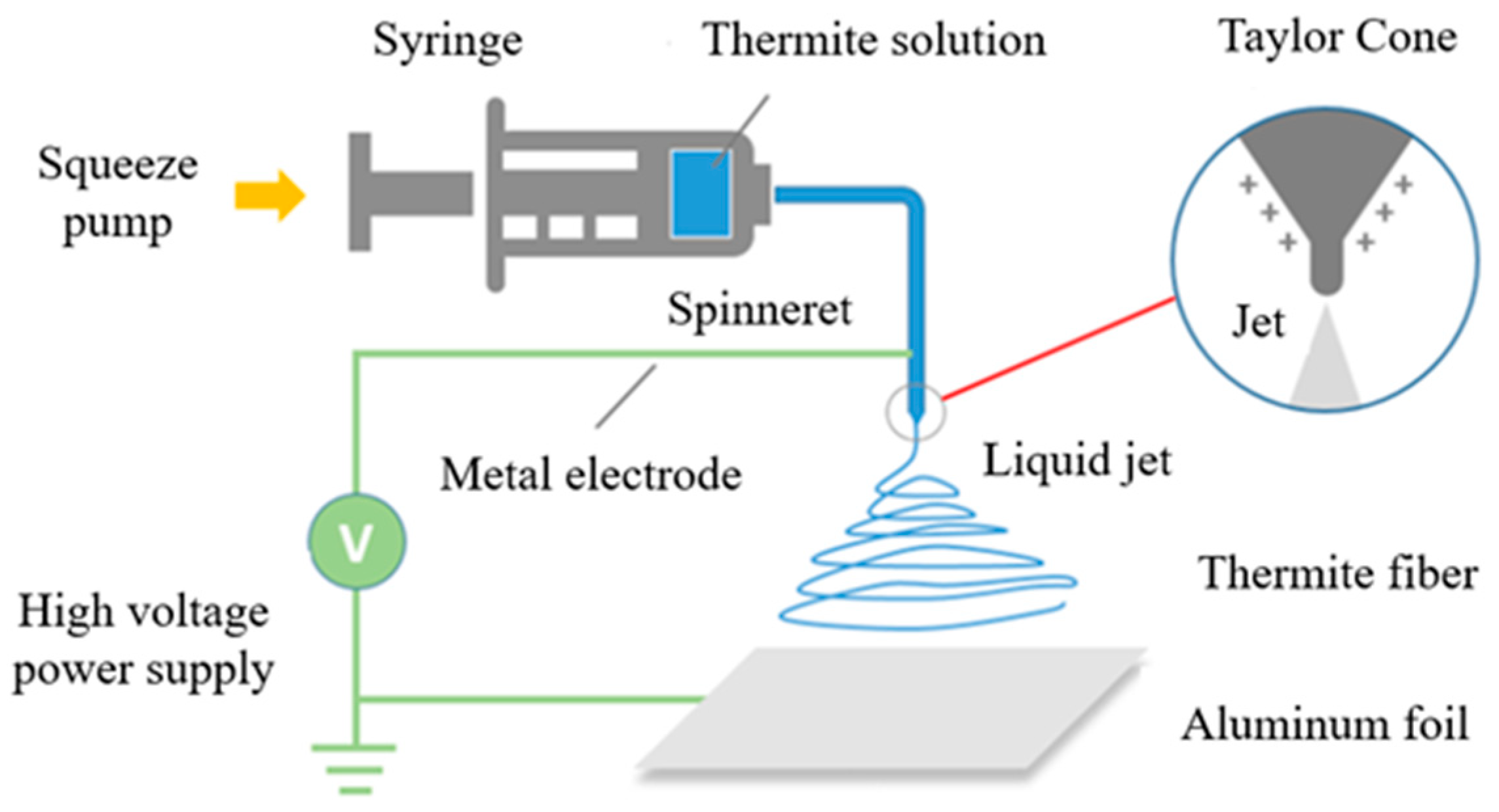
3.1. Morpology and Structure
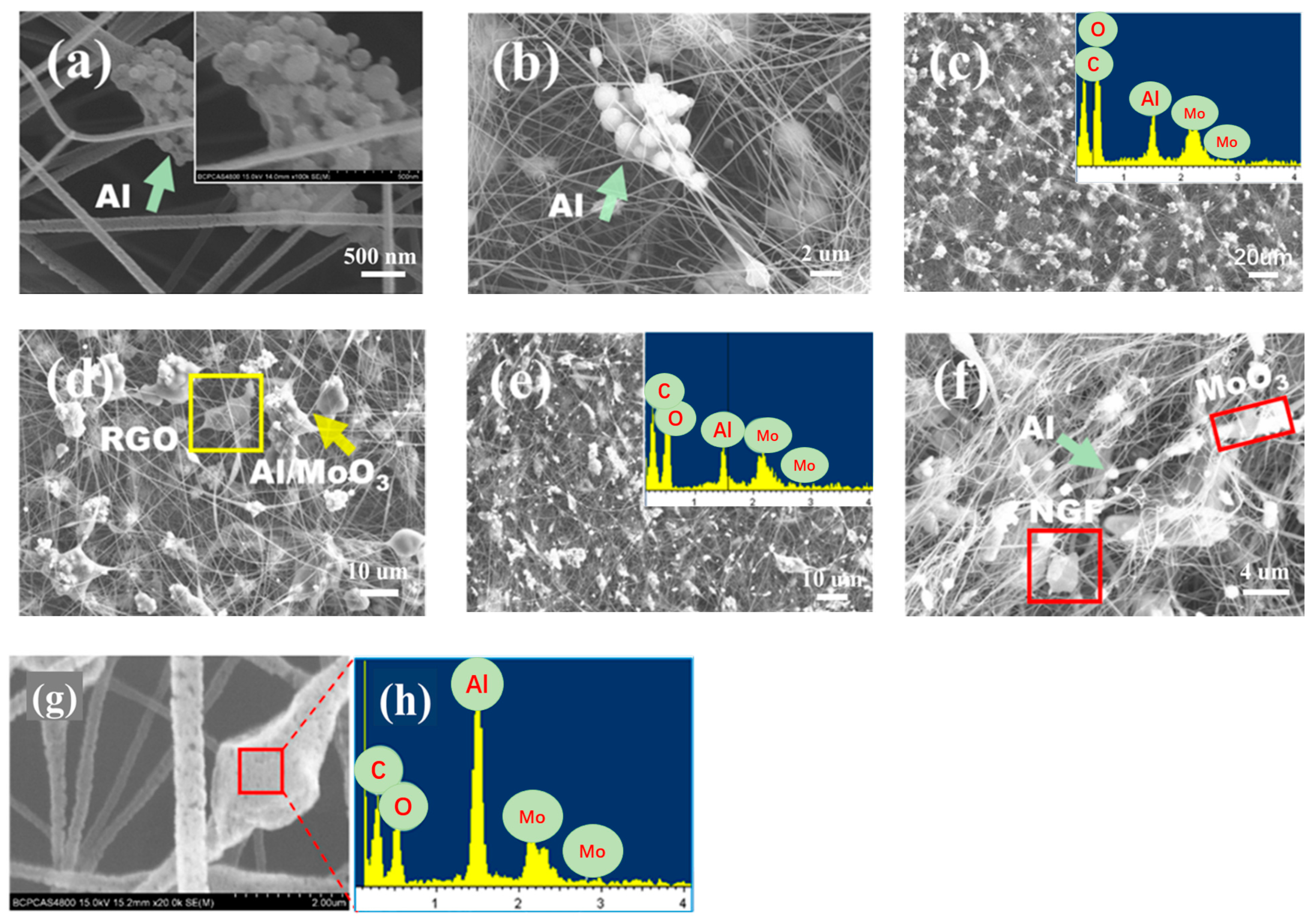
The microscopic morphology and microdomain element distributions of the Al/MoO
3/NCM composite prepared by electrospinning are shown in
Figure 2. nAl and mAl without PFPE coating will settle and aggregate due to different densities when they are slowly extruded in the syringe in
Figure 2a,b and separate from MoO
3 particles. Al particles showed obvious agglomeration; thus, they could not be effectively mixed with MoO
3 particles. This phenomenon of uneven mixing and component separation will directly affect the performance of the prepared thermite. Al and MoO
3 particles coated with PFPE are not easy to settle and separate in the thermite suspension solution, and NCM has a small density and can be stably suspended in the mixed solution; thus, uniform Al/MoO
3/NCM spinning can be obtained by electrostatic spinning. Although the particles of the micron and sub-micron Al/MoO
3/NCM thermite cannot be attached to the NC fiber, the components can be effectively dispersed and uniformly mixed in
Figure 2c,f, which can be proved from the corresponding microzone element distribution map.
Al and MoO
3 particles of the nAl/MoO
3/NCM thermite are both nanosized, and their particle size is much smaller than NC; thus, the two components can be better attached to NC fibers. However, the NCM used in the experiment is submicron or even micron in at least one dimension; thus, even if the Al/MoO
3/NCM thermite particles are used for spinning, the bead-like structure are formed or mixed particles are directly free from spinning. The micromorphology and element distribution diagrams of nAl/MoO
3/RGO spinning are shown in
Figure 2g,h. The spinning surface presents a concave–convex and intermittent structure. This is due to the fact that nAl and nMoO
3 are filled in NC, but the solvent in NC evaporates and shrinks. Microarea element analysis was performed on a similar beaded area on Al/MoO
3/RGO spinning, and the element type distribution map was obtained. It can be observed from
Figure 2h that there are four elements of C, O, Mo, and Al in the structures, which proves the existence of thermite components.
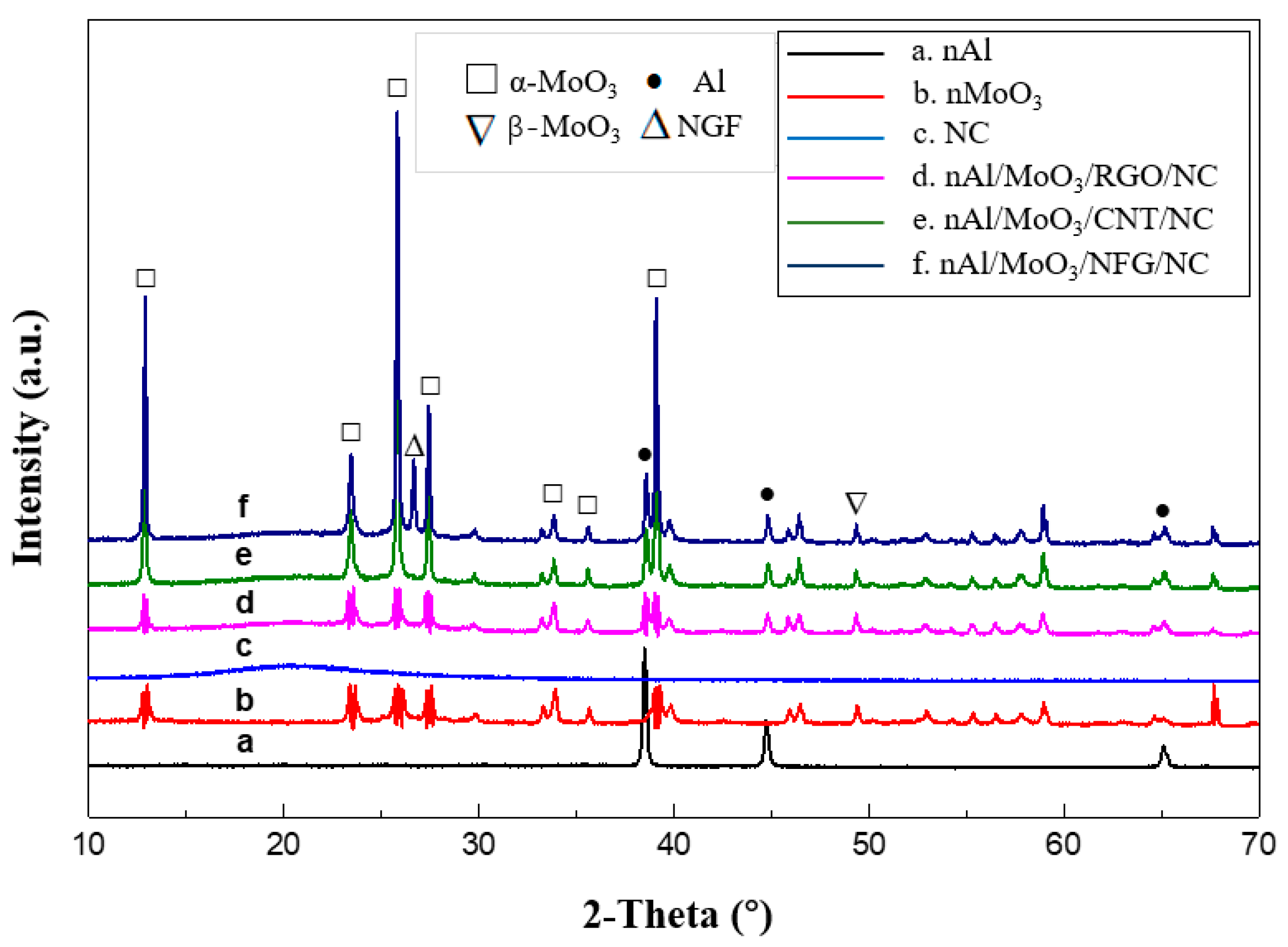
The XRD spectra of nAl/MoO
3/NCM spinning and raw materials are shown in
Figure 3.
Figure 3a is the XRD spectrum of nAl particles. There are three obvious diffraction peaks in the curve. The diffraction peaks at the diffraction angles of 38.47°, 44.78°, and 65.09° corresponded to (1 1 1), (2 0 0), and (2 2 0) crystal planes of Al crystals (JCPDS Card No. 04–0787) for the face-centered cubic system.
Figure 3b is the XRD spectrum of nMoO
3 particles. The diffraction angles 2θ in the spectrum are 12.98°, 23.58°, 25.92°, 27.48°, 33.98°, and 39.20°, corresponding to (0 2 0), (1 1 0), (0 4 0), (0 2 1), (1 1 1), and (1 5 0) crystal planes of orthorhombic system α-MoO
3 (JCPDS Card No. 21–0506), and the diffraction peak at 2θ of 49.53° is the (0 6 1) crystal plane of β-MoO
3 for the monoclinic system (JCPDS Card No. 76–1003). The XRD spectrum of pure NC shows that there is a wide amorphous dispersion peak at 2θ of about 20°, indicating that NC exists in an amorphous form.
Figure 3d–f are the XRD spectra of nano Al/MoO
3/RGO, Al/MoO
3/CNT, and Al/MoO
3/NFG spinning, respectively. The amorphous dispersion peaks of NC, crystal sharp peaks of Al, and MoO
3 particles appeared in three spectra. The diffraction peak at 26.66°in
Figure 3f is the (0 0 2) crystal plane of graphite (JCPDS Card No. 41–1487).
3.2. Thermal Conductivity
Thermal conductivity is an important thermal physical performance parameter of thermite, which is directly related to the heat transfer of thermite, the difficulty of ignition, and the ability to ignite. It can be used to qualitatively characterize the heat transfer effect of the thermite after being thermally stimulated, and it can also indirectly evaluate the ease of ignition and thermal safety of the pharmaceutical system. In order to compare the thermal conductivity of aluminum-containing thermite after adding carbon materials, the heat flow method was used to test the thermal resistance of nAl/MoO
3 and nAl/MoO
3/NCM, and the thermal conductivity and thermal resistance of the material were calculated by Formulas (1) and (2) [
27]:
where
KT is the thermal conductivity of the sample at temperature
T;
A is the area of the test plate;
Th and
Tc are the temperatures of the hot and cold plates, respectively; x is the distance between the two plates; Δ
Q/Δ
t is the amount of heat transferred by the sample in unit time, which is heat transfer rate;
RT is the thermal resistance of the sample at temperature
T; and
d is the thickness of the measured material.
The thermal conductivity test results of the thermite are shown in
Table 1, and the histogram of thermal resistance and thermal conductivity for thermite is exhibited in
Figure 4. It can be observed from
Figure 4 that the thermal conductivities of Al/MoO
3 thermite spinning of micron, submicron, and nanosize grades are similar but gradually decrease. Compared with Al/MoO
3 thermite particles, the thermal conductivity of the three particle sizes of Al/MoO
3 thermite spinning significantly decreased by about 24.8%, 27.2%, and 28.1%, respectively (the thermal conductivity of mAl/MoO
3, sub-mAl/MoO
3, and nAl/MoO
3 thermite particles are 117 W·m
−1·K
−1, 103 W·m
−1·K
−1, and 96 W·m
−1·K
−1, respectively). The thermal conductivity of nAl/MoO
3 thermite spinning is reduced the most. The thermal conductivity of Al/MoO
3 thermite spinning after NC coating or bonding is not only affected by the thermal conductivity of NC but also related to changes in internal structure. Due to the existence of gaps between the raw material component of micro–nano Al/MoO
3 thermite spinning, overall thermal conductivity is reduced. In addition, the NC with poor thermal conductivity is coated on the surface of the particles, which reduces the thermal conductivity of the system, and the spinning structure has a larger porosity. Therefore, the combined effect of these factors makes the thermal performance of Al/MoO
3 thermite spinning significantly reduced than compared to Al/MoO
3 thermite particles. For nAl/MoO
3 thermite spinning, the nanoparticles have a larger specific surface area. After the spinning process, more NC components are coated on the surface of nanoparticles, which hinders heat transfer at the interface of the nanoparticles. Compared with the spinning of micron and submicron particles, the spinning of nanoparticles has a larger porosity, and the thermal conductivity of the system has a negative correlation with the void fraction. Hence, the thermal conductivity of nAl/MoO
3 thermite spinning reduced most obviously.
Nanocarbon materials have good thermal conductivity, and adding to the thermite can improve the thermal conductivity of the thermite system. The thermal conductivity of the three particle sizes of Al/MoO3 thermite spinning has been significantly improved after adding NFG and CNT. Thermal conductivity is improved the most by sub-mAl/MoO3/NFG and nAl/MoO3/CNT, and it increased by 24.0% and 26.1%, respectively. The above two nanocarbon materials have similar effects on the thermal conductivity of micron and submicron Al/MoO3 thermite spinning, but CNT has a more significant increase in the thermal conductivity of nAl/MoO3, resulting in the thermal conductivity of nAl/MoO3/CNT being higher than nAl/MoO3/NFG. After adding RGO, the thermal conductivity of the three particle sizes of Al/MoO3 thermite spinning has been improved more significantly. In particular, the thermal conductivity of nAl/MoO3/RGO is higher than sub-mAl/MoO3/RGO and mAl/MoO3/RGO. Thermal conductivity increased by 55.7%, 61.3%, and 107.2% in the order of micron, submicron, and nanolevels compared with Al/MoO3, respectively. The addition of RGO can significantly improve the thermal conductivity of Al/MoO3 thermite spinning, especially for nAl/MoO3.
3.3. Energy Performance
According to the principle of minimum free energy, combustion temperature, combustion heat, gas-phase product volume, solid residue volume, and constant volume gas product pressure of Al/MoO
3/NCM thermite spinning with different fuel–oxygen equivalent ratios at the reaction equilibrium were calculated. The thermite is 1 kg, the environmental pressure during the reaction is 0.1 MPa, the system is an adiabatic system, and system iteration accuracy is 10
−6. The calculation results are shown in
Table 2 and
Table 3.
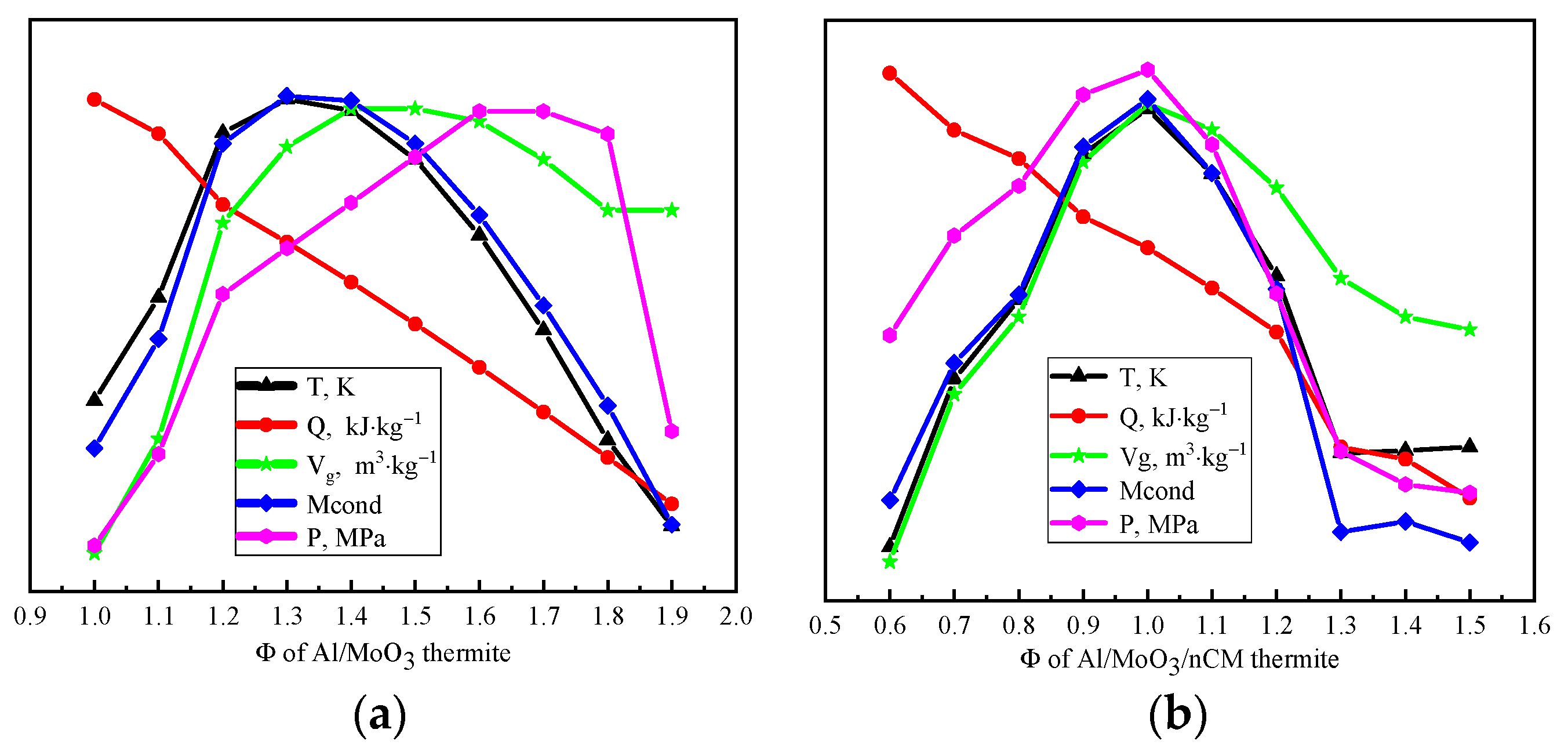
Figure 5 shows the energy parameter change curve of Al/MoO
3/NCM thermite spinning with different combustion oxygen equivalent ratios, Φ.
Combustion temperature, combustion heat, gas-phase product volume, solid residue volume, and constant volume gas product pressure reflect the comprehensive effect of each microreaction in the Al/MoO
3/NCM thermite spinning system. It can be observed from
Figure 5a that the combustion temperature and solid residue amount of Al/MoO
3 thermite spinning show a trend of increasing and then decreasing with the gradual increase in Φ value. The values of combustion temperature and solid residue amount are the highest when the value of Φ is 1.30~1.40. The change curve of the gas production volume and the constant volume gas product pressure increased and then gradually flattened, while combustion heat still shows a unilateral downward trend, indicating that the increase in the value of Φ is not conducive to the release of combustion heat. The amount of reducing agent continues to increase while the amount of oxidant continues to decrease, which is not conducive to the full progress of the reaction. In order to measure the combined effect of energy parameters, Φ = 1.30–1.40 was selected as the optimal fuel–oxygen equivalent ratio for the energy performance of Al/MoO
3 thermite spinning.
Figure 5b shows that the combustion heat of Al/MoO
3/NCM thermite spinning still shows a downward trend. The values of the other energy parameters are all at the top of the parabola when the value of Φ is 0.90~1.00. Other parameters reach the maximum except for the heat of combustion at this time. Therefore, the energy performance of Al/MoO
3/NCM thermite spinning is the best when the value of Φ is 0.90–1.00.
3.4. Combustion Performance
Before testing the burning rate of micro–nano thermite spinning, it is necessary to peel off the spinning filament attached to the aluminum foil with tweezers and then roll it into a cylindrical shape. Then, micro–nano thermites were packed into a PMMA tube with a certain inner diameter and the packing density was kept at 1.73 g·cm
−3. Use the electric ignition head to ignite the thermite spinning from one end of the PMMA tube under the action of the capacitor discharge detonator. The combustion process of thermite was recorded by a high-speed camera, and the linear burning rate of the thermite film was calculated according to the correspondence between the burning process and time. Each thermite sample was tested three times in parallel, and the standard deviation was calculated. The results are shown in
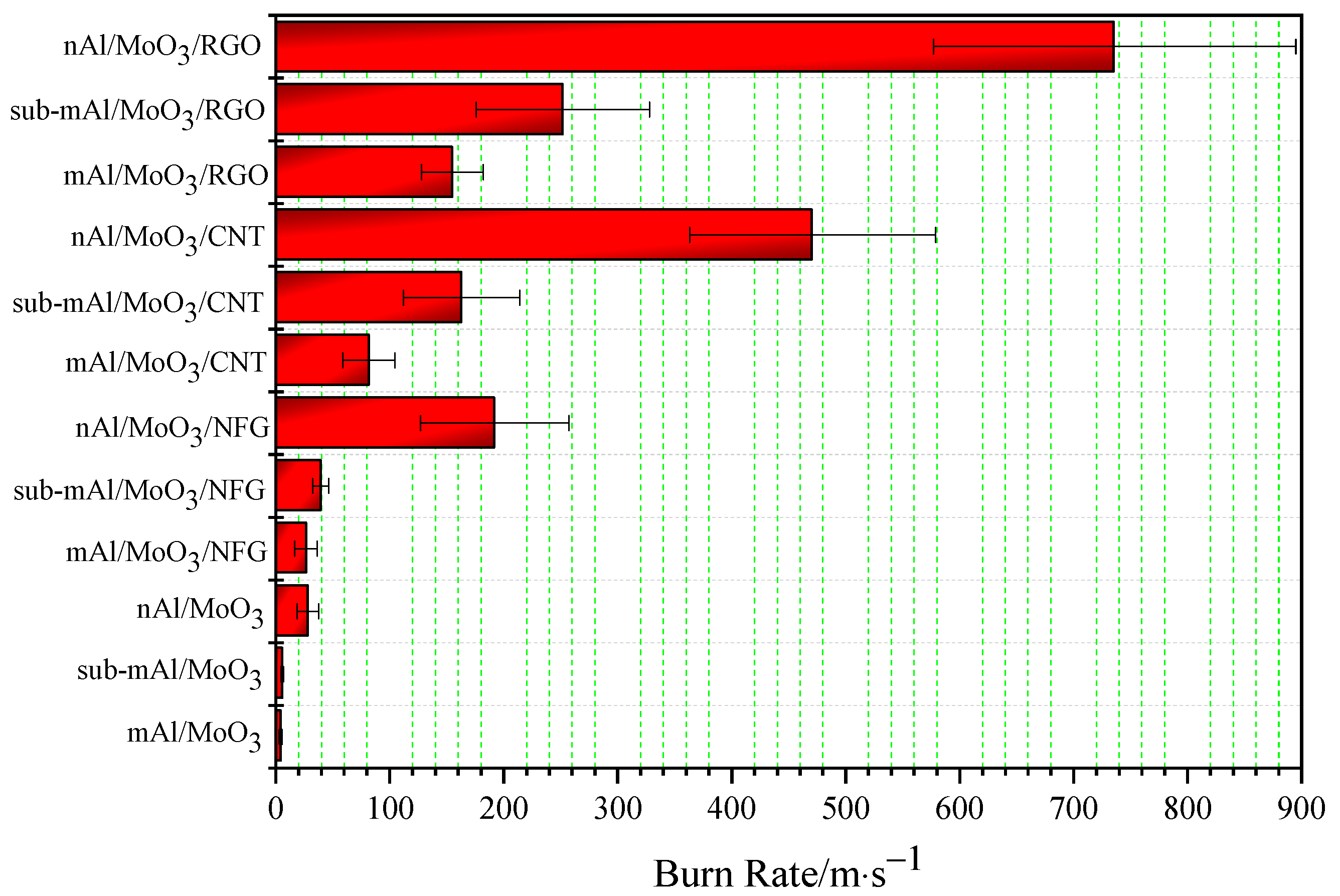
Table 4, and the histogram of the linear burning rate is exhibited in
Figure 6.
The burning rate of Al/MoO3 thermite spinning of a micron, submicron, and nanometer is low. Among them, the burning rate of mAl/MoO3 thermite spinning is the lowest, and nAl/MoO3 has the highest burning rate; that is, the smaller the particle size is, the higher the burning rate will be. The highest burning rate is about seven times the lowest burning rate. This change law is the opposite of thermal conductivity. Due to the existence of gaps between the raw material particles of thermite spinning, overall thermal conductivity is reduced. However, the particle size of nanoparticles is much smaller than microparticles, and nanoparticles have a higher specific surface area. The pore volume generated by the accumulation of nanoparticles is greater than microparticles. Therefore, when the particle size of the raw materials used in Al/MoO3 thermite spinning is smaller, the increase in its specific surface area will increase the contact area between the oxidant and reducing agent particles in the thermite, which will promote the rapid acceleration of the thermite reaction. At the same time, the reduction in particle size will increase the void fraction of the system such that a small part of the high-temperature thermite particle steam produced by the reaction acts on thermite spinning at the back end of the reaction, which promotes the decomposition reaction of NC and penetrates the thermite, thus accelerating heat transfer and heat convection in the system. The simultaneous action of the heat transfer and heat convection can significantly increase the burning rate of Al/MoO3 spinning.
The burning rate of Al/MoO3 thermite spinning for micron, submicron, and nanometer significantly increased to about 6.3, 7.1, and 6.8 times when NFG was added, but the standard deviation of the burning rate for nAl/MoO3/NFG is relatively large due to unstable combustion and increased burning rate; that is, the burning process has a large fluctuation. The burning rate of Al/MoO3 increased to about 19.3, 29.3, and 16.8 times by adding CNT. After adding RGO, the burning rate is more significantly improved than adding NFG and CNT. Compared with the three particle sizes of Al/MoO3 thermite spinning, the combustion rate of Al/MoO3/RGO increased to about 36.6, 45.3, and 26.2 times in the order of a micron, submicron, and nanometer. It can be observed that the addition of RGO can significantly improve the burning rate of Al/MoO3 spinning, especially the burning rate of nAl/MoO3. In short, the addition of nanocarbon materials can significantly increase the combustion rate of the thermite. The addition of RGO improves its combustion rate the most, followed by CNT, and NFG is the lowest. In the combustion process of thermite spinning, binder NC reacts preferentially. Under the simultaneous action of external energy stimulation, it promotes the rapid response of thermite particles in spinning. The nanocarbon material undergoes oxidation reaction under the action of hot thermite particles to generate high temperature and pressure gas products and penetrates the unreacted porous thermite spinning condensed phase interface to promote heat convection and mass transfer processes in thermite spinning. In turn, it initiates the decomposition of NC and the rapid redox reactions between the thermite particles in frontend thermite spinning. Therefore, the addition of nanocarbon materials can significantly increase the burning speed of thermite spinning.
It can be observed from the above analysis that nAl/MoO
3/RGO has the highest burning rate. To further explore the microscopic change law of the burning rate of thermite spinning with RGO, the combustion process and burning rate change law of nAl/MoO
3/RGO thermite spinning were specifically analyzed.
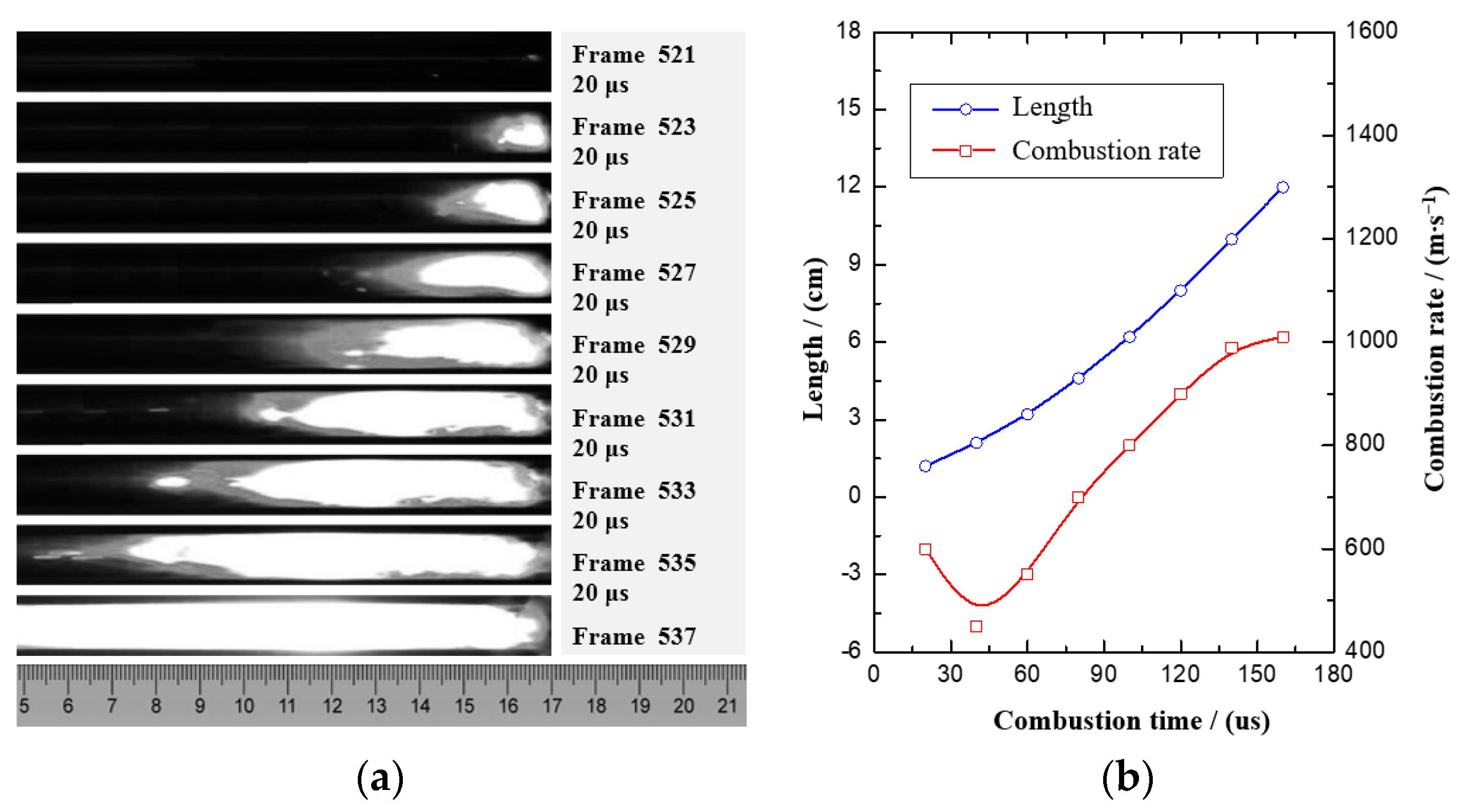
Figure 7 shows the combustion process and the graph of the combustion rate of nAl/MoO
3/RGO. The average burning rate of nAl/MoO
3/RGO thermite spinning under the weak restriction of the PMMA tube is 736 m·s
−1, and the highest burning rate is 1100 m·s
−1. Certain fluctuations at the front of the combustion flame are mainly due to the higher porosity of the spinning structure and the higher burning rate. It takes about 160 μs for the combustion wave of nAl/MoO
3/RGO thermite spinning to propagate 120 mm in the PMMA tube. At the beginning of combustion, the combustion wave has an initial step of about 20 μs, and then it undergoes a rapid rise phase of about 100 μs. The combustion rate stabilizes after the final combustion wave travels about 100 mm and maintains the highest combustion rate. The change in the burning rate indicates that nAl/MoO
3/RGO thermite spinning can quickly reach a combustion equilibrium state after starting to burn for about 140 μs under external energy stimulation.
3.5. Electrothermal Ignition Characteristics
The semiconductor bridge pyrotechnic device has high safety, fast response characteristics, low ignition energy, high reliability, and high ignition consistency. It is considered to be a revolutionary intelligent ignition device that can be combined with microelectronic circuits and digital logic. The circuit is compatible and has been successfully used in microelectromechanical systems (MEMS). In this experiment, the ignition characteristics of Al/MoO
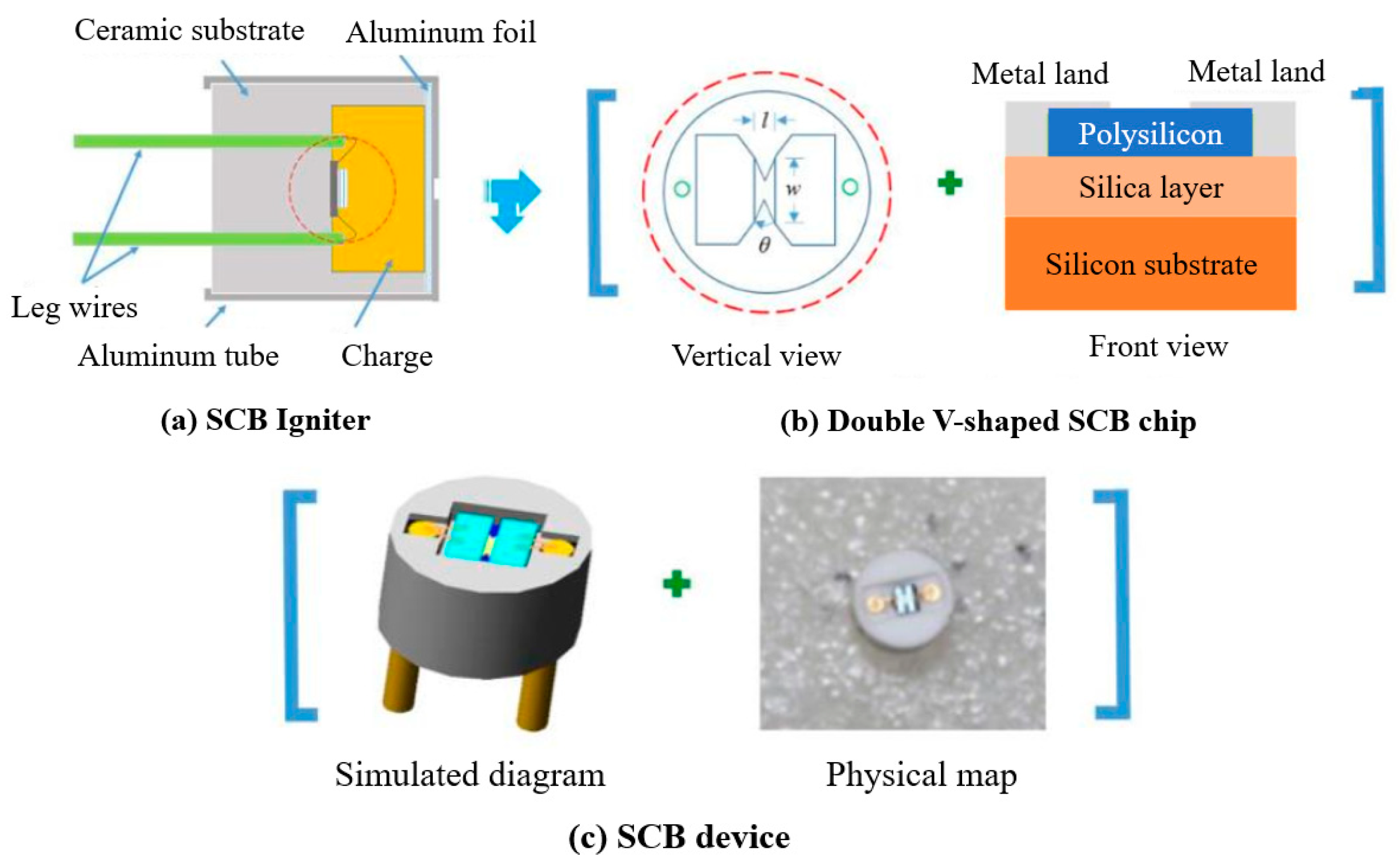
3/RGO were evaluated by semiconductor bridge ignition parts. The characteristics of the two semiconductor bridge transducer models (D1 and D2) used in the experiment are the length of the bridge area (l), the width of the bridge area (w), the number of V-shaped angles (θ), the area of the ignition area (A), and resistance (Ω) and other parameter descriptions, as shown in
Table 5. In addition, the thickness of the bridge region of these two types of semiconductor bridges is 2 μm, and the substrate is made of ceramic material. The diameter of D1 is 4.4 mm and that of D2 is 6.0 mm. Al/MoO
3/RGO thermite is uniformly packed at the bottom of the aluminum shell by pressing, and then the SCB transducer is placed. The aluminum shell, medicine, and SCB device are packaged into micro–nano thermite-charged SCB-fired parts. The pressing pressure is 7 MPa, the charge volume is 50 mg, and the charge density is 2.63 g·cm
−3. The structure of the semiconductor bridge ignition component is shown in
Figure 8.
Under the excitation of capacitor discharge, the critical ignition voltage of the semiconductor bridge ignition element containing the micro–nano thermite fiber and the film charge was tested. In order to compare the electrothermal pyrophoric performance of micro–nano thermite with the commonly used pyrophoric agents of electrothermal transducers, two types of SCB transducers were charged with neutral lead styphnate (N-LS) and combined charge (N-LS/LA) of N-LS and lead azide (LA). The results are shown in
Table 6. The ignition of the ignition element assembled by the D1 semiconductor bridge transducer is that the high temperature and pressure metal vapor generated by the explosion of the semiconductor bridge penetrate the agent and can cause the agent to ignite. This mechanism is defined as the electric explosion fire mechanism. The ignition of the ignition parts assembled from the D2 semiconductor bridge converter is caused by the semiconductor bridge heating agent, which increases the agent temperature to its ignition point and fire. This mechanism is defined as an electrothermal ignition mechanism.
It can be observed from
Table 6 that the critical ignition voltage of the D2 semiconductor bridge transducer is much larger than D1; that is, D2 requires higher energy stimulation than D1 under the excitation of capacitor discharge. The reason is that the latter has a fire zone area much larger than the former, which can disperse energy on the entire planet and requires a larger voltage to cause it to fuse or explode. For the discharge capacitor excitation and electrothermal ignition mechanism, the critical ignition voltages of the semiconductor bridge ignition components D2-nAl/MoO
3/RGO (fiber) and D2- nAl/MoO
3/RGO (membrane) are higher than the semiconductor bridge ignition components containing N-LS/LA charge, indicating that the required fire stimulation energy of the nAl/MoO
3/RGO fiber and nAl/MoO
3/RGO membrane is higher than that of N-LS/LA charge, and the required fire stimulation energy of nAl/MoO
3/RGO fiber is slightly higher than that of N-LS/LA charge. The critical ignition voltage of the three types of semiconductor bridge ignition element is lower than the critical voltage for the explosion or fusing of the semiconductor bridge transducer; that is, the semiconductor bridge transducer element will ignite without fuse. It fully shows that the three types of semiconductor bridge ignition components belong to the electrothermal ignition mechanism. By conducting the critical ignition voltage test, it can be observed that the ignition sensitivity of the agent can be adjusted by changing the form and composition of the Al/MoO
3/RGO charge and making it compatible with N-LS and N- LS/LA, which can improve its matching performance and use performance on the semiconductor bridge ignition component.
The capacitor discharge experiment was carried out on the above-mentioned semiconductor bridge ignition parts. By examining experimental phenomena and voltage-current-resistance-optical signal (VCRO) curves, the ignition situation of these igniting parts and the match between the micro–nano thermite charge and the semiconductor bridge igniting parts were analyzed. The results are shown in
Figure 9. The charging voltage of the ignition component of the D1-LS semiconductor bridge is 5.69 V, the semiconductor bridge bursts, and the ignition component ignites. The charging voltage of the ignition component of the D2-nAl/MoO
3/RGO (fiber) semiconductor bridge is 16.00 V, the ignition component ignites, but the semiconductor bridge does not burst. In
Figure 9a, the resistance value of the ignition element of the D1-LS abruptly changes to infinity at point t
3. At this time, the semiconductor bridge is transformed into high-heat steam or plasma after the explosion, and then the latter penetrates the charge and ignites the agent at the same time, which conforms to the characteristics of the electric explosion fire mechanism. In
Figure 9b, the resistance of the D2-nAl/MoO
3/RGO (fiber) semiconductor bridge ignition element has experienced the process of resistance increase and decrease and continues to increase to infinity. Between t
1 and t
2, the resistance of the ignition element decreases to a certain value, which is the resistance value of the ignition element when the polysilicon in the semiconductor bridge chip melts and is in a liquid state. However, the light signal fluctuates around t
1, indicating that the medicament ignites and burns to the end of the igniting part. Therefore, the ignition time of the medicine is prior to t
1, which is in line with the characteristics of the electrothermal ignition mechanism.