Recent Advancements in Mitochondria-Targeted Nanoparticle Drug Delivery for Cancer Therapy
Abstract
1. Introduction
2. Fundamentals of Mitochondria-Targeting
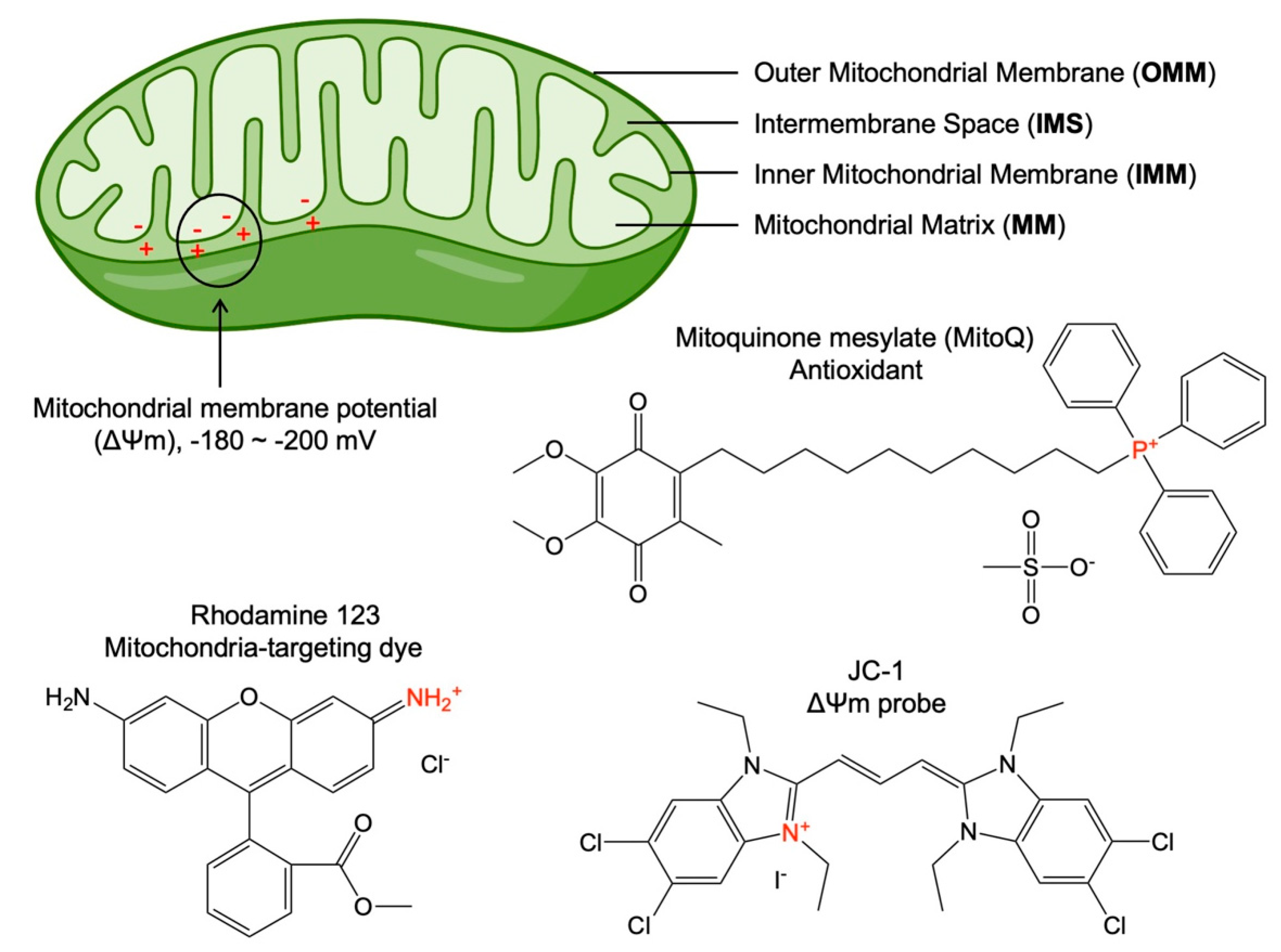
3. Mitochondria-Targeting Nanoparticles for Advancing Cancer Therapies
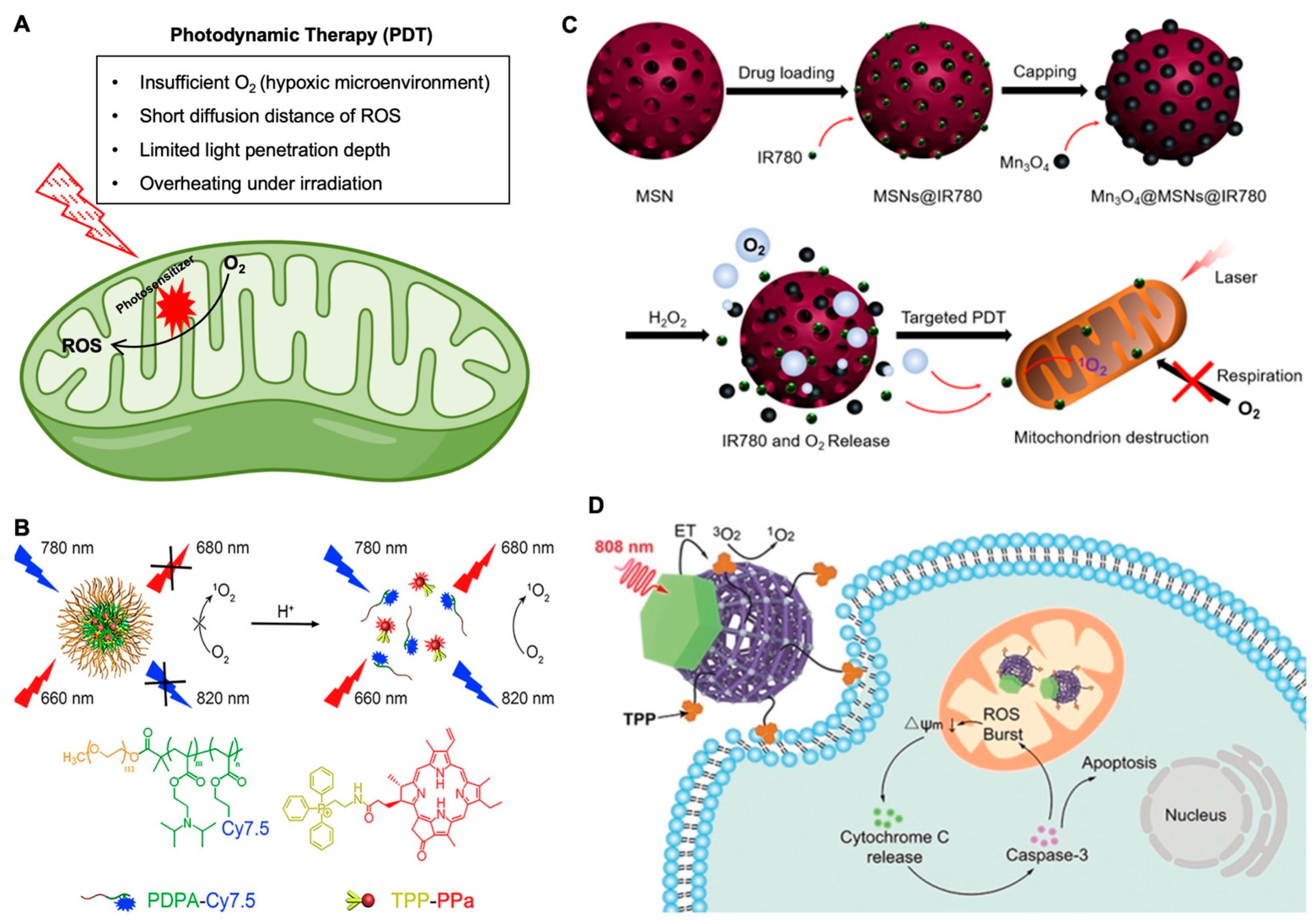
3.1. Mitochondria-Targeting in Photodynamic Therapy for Cancer
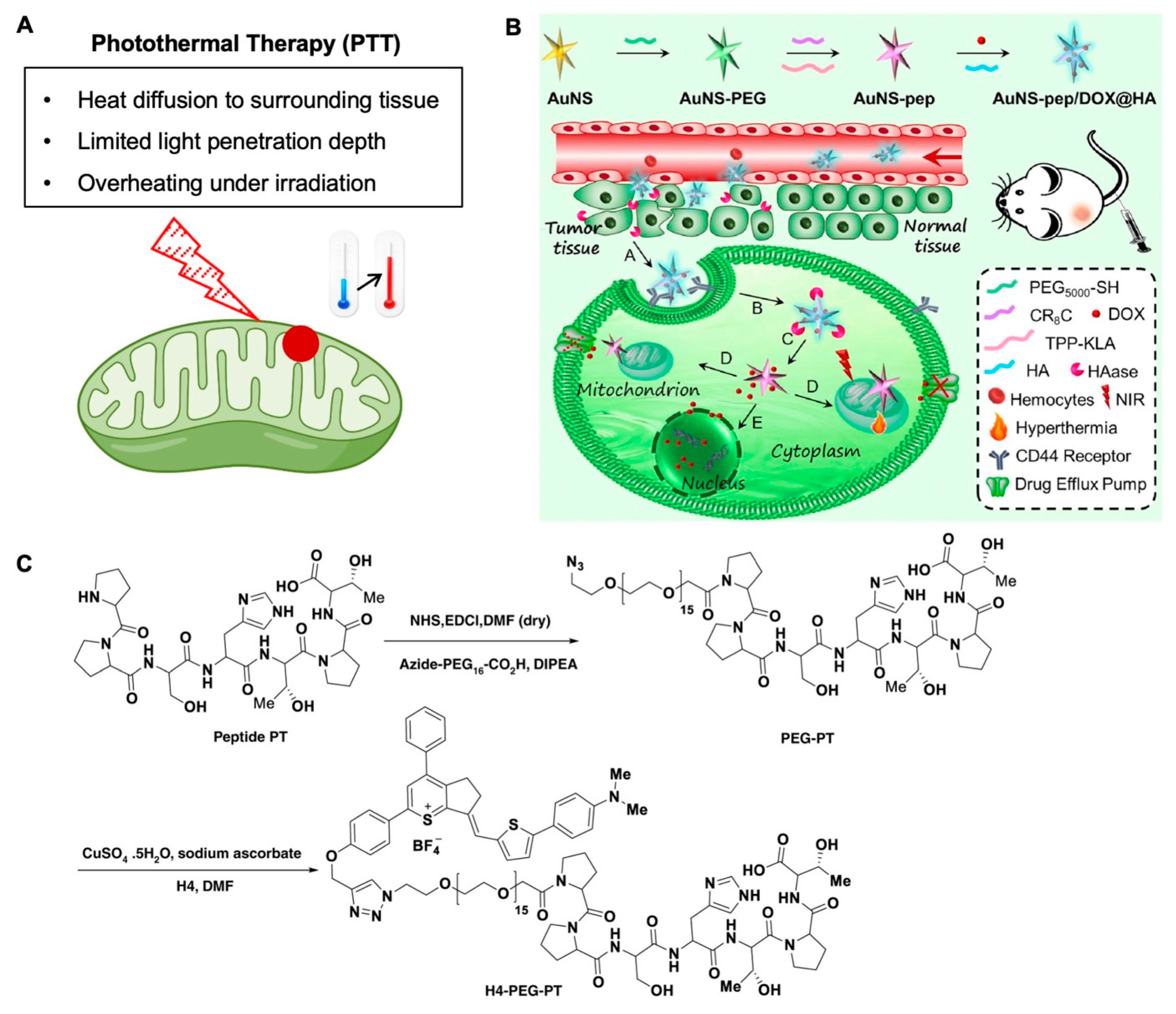
3.2. Mitochondria-Targeting in Photothermal and Magnetothermal Therapies of Cancer
3.3. Mitochondria-Targeting in Chemotherapy
4. Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Newmeyer, D.D.; Ferguson-Miller, S. Mitochondria: Releasing Power for Life and Unleashing the Machineries of Death. Cell 2003, 112, 481–490. [Google Scholar] [CrossRef]
- Chan, D.; Frank, S.; Rojo, M. Mitochondrial dynamics in cell life and death. Cell Death Differ. 2006, 13, 680–684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, S.; Zhu, D.; Huang, P. Targeting cancer cell mitochondria as a therapeutic approach. Future Med. Chem. 2013, 5, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Neuzil, J. Targeting mitochondria as an anticancer strategy. Cancer Commun. 2019, 39, 63. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef]
- Chandra, D.; Tang, D.G. Mitochondrially Localized Active Caspase-9 and Caspase-3 Result Mostly from Translocation from the Cytosol and Partly from Caspase-mediated Activation in the Organelle: LACK OF EVIDENCE FOR Apaf-1-MEDIATED PROCASPASE-9 ACTIVATION IN THE MITOCHONDRIA. J. Biol. Chem. 2003, 278, 17408–17420. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Shi, Y. Mechanisms of Caspase Activation and Inhibition during Apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell Death: Critical Control Points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Smith, R.A.J. Targeting Antioxidants to Mitochondria by Conjugation to Lipophilic Cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nature Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009, 461, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, Y.; Zheng, J.; Pan, J. Reactive Oxygen Species in Cancer Stem Cells. Antioxid. Redox Signal. 2012, 16, 1215–1228. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Rhee Sue, G. H2O2, a Necessary Evil for Cell Signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Finkel, T. Signal Transduction by Mitochondrial Oxidants. J. Biol. Chem. 2012, 287, 4434–4440. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, F.; Wu, H.; Hu, C.; Yu, C.; Wu, S. Preparation of a Mitochondria-targeted and NO-Releasing Nanoplatform and its Enhanced Pro-Apoptotic Effect on Cancer Cells. Small 2014, 10, 3750–3760. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, F.; Wu, H.; Hu, C.; Wu, S. Enhanced Photodynamic Efficiency Achieved via a Dual-Targeted Strategy Based on Photosensitizer/Micelle Structure. Biomacromolecules 2014, 15, 4249–4259. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.P.; Pasquale, N.; De, G.; Tan, T.; Ma, J.; Lee, K.-B. Core–Shell Nanoparticle-Based Peptide Therapeutics and Combined Hyperthermia for Enhanced Cancer Cell Apoptosis. ACS Nano 2014, 8, 9379–9387. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Krantz, B.A.; O’Reilly, E.M. Biomarker-Based Therapy in Pancreatic Ductal Adenocarcinoma: An Emerging Reality? Clin. Cancer Res. 2017, 24, 2241–2250. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar] [CrossRef]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer—The road to new treatment strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef]
- Mayer, E.L.; Burstein, H.J. Chemotherapy for Triple-Negative Breast Cancer: Is More Better? J. Clin. Oncol. 2016, 34, 3369–3371. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Sherlock, S.P.; Tabakman, S.M.; Xie, L.; Dai, H. Photothermally Enhanced Drug Delivery by Ultrasmall Multifunctional FeCo/Graphitic Shell Nanocrystals. ACS Nano 2011, 5, 1505–1512. [Google Scholar] [CrossRef]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, F.; Wu, H.; Wu, S. A mitochondrial-targeting and NO-based anticancer nanosystem with enhanced photo-controllability and low dark-toxicity. J. Mater. Chem. B 2015, 3, 4904–4912. [Google Scholar] [CrossRef]
- Tabish, T.A.; Narayan, R.J. Mitochondria-targeted graphene for advanced cancer therapeutics. Acta Biomater. 2021, 129, 43–56. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Venugopal, D.; Al-Dayan, N.; Ravinayagam, V.; Mohammed, A.A. Emerging insights into mitochondria-specific targeting and drug delivering strategies: Recent milestones and therapeutic implications. Saudi J. Biol. Sci. 2020, 27, 3581–3592. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Almatroudi, A.; Alsahli, M.A.; Aljaghwani, A.; El-Kady, A.M.; Rahmani, A.H.; Khan, A.A. Novel Strategies for Disrupting Cancer-Cell Functions with Mitochondria-Targeted Antitumor Drug-Loaded Nanoformulations. Int. J. Nanomed. 2021, 16, 3907–3936. [Google Scholar] [CrossRef]
- Cho, H.; Cho, Y.-Y.; Shim, M.S.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeted drug delivery in cancers. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165808. [Google Scholar] [CrossRef]
- Palade, G.E. The fine structure of mitochondria. Anat. Rec. 1952, 114, 427–451. [Google Scholar] [CrossRef]
- Frey, T.G.; Mannella, C.A. The internal structure of mitochondria. Trends Biochem. Sci. 2000, 25, 319–324. [Google Scholar] [CrossRef]
- Esteras, N.; Adjobo-Hermans, M.J.W.; Abramov, A.Y.; Koopman, W.J.H. Chapter 9—Visualization of mitochondrial membrane potential in mammalian cells. In Methods in Cell Biology; Pon, L.A., Schon, E.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 155, pp. 221–245. [Google Scholar]
- Wang, H.; Gao, Z.; Liu, X.; Agarwal, P.; Zhao, S.; Conroy, D.W.; Ji, G.; Yu, J.; Jaroniec, C.P.; Liu, Z.; et al. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat. Commun. 2018, 9, 562. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Zheng, Y.; Geng, Y.; Han, C.; Shi, Y.; Sun, H.; Zhang, C.; Chen, Y.; Zhang, L.; et al. Glycyrrhetinic Acid Functionalized Graphene Oxide for Mitochondria Targeting and Cancer Treatment In Vivo. Small 2018, 14, 1703306. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Yang, N.; Weinfeld, M.; Lemieux, H.; Montpetit, B.; Goping, I.S. Photo-activation of the delocalized lipophilic cation D112 potentiates cancer selective ROS production and apoptosis. Cell Death Dis. 2017, 8, e2587. [Google Scholar] [CrossRef]
- Wang, H.; Fang, B.; Peng, B.; Wang, L.; Xue, Y.; Bai, H.; Lu, S.; Voelcker, N.H.; Li, L.; Fu, L.; et al. Recent Advances in Chemical Biology of Mitochondria Targeting. Front. Chem. 2021, 9, 321. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, F.; Zhang, H.; Xu, J.; Qiu, J.; Wu, S. A Nanosystem Capable of Releasing a Photosensitizer Bioprecursor under Two-Photon Irradiation for Photodynamic Therapy. Adv. Sci. 2016, 3, 1500254. [Google Scholar] [CrossRef]
- Battogtokh, G.; Choi, Y.S.; Kang, D.S.; Park, S.J.; Shim, M.S.; Huh, K.M.; Cho, Y.-Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: Current strategies and future perspectives. Acta Pharm. Sin. B 2018, 8, 862–880. [Google Scholar] [CrossRef]
- Xu, J.; Shamul, J.G.; Wang, H.; Lin, J.; Agarwal, P.; Sun, M.; Lu, X.; Tkaczuk, K.H.R.; He, X. Targeted Heating of Mitochondria Greatly Augments Nanoparticle-Mediated Cancer Chemotherapy. Adv. Healthc. Mater. 2020, 9, 2000181. [Google Scholar] [CrossRef]
- Harrison, L.B.; Chadha, M.; Hill, R.J.; Hu, K.; Shasha, D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist 2002, 7, 492–508. [Google Scholar] [CrossRef]
- Fingar, V.H.; Wieman, T.J.; Park, Y.J.; Henderson, B.W. Implications of a pre-existing tumor hypoxic fraction on photodynamic therapy. J. Surg. Res. 1992, 53, 524–528. [Google Scholar] [CrossRef]
- Xiao, Z.; Halls, S.; Dickey, D.; Tulip, J.; Moore, R.B. Fractionated versus standard continuous light delivery in interstitial photodynamic therapy of dunning prostate carcinomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 7496–7505. [Google Scholar] [CrossRef][Green Version]
- Ceradini, D.J.; Kulkarni, A.R.; Callaghan, M.J.; Tepper, O.M.; Bastidas, N.; Kleinman, M.E.; Capla, J.M.; Galiano, R.D.; Levine, J.P.; Gurtner, G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004, 10, 858–864. [Google Scholar] [CrossRef]
- Vaupel, P.; Thews, O.; Hoeckel, M. Treatment resistance of solid tumors: Role of hypoxia and anemia. Med. Oncol. (Northwood Lond. Engl.) 2001, 18, 243–259. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef]
- Liang, S.; Sun, C.; Yang, P.; Ma, P.A.; Huang, S.; Cheng, Z.; Yu, X.; Lin, J. Core-shell structured upconversion nanocrystal-dendrimer composite as a carrier for mitochondria targeting and catalase enhanced anti-cancer photodynamic therapy. Biomaterials 2020, 240, 119850. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhang, X.; Liang, X.; Feng, Y.; Feng, W. Two-dimensional nanomaterials with engineered bandgap: Synthesis, properties, applications. Nano Today 2021, 37, 101059. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Yang, F.; Yuan, Z.; Wei, W.; Lu, H.; Dong, H.; Zhang, X. Near-infrared triggered Ti3C2/g-C3N4 heterostructure for mitochondria-targeting multimode photodynamic therapy combined photothermal therapy. Nano Today 2020, 34, 100919. [Google Scholar] [CrossRef]
- Qi, T.; Chen, B.; Wang, Z.; Du, H.; Liu, D.; Yin, Q.; Liu, B.; Zhang, Q.; Wang, Y. A pH-Activatable nanoparticle for dual-stage precisely mitochondria-targeted photodynamic anticancer therapy. Biomaterials 2019, 213, 119219. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Ai, S.; Sun, J.; Mai, X.; Guan, W. Self-generating oxygen enhanced mitochondrion-targeted photodynamic therapy for tumor treatment with hypoxia scavenging. Theranostics 2019, 9, 6809–6823. [Google Scholar] [CrossRef]
- Zhan, Q.; Qian, J.; Liang, H.; Somesfalean, G.; Wang, D.; He, S.; Zhang, Z.; Andersson-Engels, S. Using 915 nm Laser Excited Tm3+/Er3+/Ho3+-Doped NaYbF4 Upconversion Nanoparticles for in Vitro and Deeper in Vivo Bioimaging without Overheating Irradiation. ACS Nano 2011, 5, 3744–3757. [Google Scholar] [CrossRef]
- Heer, S.; Kömpe, K.; Güdel, H.U.; Haase, M. Highly Efficient Multicolour Upconversion Emission in Transparent Colloids of Lanthanide-Doped NaYF4 Nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar] [CrossRef]
- Johnson, N.J.J.; He, S.; Diao, S.; Chan, E.M.; Dai, H.; Almutairi, A. Direct Evidence for Coupled Surface and Concentration Quenching Dynamics in Lanthanide-Doped Nanocrystals. J. Am. Chem. Soc. 2017, 139, 3275–3282. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, D.; Schartner, E.P.; Lu, Y.; Liu, Y.; Zvyagin, A.V.; Zhang, L.; Dawes, J.M.; Xi, P.; Piper, J.A.; et al. Single-nanocrystal sensitivity achieved by enhanced upconversion luminescence. Nat. Nanotechnol. 2013, 8, 729–734. [Google Scholar] [CrossRef]
- Chen, X.; Peng, D.; Ju, Q.; Wang, F. Photon upconversion in core–shell nanoparticles. Chem. Soc. Rev. 2015, 44, 1318–1330. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Yang, X.; Zheng, X.; Wen, S.; Wang, F.; Vidal, X.; Zhao, J.; Liu, D.; Zhou, Z.; et al. Amplified stimulated emission in upconversion nanoparticles for super-resolution nanoscopy. Nature 2017, 543, 229–233. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Liu, H. Co-delivery of chitosan nanoparticles of 5-aminolevulinic acid and shGBAS for improving photodynamic therapy efficacy in oral squamous cell carcinomas. Photodiagnosis Photodyn. Ther. 2021, 34, 102218. [Google Scholar] [CrossRef]
- Liu, C.; Liu, B.; Zhao, J.; Di, Z.; Chen, D.; Gu, Z.; Li, L.; Zhao, Y. Nd3+-Sensitized Upconversion Metal–Organic Frameworks for Mitochondria-Targeted Amplified Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 2634–2638. [Google Scholar] [CrossRef]
- Park, S.Y.; Baik, H.J.; Oh, Y.T.; Oh, K.T.; Youn, Y.S.; Lee, E.S. A Smart Polysaccharide/Drug Conjugate for Photodynamic Therapy. Angew. Chem. Int. Ed. 2011, 50, 1644–1647. [Google Scholar] [CrossRef]
- Feng, G.; Qin, W.; Hu, Q.; Tang, B.Z.; Liu, B. Cellular and Mitochondrial Dual-Targeted Organic Dots with Aggregation-Induced Emission Characteristics for Image-Guided Photodynamic Therapy. Adv. Healthc. Mater. 2015, 4, 2667–2676. [Google Scholar] [CrossRef]
- Cai, X.; Wang, K.-N.; Ma, W.; Yang, Y.; Chen, G.; Fu, H.; Cui, C.; Yu, Z.; Wang, X. Multifunctional AIE iridium (III) photosensitizer nanoparticles for two-photon-activated imaging and mitochondria targeting photodynamic therapy. J. Nanobiotechnol. 2021, 19, 254. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, H.; Jiang, Z.; Guan, Y.; Zou, J.; Wang, X.; Cheng, R.; Gao, H. Low-power white light triggered AIE polymer nanoparticles with high ROS quantum yield for mitochondria-targeted and image-guided photodynamic therapy. J. Mater. Chem. B 2017, 5, 6277–6281. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Hou, Y.; Luo, Z.; Chen, Q.; Cao, H.; Huo, R.; Xue, C.; Sutrisno, L.; Hao, L.; et al. Engineering of a Nanosized Biocatalyst for Combined Tumor Starvation and Low-Temperature Photothermal Therapy. ACS Nano 2018, 12, 2858–2872. [Google Scholar] [CrossRef]
- Yuan, Z.; Lin, C.; He, Y.; Tao, B.; Chen, M.; Zhang, J.; Liu, P.; Cai, K. Near-Infrared Light-Triggered Nitric-Oxide-Enhanced Photodynamic Therapy and Low-Temperature Photothermal Therapy for Biofilm Elimination. ACS Nano 2020, 14, 3546–3562. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, W.; Dong, Z.; Chao, Y.; Xu, L.; Chen, M.; Liu, Z. 1D Coordination Polymer Nanofibers for Low-Temperature Photothermal Therapy. Adv. Mater. 2017, 29, 1703588. [Google Scholar] [CrossRef]
- Wu, J.; Bremner, D.H.; Niu, S.; Shi, M.; Wang, H.; Tang, R.; Zhu, L.-M. Chemodrug-Gated Biodegradable Hollow Mesoporous Organosilica Nanotheranostics for Multimodal Imaging-Guided Low-Temperature Photothermal Therapy/Chemotherapy of Cancer. ACS Appl. Mater. Interfaces 2018, 10, 42115–42126. [Google Scholar] [CrossRef]
- Ouyang, B.; Liu, F.; Ruan, S.; Liu, Y.; Guo, H.; Cai, Z.; Yu, X.; Pang, Z.; Shen, S. Localized Free Radicals Burst Triggered by NIR-II Light for Augmented Low-Temperature Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 38555–38567. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, K.; Liao, X.; Rees, T.W.; Chen, Y.; Zhao, Z.; Ji, L.; Chao, H. Nucleus-targeting ultrasmall ruthenium(iv) oxide nanoparticles for photoacoustic imaging and low-temperature photothermal therapy in the NIR-II window. Chem. Commun. 2020, 56, 3019–3022. [Google Scholar] [CrossRef]
- Deng, X.; Guan, W.; Qing, X.; Yang, W.; Que, Y.; Tan, L.; Liang, H.; Zhang, Z.; Wang, B.; Liu, X.; et al. Ultrafast Low-Temperature Photothermal Therapy Activates Autophagy and Recovers Immunity for Efficient Antitumor Treatment. ACS Appl. Mater. Interfaces 2020, 12, 4265–4275. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, T.; Zhang, K.; Meng, X.; Dai, W.; Wang, D.; Dong, H.; Zhang, X. Engineered Exosome-Mediated Near-Infrared-II Region V(2)C Quantum Dot Delivery for Nucleus-Target Low-Temperature Photothermal Therapy. ACS Nano 2019, 13, 1499–1510. [Google Scholar] [CrossRef]
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the Drawbacks of Photothermal and Photodynamic Cancer Therapy. Adv. Sci. 2021, 8, 2002504. [Google Scholar] [CrossRef]
- Choi, G.; Rejinold, N.S.; Piao, H.; Choy, J.-H. Inorganic–inorganic nanohybrids for drug delivery, imaging and photo-therapy: Recent developments and future scope. Chem. Sci. 2021, 12, 5044–5063. [Google Scholar] [CrossRef]
- Chitgupi, U.; Qin, Y.; Lovell, J.F. Targeted Nanomaterials for Phototherapy. Nanotheranostics 2017, 1, 38–58. [Google Scholar] [CrossRef]
- Chen, S.; Lei, Q.; Qiu, W.-X.; Liu, L.-H.; Zheng, D.-W.; Fan, J.-X.; Rong, L.; Sun, Y.-X.; Zhang, X.-Z. Mitochondria-targeting “Nanoheater” for enhanced photothermal/chemo-therapy. Biomaterials 2017, 117, 92–104. [Google Scholar] [CrossRef]
- Zhou, H.; Zeng, X.; Li, A.; Zhou, W.; Tang, L.; Hu, W.; Fan, Q.; Meng, X.; Deng, H.; Duan, L.; et al. Upconversion NIR-II fluorophores for mitochondria-targeted cancer imaging and photothermal therapy. Nat. Commun. 2020, 11, 6183. [Google Scholar] [CrossRef]
- Kenry; Duan, Y.; Liu, B. Recent Advances of Optical Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30, 1802394. [Google Scholar] [CrossRef]
- Yang, Z.; Sharma, A.; Qi, J.; Peng, X.; Lee, D.Y.; Hu, R.; Lin, D.; Qu, J.; Kim, J.S. Super-resolution fluorescent materials: An insight into design and bioimaging applications. Chem. Soc. Rev. 2016, 45, 4651–4667. [Google Scholar] [CrossRef]
- Dean, K.M.; Palmer, A.E. Advances in fluorescence labeling strategies for dynamic cellular imaging. Nat. Chem. Biol. 2014, 10, 512–523. [Google Scholar] [CrossRef]
- Sauer, M.; Heilemann, M. Single-Molecule Localization Microscopy in Eukaryotes. Chem. Rev. 2017, 117, 7478–7509. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, F. Molecular Engineering of NIR-II Fluorophores for Improved Biomedical Detection. Angew. Chem. Int. Ed. 2021, 60, 16294–16308. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, J. Far-red and near infrared BODIPY dyes: Synthesis and applications for fluorescent pH probes and bio-imaging. Org. Biomol. Chem. 2014, 12, 3774–3791. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Jose, J.; Burgess, K. Benzophenoxazine-based fluorescent dyes for labeling biomolecules. Tetrahedron 2006, 62, 11021–11037. [Google Scholar] [CrossRef]
- Levitus, M.; Ranjit, S. Cyanine dyes in biophysical research: The photophysics of polymethine fluorescent dyes in biomolecular environments. Q. Rev. Biophys. 2010, 44, 123–151. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2012. [Google Scholar] [CrossRef]
- Panigrahi, M.; Dash, S.; Patel, S.; Mishra, B.K. Syntheses of cyanines: A review. Tetrahedron 2012, 68, 781–805. [Google Scholar] [CrossRef]
- Henary, M.; Levitz, A. Synthesis and applications of unsymmetrical carbocyanine dyes. Dye. Pigment. 2013, 99, 1107–1116. [Google Scholar] [CrossRef]
- Bricks, J.L.; Kachkovskii, A.D.; Slominskii, Y.L.; Gerasov, A.O.; Popov, S.V. Molecular design of near infrared polymethine dyes: A review. Dye. Pigment. 2015, 121, 238–255. [Google Scholar] [CrossRef]
- Gorka, A.P.; Nani, R.R.; Schnermann, M.J. Cyanine polyene reactivity: Scope and biomedical applications. Org. Biomol. Chem. 2015, 13, 7584–7598. [Google Scholar] [CrossRef]
- Beija, M.; Afonso, C.A.M.; Martinho, J.M.G. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410–2433. [Google Scholar] [CrossRef]
- Jia, K.; Wan, Y.; Xia, A.; Li, S.; Gong, F.; Yang, G. Characterization of Photoinduced Isomerization and Intersystem Crossing of the Cyanine Dye Cy3. J. Phys. Chem. A 2007, 111, 1593–1597. [Google Scholar] [CrossRef]
- Nunnally, B.K.; He, H.; Li, L.-C.; Tucker, S.A.; McGown, L.B. Characterization of Visible Dyes for Four-Decay Fluorescence Detection in DNA Sequencing. Anal. Chem. 1997, 69, 2392–2397. [Google Scholar] [CrossRef]
- Shin, J.; Xu, Y.; Koo, S.; Lim, J.H.; Lee, J.Y.; Sharma, A.; Sun, Y.; Kim, J.S. Mitochondria-targeted nanotheranostic: Harnessing single-laser-activated dual phototherapeutic processing for hypoxic tumor treatment. Matter 2021, 4, 2508–2521. [Google Scholar] [CrossRef]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.-F.; Zhao, Y.; Zhang, H.; et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.-F.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Zhu, J.; Xue, L.; Ou, C.; Wang, W.; Lu, M.; Song, X.; Dong, X. Functional black phosphorus nanosheets for mitochondria-targeting photothermal/photodynamic synergistic cancer therapy. Chem. Sci. 2019, 10, 3779–3785. [Google Scholar] [CrossRef]
- Ju, K.-Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.-K. Bioinspired Polymerization of Dopamine to Generate Melanin-Like Nanoparticles Having an Excellent Free-Radical-Scavenging Property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, T.; Liu, B.; Tian, J.; Wu, H.; Liu, Z.; Liu, Z.; Zhang, L.; Bao, H.; Liu, T. TB@PLGA Nanoparticles for Photodynamic/Photothermal Combined Cancer Therapy with Single Near-Infrared Irradiation. Int. J. Nanomed. 2021, 16, 4863–4871. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Teng, X.; Yan, M.; Bi, H.; Morais, P.C. Mitochondria-Targeting Nanoplatform with Fluorescent Carbon Dots for Long Time Imaging and Magnetic Field-Enhanced Cellular Uptake. ACS Appl. Mater. Interfaces 2015, 7, 10201–10212. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Q.; Wang, H.; Zha, Y.; Zheng, P.; Yang, T.; Ma, D.; Qiu, L.; Xu, X.; Hu, Y.; et al. Mitochondria-targeted magnetic gold nanoheterostructure for multi-modal imaging guided photothermal and photodynamic therapy of triple-negative breast cancer. Chem. Eng. J. 2021, 403, 126364. [Google Scholar] [CrossRef]
- Yan, H.; Shang, W.; Sun, X.; Zhao, L.; Wang, J.; Xiong, Z.; Yuan, J.; Zhang, R.; Huang, Q.; Wang, K.; et al. “All-in-One” Nanoparticles for Trimodality Imaging-Guided Intracellular Photo-magnetic Hyperthermia Therapy under Intravenous Administration. Adv. Funct. Mater. 2018, 28, 1705710. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef]
- Shen, J.; Rees, T.W.; Zhou, Z.; Yang, S.; Ji, L.; Chao, H. A mitochondria-targeting magnetothermogenic nanozyme for magnet-induced synergistic cancer therapy. Biomaterials 2020, 251, 120079. [Google Scholar] [CrossRef]
- Kang, B.H.; Plescia, J.; Song, H.Y.; Meli, M.; Colombo, G.; Beebe, K.; Scroggins, B.; Neckers, L.; Altieri, D.C. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J. Clin. Investig. 2009, 119, 454–464. [Google Scholar] [CrossRef]
- Sibrian-Vazquez, M.; Nesterova, I.V.; Jensen, T.J.; Vicente, M.G.H. Mitochondria Targeting by Guanidine− and Biguanidine−Porphyrin Photosensitizers. Bioconjugate Chem. 2008, 19, 705–713. [Google Scholar] [CrossRef]
- Xiang, C.; Li, D.-W.; Qi, Z.-D.; Jiang, F.-L.; Ge, Y.-S.; Liu, Y. Synthesis of F16 conjugated with 5-fluorouracil and biophysical investigation of its interaction with bovine serum albumin by a spectroscopic and molecular modeling approach. Luminescence 2013, 28, 865–872. [Google Scholar] [CrossRef]
- Vivès, E.; Schmidt, J.; Pèlegrin, A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim. Et Biophys. Acta (BBA) -Rev. Cancer 2008, 1786, 126–138. [Google Scholar] [CrossRef]
- Mourtada, R.; Fonseca, S.B.; Wisnovsky, S.P.; Pereira, M.P.; Wang, X.; Hurren, R.; Parfitt, J.; Larsen, L.; Smith, R.A.J.; Murphy, M.P.; et al. Re-Directing an Alkylating Agent to Mitochondria Alters Drug Target and Cell Death Mechanism. PLoS ONE 2013, 8, e60253. [Google Scholar]
- D’Souza, G.G.M.; Weissig, V. Subcellular targeting: A new frontier for drug-loaded pharmaceutical nanocarriers and the concept of the magic bullet. Expert Opin. Drug Deliv. 2009, 6, 1135–1148. [Google Scholar] [CrossRef]
- Jung, K.; Reszka, R. Mitochondria as subcellular targets for clinically useful anthracyclines. Adv. Drug Deliv. Rev. 2001, 49, 87–105. [Google Scholar] [CrossRef]
- Salvi, M.; Fiore, C.; Armanini, D.; Toninello, A. Glycyrrhetinic acid-induced permeability transition in rat liver mitochondria. Biochem. Pharmacol. 2003, 66, 2375–2379. [Google Scholar] [CrossRef]
- Battaglia, V.; Salvi, M.; Toninello, A. Oxidative Stress Is Responsible for Mitochondrial Permeability Transition Induction by Salicylate in Liver Mitochondria. J. Biol. Chem. 2005, 280, 33864–33872. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Iranshahy, M.; Iranshahi, M. Glycyrrhetinic Acid and Its Derivatives: Anti-Cancer and Cancer Chemopreventive Properties, Mechanisms of Action and Structure- Cytotoxic Activity Relationship. Curr. Med. Chem. 2016, 23, 498–517. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, F.; Wu, H.; Yu, C.; Wu, S. Dual-Targeting Nanosystem for Enhancing Photodynamic Therapy Efficiency. ACS Appl. Mater. Interfaces 2015, 7, 9287–9296. [Google Scholar] [CrossRef]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.M.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef]
- Wei, H.; Hu, Y.; Wang, J.; Gao, X.; Qian, X.; Tang, M. Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int. J. Nanomed. 2021, 16, 6097–6113. [Google Scholar] [CrossRef]
- Jahanbani, J.; Ghotbi, M.; Shahsavari, F.; Seydi, E.; Rahimi, S.; Pourahmad, J. Selective anticancer activity of superparamagnetic iron oxide nanoparticles (SPIONs) against oral tongue cancer using In Vitro methods: The key role of oxidative stress on cancerous mitochondria. J. Biochem Mol. Toxicol. 2020, 34, e22557. [Google Scholar] [CrossRef]
- Afrasiabi, M.; Seydi, E.; Rahimi, S.; Tahmasebi, G.; Jahanbani, J.; Pourahmad, J. The selective toxicity of superparamagnetic iron oxide nanoparticles (SPIONs) on oral squamous cell carcinoma (OSCC) by targeting their mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, e22769. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, F.; Wen, H.; Shi, W.; Huang, Q.; Huang, Y.; Xie, J.; Li, P.; Chen, J.; Qin, L.; et al. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J. Nanobiotechnol. 2020, 18, 8. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasileva, L.A.; Sibgatullina, G.V.; Samigullin, D.V.; Sapunova, A.S.; Voloshina, A.D.; Galkina, I.V.; Petrov, K.A.; Zakharova, L.Y. Mitochondria-targeted cationic liposomes modified with alkyltriphenylphosphonium bromides loaded with hydrophilic drugs: Preparation, cytotoxicity and colocalization assay. J. Mater. Chem. B 2019, 7, 7351–7362. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Feng, G.; Liu, J.; Zhang, C.-J.; Liu, B. Artemisinin and AIEgen Conjugate for Mitochondria-Targeted and Image-Guided Chemo- and Photodynamic Cancer Cell Ablation. ACS Appl. Mater. Interfaces 2018, 10, 11546–11553. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, S.; Shi, L.; Teh, C.; Qi, G.; Liu, B. Cancer-Cell-Activated in situ Synthesis of Mitochondria-Targeting AIE Photosensitizer for Precise Photodynamic Therapy. Angew. Chem. Int. Ed. 2021, 60, 14945–14953. [Google Scholar] [CrossRef]
- Li, M.; Zhong, Z.; Zhu, J.; Xiang, D.; Dai, N.; Cao, X.; Qing, Y.; Yang, Z.; Xie, J.; Li, Z.; et al. Identification and Characterization of Mitochondrial Targeting Sequence of Human Apurinic/Apyrimidinic Endonuclease. J. Biol. Chem. 2010, 285, 14871–14881. [Google Scholar] [CrossRef]
- Cheng, T.-L.; Liao, C.-C.; Tsai, W.-H.; Lin, C.-C.; Yeh, C.-W.; Teng, C.-F.; Chang, W.-T. Identification and characterization of the mitochondrial targeting sequence and mechanism in human citrate synthase. J. Cell. Biochem. 2009, 107, 1002–1015. [Google Scholar] [CrossRef]
- Kang, Y.C.; Son, M.; Kang, S.; Im, S.; Piao, Y.; Lim, K.S.; Song, M.-Y.; Park, K.-S.; Kim, Y.-H.; Pak, Y.K. Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson’s disease models. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef]
- Bai, L.; Li, Z.; Chen, J.; Chung, N.N.; Wilkes, B.C.; Li, T.; Schiller, P.W. [Dmt1]DALDA analogues with enhanced μ opioid agonist potency and with a mixed μ/κ opioid activity profile. Bioorg. Med. Chem. 2014, 22, 2333–2338. [Google Scholar] [CrossRef]
- Szeto, H.H. Mitochondria-Targeted Cytoprotective Peptides for Ischemia–Reperfusion Injury. Antioxid. Redox Signal. 2007, 10, 601–620. [Google Scholar] [CrossRef]
- Szeto, H.H. Development of Mitochondria-targeted Aromatic-cationic Peptides for Neurodegenerative Diseases. Ann. N. Y. Acad. Sci. 2008, 1147, 112–121. [Google Scholar] [CrossRef]
- Szeto, H.H. Mitochondria-targeted peptide antioxidants: Novel neuroprotective agents. AAPS J. 2006, 8, E521–E531. [Google Scholar] [CrossRef]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Shamul, J.G.; Kwizera, E.A.; He, X. Recent Advancements in Mitochondria-Targeted Nanoparticle Drug Delivery for Cancer Therapy. Nanomaterials 2022, 12, 743. https://doi.org/10.3390/nano12050743
Xu J, Shamul JG, Kwizera EA, He X. Recent Advancements in Mitochondria-Targeted Nanoparticle Drug Delivery for Cancer Therapy. Nanomaterials. 2022; 12(5):743. https://doi.org/10.3390/nano12050743
Chicago/Turabian StyleXu, Jiangsheng, James G. Shamul, Elyahb Allie Kwizera, and Xiaoming He. 2022. "Recent Advancements in Mitochondria-Targeted Nanoparticle Drug Delivery for Cancer Therapy" Nanomaterials 12, no. 5: 743. https://doi.org/10.3390/nano12050743
APA StyleXu, J., Shamul, J. G., Kwizera, E. A., & He, X. (2022). Recent Advancements in Mitochondria-Targeted Nanoparticle Drug Delivery for Cancer Therapy. Nanomaterials, 12(5), 743. https://doi.org/10.3390/nano12050743






