Continuous Hydrothermal Flow Synthesis and Characterization of ZrO2 Nanoparticles Doped with CeO2 in Supercritical Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
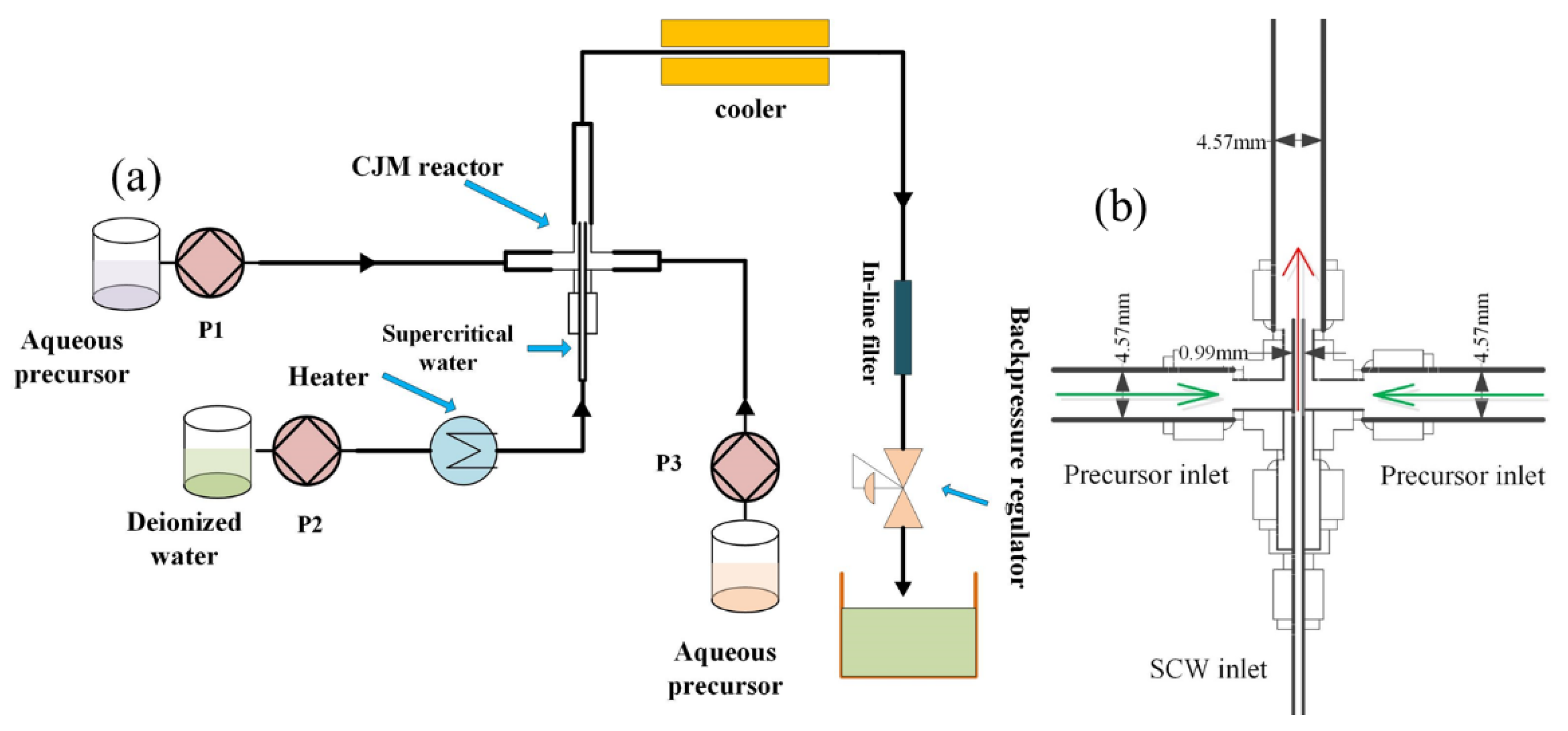
2.2. CHFS System
2.3. CZ Characterization
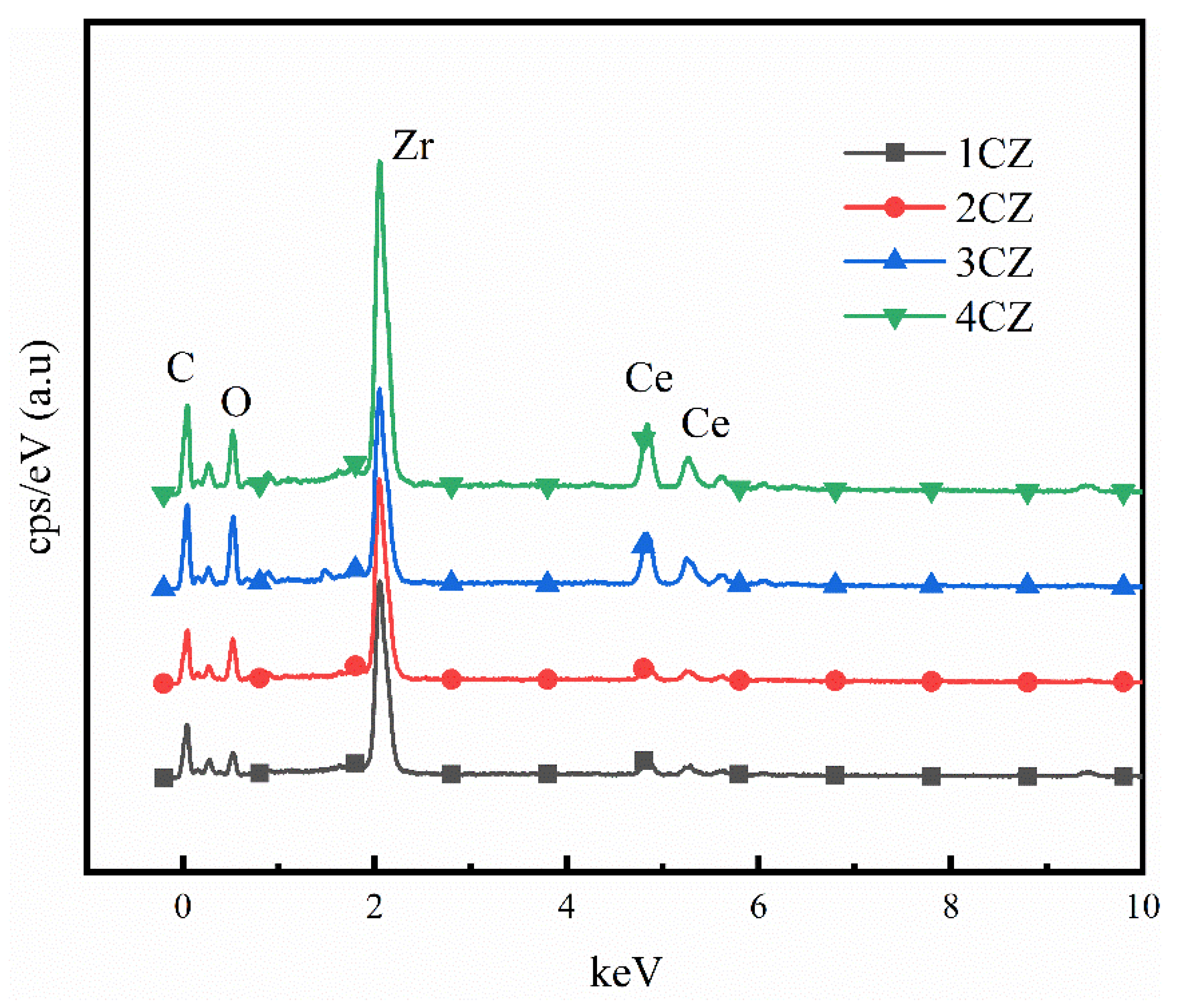
2.3.1. SEM-EDS
2.3.2. ICP-AES
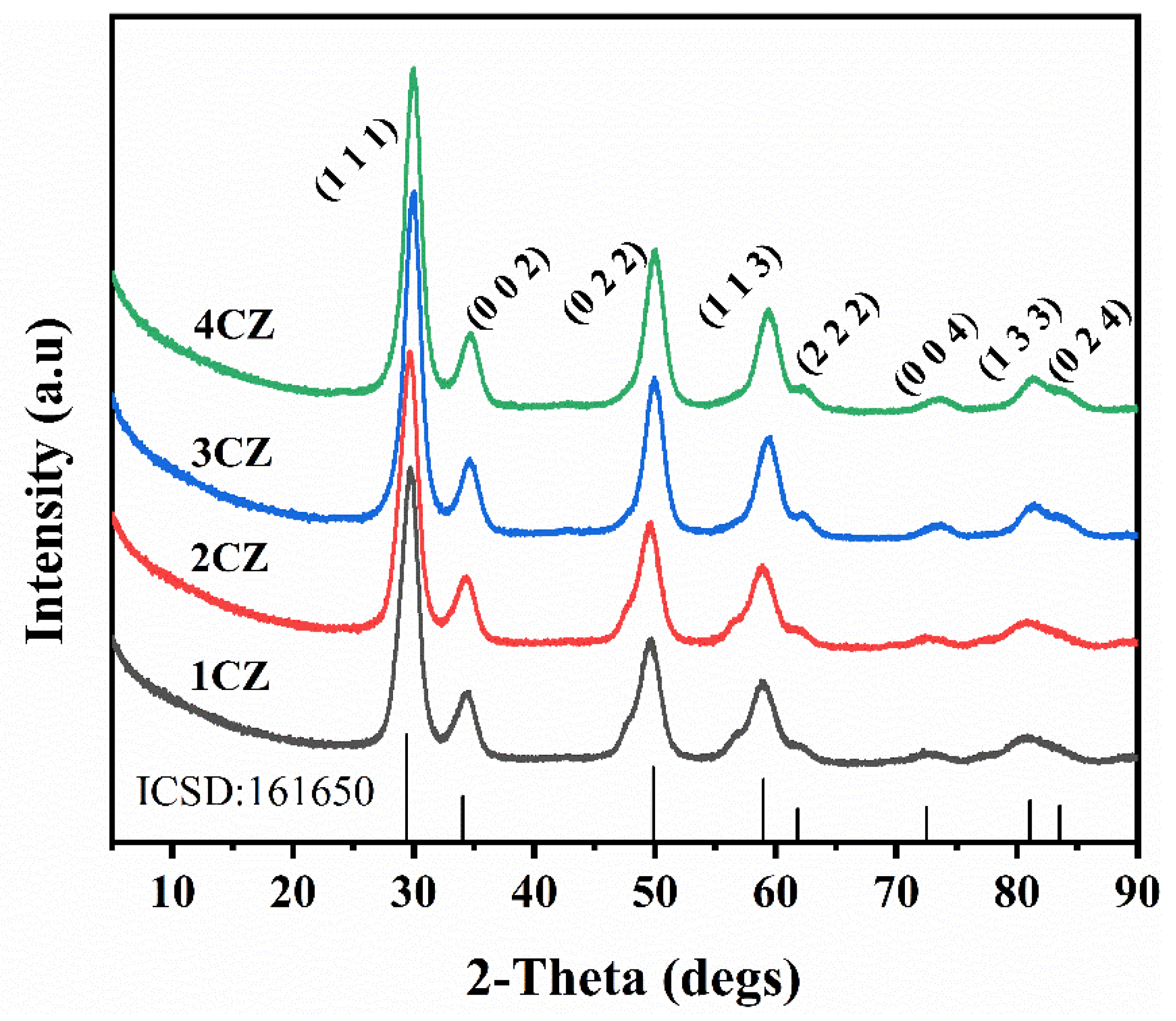
2.3.3. X-ray Diffraction
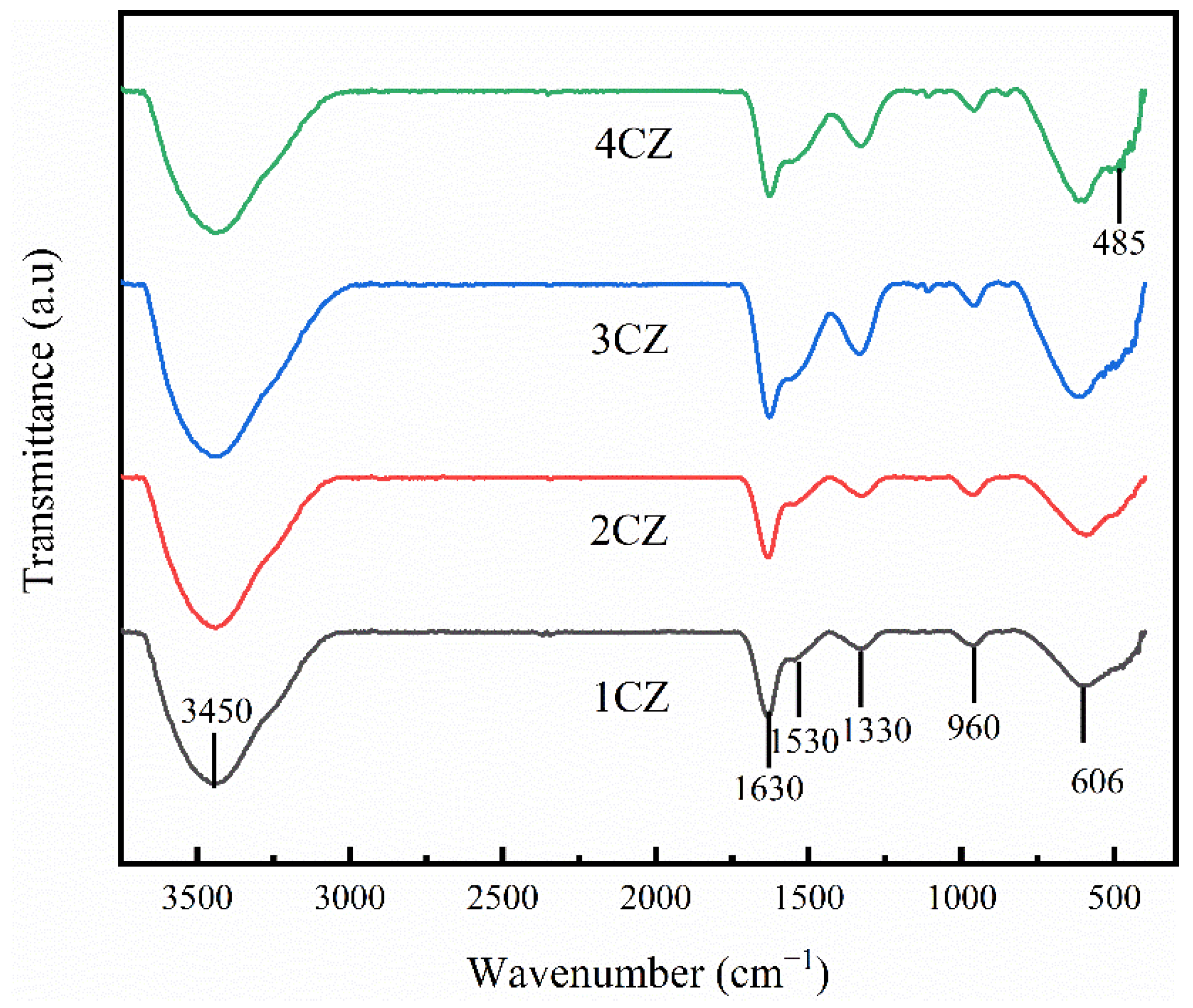
2.3.4. FTIR
2.3.5. RAMAN
2.3.6. TEM-SEAD
2.3.7. BET-Specific Surface Area Test
2.3.8. Oxygen Storage Capacity (OSC) Test
2.3.9. H2-TPR Test
3. Results and Discussion
3.1. Elemental Analysis
3.2. X-ray Diffraction
3.3. FTIR
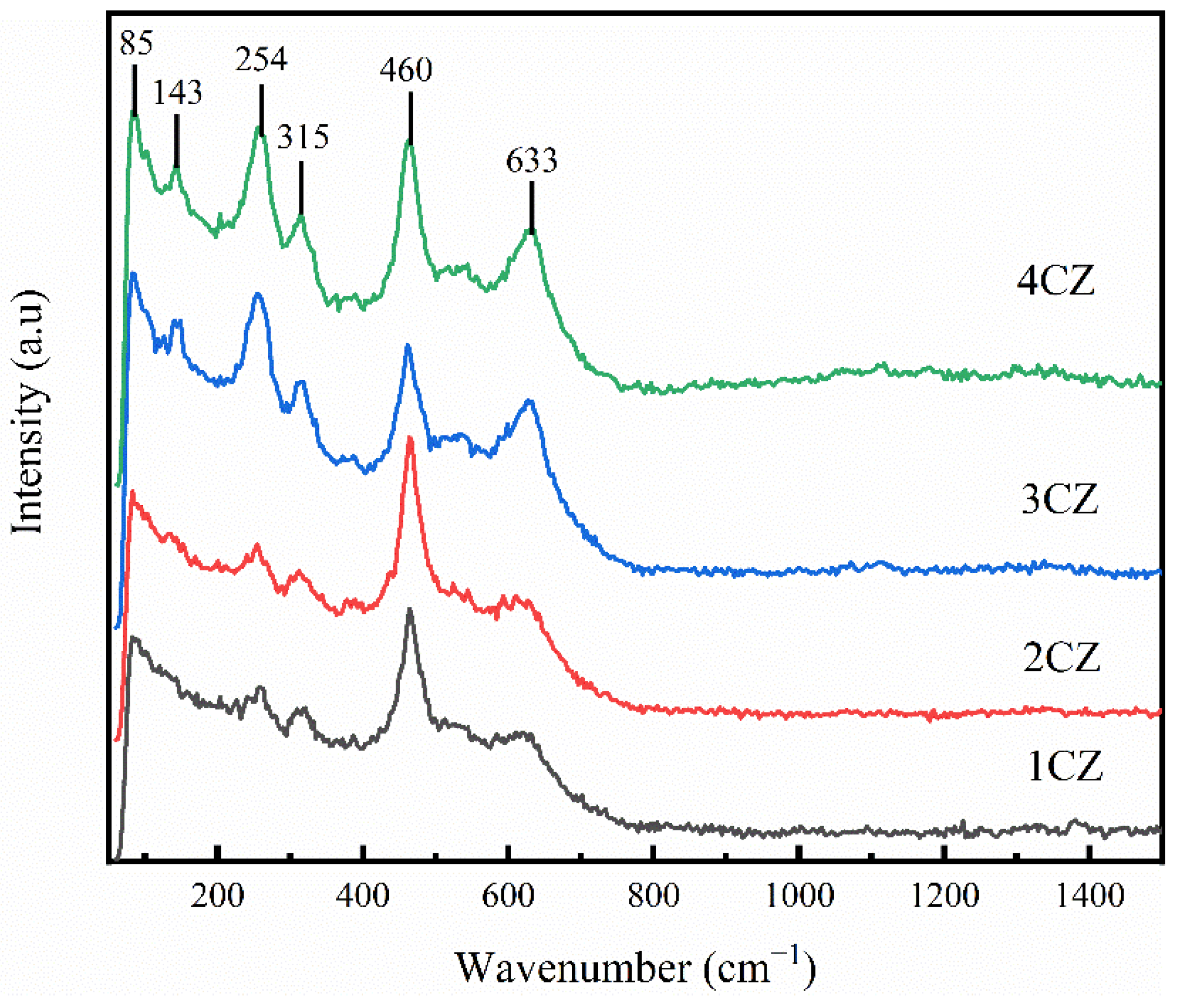
3.4. RAMAN
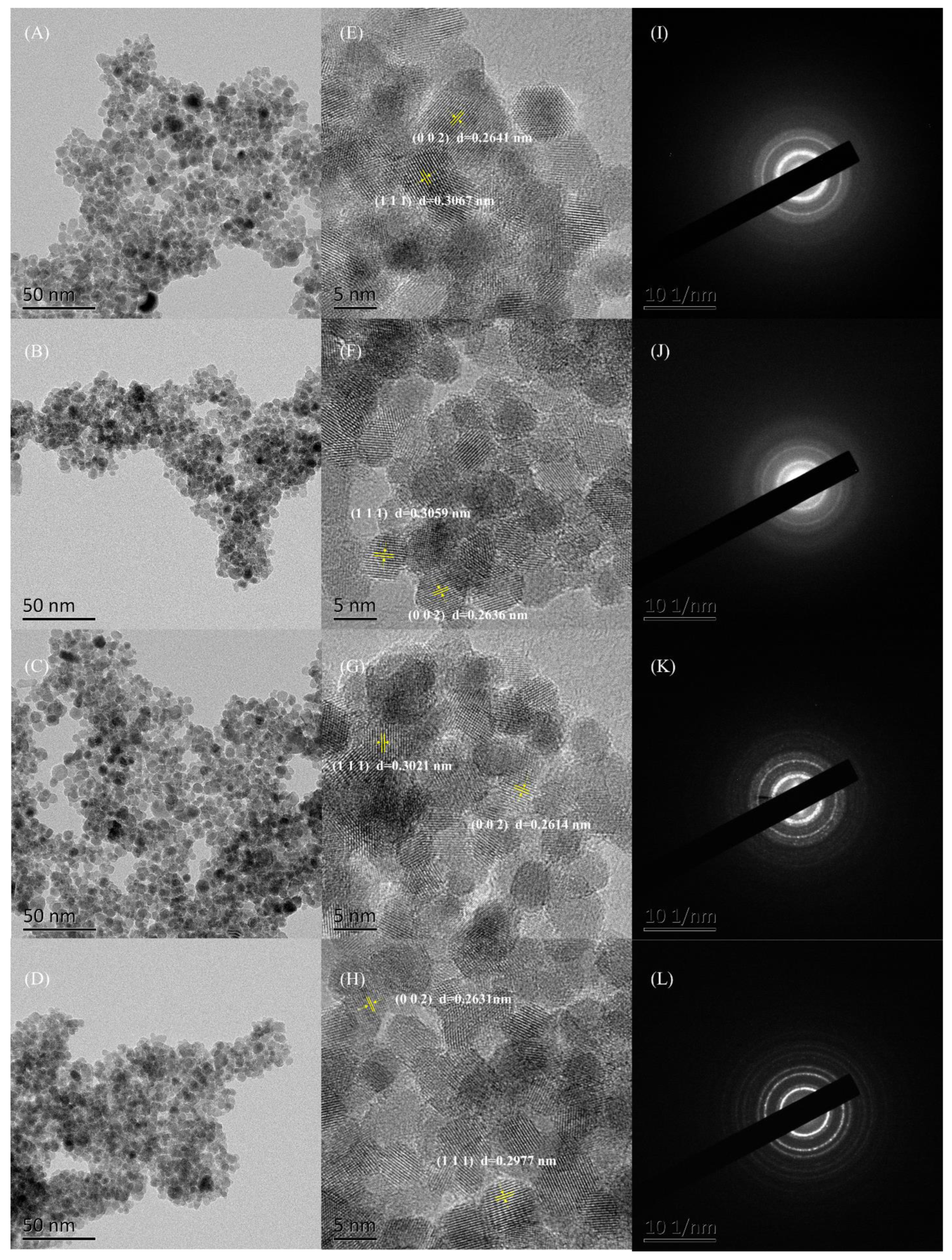
3.5. TEM-SEAD
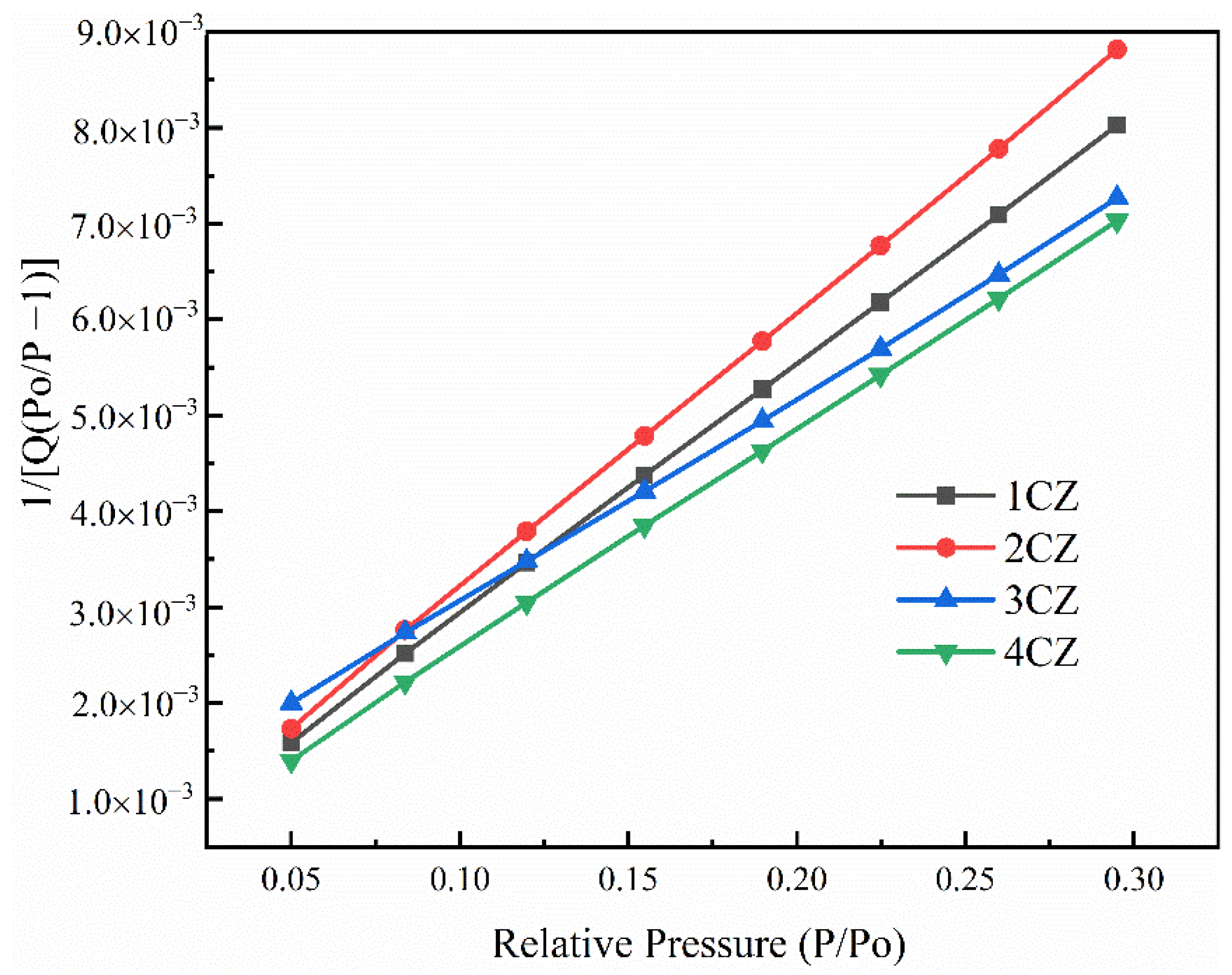
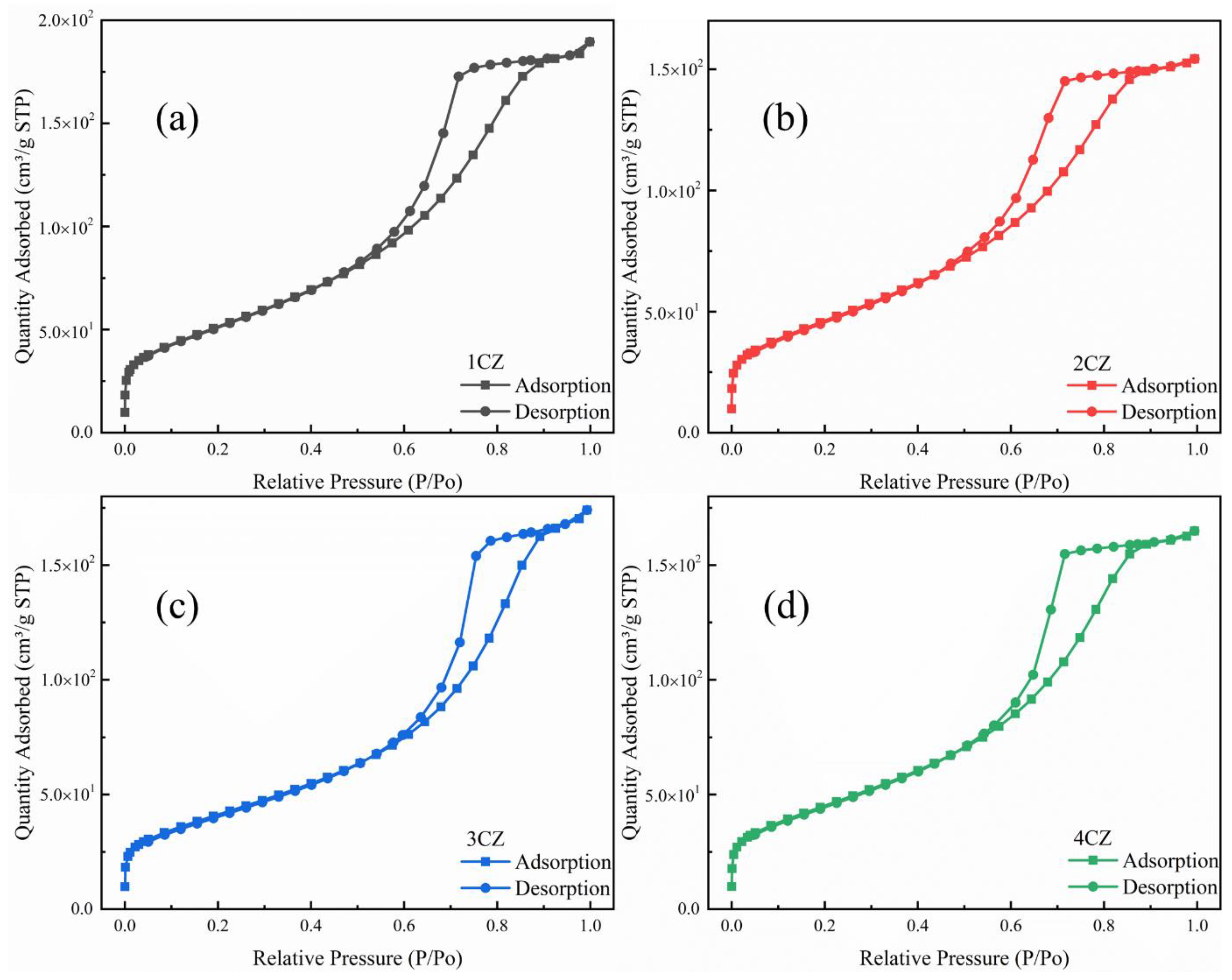
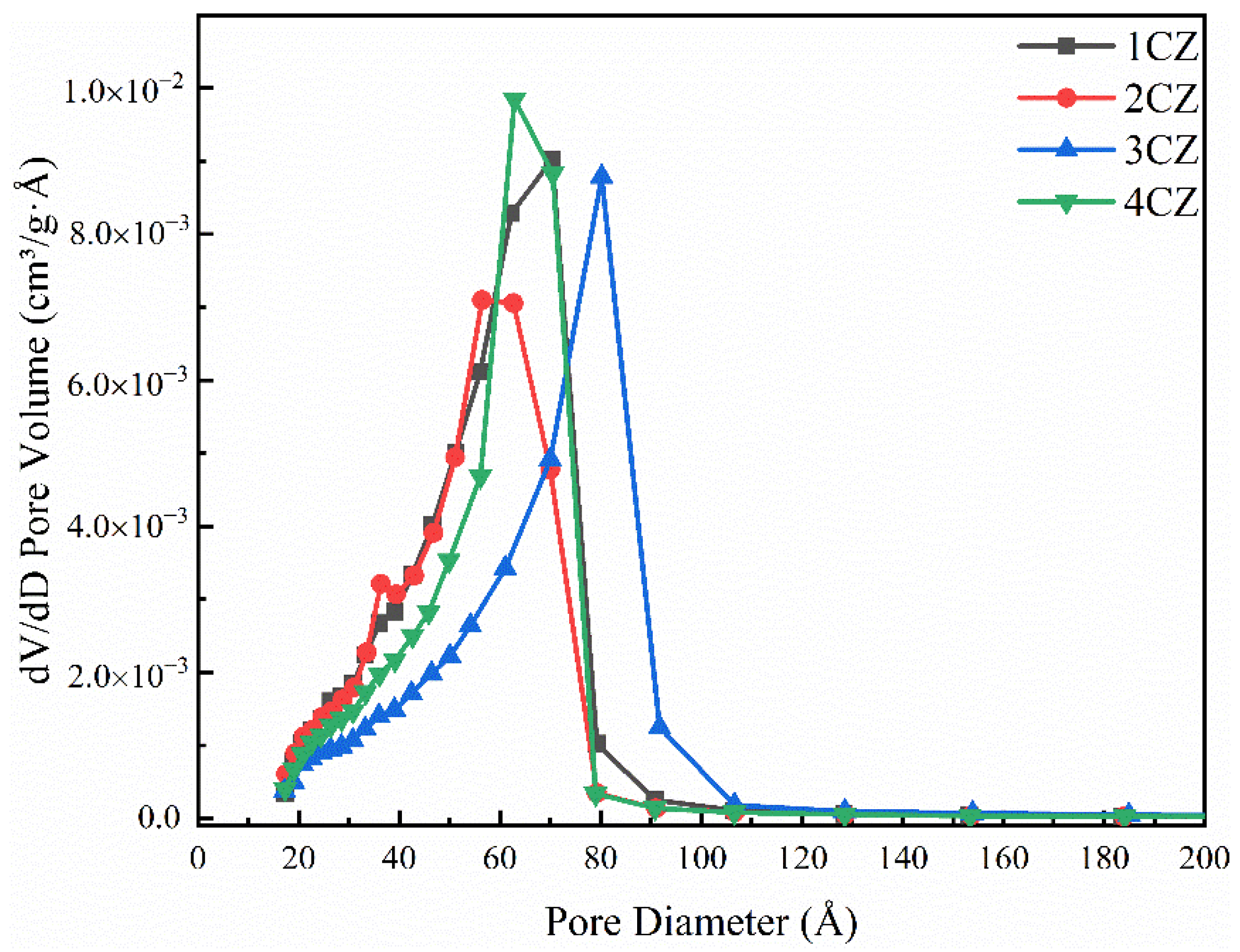
3.6. Specific Surface and Pore Analysis
3.7. Oxygen Storage Capacity (OSC) Test
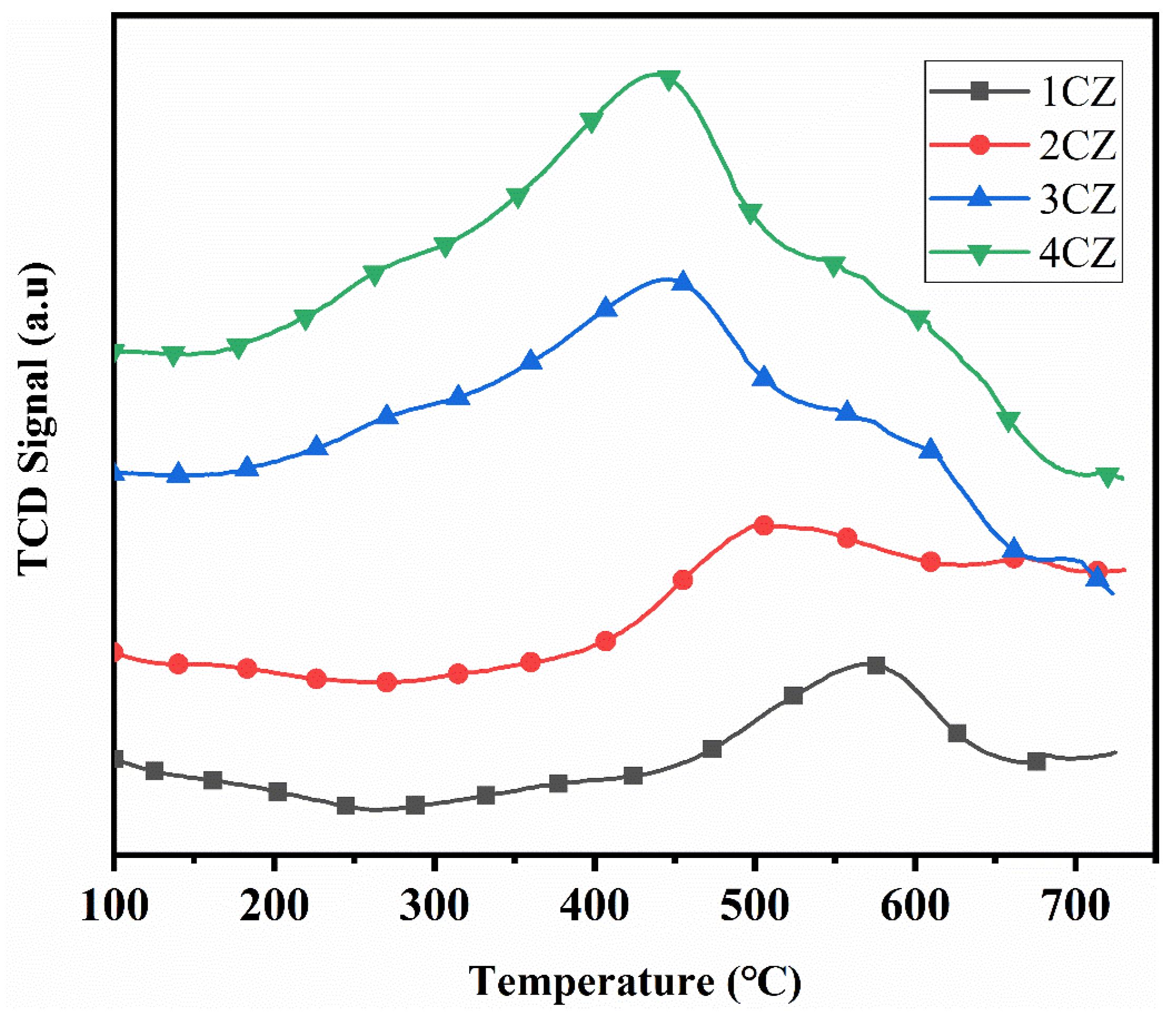
3.8. H2-TPR Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diagne, C.; Idriss, H.; Kiennemann, A. Hydrogen production by ethanol reforming over Rh/CeO2–ZrO2 catalysts. Catal. Commun. 2002, 3, 565–571. [Google Scholar] [CrossRef]
- Hori, C.E.; Permana, H.; Ng, K.Y.S.; Brenner, A.; More, K.; Rahmoeller, K.M.; Belton, D. Thermal stability of oxygen storage properties in a mixed CeO2-ZrO2 system. Appl. Catal. B Environ. 1998, 16, 105–117. [Google Scholar] [CrossRef]
- Hosoya, A.; Tamura, S.; Imanaka, N. Catalytic combustion-type CO sensor applying Pt loaded CeO2-ZrO2-ZnO solid solution. J. Ceram. Soc. Jpn. 2014, 122, 601–603. [Google Scholar] [CrossRef]
- Arsent’ev, M.Y.; Kalinina, M.V.; Tikhonov, P.A.; Morozova, L.V.; Kovalenko, A.S.; Koval’ko, N.Y.; Khlamov, I.I.; Shilova, O.A. Synthesis and study of sensor oxide nanofilms in a ZrO2-CeO2 system. Glass Phys. Chem. 2014, 40, 362–366. [Google Scholar] [CrossRef]
- Izu, N.; Shin, W.; Matsubara, I.; Murayama, N. Resistive oxygen gas sensors using ceria-zirconia thick films. J. Ceram. Soc. Jpn. 2004, 112, S535–S539. [Google Scholar]
- Ahn, K.Y.; He, H.P.; Vohs, J.M.; Gorte, R.J. Enhanced thermal stability of SOFC anodes made with CeO2-ZrO2 solutions. Electrochem. Solid State Lett. 2005, 8, A414–A417. [Google Scholar] [CrossRef]
- Xia, C.; Liu, M. Low-temperature SOFCs based on Gd0.1Ce0.9O1.95 fabricated by dry pressing. Solid State Ion. 2001, 144, 249–255. [Google Scholar] [CrossRef]
- Celik, E.; Akın, Y.; Avci, E.; Sigmund, W.; Hascicek, Y. CeO2-ZrO2 insulation coatings on Ag/AgMg sheathed Bi-2212 superconducting tapes by sol-gel process for magnet technology. Appl. Supercond. IEEE Trans. 2002, 12, 1223–1226. [Google Scholar] [CrossRef]
- Terribile, D.; Trovarelli, A.; Llorca, J.; de Leitenburg, C.; Dolcetti, G. The preparation of high surface area CeO2–ZrO2 mixed oxides by a surfactant-assisted approach. Catal. Today 1998, 43, 79–88. [Google Scholar] [CrossRef]
- Daturi, M.; Finocchio, E.; Binet, C.; Lavalley, J.C.; Fally, F.; Perrichon, V.; Vidai, H.; Hickey, N.; Kašpar, J. Reduction of high surface area CeO2-ZrO2 mixed oxides. J. Phys. Chem. B 2000, 104, 9186–9194. [Google Scholar] [CrossRef]
- Jiang, P.; Lu, G.; Li, Y.; Guo, Y.; Guo, Y.; Wang, X. Preparation of La2O3-doped CeO2–ZrO2 Solid Solution with High Thermal Stability by Water-in-Oil Microemulsion. Chem. Lett. 2004, 33, 1064–1065. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Wang, Q.; Hu, Z.; Gu, G. Preparation, Characterization of CeO2-ZrO2 Composite Hollow Microspheres and Their Application as Electrocatalysis Materials for Hemoglobin in Biosensor. J. Dispers. Sci. Technol. 2009, 30, 178–184. [Google Scholar] [CrossRef]
- Bhosale, A.K.; Shinde, P.; Tarwal, D.N.L.; Pawar, R.; Kadam, P.M.; Patil, P. Synthesis and characterization of highly stable optically passive CeO2–ZrO2 counter electrode. Electrochim. Acta 2010, 55, 1900–1906. [Google Scholar] [CrossRef]
- Hirano, M.; Miwa, T.; Inagaki, M. Low-Temperature Direct Synthesis of Nanoparticles of Fluorite-Type Ceria–Zirconia Solid Solutions by “Forced Cohydrolysis” at 100 °C. J. Solid State Chem. 2001, 158, 112–117. [Google Scholar] [CrossRef]
- Hirano, M.; Miwa, T.; Inagaki, M. Effect of the Presence of Ammonium Peroxodisulfate on the Direct Precipitation of Ceria and Ceria–Zirconia Solid Solutions from Acidic Aqueous Solutions. J. Am. Ceram. Soc. 2001, 84, 1728–1732. [Google Scholar] [CrossRef]
- Eslamian, M.; Ahmed, M.; Ashgriz, N. Modeling of Nano-particle Formation during Spray Pyrolysis. Nanotechnology 2006, 17, 1674–1685. [Google Scholar] [CrossRef]
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef]
- Kawasaki, S.-i.; Xiuyi, Y.; Sue, K.; Hakuta, Y.; Suzuki, A.; Arai, K. Continuous supercritical hydrothermal synthesis of controlled size and highly crystalline anatase TiO2 nanoparticles. J. Supercrit. Fluids 2009, 50, 276–282. [Google Scholar] [CrossRef]
- Lester, E.; Blood, P.; Denyer, J.; Giddings, D.; Azzopardi, B.; Poliakoff, M. Reaction engineering: The supercritical water hydrothermal synthesis of nano-particles. J. Supercrit. Fluids 2006, 37, 209–214. [Google Scholar] [CrossRef]
- Zhang, Z.; Brown, S.; Goodall, J.B.M.; Weng, X.; Thompson, K.; Gong, K.; Kellici, S.; Clark, R.J.H.; Evans, J.R.G.; Darr, J.A. Direct continuous hydrothermal synthesis of high surface area nanosized titania. J. Alloys Compd. 2009, 476, 451–456. [Google Scholar] [CrossRef]
- Zielke, P.; Xu, Y.; Simonsen, S.B.; Norby, P.; Kiebach, R. Simulation, design and proof-of-concept of a two-stage continuous hydrothermal flow synthesis reactor for synthesis of functionalized nano-sized inorganic composite materials. J. Supercrit. Fluids 2016, 117, 1–12. [Google Scholar] [CrossRef][Green Version]
- Sue, K.; Suzuki, M.; Arai, K.; Ohashi, T.; Ura, H.; Matsui, K.; Hakuta, Y.; Hayashi, H.; Watanabe, M.; Hiaki, T. Size-controlled synthesis of metal oxide nanoparticles with a flow-through supercritical water method. Green Chem. 2006, 8, 634–638. [Google Scholar] [CrossRef]
- Loppinet-Serani, A.; Aymonier, C.; Cansell, F. Current and foreseeable applications of supercritical water for energy and the environment. ChemSusChem 2008, 1, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Adschiri, T.; Lee, Y.-W.; Goto, M.; Takami, S. Green materials synthesis with supercritical water. Green Chem. 2011, 13, 1380–1390. [Google Scholar] [CrossRef]
- Adschiri, T.; Yoko, A. Supercritical fluids for nanotechnology. J. Supercrit. Fluids 2018, 134, 167–175. [Google Scholar] [CrossRef]
- Kim, J.-R.; Lee, K.-Y.; Suh, M.-J.; Ihm, S.-K. Ceria–zirconia mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as catalyst support. Catal. Today 2012, 185, 25–34. [Google Scholar] [CrossRef]
- Kim, J.-R.; Myeong, W.-J.; Ihm, S.-K. Characteristics of CeO2–ZrO2 mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as support of Rh catalyst for catalytic reduction of NO by CO. J. Catal. 2009, 263, 123–133. [Google Scholar] [CrossRef]
- Weng, X.; Cockcroft, J.K.; Hyett, G.; Vickers, M.; Boldrin, P.; Tang, C.C.; Thompson, S.P.; Parker, J.E.; Knowles, J.C.; Rehman, I.; et al. High-Throughput Continuous Hydrothermal Synthesis of an Entire Nanoceramic Phase Diagram. J. Comb. Chem. 2009, 11, 829–834. [Google Scholar] [CrossRef]
- Weng, X.; Perston, B.; Wang, X.Z.; Abrahams, I.; Lin, T.; Yang, S.; Evans, J.R.G.; Morgan, D.J.; Carley, A.F.; Bowker, M.; et al. Synthesis and characterization of doped nano-sized ceria–zirconia solid solutions. Appl. Catal. B Environ. 2009, 90, 405–415. [Google Scholar] [CrossRef]
- Ma, C.Y.; Chen, M.; Wang, X.Z. Modelling and simulation of counter-current and confined jet reactors for hydrothermal synthesis of nano-materials. Chem. Eng. Sci. 2014, 109, 26–37. [Google Scholar] [CrossRef]
- Ma, C.Y.; Liu, J.J.; Zhang, Y.; Wang, X.Z. Simulation for scale-up of a confined jet mixer for continuous hydrothermal flow synthesis of nanomaterials. J. Supercrit. Fluids 2015, 98, 211–221. [Google Scholar] [CrossRef]
- Gruar, R.I.; Tighe, C.J.; Darr, J.A. Scaling-up a Confined Jet Reactor for the Continuous Hydrothermal Manufacture of Nanomaterials. Ind. Eng. Chem. Res. 2013, 52, 5270–5281. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [PubMed]
- Renuka, L.; Anantharaju, K.S.; Sharma, S.C.; Nagabhushana, H.; Vidya, Y.S.; Nagaswarupa, H.P.; Prashantha, S.C. A comparative study on the structural, optical, electrochemical and photocatalytic properties of ZrO2 nanooxide synthesized by different routes. J. Alloys Compd. 2017, 695, 382–395. [Google Scholar] [CrossRef]
- Guo, G.-Y.; Chen, Y.-L.; Ying, W.-J. Thermal, spectroscopic and X-ray diffractional analyses of zirconium hydroxides precipitated at low pH values. Mater. Chem. Phys. 2004, 84, 308–314. [Google Scholar] [CrossRef]
- Kanade, K.G.; Baeg, J.O.; Apte, S.K.; Prakash, T.L.; Kale, B.B. Synthesis and characterization of nanocrystallined zirconia by hydrothermal method. Mater. Res. Bull. 2008, 43, 723–729. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Liu, J.; Jiang, F.; Tang, H.; Feng, G.; Jiang, W. Preparation, characterization and growth mechanism of ZrO2 nanosheets. Ceram. Int. 2020, 46, 4864–4869. [Google Scholar] [CrossRef]
- Gurushantha, K.; Anantharaju, K.S.; Nagabhushana, H.; Sharma, S.C.; Vidya, Y.S.; Shivakumara, C.; Nagaswarupa, H.P.; Prashantha, S.C.; Anilkumar, M.R. Facile green fabrication of iron-doped cubic ZrO2 nanoparticles by Phyllanthus acidus: Structural, photocatalytic and photoluminescent properties. J. Mol. Catal. A Chem. 2015, 397, 36–47. [Google Scholar] [CrossRef]
- Wang, S.F.; Gu, F.; Lü, M.K.; Yang, Z.S.; Zhou, G.J.; Zhang, H.P.; Zhou, Y.; Wang, S.M. Structure evolution and photoluminescence properties of ZrO2:Eu3+ nanocrystals. Opt. Mater. 2006, 28, 1222–1226. [Google Scholar] [CrossRef]
- Reddy, C.V.; Babu, B.; Reddy, I.N.; Shim, J. Synthesis and characterization of pure tetragonal ZrO2 nanoparticles with enhanced photocatalytic activity. Ceram. Int. 2018, 44, 6940–6948. [Google Scholar] [CrossRef]
- Davar, F.; Hassankhani, A.; Loghman-Estarki, M.R. Controllable synthesis of metastable tetragonal zirconia nanocrystals using citric acid assisted sol–gel method. Ceram. Int. 2013, 39, 2933–2941. [Google Scholar] [CrossRef]
- Buttersack, C. Modeling of type IV and V sigmoidal adsorption isotherms. Phys. Chem. Chem. Phys. PCCP 2019, 21, 5614–5626. [Google Scholar] [CrossRef] [PubMed]
- Scherdel, C.; Reichenauer, G.; Wiener, M. Relationship between pore volumes and surface areas derived from the evaluation of N2-sorption data by DR-, BET- and t-plot. Microporous Mesoporous Mater. 2010, 132, 572–575. [Google Scholar] [CrossRef]
- Harkins, W.D.; Jura, G. Surfaces of Solids. XIII. A Vapor Adsorption Method for the Determination of the Area of a Solid without the Assumption of a Molecular Area, and the Areas Occupied by Nitrogen and Other Molecules on the Surface of a Solid. J. Am. Chem. Soc. 1944, 66, 1366–1373. [Google Scholar] [CrossRef]
- Haghighatju, F.; Hashemipour Rafsanjani, H.; Esmaeilzadeh, F. Estimation of the dimension of micropores and mesopores in single walled carbon nanotubes using the method Horvath–Kawazoe, Saito and Foley and BJH equations. Micro Nano Lett. 2017, 12, 1–5. [Google Scholar] [CrossRef]
- Aneggi, E.; Boaro, M.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Insights into the redox properties of ceria-based oxides and their implications in catalysis. J. Alloys Compd. 2006, 408, 1096–1102. [Google Scholar] [CrossRef]

| 1CZ | 2CZ | 3CZ | 4CZ | |
|---|---|---|---|---|
| ZrO(NO3)2·xH2O (g) | 20.8107 | 18.4984 | 16.1861 | 13.8738 |
| Ce(NO3)3·6H2O (g) | 4.3422 | 8.6844 | 13.0266 | 17.3688 |
| Ce: Zr (molar ratio) | 1:9 | 2:8 | 3:7 | 4:6 |
| Ce:Zr (Molar Ratio) | 1CZ | 2CZ | 3CZ | 4CZ |
|---|---|---|---|---|
| EDS | 1.76:18.72 | 3.43:15.46 | 5.71:14.61 | 6.84:11.26 |
| ICP-AES | 2.16:20.47 | 4.61:19.4 | 6.22:15.51 | 7.69:12.54 |
| Date | 1CZ | 2CZ | 3CZ | 4CZ |
|---|---|---|---|---|
| BET surface area (m2/g) | 147.7 | 164.4 | 153.6 | 161.1 |
| C | 92.75 | 83.88 | 83.07 | 96.14 |
| Correlation coefficient | 0.9999539 | 0.9999827 | 0.9999418 | 0.9999625 |
| Volume (cm3/g) | 1CZ | 2CZ | 3CZ | 4CZ |
|---|---|---|---|---|
| t-Plot | 0.005626 | 0.006665 | 0.002708 | 0.003913 |
| BJH | 0.289700 | 0.245361 | 0.273827 | 0.260834 |
| Samples | 1CZ | 2CZ | 3CZ | 4CZ |
|---|---|---|---|---|
| OSC (μmol/g) | 433.7 | 495.6 | 535.7 | 631.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, L.; Wang, Z.; Wang, X. Continuous Hydrothermal Flow Synthesis and Characterization of ZrO2 Nanoparticles Doped with CeO2 in Supercritical Water. Nanomaterials 2022, 12, 668. https://doi.org/10.3390/nano12040668
Li Q, Liu L, Wang Z, Wang X. Continuous Hydrothermal Flow Synthesis and Characterization of ZrO2 Nanoparticles Doped with CeO2 in Supercritical Water. Nanomaterials. 2022; 12(4):668. https://doi.org/10.3390/nano12040668
Chicago/Turabian StyleLi, Qingyun, Lingyu Liu, Zihua Wang, and Xuezhong Wang. 2022. "Continuous Hydrothermal Flow Synthesis and Characterization of ZrO2 Nanoparticles Doped with CeO2 in Supercritical Water" Nanomaterials 12, no. 4: 668. https://doi.org/10.3390/nano12040668
APA StyleLi, Q., Liu, L., Wang, Z., & Wang, X. (2022). Continuous Hydrothermal Flow Synthesis and Characterization of ZrO2 Nanoparticles Doped with CeO2 in Supercritical Water. Nanomaterials, 12(4), 668. https://doi.org/10.3390/nano12040668





