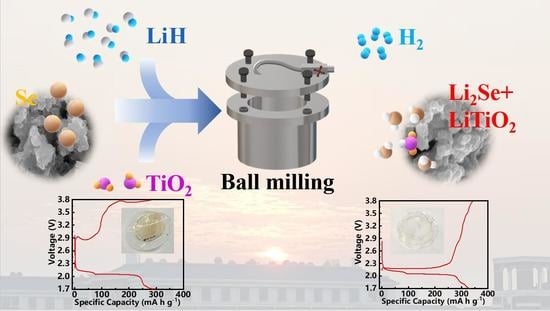
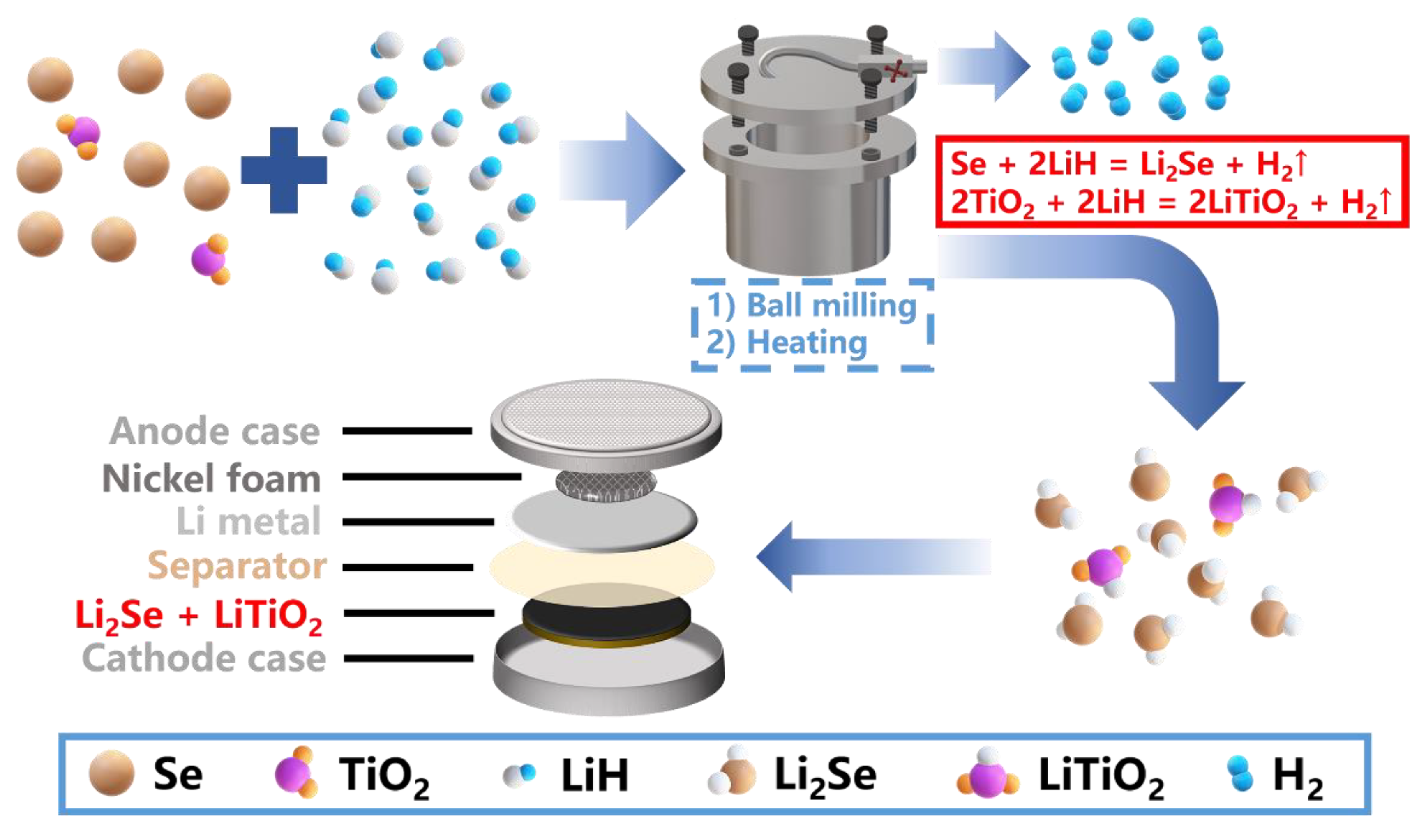
A Facile Pre-Lithiated Strategy towards High-Performance Li2Se-LiTiO2 Composite Cathode for Li-Se Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Li2Se-LiTiO2 Composites
2.2. Materials Characterization
2.3. Electrochemical Measurements
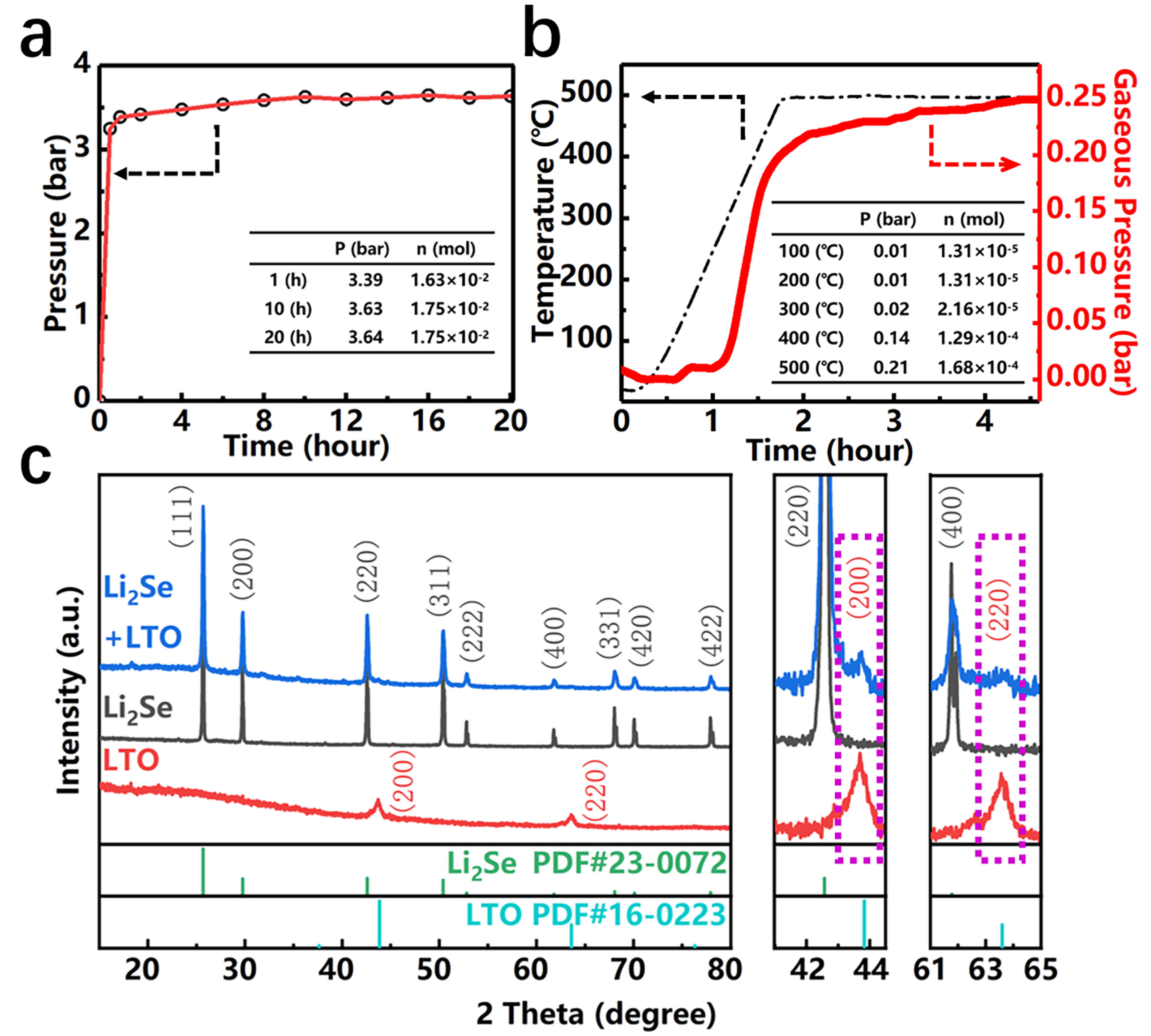
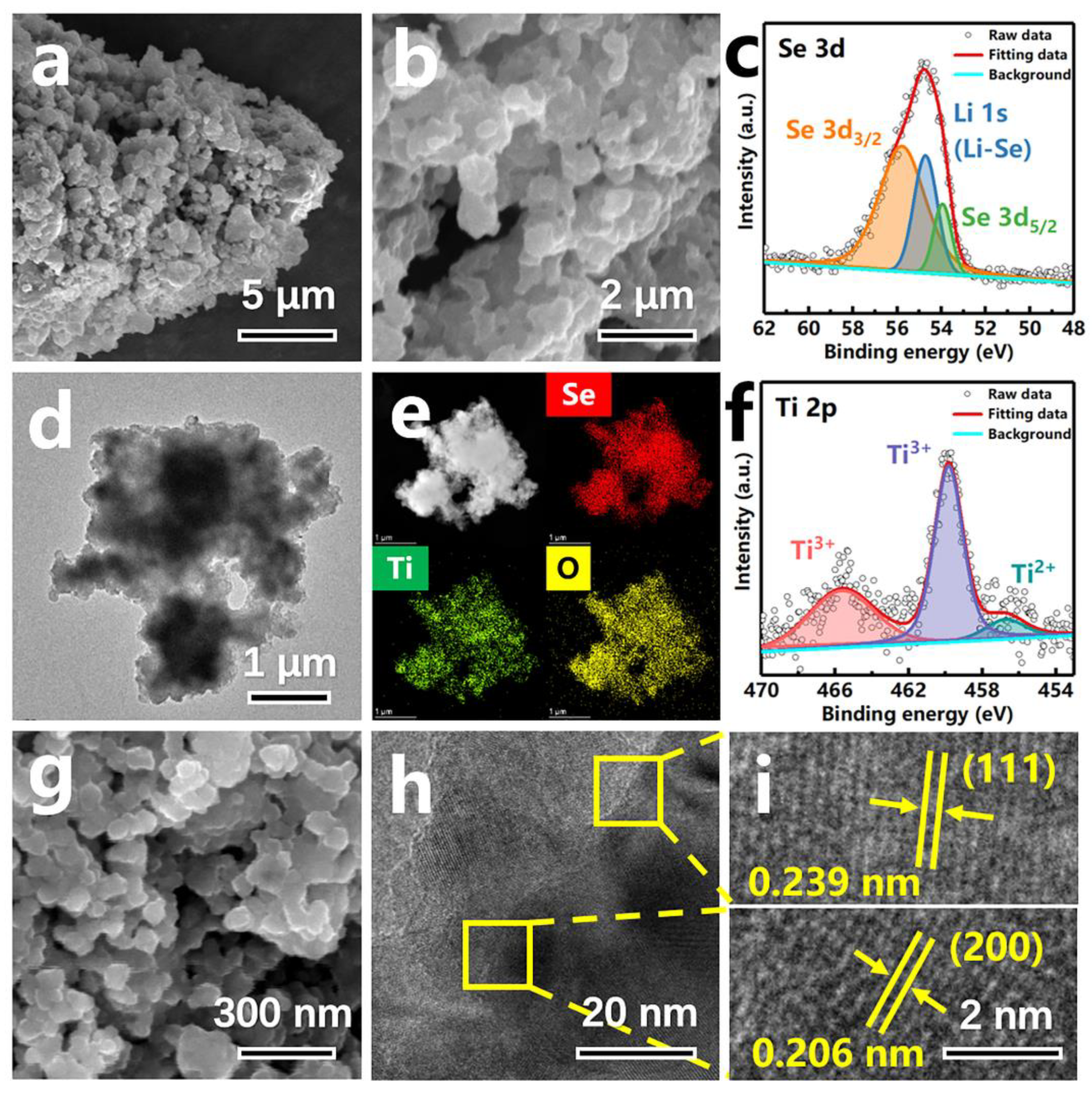
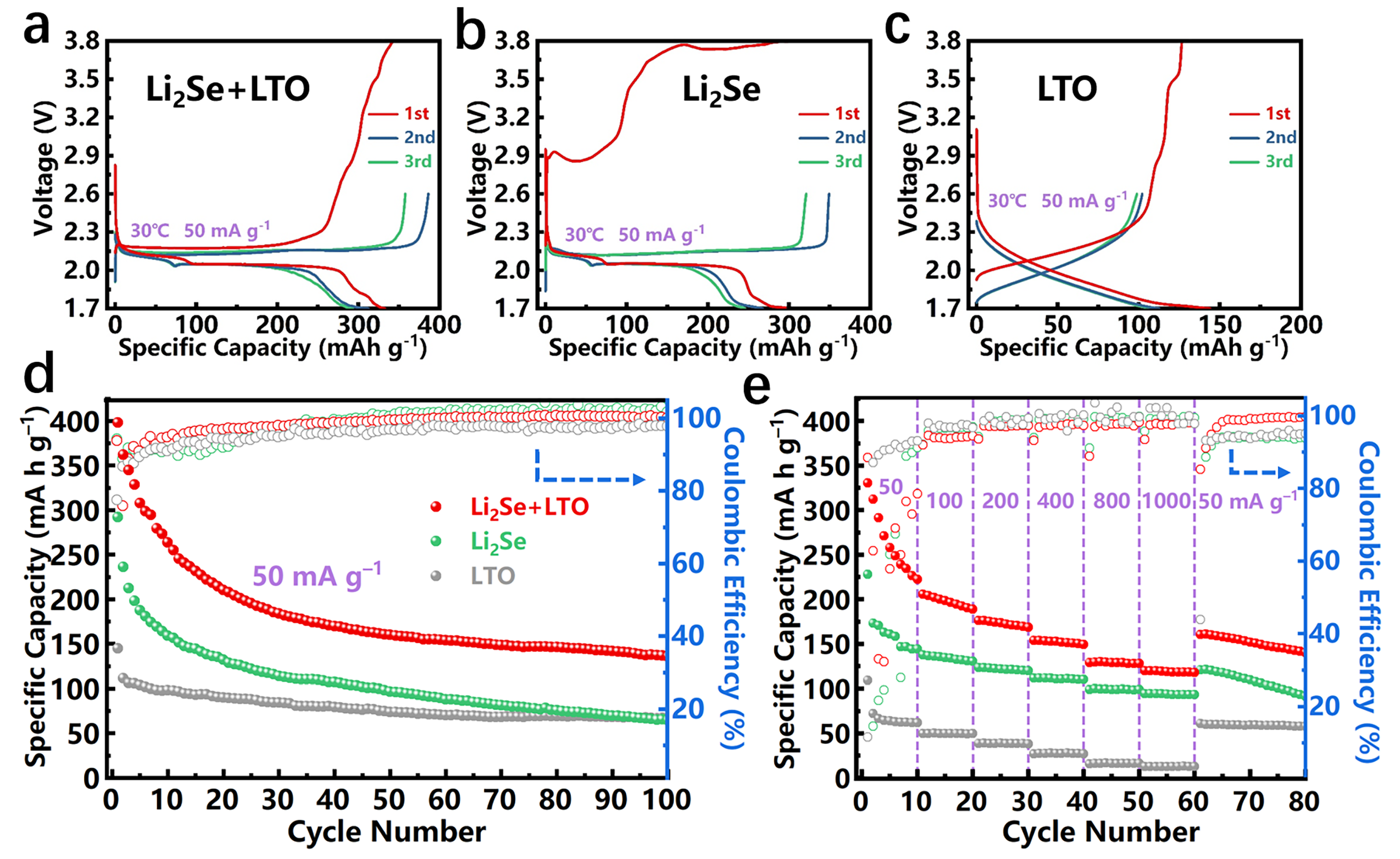
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghosh, A.; Cherepanov, P.; Nguyen, C.; Ghosh, A.; Kumar, A.; Ahuja, A.; Kar, M.; MacFarlane, D.R.; Mitra, S. Simple route to lithium dendrite prevention for long cycle-life lithium metal batteries. Appl. Mater. Today 2021, 23, 101062. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Z.; Shi, L.; Jungsuttiwong, S.; Yuan, S. Ionic liquids for high performance lithium metal batteries. J. Energy Chem. 2021, 59, 320–333. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Tang, P.; Wang, H.; Li, K.; Yin, Y.; Yang, S. A novel strategy for improving performance of lithium-oxygen batteries. J. Colloid Interface Sci. 2021, 584, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in Lithium–Sulfur Batteries: From Academic Research to Commercial Viability. Adv. Mater. 2021, 33, e2003666. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jia, D.; Wu, Y.; Song, D.; Sun, X.; Wang, C.; Yang, L.; Zhang, Y.; Gao, J.; Ohsaka, T.; et al. Novel lithium-chalcogenide batteries combining S, Se and C characteristics supported by chitosan-derived carbon intertwined with CNTs. Chem. Eng. J. 2022, 427, 131790. [Google Scholar] [CrossRef]
- Atin, P.; Sandipan, M.; Sourindra, M. Metal hydroxides as a conversion electrode for lithium-ion batteries: A case study with a Cu(OH)2 nanoflower array. J. Mater. Chem. A 2014, 2, 18515–18522. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-Performance Anode Materials for Rechargeable Lithium-Ion Batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Naoki, N.; Wu, F.; Jung, T.L.; Gleb, Y. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Atin, P.; Shreyasi, C.; Goutam, D.; Sourindra, M. Efficient energy storage in mustard husk derived porous spherical carbon nanostructures. Mater. Adv. 2021, 2, 7463–7472. [Google Scholar] [CrossRef]
- Liu, Y.; Chatterjee, A.; Rusch, P.; Wu, C.; Nan, P.; Peng, M.; Bettels, F.; Li, T.; Ma, C.; Zhang, C.; et al. Monodisperse Molybdenum Nanoparticles as Highly Efficient Electrocatalysts for Li-S Batteries. ACS Nano 2021, 15, 15047–15056. [Google Scholar] [CrossRef]
- Tang, T.; Hou, Y. Chemical Confinement and Utility of Lithium Polysulfides in Lithium Sulfur Batteries. Small Methods 2019, 4, 1900001. [Google Scholar] [CrossRef]
- Baek, M.; Shin, H.; Char, K.; Choi, J.W. New High Donor Electrolyte for Lithium–Sulfur Batteries. Adv. Mater. 2020, 32, e2005022. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Amine, K. Nanotechnology for Sulfur Cathodes. ACS Nano 2021, 15, 8087–8094. [Google Scholar] [CrossRef]
- Zhao, K.; Jin, Q.; Zhang, L.; Li, L.; Wu, L.; Zhang, X. Achieving dendrite-free lithium deposition on the anode of Lithium–Sulfur battery by LiF-rich regulation layer. Electrochim. Acta 2021, 393, 138981. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Y.; Wang, Y.; Cai, Z.; Xiao, J.; Muhmood, T.; Hu, X. Three-Dimensional Ordered Porous Nanostructures for Lithium–Selenium Battery Cathodes That Confer Superior Energy-Storage Performance. ACS Appl. Mater. Interfaces 2021, 13, 9955–9964. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Zhang, S.; Hou, Y. Sulfur Hosts against the Shuttle Effect. Small Methods 2018, 2, 1700345. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, L.; Ma, C.; Cheng, L.; Wang, C.; Chen, G.; Yue, H.; Zhang, D. The synergistic effects of nanoporous fiber TiO2 and nickel foam interlayer for ultra-stable performance in lithium-selenium batteries. J. Power Sources 2021, 490, 229534. [Google Scholar] [CrossRef]
- Xiang, H.; Deng, N.; Zhao, H.; Wang, X.; Wei, L.; Wang, M.; Cheng, B.; Kang, W. A review on electronically conducting polymers for lithium-sulfur battery and lithium-selenium battery: Progress and prospects. J. Energy Chem. 2021, 58, 523–556. [Google Scholar] [CrossRef]
- Cao, Y.; Lei, F.; Li, Y.; Qiu, S.; Wang, Y.; Zhang, W.; Zhang, Z. A MOF-derived carbon host associated with Fe and Co single atoms for Li–Se batteries. J. Mater. Chem. A 2021, 9, 16196–16207. [Google Scholar] [CrossRef]
- Deng, N.; Feng, Y.; Wang, G.; Wang, X.; Wang, L.; Li, Q.; Zhang, L.; Kang, W.; Cheng, B.; Liu, Y. Rational structure designs of 2D materials and their applications toward advanced lithium-sulfur battery and lithium-selenium battery. Chem. Eng. J. 2020, 401, 125976. [Google Scholar] [CrossRef]
- Jin, J.; Tian, X.; Srikanth, N.; Kong, L.B.; Zhou, K. Advances and challenges of nanostructured electrodes for Li–Se batteries. J. Mater. Chem. A 2017, 5, 10110–10126. [Google Scholar] [CrossRef]
- Huang, X.L.; Guo, Z.; Dou, S.X.; Wang, Z.M. Rechargeable Potassium–Selenium Batteries. Adv. Funct. Mater. 2021, 31, 2102326. [Google Scholar] [CrossRef]
- Tang, S.; Liu, C.; Sun, W.; Zhang, X.; Shen, D.; Dong, W.; Yang, S. Understanding the anchoring and catalytic effect of the Co@C2N monolayer in lithium–selenium batteries: A first-principles study. Nanoscale 2021, 13, 16316–16323. [Google Scholar] [CrossRef]
- Fang, Y.; Luan, D.; Gao, S.; Lou, X.W. Rational Design and Engineering of One-Dimensional Hollow Nanostructures for Efficient Electrochemical Energy Storage. Angew. Chem. Int. Ed. 2021, 60, 20102–20118. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Zhou, C.; He, W.; Sun, S.; Chueh, Y.-L.; Wang, Z.M.; Liu, H.K.; Dou, S.X. An Emerging Energy Storage System: Advanced Na–Se Batteries. ACS Nano 2021, 15, 5876–5903. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Li, H.; Liu, J.; Song, J.; Fusaro, L.; Hu, Z.-Y.; Chen, Y.; Li, Y.; Su, B.-L. Weaving 3D highly conductive hierarchically interconnected nanoporous web by threading MOF crystals onto multi walled carbon nanotubes for high performance Li–Se battery. J. Energy Chem. 2021, 59, 396–404. [Google Scholar] [CrossRef]
- Wu, K.; Wang, J.; Xu, C.; Jiao, X.; Hu, X.; Guan, W. Hollow Spherical α-MoO3: An Effective Electrocatalyst of Polyselenides for Lithium–Selenium Batteries. ACS Appl. Energy Mater. 2021, 4, 10203–10212. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Zhang, K.; Yang, X.; Li, Q. Improvement of electrochemical performance of rechargeable lithium–selenium batteries by inserting a free-standing carbon interlayer. RSC Adv. 2014, 4, 15489–15492. [Google Scholar] [CrossRef]
- Liu, N.; Ma, H.; Wang, L.; Zhao, Y.; Bakenov, Z.; Wang, X. Dealloying-derived nanoporous deficient titanium oxide as high-performance bifunctional sulfur host-catalysis material in lithium-sulfur battery. J. Mater. Sci. Technol. 2021, 84, 124–132. [Google Scholar] [CrossRef]
- Gui, Y.; Chen, P.; Liu, D.; Fan, Y.; Zhou, J.; Zhao, J.; Liu, H.; Guo, X.; Liu, W.; Cheng, Y. TiO2 nanotube/RGO modified separator as an effective polysulfide-barrier for high electrochemical performance Li-S batteries. J. Alloys Compd. 2021, 895, 162495. [Google Scholar] [CrossRef]
- Xia, Y.; Ren, Q.; Lu, C.; Zhu, J.; Zhang, J.; Liang, C.; Huang, H.; Gan, Y.; He, X.; Zhu, D.; et al. Graphene/TiO2 decorated N-doped carbon foam as 3D porous current collector for high loading sulfur cathode. Mater. Res. Bull. 2021, 135, 111129. [Google Scholar] [CrossRef]
- Lei, Z.; Lei, Y.; Liang, X.; Yang, L.; Feng, J. High stable rate cycling performances of microporous carbon spheres/selenium composite (MPCS/Se) cathode as lithium–selenium battery. J. Power Sources 2020, 473, 228611. [Google Scholar] [CrossRef]
- Wu, F.; Lee, J.T.; Xiao, Y.; Yushin, G. Nanostructured Li2Se cathodes for high performance lithium-selenium batteries. Nano Energy 2016, 27, 238–246. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, W.; Fang, R.; Xiao, Z.; Huang, H.; Gan, Y.; Zhang, J.; He, X.; Liang, C.; Zhu, D.; et al. Facile and efficient synthesis of Li2Se particles towards high-areal capacity Li2Se cathode for advanced Li–Se battery. Sustain. Mater. Technol. 2021, 29, e00288. [Google Scholar] [CrossRef]
- Bui, H.T.; Jang, H.; Ahn, D.; Han, J.; Sung, M.; Kutwade, V.; Patil, M.; Sharma, R.; Han, S.-H. High-performance Li–Se battery: Li2Se cathode as intercalation product of electrochemical in situ reduction of multilayer graphene-embedded 2D-MoSe2. Electrochim. Acta 2020, 368, 137556. [Google Scholar] [CrossRef]
- Koudriachova, M. Ramsdellite-structured LiTiO2: A new phase predicted from ab initio calculations. Chem. Phys. Lett. 2008, 458, 108–112. [Google Scholar] [CrossRef]
- Yang, H.-D.; Kang, Y.-Y.; Zhu, P.-P.; Chen, Q.-W.; Yang, L.; Zhou, J.-P. Facile hydrothermal preparation, characterization and multifunction of rock salt-type LiTiO2. J. Alloys Compd. 2021, 872, 159759. [Google Scholar] [CrossRef]
- Mackrodt, W. First Principles Hartree–Fock Description of Lithium Insertion in Oxides: I. The End Members TiO2 and LiTiO2 of the System LixTiO2. J. Solid State Chem. 1999, 142, 428–439. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, G.; Shan, Z.; Zhang, T.; Tian, J. Hierarchically Micro-/Nanostructured TiO2/Micron Carbon Fibers Composites for Long-Life and Fast-Charging Lithium-Ion Batteries. ChemElectroChem 2018, 5, 540–545. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, W.; Wang, Y.; Liang, S.; Gan, Y.; Huang, H.; Zhang, J.; Xia, Y.; Liang, C. Lithium Sulfide as Cathode Materials for Lithium-Ion Batteries: Advances and Challenges. J. Chem. 2020, 2020, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Hong, J.; Park, Y.R.; Kim, K.J. The origin of oxygen vacancy induced ferromagnetism in undoped TiO2. J. Phys. Condens. Matter 2009, 21, 195405. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, Y.; Chen, C.; Feng, X.; Zhang, Z. High-performance polymer electrolyte membrane modified with isocyanate-grafted Ti3+ doped TiO2 nanowires for lithium batteries. Appl. Surf. Sci. 2021, 563, 150248. [Google Scholar] [CrossRef]
- Zhu, W.-D.; Wang, C.-W.; Chen, J.-B.; Li, Y.; Wang, J. Enhanced field emission from Ti3+ self-doped TiO2 nanotube arrays synthesized by a facile cathodic reduction process. Appl. Surf. Sci. 2014, 301, 525–529. [Google Scholar] [CrossRef]
- Singh, A.; Kalra, V. Electrospun nanostructures for conversion type cathode (S, Se) based lithium and sodium batteries. J. Mater. Chem. A 2019, 7, 11613–11650. [Google Scholar] [CrossRef]
- Jin, W.-W.; Li, H.-J.; Zou, J.-Z.; Inguva, S.; Zhang, Q.; Zeng, S.-Z.; Xu, G.-Z.; Zeng, X.-R. Metal organic framework-derived carbon nanosheets with fish-scale surface morphology as cathode materials for lithium–selenium batteries. J. Alloys Compd. 2020, 820, 153084. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Zhao, X.; Wang, X.; Miao, Y.; Cheng, L.; Wang, C.; Wang, L.; Yue, H.; Zhang, D. Porous Bamboo-Derived Carbon as Selenium Host for Advanced Lithium/Sodium–Selenium Batteries. Energy Technol.-Ger. 2020, 8, 1901445. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, C.; Zhou, J.; Qin, L.; Lin, X.; Cheng, Q.; Liu, J. N-doped C/Se derived from a Cu-based coordination polymer as cathode for lithium-selenium batteries. Inorg. Chem. Commun. 2019, 108, 107537. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Fang, Z.; Lu, C.; Xiao, Z.; He, X.; Gan, Y.; Huang, H.; Wang, G.; Zhang, W. A Facile Pre-Lithiated Strategy towards High-Performance Li2Se-LiTiO2 Composite Cathode for Li-Se Batteries. Nanomaterials 2022, 12, 815. https://doi.org/10.3390/nano12050815
Xia Y, Fang Z, Lu C, Xiao Z, He X, Gan Y, Huang H, Wang G, Zhang W. A Facile Pre-Lithiated Strategy towards High-Performance Li2Se-LiTiO2 Composite Cathode for Li-Se Batteries. Nanomaterials. 2022; 12(5):815. https://doi.org/10.3390/nano12050815
Chicago/Turabian StyleXia, Yang, Zheng Fang, Chengwei Lu, Zhen Xiao, Xinping He, Yongping Gan, Hui Huang, Guoguang Wang, and Wenkui Zhang. 2022. "A Facile Pre-Lithiated Strategy towards High-Performance Li2Se-LiTiO2 Composite Cathode for Li-Se Batteries" Nanomaterials 12, no. 5: 815. https://doi.org/10.3390/nano12050815
APA StyleXia, Y., Fang, Z., Lu, C., Xiao, Z., He, X., Gan, Y., Huang, H., Wang, G., & Zhang, W. (2022). A Facile Pre-Lithiated Strategy towards High-Performance Li2Se-LiTiO2 Composite Cathode for Li-Se Batteries. Nanomaterials, 12(5), 815. https://doi.org/10.3390/nano12050815