Magnetic Nanoparticles in Bone Tissue Engineering
Abstract
:1. Introduction
Bone Tissue Engineering
2. Magnetic Nanoparticles and Bone Tissue Engineering
2.1. Magnetic Nanoparticles
2.2. The Influence of Magnetic Nanoparticles on Bone Tissue Engineering
2.2.1. Magnetic Nanoparticles and Cells
Cell Induction
Cell Guidance
Cell-Based Tissue Engineering
Delivery of Bioactive Agents Using Magnetic Nanoparticles
3. Magnetic Nanoparticles in Scaffolds for Bone Tissue Engineering
3.1. Impact on Osteogenesis
| Scaffold Material | MNP Composition | MNP Content within Scaffold | Magnetism Intensity (emu/g) | Osteogenic Impact | Mechanism |
|---|---|---|---|---|---|
| HA and Collagen [61] | NI | 2.65% | NI | Enhanced bone maturity in-vivo, identified by improved mechanical properties. | Incongruous magnetic moment created by the distribution of MNPs within the scaffold. |
| PCL [59] | Maghemite | 7.9% | NI | Improved cell adhesion, proliferation and osteogenic differentiation (elevated ALP) of MSCs. | MNP incorporation generates a magnetic microenvironment. |
| PCL [52] | GdHA | 2.67% | NI | Greater cell attachment, spreading, proliferation and osteogenic differentiation (higher ALP, RUNX2) of MSCs. Improved mechanical properties. | Gadolinium released entered cells and promoted cell cycle progression. Greater hydrophilicity and surface area facilitate protein adsorption. Reduced PCL fibre diameter increases scaffold strength. |
| PCL [62] | FeHA | 4.5% | NI | Improved cell growth. Scaffold filled with new bone after just 4 weeks in-vivo. | MNP incorporation generates a magnetic microenvironment. |
| PCL [49] | Magnetite | 5% 10% | 5%—1.6 10%—3.1 | Greater cell adhesion, proliferation and osteogenic differentiation (enhanced cellular mineralisation) of MSCs. | Elevated hydrophilicity improved cell adhesion that facilitated proliferation and differentiation to follow. MNP incorporation generates a magnetic microenvironment. |
| PCL [56] | Magnetite | 5%, 10%, 15%, 20% | 5%—1.0 20%—11.2 | Better cell adhesion, spreading, penetration and osteogenic differentiation (ALP, COL-1, OPN, BSP) of MSCs. Histology showed higher blood vessel formation and better integration with the host tissue in-vivo. Enhanced mechanical properties. | MNP incorporation generates a magnetic microenvironment. Controlled degradation rate allows ingrowth of cells and vascularisation. Strong chemical interaction between MNPs and polymer chains. |
| PCL and PLGA [48] | Maghemite | 16.4% | 3.56 | Improved cell adhesion, spreading and osteogenic differentiation (higher ALP, RUNX2, OCN, COL-1 and bone mineralisation) of ADSCs. Better mechanical properties. | Greater hydrophilicity and protein adsorptions facilitate cell attachment. Higher gene expression of a transmembrane magnetoreceptor ISCA1-osteogenic enhancement as a result of transmembrane effect of MNPs. |
| PLLA and PGA [60] | Magnetite | 2.5%, 5%, 7.5%, 10% | 2.5%—1.66 10%—8.51 | Greater cell adhesion, spreading, proliferation and osteogenic differentiation (ALP) of MG63 cells. Improved mechanical properties. Better BMD, BVF, fusion and blood vessel formation in-vivo. | Improved hydrophilicity and magnetic microenvironments facilitate improved cellular activity. MNPs resist deformation of the polymer chains. Microenvironment promoted adhesion, migration and differentiation of osteocytes in-vivo. |
| PCL and Mesoporous Bioactive glass [58] | Magnetite | 5%, 10%, 15% | 5%—3.1 10%—6.2 15%—9.3 | Increased cell adhesion, proliferation and osteogenic differentiation (elevated ALP, RUNX2, OCN, BMP-2 and COL-1) of MSCs. | Improved hierarchal pore structure. MNP incorporation generates a magnetic microenvironment. |
| CPC [51] | Magnetite | 0.05–5% | 0.1%—0.05 1%—0.35 | Greater cell adhesion, spreading, proliferation and osteogenic differentiation (increased ALP) of BMSCs. Improved mechanical properties. | Altered surface morphology- change in crystal shape and reduced size increased the surface area for adhesion of proteins involved in cell adhesion. MNP incorporation generates a magnetic microenvironment. |
| CPC [50] | Maghemite | NI | NI | Enhanced cell attachment, spreading, proliferation and osteogenic differentiation (increased ALP, RUNX2, OCN, COL-1) of DPSCs. | Altered surface morphology-reduced crystal size increased the surface area for adhesion of proteins involved in cell adhesion. MNPs released by the degrading scaffolds and interact with cells via membrane adsorption and internalisation. |
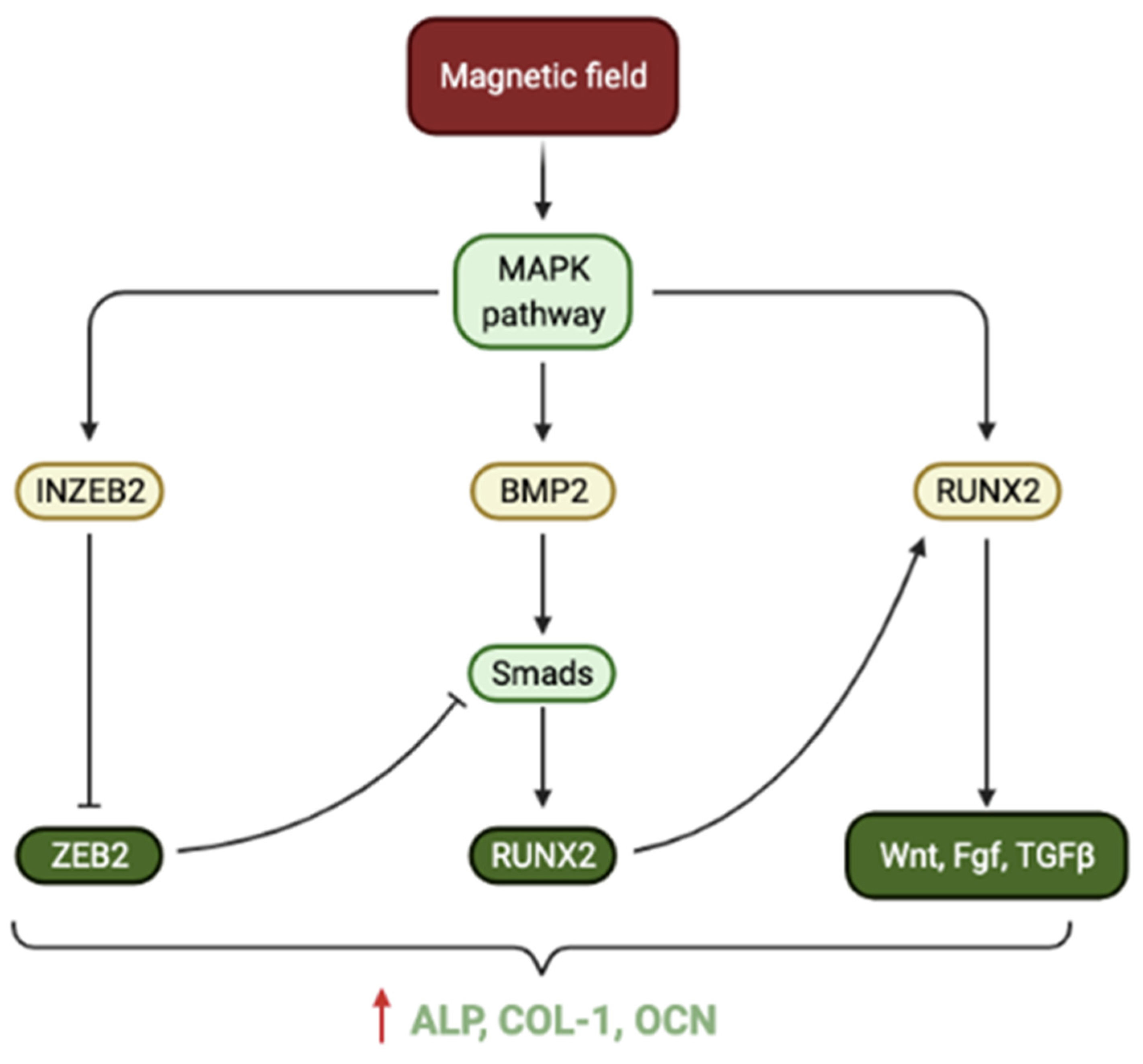
| CPC [53] | Maghemite | 1–6% | NI | Improved cell adhesion, spreading, proliferation and osteogenic differentiation (increased ALP, RUNX2, OCN, COL-1) of DPSCs. Enhanced the mechanical properties. | Greater hydrophilicity and improved nanostructure facilitated cell adhesion and spreading. The WNT signalling pathway is activated and mediates proliferation osteogenic differentiation upon magnetic stimulation. Cells internalise released MNPs. |
| Gelatin and Siloxane [54] | Magnetite | 1–3% | 1%—0.24 3%—0.64 | Greater cell adhesion, proliferation and osteogenic differentiation (greater ALP and mineralisation) of MSCs. Improved mechanical properties. | Improved hydrophilicity allowed better cell adhesion. MNP incorporation generates a magnetic microenvironment. |
| Bioglass and Chitosan [57] | SrFe12O19 | 1:7, 1:3 (ratio of SrFe12O19 to Bioglass) | 1:7–4.44 1:3–7.68 | Enhanced cell adhesion, spreading, proliferation and osteogenic differentiation (increased ALP, RUNX2, OCN, COL-1, BMP-2) of BMSCs. Greater bone mineralisation, BMD and BV/TV in-vivo. | Proliferation and osteogenic differentiation are mediated by BMP-2/Smad/RUNX2 pathway upon magnetic stimulation. |
| Chitosan and Collagen [55] | Magnetite | NI | 0.025 | Improved cell adhesion, proliferation and osteogenic differentiation (better mineralisation) in pre-osteoblasts. Enhanced bony ingrowth, BMD and BVF in-vivo. Better mechanical properties. | Improved hierarchical nanostructure- surface roughness and interconnected porosity. This can improve cell adhesion, cell penetration as well as nutrient transfer and flow transportation in the scaffold. |
3.2. Effect of MNPs on Angiogenesis
3.3. External Magnetic Stimulation
4. Toxicity of Magnetic Nanoparticles
4.1. Magnetic Nanoparticle-Induced Toxicity
4.2. The Significance of Toxicity on Bone Tissue Engineering Applications
| Cell Type | SPION Core-Coating (Name If Given) | SPION Diamete (nm) | SPION Incubation Concentration (μg/mL) | Incubation Period | Iron Content per Cell (pg) | Experiment Duration (Days) | Impact on Osteogenic Differentiation | Other Experiments |
|---|---|---|---|---|---|---|---|---|
| Rat BMSCs [88] | Iron oxide- citric acid | 96 | 50 | 72 h | 13 | 14 | Impaired | Reduced cell viability with increasing concentration. |
| Rat ADSCs [88] | Iron oxide- citric acid | 96 | 50 | 72 h | 13 | 14 | Impaired | Reduced cell viability with increasing concentration. |
| hMSCs [91] | Magnetite- amine (NH3+) | 6 | 50 | 72 h | 200 | 21 | Impaired | Improved cell proliferation. |
| hMSCs [92] | Iron oxide- carboxydextran (Ferucarbotran) | 62 | 100 | 60 min | NI | 7 | Impaired | Cell mobilisation was promoted. |
| hMSCs [95] | Iron oxide- silica | 4.5 | 50 | 4 days | 4 | 14 | Unaffected | Cell viability and proliferation was unimpacted. No changes in gene expression of VEGF or anti-inflammatory factors. No tissue damage or blood toxicity in-vivo after 7 weeks. |
| hMSCs [88] | Magnetite- citric acid | 48 | 100 | 72 h | NI | 14 | Unaffected | Cell viability was unaffected. |
| Canine ADSCs [89] | Magnetite | 10 | 50 | 12 h | 28 | 21 | Unaffected | Cell viability and proliferation were unimpacted. |
| hMSCs [93] | Magnetite- PDA | 57 | 50 | 24 h | NI | 21 | Unaffected | Cell viability and proliferation were unaffected. |
| hMSCs [90] | Iron oxide- citrate | 98 | 25 | 24 h | 70 | NI | Unaffected | No cytotoxicity was observed. |
| hMSCs [90] | Iron oxide- dextran (Ferumoxide) | 157 | 500 | 24 h | 26 | NI | Unaffected | No cytotoxicity was observed. |
| hMSCs [20] | Maghemite- PSC | 30 | 100 | 72 h | NI | 21 | Promoted | Cell viability was unimpacted. |
| hMSCs [18] | Magnetite- silica | 55 | 100 | 24 h | NI | 14 | Promoted | Cell viability and proliferation were unaffected. |
| hMSCs [28] | Maghemite- PSC | 30 | 100 | 48 h | 0.9 | 21 | Promoted | Cell viability was unimpacted. |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Sanders, R. Bone Graft Substitutes. J. Bone Jt. Surg. 2007, 89, 469. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Maia, F.R.; Bastos, A.R.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Recent approaches towards bone tissue engineering. Bone 2021, 154, 116256. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Awad, H.A.; O’Keefe, R.J.; Lee, C.H.; Mao, J.J. Bone Tissue Engineering. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1733–1743. [Google Scholar]
- Shahri, M.M. Magnetic Materials and Magnetic Nanocomposites for Biomedical Application. Harnessing Nanoscale Surface Interactions: Contemporary Synthesis, Applications and Theory; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–95. [Google Scholar] [CrossRef]
- Savliwala, S.; Chiu-Lam, A.; Unni, M.; Rivera-Rodriguez, A.; Fuller, E.; Sen, K.; Threadcraft, M.; Rinaldi, C. Magnetic Nanoparticles. Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 195–221. [Google Scholar]
- Hinge, N.; Pandey, M.M.; Singhvi, G.; Gupta, G.; Mehta, M.; Satija, S.; Gulati, M.; Dureja, H.; Dua, K. Nanomedicine Advances in Cancer Therapy. Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 219–253. [Google Scholar]
- Singh, A.V.; Dad Ansari, M.H.; Dayan, C.B.; Giltinan, J.; Wang, S.; Yu, Y.; Kishore, V.; Laux, P.; Luch, A.; Sitti, M. Multifunctional magnetic hairbot for untethered osteogenesis, ultrasound contrast imaging and drug delivery. Biomaterials 2019, 219, 119394. [Google Scholar] [CrossRef]
- Ni, J.-S.; Li, Y.; Yue, W.; Liu, B.; Li, K. Nanoparticle-based Cell Trackers for Biomedical Applications. Theranostics 2020, 10, 1923–1947. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Navas, P.M.; Garcia-Martin, M.L. Application of Inorganic Nanoparticles for Diagnosis Based on MRI. Front. Nanosci. 2012, 4, 233–245. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, L.; Bao, G. Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Curr. Opin. Biomed. Eng. 2021, 20, 100330. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Sharma, P.K.; Sood, N.; Bhalla, N. Designing magnetic nanoparticles for in vivo applications and understanding their fate inside human body. Coord. Chem. Rev. 2021, 445, 214082. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, P.; Sun, Y.; Kang, Q.; Xu, J.; Zhang, C.; Chai, Y. Regeneration of large bone defects using mesoporous silica coated magnetic nanoparticles during distraction osteogenesis. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102040. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, Y.; Zhao, Y.; Xu, Y.; Zhang, F.; Gu, N.; Ma, J.; Reynolds, M.A.; Xia, Y.; Xu, H.H. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018, 12, e2085–e2098. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Cao, M.; Sun, J.; Wu, H.; Zhao, P.; Xing, J.; Yang, Y.; Zhang, X.; Ji, M.; et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 2016, 86, 11–20. [Google Scholar] [CrossRef]
- Nikukar, H.; Reid, S.; Tsimbouri, P.M.; Riehle, M.O.; Curtis, A.S.G.; Dalby, M.J. Osteogenesis of Mesenchymal Stem Cells by Nanoscale Mechanotransduction. ACS Nano 2013, 7, 2758–2767. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [Green Version]
- Håkelien, A.-M.; Bryne, J.C.; Harstad, K.G.; Lorenz, S.; Paulsen, J.; Sun, J.; Mikkelsen, T.S.; Myklebost, O.; Meza-Zepeda, L.A. The Regulatory Landscape of Osteogenic Differentiation. Stem Cells 2014, 32, 2780–2793. [Google Scholar] [CrossRef]
- He, L.; Montell, D. A cellular sense of touch. Nat. Cell Biol. 2012, 14, 902–903. [Google Scholar] [CrossRef]
- Yi, C.; Liu, D.; Fong, C.-C.; Zhang, J.; Yang, M. Gold Nanoparticles Promote Osteogenic Differentiation of Mesenchymal Stem Cells through p38 MAPK Pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Z.; Hou, Y.; Fang, W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 2527–2535. [Google Scholar] [PubMed]
- Jang, W.-G.; Kim, E.-J.; Kim, D.-K.; Ryoo, H.-M.; Lee, K.-B.; Kim, S.-H.; Choi, H.-S.; Koh, J.-T. BMP2 Protein Regulates Osteocalcin Expression via Runx2-mediated Atf6 Gene Transcription. J. Biol. Chem. 2012, 287, 905–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Chen, B.; Ma, F.; Lin, S.; Cao, M.; Li, Y.; Gu, N. Magnetic iron oxide nanoparticles accelerate osteogenic differentiation of mesenchymal stem cells via modulation of long noncoding RNA INZEB2. Nano Res. 2016, 10, 626–642. [Google Scholar] [CrossRef]
- Huang, D.-M.; Hsiao, J.-K.; Chen, Y.-C.; Chien, L.-Y.; Yao, M.; Chen, Y.-K.; Ko, B.-S.; Hsu, S.-C.; Tai, L.-A.; Cheng, H.-Y.; et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645–3651. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Henstock, J.R.; Rotherham, M.; El Haj, A.J. Magnetic ion channel activation of TREK1 in human mesenchymal stem cells using nanoparticles promotes osteogenesis in surrounding cells. J. Tissue Eng. 2018, 9, 2041731418808695. [Google Scholar] [CrossRef] [Green Version]
- Markides, H.; McLaren, J.S.; Telling, N.D.; Alom, N.; Al-Mutheffer, E.A.; Oreffo, R.O.C.; Zannettino, A.; Scammell, B.E.; White, L.J.; El Haj, A.J. Translation of remote control regenerative technologies for bone repair. NPJ Regen. Med. 2018, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wei, Z.; Lv, H.; Wu, L.; Cui, Y.; Yao, H.; Li, J.; Zhang, H.; Yang, B.; Jiang, J. Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites. Int. J. Nanomed. 2019, 14, 573–589. [Google Scholar] [CrossRef] [Green Version]
- Oshima, S.; Ishikawa, M.; Mochizuki, Y.; Kobayashi, T.; Yasunaga, Y.; Ochi, M. Enhancement of bone formation in an experimental bony defect using ferumoxide-labelled mesenchymal stromal cells and a magnetic targeting system. J. Bone Jt. Surgery. Br. Vol. 2010, 92, 1606–1613. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, E.E.; Kamei, G.; Harada, Y.; Shimizu, R.; Kamei, N.; Adachi, N.; Misk, N.A.; Ochi, M. Cell Magnetic Targeting System for Repair of Severe Chronic Osteochondral Defect in a Rabbit Model. Cell Transplant. 2016, 25, 1073–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alblawi, A.; Ranjani, A.S.; Yasmin, H.; Gupta, S.; Bit, A.; Rahimi-Gorji, M. Scaffold-free: A developing technique in field of tissue engineering. Comput. Methods Programs Biomed. 2020, 185, 105148. [Google Scholar] [CrossRef] [PubMed]
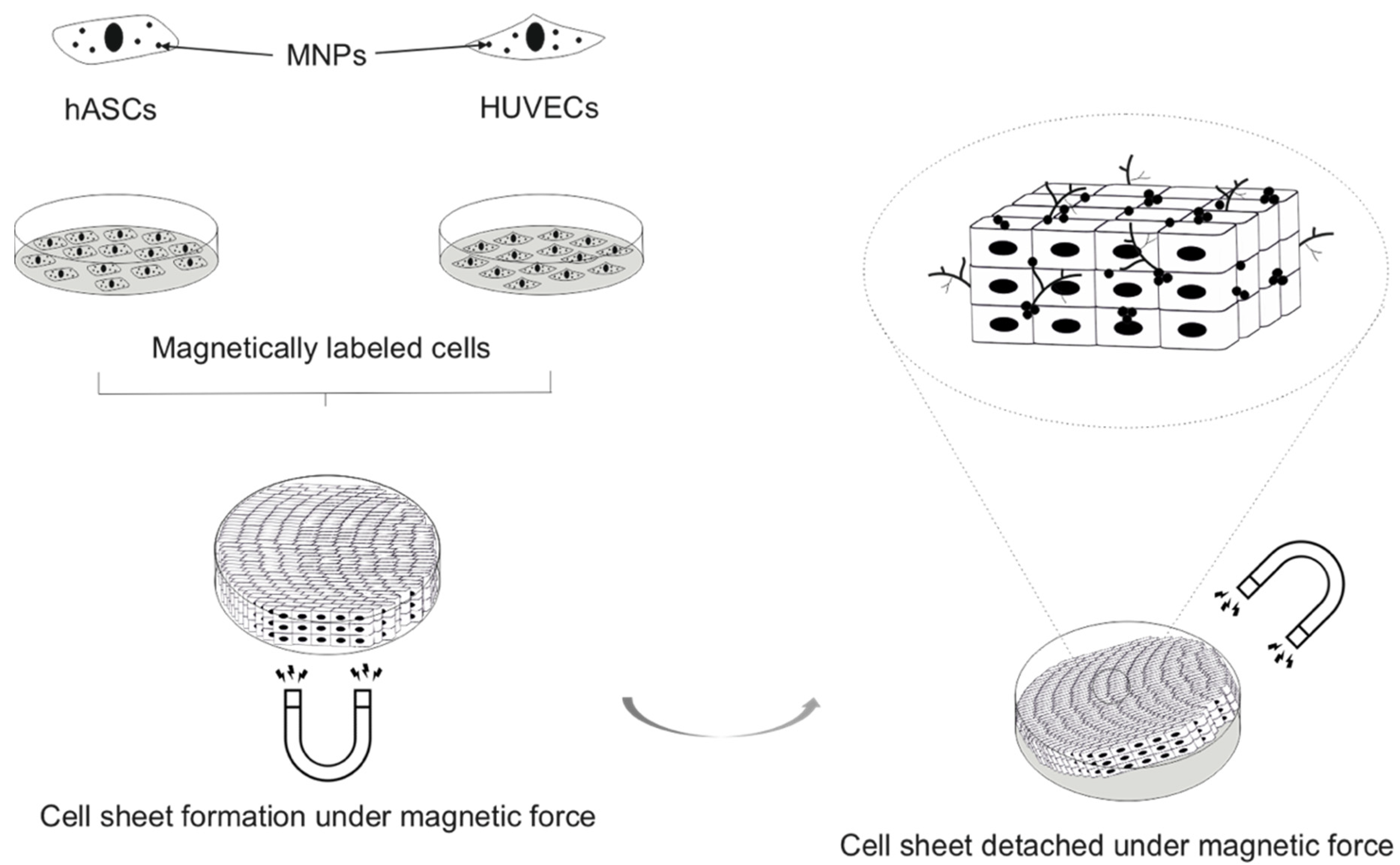
- Moschouris, K.; Firoozi, N.; Kang, Y. The application of cell sheet engineering in the vascularization of tissue regeneration. Regen. Med. 2016, 11, 559–570. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.; Miranda, M.; Rodrigues, M.; Reis, R.L.; Gomes, M.E. Magnetic responsive cell-based strategies for diagnostics and therapeutics. Biomed. Mater. 2018, 13, 054001. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Santos, L.F.; Mendes, M.C.; Mano, J.F. Multi-layer pre-vascularized magnetic cell sheets for bone regeneration. Biomaterials 2020, 231, 119664. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef]
- Dzamukova, M.R.; Naumenko, E.A.; Rozhina, E.V.; Trifonov, A.A.; Fakhrullin, R.F. Cell surface engineering with polyelectrolyte-stabilized magnetic nanoparticles: A facile approach for fabrication of artificial multicellular tissue-mimicking clusters. Nano Res. 2015, 8, 2515–2532. [Google Scholar] [CrossRef] [Green Version]
- Dzamukova, M.R.; Naumenko, E.A.; Lannik, N.I.; Fakhrullin, R.F. Surface-modified magnetic human cells for scaffold-free tissue engineering. Biomater. Sci. 2013, 1, 810–813. [Google Scholar] [CrossRef]
- Guryanov, I.; Naumenko, E.; Konnova, S.; Lagarkova, M.; Kiselev, S.; Fakhrullin, R. Spatial manipulation of magnetically-responsive nanoparticle engineered human neuronal progenitor cells. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102038. [Google Scholar] [CrossRef]
- Denyer, S.; Bhimani, A.; Papastefan, S.; Kheirkhah, P.; Aguilar, T.; Zakrzewski, J.; Rosinski, C.L.; Patel, A.S.; Patel, S.; Zakrzewski, V.; et al. Magnetic kyphoplasty: A novel drug delivery system for the spinal column. PLoS ONE 2018, 13, e0201402. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, H.; Liu, J.; Tao, S.; Chai, G.; Wang, J.; Hu, F. Tetracycline-grafted PLGA nanoparticles as bone-targeting drug delivery system. Int. J. Nanomed. 2015, 10, 5671–5685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, I.; Sher, I.; Corem-Salkmon, E.; Ziv-Polat, O.; Meir, A.; Treves, A.J.; Nagler, A.; Kalter-Leibovici, O.; Margel, S.; Rotenstreich, Y. Bioactive magnetic near Infra-Red fluorescent core-shell iron oxide/human serum albumin nanoparticles for controlled release of growth factors for augmentation of human mesenchymal stem cell growth and differentiation. J. Nanobiotechnol. 2015, 13, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Armstrong, J.P.; Pence, I.; Kit-Anan, W.; Puetzer, J.L.; Carreira, S.C.; Moore, A.; Stevens, M.M. Glycosylated superparamagnetic nanoparticle gradients for osteochondral tissue engineering. Biomaterials 2018, 176, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, J.; Wang, Z.; Zhou, Y.; Lou, Z.; Chen, B.; Wang, P.; Guo, Z.; Tang, H.; Ma, J.; et al. Magnetic Cell–Scaffold Interface Constructed by Superparamagnetic IONP Enhanced Osteogenesis of Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 44279–44289. [Google Scholar] [CrossRef]
- Kim, J.-J.; Singh, R.K.; Seo, S.-J.; Kim, T.-H.; Kim, J.-H.; Lee, E.-J.; Kim, H.-W. Magnetic scaffolds of polycaprolactone with functionalized magnetite nanoparticles: Physicochemical, mechanical, and biological properties effective for bone regeneration. RSC Adv. 2014, 4, 17325–17336. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhang, F.; Wang, L.; Chen, B.; Reynolds, M.A.; Ma, J.; Schneider, A.; Gu, N.; Xu, H.H.K. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.A.; Patel, K.D.; Kim, H.-W. Novel magnetic nanocomposite injectables: Calcium phosphate cements impregnated with ultrafine magnetic nanoparticles for bone regeneration. RSC Adv. 2015, 5, 13411–13419. [Google Scholar] [CrossRef]
- Ganesh, N.; Ashokan, A.; Rajeshkannan, R.; Chennazhi, K.; Koyakutty, M.; Nair, S.V. Magnetic Resonance Functional Nano-Hydroxyapatite Incorporated Poly(Caprolactone) Composite Scaffolds for In Situ Monitoring of Bone Tissue Regeneration by MRI. Tissue Eng. Part A 2014, 20, 2783–2794. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Guo, Y.; Yang, Z.; Chen, H.; Ren, K.; Weir, M.D.; Chow, L.C.; Reynolds, M.A.; Zhang, F.; Gu, N.; et al. Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/β-catenin signaling. Mater. Sci. Eng. C 2019, 104, 109955. [Google Scholar] [CrossRef]
- Dashnyam, K.; Perez, R.A.; Singh, R.K.; Lee, E.-J.; Kim, H.-W. Hybrid magnetic scaffolds of gelatin–siloxane incorporated with magnetite nanoparticles effective for bone tissue engineering. RSC Adv. 2014, 4, 40841–40851. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.; Chen, J.; Su, J.; Zhi, X.; Pan, P.; Zou, L.; Zhang, Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Patel, K.D.; Lee, J.H.; Lee, E.-J.; Kim, J.-H.; Kim, T.-H.; Kim, H.-W. Potential of Magnetic Nanofiber Scaffolds with Mechanical and Biological Properties Applicable for Bone Regeneration. PLoS ONE 2014, 9, e91584. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.-W.; Yang, F.; Ke, Q.-F.; Xie, X.-T.; Guo, Y.-P. Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumors. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef] [PubMed]
- Daňková, J.; Buzgo, M.; Vejpravová, J.; Kubíčková, S.; Sovková, V.; Vysloužilová, L.; Mantlíková, A.; Amler, E.; Nečas, A. Highly efficient mesenchymal stem cell proliferation on poly-ε-caprolactone nanofibers with embedded magnetic nanoparticles. Int. J. Nanomed. 2015, 10, 7307–7317. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Yang, W.; He, C.; Peng, S.; Gao, C.; Yang, Y.; Qi, F.; Feng, P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2020, 185, 108275. [Google Scholar] [CrossRef]
- Bianchi, M.; Boi, M.; Sartori, M.; Giavaresi, G.; Lopomo, N.F.; Fini, M.; Dediu, A.; Tampieri, A.; Marcacci, M.; Russo, A. Nanomechanical mapping of bone tissue regenerated by magnetic scaffolds. J. Mater. Sci. Mater. Electron. 2015, 26, 35. [Google Scholar] [CrossRef]
- De Santis, R.; Russo, A.; Gloria, A.; D’Amora, U.; Russo, T.; Panseri, S.; Sandri, M.; Tampieri, A.; Marcacci, M.; Dediu, V.A.; et al. Towards the Design of 3D Fiber-Deposited Poly(-caprolactone)/Iron-Doped Hydroxyapatite Nanocomposite Magnetic Scaffolds for Bone Regeneration. J. Biomed. Nanotechnol. 2015, 11, 1236–1246. [Google Scholar] [CrossRef]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- Rather, H.; Jhala, D.; Vasita, R. Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Mater. Sci. Eng. C 2019, 103, 109761. [Google Scholar] [CrossRef]
- Barati, D.; Shariati, S.R.P.; Moeinzadeh, S.; Melero-Martin, J.M.; Khademhosseini, A.; Jabbari, E. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J. Control. Release 2016, 223, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Effect of Chemistry on Osteogenesis and Angiogenesis Towards Bone Tissue Engineering Using 3D Printed Scaffolds. Ann. Biomed. Eng. 2017, 45, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulens-Arias, V.; Rojas, J.M.; Sanz-Ortega, L.; Portilla, Y.; Pérez-Yagüe, S.; Barber, D.F. Polyethylenimine-coated superparamagnetic iron oxide nanoparticles impair in vitro and in vivo angiogenesis. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102063. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.-M.; Ahn, S.-J.; Park, K.-R.; Kim, M.-J.; Kim, J.-J.; Jin, G.-Z.; Kim, H.-W.; Kim, E.-C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Hao, S.; Meng, J.; Zhang, Y.; Liu, J.; Nie, X.; Wu, F.; Yang, Y.; Wang, C.; Gu, N.; Xu, H. Macrophage phenotypic mechanomodulation of enhancing bone regeneration by superparamagnetic scaffold upon magnetization. Biomaterials 2017, 140, 16–25. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Xiong, Y.; Lai, W.; Xu, H.; Wei, H. Size dependent biodistribution and toxicokinetics of iron oxide magnetic nanoparticles in mice. Nanoscale 2015, 7, 625–636. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Yang, X.; Ma, P.; Luo, Q.; Chen, J.; Gan, Y.; Du, J.; Ding, S.; Xi, Z. Intraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. Int. J. Nanomed. 2012, 7, 4809–4818. [Google Scholar] [CrossRef] [Green Version]
- Pham, B.T.T.; Colvin, E.K.; Pham, N.T.H.; Kim, B.J.; Fuller, E.S.; Moon, E.A.; Barbey, R.; Yuen, S.; Rickman, B.H.; Bryce, N.S.; et al. Biodistribution and Clearance of Stable Superparamagnetic Maghemite Iron Oxide Nanoparticles in Mice Following Intraperitoneal Administration. Int. J. Mol. Sci. 2018, 19, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storey, P.; Lim, R.P.; Chandarana, H.; Rosenkrantz, A.B.; Kim, D.; Stoffel, D.R.; Lee, V.S. MRI Assessment of Hepatic Iron Clearance Rates After USPIO Administration in Healthy Adults. Investig. Radiol. 2012, 47, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Jarockyte, G.; Daugelaite, E.; Stasys, M.; Statkute, U.; Poderys, V.; Tseng, T.-C.; Hsu, S.-H.; Karabanovas, V.; Rotomskis, R. Accumulation and Toxicity of Superparamagnetic Iron Oxide Nanoparticles in Cells and Experimental Animals. Int. J. Mol. Sci. 2016, 17, 1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef]
- Rajiv, S.; Jerobin, J.; Saranya, V.; Nainawat, M.; Sharma, A.; Makwana, P.; Gayathri, C.; Bharath, L.; Singh, M.; Kumar, M.; et al. Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro. Hum. Exp. Toxicol. 2016, 35, 170–183. [Google Scholar] [CrossRef]
- Wu, J.; Sun, J. Investigation on mechanism of growth arrest induced by iron oxide nanoparticles in PC12 cells. J. Nanosci. Nanotechnol. 2011, 11, 11079–11083. [Google Scholar] [CrossRef]
- Berman, S.M.C.; Kshitiz; Wang, C.J.; Orukari, I.; Levchenko, A.; Bulte, J.W.M.; Walczak, P. Cell motility of neural stem cells is reduced after SPIO-labeling, which is mitigated after exocytosis. Magn. Reson. Med. 2013, 69, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Valdiglesias, V.; Kiliç, G.; Costa, C.; Bertólez, N.F.; Pasaro, E.; Teixeira, J.P.; Laffon, B. Effects of iron oxide nanoparticles: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ. Mol. Mutagen. 2015, 56, 125–148. [Google Scholar] [CrossRef]
- Van De Walle, A.; Fromain, A.; Sangnier, A.P.; Curcio, A.; Lenglet, L.; Motte, L.; Lalatonne, Y.; Wilhelm, C. Real-time in situ magnetic measurement of the intracellular biodegradation of iron oxide nanoparticles in a stem cell-spheroid tissue model. Nano Res. 2020, 13, 467–476. [Google Scholar] [CrossRef]
- Van de Walle, A.; Perez, J.; Abou-Hassan, A.; Hémadi, M.; Luciani, N.; Wilhelm, C. Magnetic nanoparticles in regenerative medicine: What of their fate and impact in stem cells? Mater. Today Nano 2020, 11, 100084. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [Green Version]
- Han, D.-W.; Hong, S.C.; Lee, J.H.; Lee, J.; Kim, H.Y.; Park, J.Y.; Cho, J.; Lee, J. Subtle cytotoxicity and genotoxicity differences in superparamagnetic iron oxide nanoparticles coated with various functional groups. Int. J. Nanomed. 2011, 6, 3219–3231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moise, S.; Céspedes, E.; Soukup, D.; Byrne, J.M.; El Haj, A.J.; Telling, N.D. The cellular magnetic response and biocompatibility of biogenic zinc- and cobalt-doped magnetite nanoparticles. Sci. Rep. 2017, 7, 39922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Tan, Y.; Jie, L.; Wu, X.; Yu, R.; Zhang, M. Biological activity and magnetic resonance imaging of superparamagnetic iron oxide nanoparticles-labeled adipose-derived stem cells. Stem Cell Res. Ther. 2013, 4, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Yin, T.; Zou, Q.; Zhang, K.; Gao, G.; Shapter, J.G.; Huang, P.; Fu, Q. Labeling adipose derived stem cell sheet by ultrasmall super-paramagnetic Fe3O4 nanoparticles and magnetic resonance tracking in vivo. Sci. Rep. 2017, 7, 42793. [Google Scholar] [CrossRef] [Green Version]
- Andreas, K.; Georgieva, R.; Ladwig, M.; Mueller, S.; Notter, M.; Sittinger, M.; Ringe, J. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials 2012, 33, 4515–4525. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Liu, Y.-P.; Ho, J.H.; Hsu, S.-C.; Lee, O.K. Amine-surface-modified superparamagnetic iron oxide nanoparticles interfere with differentiation of human mesenchymal stem cells. J. Orthop. Res. 2012, 30, 1499–1506. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Hsiao, J.-K.; Liu, H.-M.; Lai, I.-Y.; Yao, M.; Hsu, S.-C.; Ko, B.-S.; Chen, Y.-C.; Yang, C.-S.; Huang, D.-M. The inhibitory effect of superparamagnetic iron oxide nanoparticle (Ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol. Appl. Pharmacol. 2010, 245, 272–279. [Google Scholar] [CrossRef]
- Duan, L.; Zuo, J.; Zhang, F.; Li, B.; Xu, Z.; Zhang, H.; Yang, B.; Song, W.; Jiang, J. Magnetic Targeting of HU-MSCs in the Treatment of Glucocorticoid-Associated Osteonecrosis of the Femoral Head Through Akt/Bcl2/Bad/Caspase-3 Pathway. Int. J. Nanomed. 2020, 15, 3605–3620. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gu, L.; Gong, Q.; Sun, J.; Ma, Y.; Wu, H.; Wang, Y.; Guo, G.; Li, X.; Zhu, H. Strategies to reduce the intracellular effects of iron oxide nanoparticle degradation. Nanomedicine 2017, 12, 555–570. [Google Scholar] [CrossRef]
- Ledda, M.; Fioretti, D.; Lolli, M.G.; Papi, M.; Di Gioia, C.; Carletti, R.; Ciasca, G.; Foglia, S.; Palmieri, V.; Marchese, R.; et al. Biocompatibility assessment of sub-5 nm silica-coated superparamagnetic iron oxide nanoparticles in human stem cells and in mice for potential application in nanomedicine. Nanoscale 2020, 12, 1759–1778. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasari, A.; Xue, J.; Deb, S. Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials 2022, 12, 757. https://doi.org/10.3390/nano12050757
Dasari A, Xue J, Deb S. Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials. 2022; 12(5):757. https://doi.org/10.3390/nano12050757
Chicago/Turabian StyleDasari, Akshith, Jingyi Xue, and Sanjukta Deb. 2022. "Magnetic Nanoparticles in Bone Tissue Engineering" Nanomaterials 12, no. 5: 757. https://doi.org/10.3390/nano12050757
APA StyleDasari, A., Xue, J., & Deb, S. (2022). Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials, 12(5), 757. https://doi.org/10.3390/nano12050757






