implications for surface adsorption and interfacial tension reduction. Pet. Sci. 2020, 17, 1037–1055. [Google Scholar] [CrossRef] [Green Version]
- Molchanov, V.S.; Pletneva, V.A.; Klepikov, I.A.; Razumovskaya, I.V.; Philippova, O.E. Soft magnetic nanocomposites based on adaptive matrix of wormlike surfactant micelles. RSC Adv. 2018, 8, 11589–11597. [Google Scholar] [CrossRef] [Green Version]
- Claracq, J.; Sarrazin, J.; Montfort, J.P. Viscoelastic properties of magnetorheological fluids. Rheol. Acta 2004, 43, 38–49. [Google Scholar] [CrossRef]
- Christus, A.; Ravikumar, A.; Panneerselvam, P.; Radhakrishnan, K. A novel Hg(II) sensor based on Fe3O4@ZnO nanocomposite as peroxidase mimics. Appl. Surf. Sci. 2018, 449, 669–676. [Google Scholar] [CrossRef]
- Nadafan, M.; Sabbaghan, M.; Sofalgar, P.; Anvari, J.Z. Comparative study of the third-order nonlinear optical properties of ZnO/Fe3O4 nanocomposites synthesized with or without Ionic Liquid. Opt. Laser Technol. 2020, 131, 106435. [Google Scholar] [CrossRef]
- Croce, V.; Cosgrove, T.; Dreiss, C.A.; King, S.; Maitland, G.; Hughes, T. Giant micellar worms under shear: A rheological study using SANS. Langmuir 2005, 21, 6762–6768. [Google Scholar] [CrossRef]
- Chu, Z.L.; Feng, Y.J.; Su, X.; Han, Y.X. Wormlike micelles and solution properties of a C22-tailed amidosulfobetaine surfactant. Langmuir 2010, 26, 7783–7791. [Google Scholar] [CrossRef]
- Gao, Z.B.; Dai, C.L.; Sun, X.; Huang, Y.P.; Gao, M.W.; Zhao, M.W. Investigation of cellulose nanofiber enhanced viscoelastic fracturing fluid system: Increasing viscoelasticity and reducing filtration. Colloids Surf. A 2019, 582, 123938. [Google Scholar] [CrossRef]
- Yin, H.Y.; Feng, Y.J.; Li, P.X.; Doutch, J.; Han, Y.X.; Mei, Y.J. Cryogenic viscoelastic surfactant fluids: Fabrication and application in a subzero environment. J. Colloid Interface Sci. 2019, 551, 89–100. [Google Scholar] [CrossRef]
- Fanzatovich, I.I.; Aleksandrovich, K.D.; Rinatovich, I.A. Supramolecular system based on cylindrical micelles of anionic surfactant and silica nanoparticles. Colloids Surf. A 2016, 507, 255–260. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Yang, Z.Z.; Li, X.G.; Hou, L.L.; Xie, S.Y. Experimental study on the microscopic characteristics of foams stabilized by viscoelastic surfactant and nanoparticles. Colloids Surf. A 2019, 572, 88–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, C.L.; Wu, X.P.; Wu, Y.N.; Li, Y.Y.; Huang, Y.P. The construction of anhydride-modified silica nanoparticles (AMSNPs) strengthened wormlike micelles based on strong electrostatic and hydrogen bonding interactions. J. Mol. Liq. 2019, 277, 372–379. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Hodgdon, T.K.; Kaler, E.W.; Wagner, N.J.; Vethamuthu, M.; Ananthapadmanabhan, K.P. Formation and rheology of viscoelastic “double networks” in wormlike micelle−nanoparticle mixtures. Langmuir 2010, 26, 8049–8060. [Google Scholar] [CrossRef]
- Jiao, W.X.; Wang, Z.; Liu, T.Q.; Li, X.F.; Dong, J.F. pH and light dual stimuli-responsive wormlike micelles with a novel Gemini surfactant. Colloids Surf. A 2021, 618, 126505. [Google Scholar] [CrossRef]
- Fu, H.R.; Duan, W.M.; Zhang, T.L.; Xu, K.; Zhao, H.F.; Yang, L.; Zheng, C.C. Preparation and mechanism of pH and temperature stimulus-responsive wormlike micelles. Colloids Surf. A 2021, 624, 126788. [Google Scholar] [CrossRef]
- Liu, F.; Liu, D.J.; Zhou, W.J.; Wang, S.; Chen, F.; Wei, J.J. Weakening or losing of surfactant drag reduction ability: A coarse-grained molecular dynamics study. Chem. Eng. Sci. 2020, 219, 115610. [Google Scholar] [CrossRef]
- Wu, H.R.; Zhou, Q.; Xu, D.R.; Sun, R.X.; Wang, P.Y. SiO2 nanoparticle-assisted low-concentration viscoelastic cationic surfactant fracturing fluid. J. Mol. Liq. 2018, 266, 864–869. [Google Scholar] [CrossRef]
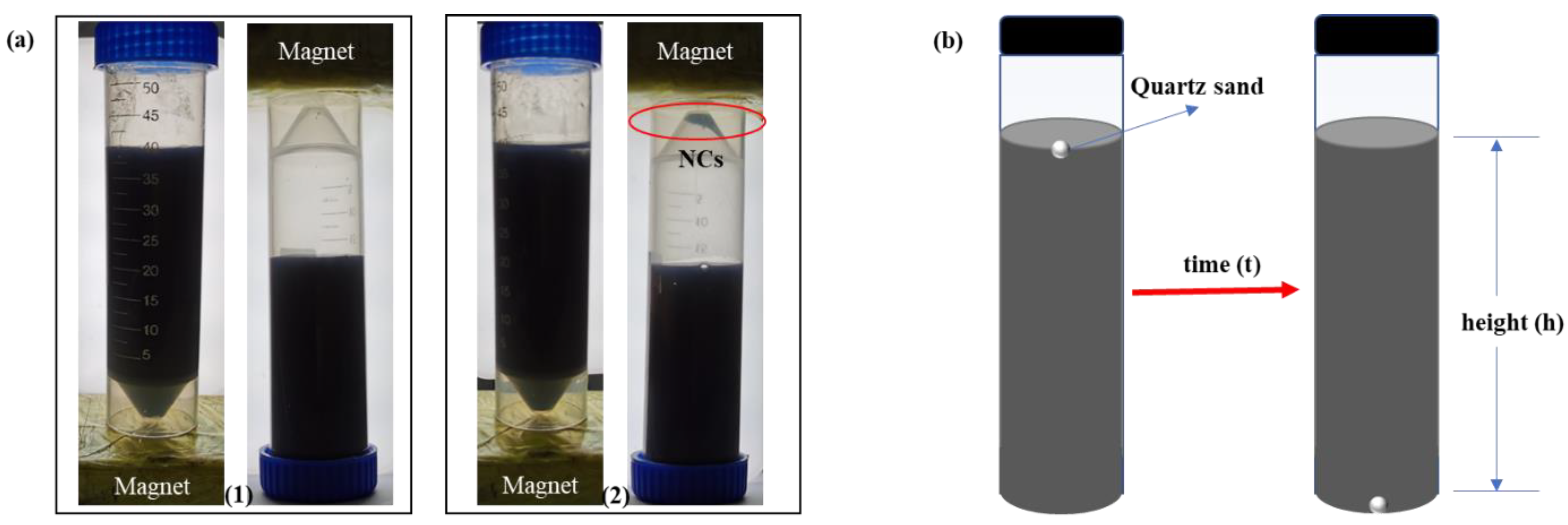
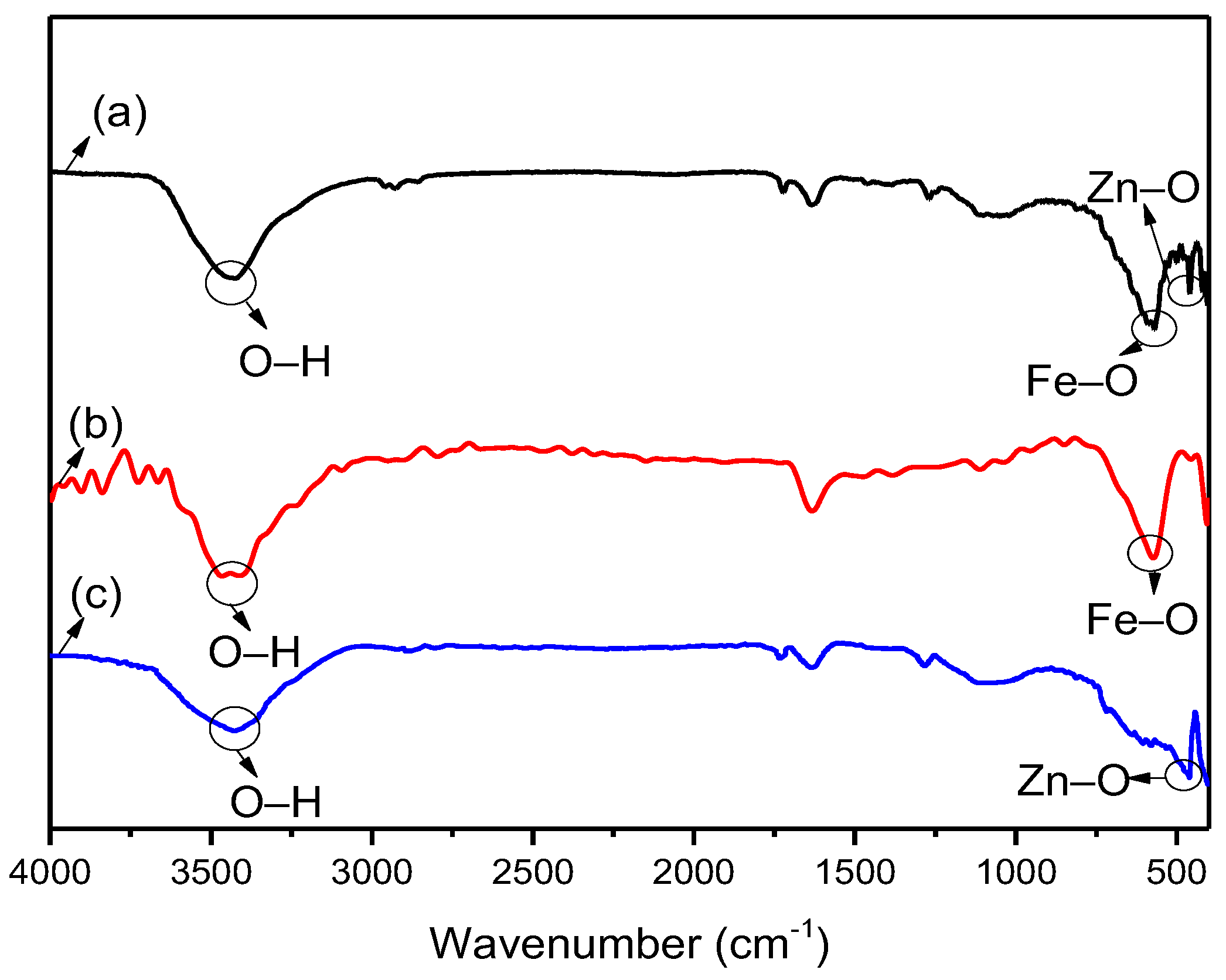
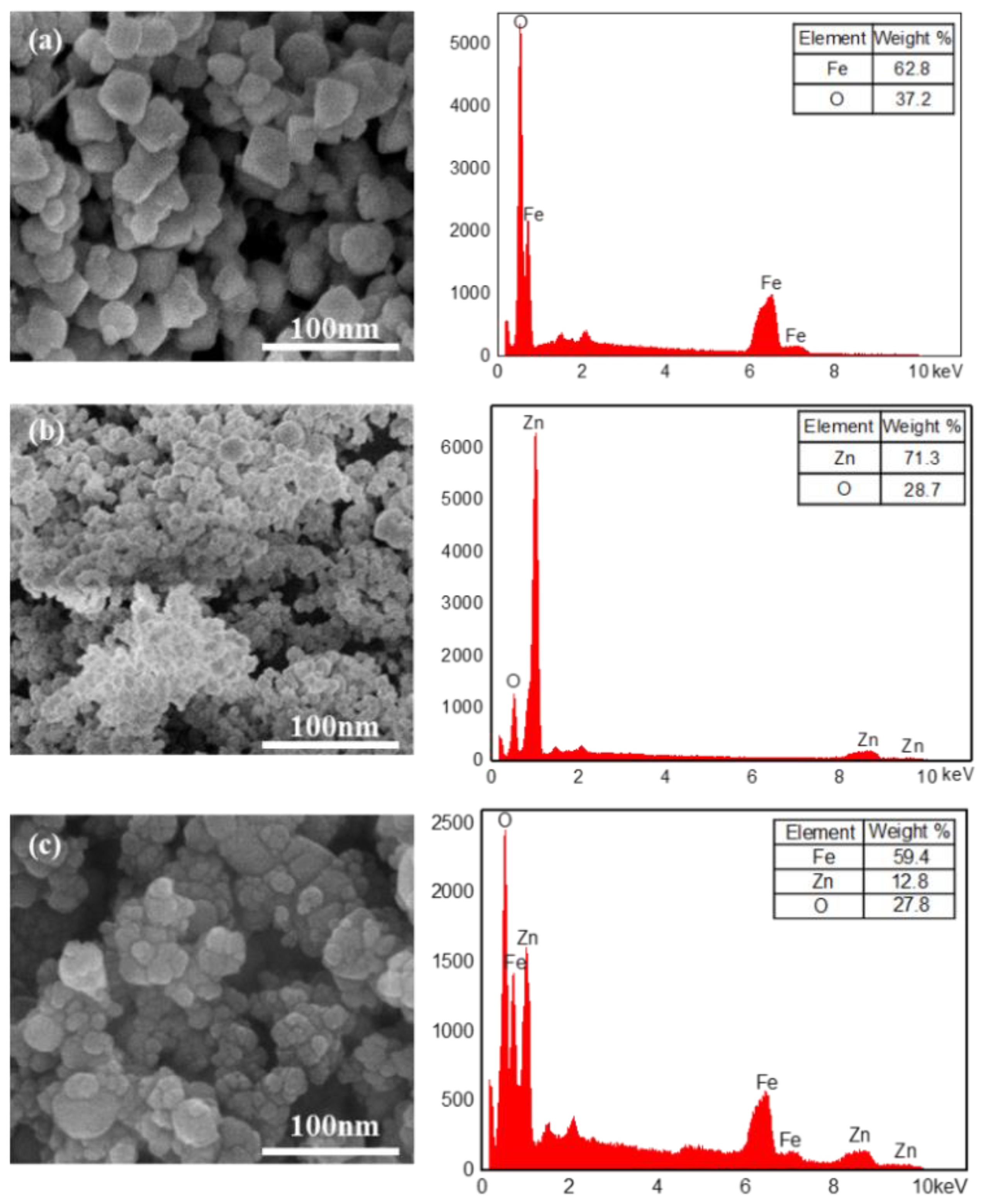
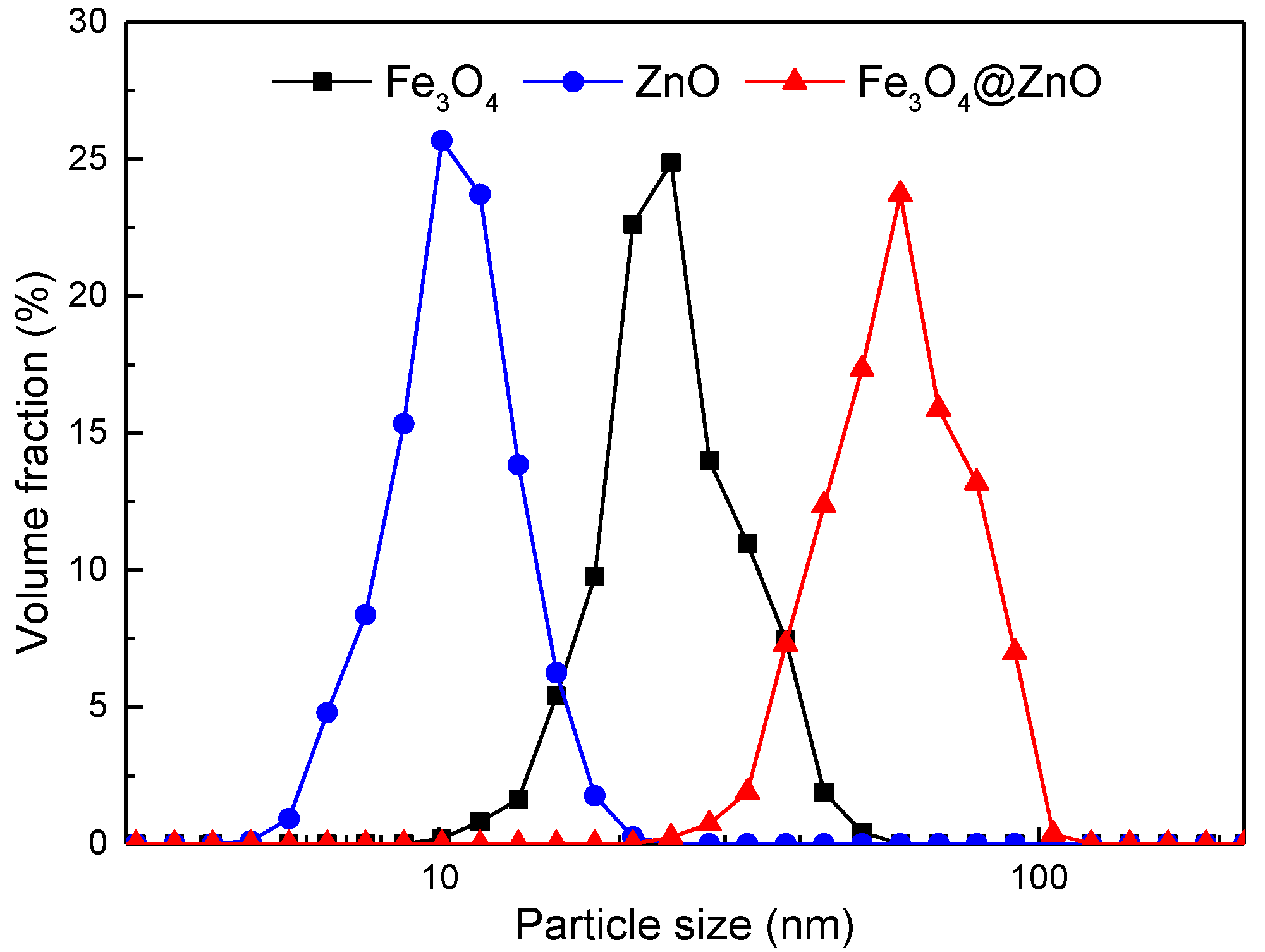




| Samples | Constituents (per 100 mL Water) | Viscosity (mPa·s) | Settlement Velocity (cm/s) |
|---|---|---|---|
| NC-VES | 1 wt.% OTAC + 0.5 wt.% NaSal + 0.1 wt.% NCs | 158 | 0.52 × 10−3 |
| VESFF | 1 wt.% OTAC + 0.5 wt.% NaSal | 130 | 7.29 × 10−3 |
| Guar FF | 0.35 wt.% Guar | 51 | 0.18 |
| PAM FF | 0.35 wt.% PAM | 59 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, X.; Luo, M.; Li, M.; Ma, Y.; Huang, Y.; Pu, J. Experimental Study on the Stability of a Novel Nanocomposite-Enhanced Viscoelastic Surfactant Solution as a Fracturing Fluid under Unconventional Reservoir Stimulation. Nanomaterials 2022, 12, 812. https://doi.org/10.3390/nano12050812
Si X, Luo M, Li M, Ma Y, Huang Y, Pu J. Experimental Study on the Stability of a Novel Nanocomposite-Enhanced Viscoelastic Surfactant Solution as a Fracturing Fluid under Unconventional Reservoir Stimulation. Nanomaterials. 2022; 12(5):812. https://doi.org/10.3390/nano12050812
Chicago/Turabian StyleSi, Xiaodong, Mingliang Luo, Mingzhong Li, Yuben Ma, Yige Huang, and Jingyang Pu. 2022. "Experimental Study on the Stability of a Novel Nanocomposite-Enhanced Viscoelastic Surfactant Solution as a Fracturing Fluid under Unconventional Reservoir Stimulation" Nanomaterials 12, no. 5: 812. https://doi.org/10.3390/nano12050812
APA StyleSi, X., Luo, M., Li, M., Ma, Y., Huang, Y., & Pu, J. (2022). Experimental Study on the Stability of a Novel Nanocomposite-Enhanced Viscoelastic Surfactant Solution as a Fracturing Fluid under Unconventional Reservoir Stimulation. Nanomaterials, 12(5), 812. https://doi.org/10.3390/nano12050812






