1. Introduction
Process emissions are a point-of-departure for subsequent exposure and health effects, thus, the quantification of them is foundational for effective risk control solutions [
1,
2] and communication [
3]. When process emissions are characterized according to process parameters it is possible to optimize the process according to exposure and risk. For example, Wang et al. [
4] showed that, in plasma cutting of stainless steel when arc current was increased from 35 to 42 and 50 A, it increased Cr
6+ emissions by ca. 60%. Such knowledge can be used to optimize the process according to production rate and Cr
6+ emissions.
Exposure modeling tools designed for occupational nano-safety are mainly relying on qualitative control banding exposure assessment tools [
5,
6,
7,
8]. Control banding tools are either based on the emission potential or on the exposure estimates and do not require quantitative concentration levels or emission factors [
9]. This makes them less accurate while providing different hazard and exposure outputs when compared with each other and with experimental data [
10,
11,
12]. Inconsistent results limit their applicability in safety decision making and there is a need for quantitative exposure assessment tools that can effectively address the existing uncertainties in a model’s output accuracy and precision [
12]. Unlike control banding tools, mechanistic exposure models (e.g., [
13,
14]) require quantitative information about exposure determinants where the emission source is the most relevant exposure determinant [
3]. However, nanomaterial’s (NM) process emissions are rarely reported, which limits their use [
15].
Commonly used mechanistic models in worker inhalation exposure assessment are single compartment model (fully mixed concentrations) or a Near-Field/Far-Field (NF/FF) model that simulates a concentration gradient near the source [
16,
17]. The NF/FF model is validated in numerous studies and accepted for regulatory occupational exposure assessment when properly applied [
17]. Abattan et al. [
16] showed that the NF/FF model predictive performance for solvents was within a factor of 0.3–3.7 times the measured concentrations with 93% of the values between 0.5 and 2. Similar results are obtained for various industrial processes [
18]. Functioning models can be used to extrapolate the observed concentrations or exposure levels in one operational condition to another. Such extrapolation is needed when production is scaled up or if operational conditions vary significantly within the company or between different facilities [
3]. Especially in the nanotechnology industry, scaling up the exposure measurements is needed because many of the processes are not yet adopted to full production scale and full-scale exposure measurements may not be feasible to perform.
Recently, Koivisto et al. [
3] demonstrated with pigment and filler pouring in a paint factory how emission source characterization and a probabilistic NF/FF exposure model can be used to answer common chemical safety questions, such as “Is the process sufficiently safe?” and “What are the most efficient risk mitigation method?”. This methodology can be used to quantify safe Conditions of Use (CoU), which describe the operational conditions and risk management measures that are needed to maintain the exposure under well controlled level [
3]. CoU are required in the European Chemicals Agency (ECHA) Chapter R.14 [
19] and it is applied for efficient safety communication between workers and manufacturers [
3]. According to authors knowledge, CoU are not earlier quantified for occupational processes manufacturing or fabricating NMs [
5,
6,
7,
8].
In this study, we demonstrate how the concept by Koivisto et al. [
3] can be used to quantify safe CoU for an industrial nano-coating process performed with air spray guns in a ventilated chamber. Setting CoU requires quantification of process specific emission rates, identification of relevant exposure determinants, identification of relevant reference exposure/dose values, setting Reasonable Worst-Case (RWC) operational conditions, and specifying emission control needs. Here is explained how this assessment can be performed in an occupational environment by analyzing measured work-area concentration levels with a probabilistic mass balance model. The work is two-fold; first emission rates are calculated according to process parameters by predicting similar concentration levels as measured, and then, extrapolating the measured concentrations in RWC conditions where the process times for example corresponds to a full production of an 8-h workday. A safety call and setting CoU can be made by comparing the exposure potential with a reference exposure/dose value [
20,
21]. This method is applicable to any process where emissions are known and when there is a reference value for acceptable exposure/dose level.
2. Methods
This work is based on the measurements reported by Del Secco et al. [
22] where details related to materials, processes, work environment, instrumentation, and concentration measurements are reported. A video from the process is available by ASINA project [
23]. The concentration measurements were used to characterize the process emissions from polymethyl methacrylate (PMMA), and textile substrates coated with titanium dioxide doped with nitrogen (TiO
2N) and silver capped by hydroxyethylcellulose (AgHEC) Nano Particles (NPs) using air spray guns. Spray process is known to have a limited transfer efficiency, i.e., a fraction of sprayed product is released to the air [
15,
24]. Here, we quantified the transfer efficiency for the spraying process that was performed in a ventilated chamber. The fraction of NPs released to spray chamber air are escaped to the process room and further from the room ventilated to outdoor air via local exhaust ventilation (LEV). Emission factors (mg-NP/g-NP; mg of NPs per g of sprayed NPs) were quantified for the coating process by performing measurements in the spray chamber, near the chamber (NF) and in the room (FF). The emission rate from the spray chamber to the room was estimated using a NF/FF model. Then, the model was used to predict how different exposure determinants effect the worker exposure (see exposure determinants form [
3]). CoU were estimated by comparing the predicted 8-h Time Weighted Average (TWA) exposure levels under Reasonable Worst-Case (RWC) conditions with the Recommended Exposure Limit (REL) values for TiO
2 at 300 μg/m
3; [
25]) and Ag at 0.9 μg/m
3; [
26]. Here, the CoU was considered adequate when the 95th percentile of the lognormal distribution of 8-h exposure is below 0.1×REL. According to the human exposure categorization scheme by American Industrial Hygiene Association (AIHA), this limit correspond to exposure category D with “Minimal exposure in the workplace, no effects are expected, based on available information” [
20] or highly controlled exposure rating category [
21].
2.1. Coating Process
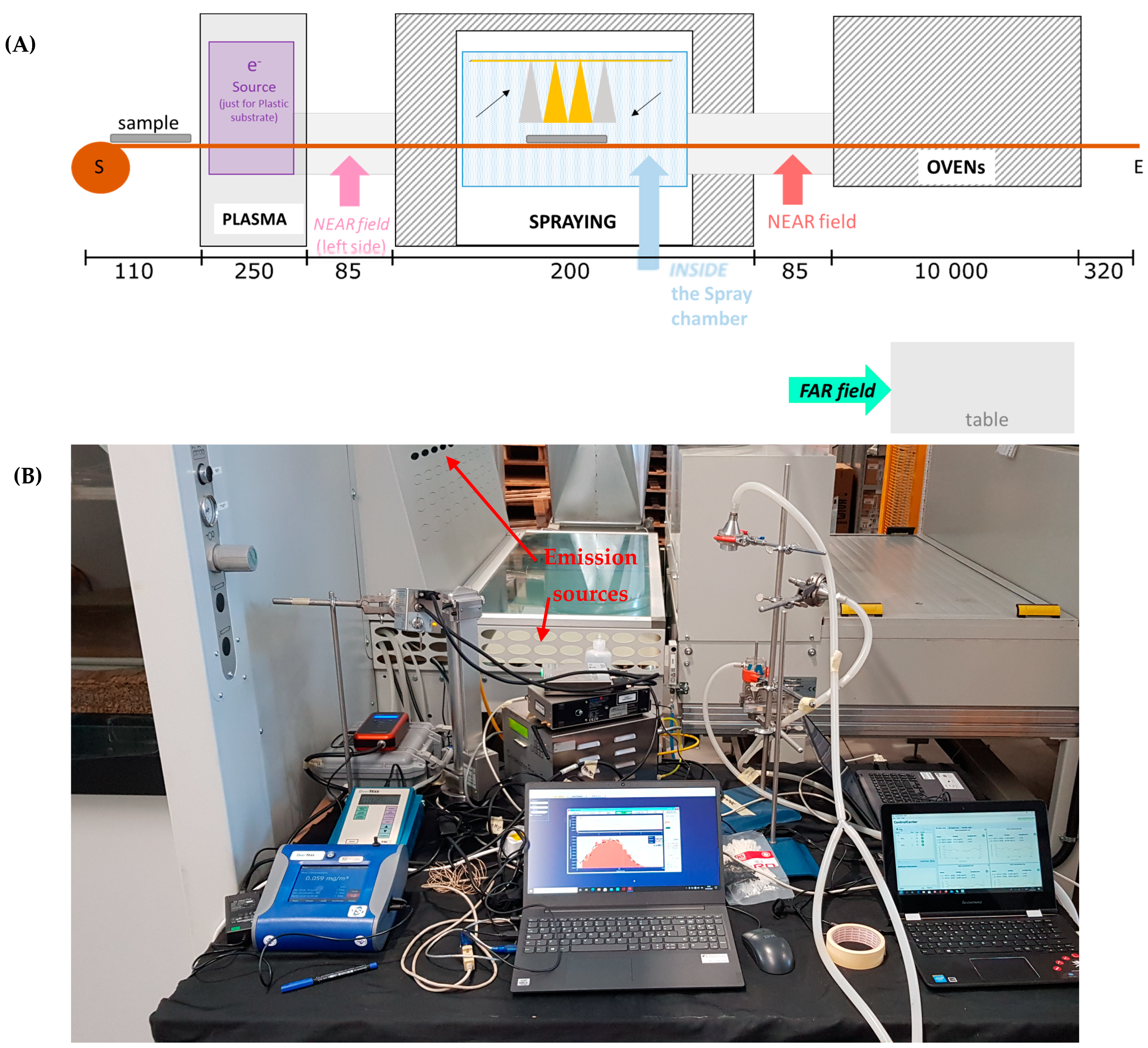
The coating machine (
Figure 1) is conveyor belt operated and transfers the substrate through a plasma neutralizer to the spray chamber and then to a drying oven. The machine is designed for coating up to 120 cm wide textile and plastic substrates. The plasma neutralizer is optionally used to remove surface charge from the substrate. Spraying is performed inside a ventilated chamber. The fully automated sprayer consists of four spray nozzles and is capable of operating with either one, two, or four nozzles simultaneously. The four nozzles are attached to a mobile element that moves horizontally on the substrate, always at the same height. The spray nozzle operates with 270 normal L/min air flow atomizing the coating suspension, which is fed at a flow rate of 200 mL/min per nozzle. After spraying, the substrate is dried in the drying oven at low temperature.
The process parameters that were expected to affect the emissions were investigated:
- (1)
Suspension: ethanol containing 1 wt.% TiO2N and water containing 0.01, 0.05 and 0.1 wt.% AgHEC.
- (2)
Substrate: textile and PMMA for TiO2N and textile for AgHEC.
- (3)
Number of spray nozzles: 1, 2, and 4 nozzles corresponding to coating suspension flow rates of 200, 400, and 800 mL/min, respectively.
2.2. Measurements
Measurements took place in the period 15–18 of February 2021. On 15 February, instruments were set-up and background concentration measurements were carried out with offline particulate matter (PM) samplers. On 16 February, TiO
2N NPs were sprayed both on PMMA and textile substrates, while on 17 February, AgHEC NPs were sprayed on textiles. On 18 February, additional experiments were carried out (data not shown), and instruments were packed off. During the campaign, measurements were obtained at three different positions: NF, FF, and inside the spray chamber (
Figure 1A,B shows NF instrumentation; see also additional photos from [
22]).
Size resolved particle concentrations and mass concentrations were measured at NF and FF at heights from 1 to 1.3 m. Details of the instrumentation is given in [
22]. The real time NF particle measurement position included:
NF particle mobility size distributions (1/cm3) measured by Scanning Mobility Particle Sizer (SMPS) in the range from 10 nm to 1 μm. SMPS scan time was ca 4.5 min with a 1.5 min retrace time.
NF particle optical size distributions (1/cm3) measured by an optical particles counter (OPC) in size range from 0.3 to 30 μm (in 32 channels) with a time resolution of 6 sec.
FF particle optical size distributions (1/cm3) measured by another OPC in size range from 0.3 to 30 μm (in 32 channels) with a time resolution of 6 sec.
All instruments were calibrated by the manufacturer prior to the campaign. OPC NF and FF were also intercompared at CNR-ISAC by sampling in parallel aerosol particles at different concentrations. The OPC FF concentration was 1.14 OPC NF in the particle concentration range from ca. 600 to 1600 1/cm3 with coefficient of determination R2 of 0.962.
OFF-line gravimetric PM samples (total fraction) were taken simultaneously inside the chamber and at NF by collecting particles on filters (PTFE, 1 µm porosity) at 50 L/min flow rate (Bravo H-Plus, TCR Tecora, Cogliate, Italy). Air flow velocities were measured with a hot wire anemometer (Terman ANM-0/B, LSI spa, Milan, Italy).
2.3. Combining the NF SMPS and OPC Particle Size Distribution
The particle size distributions by SMPS and OPC were combined to one d
N/dLog(
Dp) particle number size distribution. The combined particle number size distribution,
NNF (1/cm
3), was based on the mobility size from 0.010 to 0.74 µm and optical size from 0.87 to 8.69 µm. The number count in overlapping size bins of 0.74 and 0.87 μm were approximated as an average number count counted by SMPS and OPC. The correction due to the soft X-Ray (TSI, model 3087) neutralizer instead of the original one
241Am (Grimm Mod. 5522) was applied according to Nicosia et al. [
27]. Logarithmic width of the bins were calculated as 10-based logarithm [
28]. It was assumed that the mobility and optical particle diameters are the same when using the OPC default refractive index of 1.59 + 0
i for PSL particles at 632.8 nm. The sampling diffusion losses of SMPS were corrected according to Cheng [
29].
2.4. Assessment of Test Specific Process Mass Emission Rates from the Spray Chamber to the NF
Test specific mass emission rates from the spray chamber to NF can be estimated from the time resolved NF concentrations measured by SMPS, OPC, and the integrative mass concentration measurement by the gravimetric PM sampler, over all experiments. A combined instrumentation is needed because PM sampler requires long collection times compared to individual tests to reach the limit of quantification. SMPS and OPC measure size resolved number concentrations where the effective density is needed to translate the number to mass concentrations. Mass concentration time series are used to calculate test specific mass concentrations, which further are used to calculate mass emission rates from the spray chamber to NF using a NF/FF model. The assessment consists of following steps:
Specify number concentration levels for SMPS and OPC and mass concentration level for PM sampler.
Calculate process concentrations where background particles are subtracted for SMPS, OPC, and PM sampler.
Calculate effective densities using process particle mass concentrations measured by PM sampler and process particle mass size distributions averaged over PM sampling period.
Calculate test specific process particle number concentration averages measured by the SMPS and OPC in NF, NP,i (1/cm3), where i is from 1 to 12 according to the test number.
Calculate test specific mass concentrations in the NF, mP,i (μg/m3), using Np,i and the effective densities of both NP’s process emission particles.
Develop a model to describe the mass flows in the work environment.
Use the model to calculate the emission rates, G (mg/min), that reproduce the test specific mass concentrations mP,i in the NF.
Use the model to simulate the processes performed during the PM sampling period and compare the measured and simulated concentrations to evaluate the model goodness.
Use the model to identify relevant exposure determinants and quantify CoU.
2.4.1. Process Specific Concentration
Process concentration is solely caused by the process emissions, i.e., the contribution of background concentrations is subtracted. Test specific process concentrations were calculated by subtracting background concentrations from the concentration measured during the test cycle, which in this experiment included four sprayings and four pauses, i.e., averaging time was ca. 40 min per test. Here, the pre-process particle size distribution measured before the spray test was used as a background concentration. The process particle concentrations for test i, NP,i (1/cm3), were calculated separately for NF and FF.
Process mass concentration measured by the PM sampler, mP,PM (μg/m3), was calculated by subtracting the PM sampler background concentration measured on day 1 from the TiO2N and AgHEC coating process concentrations measured during days 2 and 3, respectively. The mP,PM was specified over tests 1–6 for TiO2N experiments and 7–12 for AgHEC experiments.
The background concentration was assumed to be constant when calculating the process particle concentrations.
2.4.2. Effective Densities of TiO2N and AgHEC Process Particles
The particle density was calculated for both NPs process emissions from the respective NF
NP,i and
mP,PM process concentrations. Effective density takes into account particle porosity and shape [
30] and it can be calculated from mass and volume concentrations [
31]. First,
NP,i concentrations were averaged over all tests, converted first to volume size distribution and then to a mass distribution
mP (μg/m
3). The density was adjusted so that the averaged mass concentration over PM sampling period was similar as the
mP,PM mass concentration measured from the NF. The following assumptions were used:
Spherical particles
Constant density across the particle size distribution
Constant background concentration
Homogenous concentration at the SMPS, OPC, and PM sampling locations at NF
Mobility and optical diameters are the same
This method calculates an effective density related to particles mobility size, optical size classification, and mass measured by weighting the collected particles by PM sampler (see e.g., [
32]).
2.4.3. Test Specific Mass Concentrations at the NF
The NF mass concentrations for individual tests, mP,i, were calculated from NP,i process concentrations and NP’s process respective effective densities. It is assumed that the density of emitted particles is fixed.
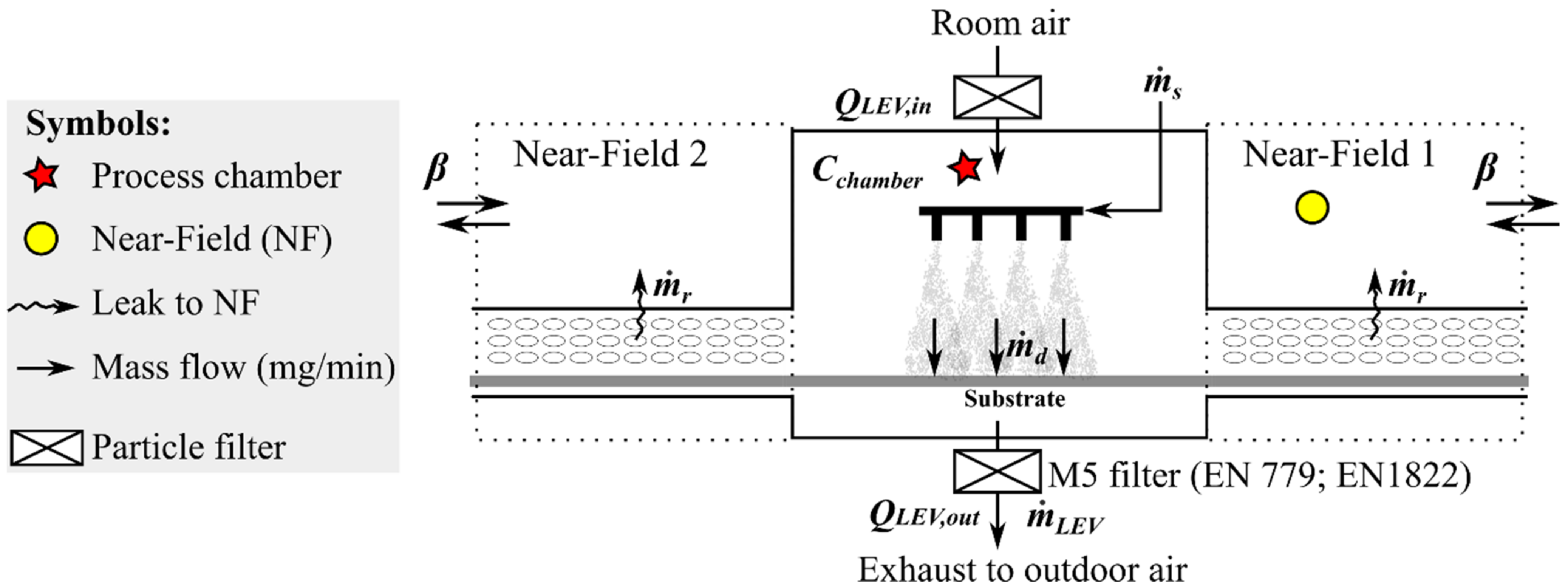
2.4.4. A NF/FF Model for the Spray Chamber
The spray chamber (3.5 × 2.0 × 1.0 m and volume ca. 5.3 m
3) has two openings for a conveyor belt where the substrate is transferred to the spraying area (
Figure 2). The chamber was mechanically ventilated with filtered air extracted from the room,
QLEV,in (m
3/min), at a flow rate of 48.6 m
3/min. The air velocity measured at the spray chamber front screen slit was up to 0.03 m/s (<0.01 m
3/min) and from the conveyor belt screen to the NF was ca. 0.1 m/s (ca. 1.34 m
3/min per NF). The flow direction was not possible to define. The spray chamber ventilation flow rate depends also on the ambient pressure and number of spray nozzles air flows. Thus, the flow from conveyor belt screen to the NF volume was assumed to be in balance.
NPs enter the chamber via atomization of the suspension by the spray nozzles. The NP suspension mass flow,
(mg-NP/min) is calculated from the suspension feed rate,
(mL/min), and NP concentration
CNP (wt.%). Atomized NPs are transferred to the substrate,
(mg-NP/min) and a fraction is released to the chamber air
Cchamber,NP (mg-NP/m
3). The released NPs from the spraying process are mainly removed by LEV and exhausted through a filter to outdoors
(mg-NP/min). However, a small fraction is leaked into NF from the chamber and conveyor belt cover openings
(mg-NP/min). Another fraction is escaped to the pre-heater and oven
(mg-NP/min) and deposited onto chamber surfaces
(mg-NP/min). Assuming small NPs escape and surface deposition, i.e.,
mg-NP/min, the NP mass balance inside the chamber is:
The particles escaping are assumed to be fully mixed with NF volumes, VNF (m3) in NF1 (spray chamber exit with aerosol instrumentation) and NF2 (spray chamber entrance). A fraction of NF concentrations, CNF, (μg/m3), enters back to the chamber via QNF,in, which is assumed to be insignificant if the spray chamber is QLEV,in = QLEV,out. A fraction escapes to FF by an air mixing between NF and FF, β (m3/min). Due to NF’ symmetry in entrance and exit of the spray chamber, the NF properties are assumed to be the same.
Assuming that
CFF CNF, the NF concentration can be estimated with a NF/FF model (see Model 205-2Box.CE.Gv in [
33]. Modellings were performed using a probabilistic NF/FF model (TEAS, Exposure Assessment Solutions, Inc., Morgantown, MI, USA).
Table 1 shows the common parameters for the spray chamber model NF1 that is assumed to be symmetric with NF2 (
Figure 2) and was used to calculate particle emissions from the spray chamber to the NF1 by using the task specific NF mass concentrations.
2.4.5. Transfer Efficiency and Emission Factors
A transfer efficiency,
Peff (-), describes the fraction that is deposited onto the substrate during spraying. Assuming fully mixed concentrations inside the spray chamber the transfer efficiency is:
The environmental emission of NPs to outdoor air via LEV exhaust,
(mg-NP/min), can be calculated using the suspension feed rate, transfer efficiency and exhaust air filter filtration efficiency,
(-), as:
According to EN 779:2012 [
36] and EN 1822:2019 [
37] standards a M5 type filter has an efficiency at 0.4 μm between 40% and 60%.
Emission factors were defined for NF,
EFNF, NP (mg-NP/g-NP), and LEV
EFLEV, NP (mg-NP/g-NP) as the ratio of the NP release and use as:
Here, the LEV filter filtration efficiency is not included in LEV emission.
2.4.6. Particle Emission Rates
Spray process particle emission rates from the chamber to NF were quantified by setting the emission rate so that the modelled mass concentration during the individual spray test was similar to the respective measured NF mass concentration. The modellings were performed using the spray chamber model,
Table 1 parameters, and the
mP,i mass concentrations. The method has the following assumptions:
A NF/FF model describes the dispersion of the emitted particles, i.e.,:
- ○
All mass entering the model volume is created at a source inside the NF volume or from incoming ventilation air;
- ○
Particles are fully mixed at all times in NF and FF;
- ○
There is limited air exchange between NF and FF volumes;
- ○
There are no other particle losses from the room air than the FF ventilation.
Background particle concentration is constant.
Particle properties are independent of number of spray nozzles and substrate.
FF concentration is significantly lower than NF during the process, i.e., NF2 concentration has an insignificant effect on NF1 concentration.
Particle emissions from the spray chamber to NF2 are assumed to be the same as in NF1.
2.5. Setting Conditions of Use (CoU) for the Spray Process
The CoU was evaluated using emission rates and by simulating the exposure levels under different operational conditions [
3]. Effects of the general ventilation, room volume, and air mixing between NF and FF were studied. The effect of LEV was modelled using Model 205-2Box.CE.Gv described by Ganser and Hewett [
33]. Setting CoU requires exposure limit values, which are currently not available for NPs. Thus, the evaluation was performed using REL values that may not reflect national regulatory occupational exposure limit values based on the materials CAS number.
2.6. Worker Airway Deposition Model
The airway deposition (in head, tracheobronchial, and pulmonary region) for workers in the NF conditions for the 12 spray tests was estimated by the Multi Path Particle Deposition Model (MPPD V3.04; [
38]). The combined size distribution from SMPS and OPC (d
N/dLog(
Dp)) and the effective densities were used as inputs for the model. Additionally, the following parameters were defined in the model:
The human Yeh/Schum Symmetric lung model [
39].
Breathing parameters for adult males were selected to reflect moderate worker activity: 3300 mL for functional residual capacity, 50 mL for extrathoracic volume, 20 breaths/minute for breathing frequency, and 1100 mL for tidal volume. This corresponds to an inhalation rate of 1.36 m
3/h, which is between light (0.6 m
3/h) to moderate (1.7 m
3/h) activity for a 81 kg male between the ages 35 and 64 [
40].
A polydisperse particle distribution for particle diameter as CMD (count median distribution) and the calculated GSD.
Aspect ratio was set to “1” (assuming spherical particles).
The oronasal (normal augmenter) assuming most humans combine nose and mouth breathing.
The “inhalability adjustment” was “off” given that this is only relevant for particle sizes larger than about 8 μm for humans.
Up-right body orientation was selected.
Test specific mass concentrations, mP,i, as input for “Aerosol concentration”.
Constant exposure conditions and default exposure values (i.e., for acceleration of gravity, breathing frequency, tidal volume, inspiration fraction, and pause fraction).
Deposited dose of TiO
2N NPs was compared with the no significant dose level of 300 µg/day for particle overload, chronic inflammation, and cell proliferation and with the no significant dose level of 44 µg/day for tumor incidence [
41].
3. Results
In total, 12 spray tests were conducted. A single test cycle consisted of four spraying events where a single spray includes spraying from 1.5 to 3 min and ca. 8-min pause. Between the tests, a 14–28 min break occurs to prepare for a new test. The overall cycle including a break is ca. 60 min. A single test cycle is demonstrated in
Figure 3, and the individual spray times are presented in
Table S1, Supplementary Material S2.
Figure S1, Supplementary Material S2, shows an example how the measurement cycle in Test 1 is related to the measurements. The experimental variables for each test are shown in
Table 2 and the experiment duration defined as the PM sampler sampling duration and spraying times are shown in
Table S1, Supplementary Material S2.
3.1. PM Sampler Mass Concentrations
Background concentration measured on day 1 was 28 ± 3 and 19 ± 2 μg/m
3 at NF and inside spray chamber, respectively, when measured without chamber ventilation (
Table S2, Supplementary Material S2). According to Inductively Coupled Plasma - Optical Emission Spectrometer (ICP-OES) analysis, background concentration did not contain Ti or Ag at detectable quantities.
The concentration during TiO
2N spraying processes on day 2 was 120 ± 2 and 1217 ± 1 μg/m
3 at NF and inside the spray chamber, respectively (
Table S2, Supplementary Material S2). Process specific concentrations with subtracted background concentrations were 92 and 1180 μg/m
3, respectively. TiO
2 concentration calculated from Ti concentration and Ti and O elemental masses was 40.9 and 776.7 TiO
2-μg/m
3, respectively, indicating that 44% and 65% of the NF and chamber concentrations was TiO
2, respectively. Note that nitrate doping is not included in the mass concentrations.
The concentration during AgHEC spraying processes on day 3 was 65 ± 2 and 191 ± 2 μg/m
3 at NF and inside the spray chamber, respectively (
Table S2, Supplementary Material S2). Process specific concentrations with subtracted background concentrations were 37 and 178 μg/m
3, respectively. Ag concentration was 0.4 and 13.2 Ag-μg/m
3, respectively, indicating that 1.1% and 8% of the NF and chamber concentrations was Ag, respectively. Note that HEC doping is not included in the mass concentrations.
3.2. Particle Number Concentrations
Figure 4 shows an overview of the temporal trend of the particle number concentrations measured by OPCs in NF and FF for TiO
2N and AgHEC spray tests. The background concentration was between 250 and 500 1/cm
3 on day 1 and ca. 100 1/cm
3 on day 2 until ca. 2:00 PM. The background concentration increased to ca. 300 1/cm
3 between 2:00 and 3:30 PM. This was caused by a combustion engine exhaust outside the warehouse. Ultrafine particles released from the combustion engine are not expected to significantly affect the background mass concentration.
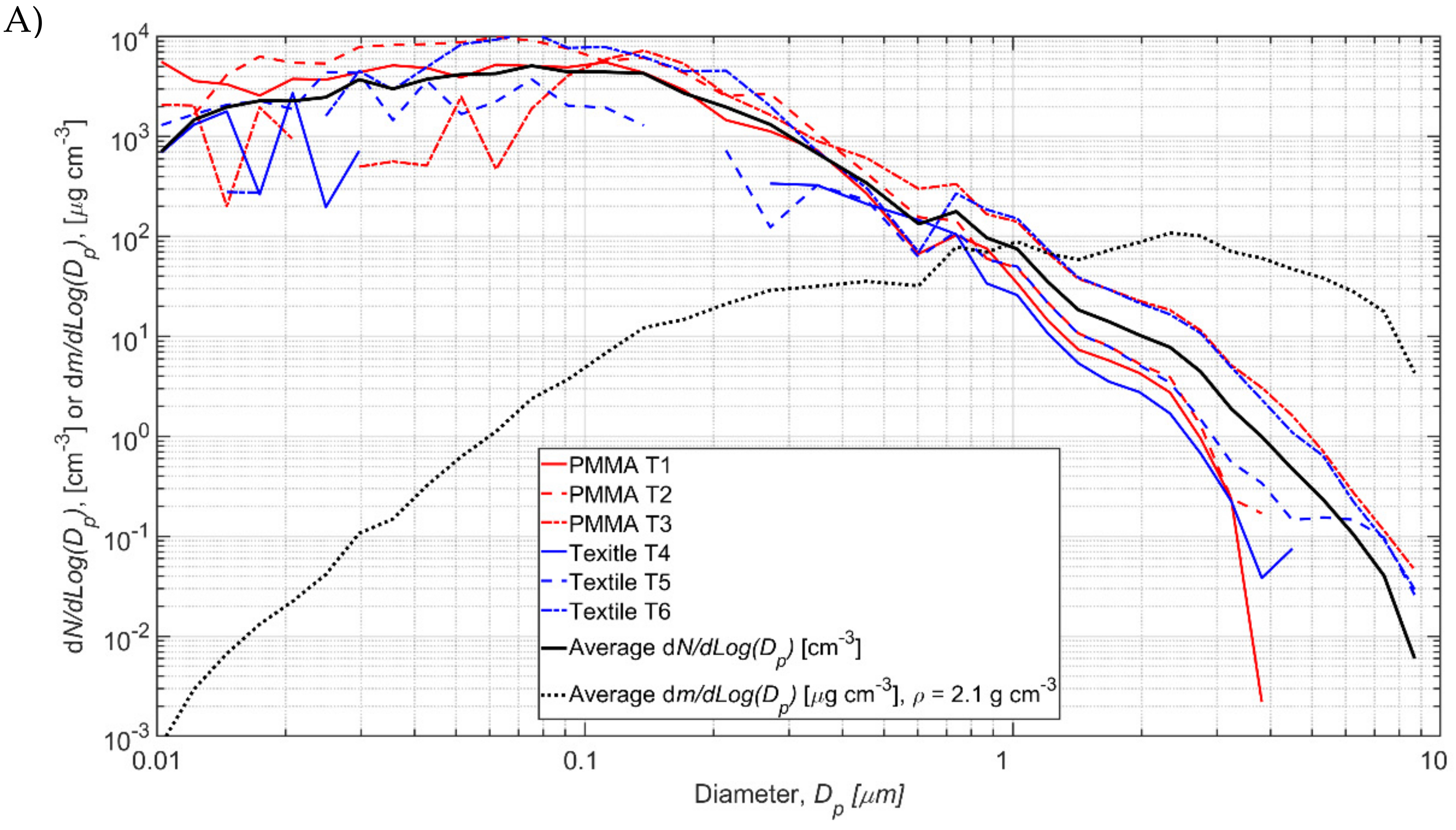
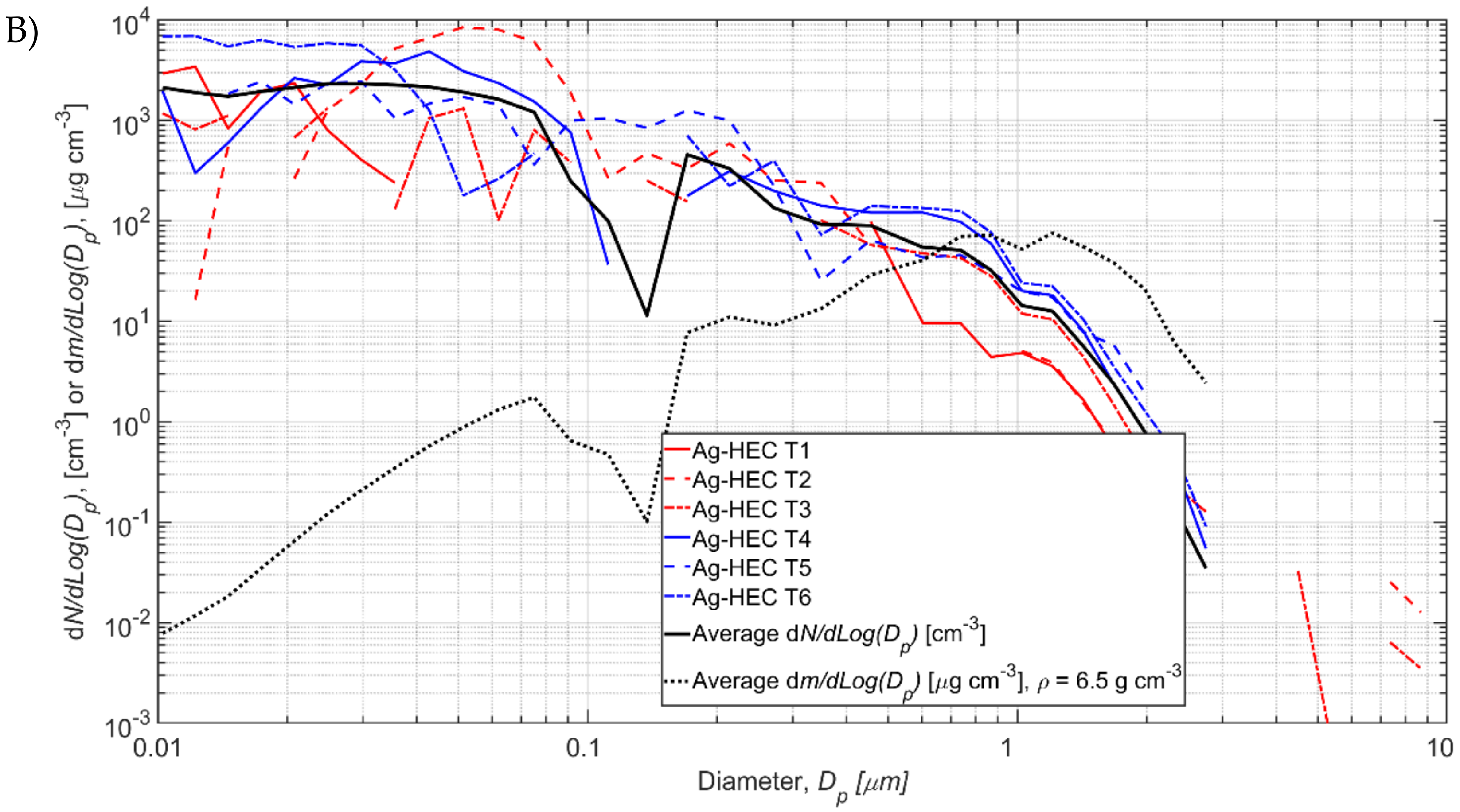
Test specific process particle number size distributions
NP,i are shown in
Figure 5 and the values are given in
Tables S3.1 and S3.2, Supplementary Material S2. The background concentration is occasionally higher than the measured during the process resulting in negative concentration values in some size channels (
Figure 5). Total process number concentrations varied from 516 to 8371 1/cm
3 for TiO
2N tests and from 38 to 3708 1/cm
3 for AgHEC tests (
Table 2). The process number concentration
NP averaged over TiO
2 tests 1–6 was 4740 1/cm
3 and for AgHEC averaged over tests 7–12 was 2020 1/cm
3.
The FF particle number concentrations increased slightly during some spraying tests (
Figure 5). The FF particle concentrations and size distributions measured by the OPC are shown in
Tables S4.1 and S4.2, and Figures S4.1–S4.2, Supplementary Material S2. Total process number concentrations in FF varied from −9 to 81 1/cm
3 for TiO
2N tests and from −4 to 58 1/cm
3 for AgHEC tests. The FF total mass concentrations were mainly negative, which was caused by the higher background particle concentrations in the size larger than ca. 2 μm (
Tables S4.1 and S4.2, Supplementary Material S2). The physical interpretation of negative concentrations is that the background concentration was not constant, and the process concentration was below limit of quantification (not specified).
3.3. TiO2N and AgHEC Process Particles Effective Densities
TiO
2N tests 1–6 and AgHEC tests 7–12 average
NP,i concentrations are shown with black solid lines in
Figure 5A and
Figure 5B, respectively. Those were used to calculate densities. The average mass concentration calculated from SMPS and OPS measurements is 92.4 μg/m
3 for TiO
2N experiments 1–6 at density of 2.1 g/cm
3 and 36.9 μg/m
3 for AgHEC experiments 7–12 at density of 6.5 g/cm
3. The calculated average mass concentrations,
mp, are shown in
Figure 5 (black dashed lines). The elemental densities of TiO
2, Ag, and HEC are 4.23, 10.49, and ca. 0.8 g/cm
3, respectively. Composition and density of the other process emission particles were not evaluated.
3.4. Task Specific Mass Concentrations
Task specific mass concentrations,
mp,i, were calculated from task specific average number distributions (
Table S3.1, Supplementary Material S2) using TiO
2N and AgHEC process particle effective densities. The NF mass concentrations varied in TiO
2N tests 1–6 from 25.1 to 202.6 μg/m
3 and in AgHEC tests 7–12 from −4.9 to 71.3 μg/m
3 (
Table 2 and
Table S3.2, Supplementary Material S2). The negative mass concentration (test 7) means background concentration was higher than the concentration measured during the process.
3.5. Emission Rates
Modelled task specific mass concentrations were simulated using the NF/FF model. Parametrization of the work environment (
Table 1) was the same for all simulations and the task specific process times are given in
Table S5.1, Supplementary Material S2. Because the PM sampler is an integrative sampler it cannot distinguish the individual breaks between the tests and the break before and after the first and last spray test. Thus, the sum of the break times, 111 min for TiO
2N experiments and 115 min for AgHEC experiments, were divided equally with the individual tests, resulting to 18.5 min break for a TiO
2N test and 19 min break for an AgHEC test.
Individual simulation results and deviation from the
mP,i concentrations are shown in
Table S5.2, Supplementary Material S2, and an example for test 1 is shown in
Figure 3. The ratio of the measured mass concentration
mP,i and the respective modelled mass concentration varied in TiO
2N tests 1–6 from 97% to 100% and in AgHEC tests 7–12 from 94% to 102%. The emission rates varied from 0.9 to 5.9 mg/min in TiO
2N and from 0.8 to 2.6 mg/min for AgHEC experiments (
Table 2).
3.6. Model Comparison with the Measurements
The experiments were simulated 10,000 times using
Table 1 parametrization with the mass emission rates quantified for individual tasks as total process emissions and NPs (
Table 2) and the testing times presented in
Table S1, Supplementary Material S2. The number of simulations was selected so that the results variation was insignificant when simulation was repeated by the same scenario. Task 7 emission rate was set to 0 mg/min. Simulation time was the same as the PM sampler sampling time.
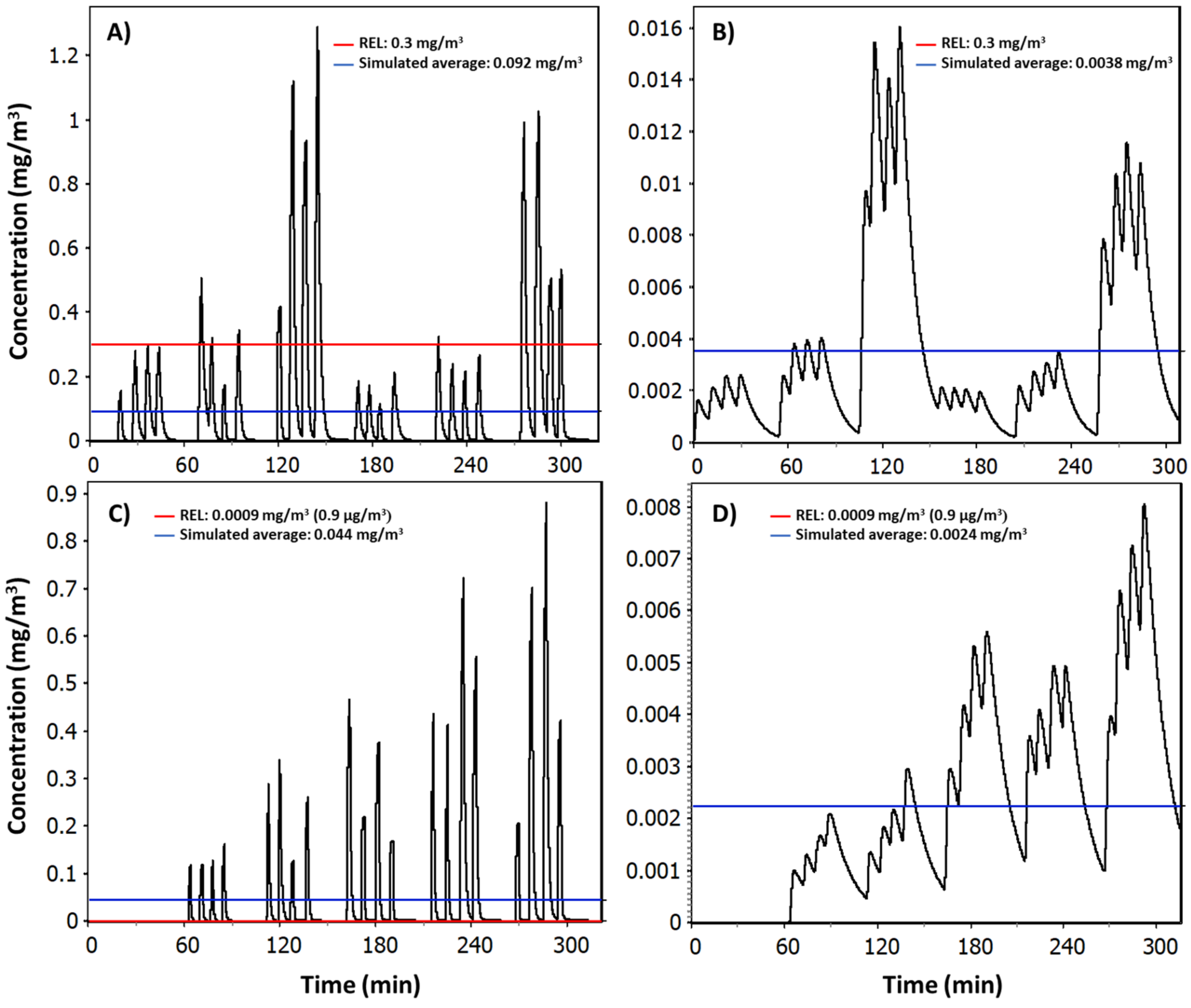
Reports are given in
Texts S3–S6, Supplementary Material S1, showing individual model parametrization, exposure distributions, exposure statistics, and job sensitivity analyses for NF and FF concentrations. Simulated random day mass concentration time series in the NF (
Figure 6) reproduced similar concentration peaks as measured particle number concentrations shown in
Figure 4 for TiO
2N and AgHEC experiments, except test 7. The measured geometric mean NF concentration for TiO
2N experiment was 94 μg/m
3 (GSD 1.111) and for AgHEC experiment was 39 μg/m
3 (GSD 1.106). Emission rates were adjusted to reproduce test specific mass concentrations (
Table S5.1, Supplementary Material S2). Test specific emission rates were used to reproduce the full day of NF measurements (
Texts S3 and S5, Supplementary Material S1). The ratio of the measured mass concentration
mP,PM and the respective modelled mass concentration was 98% for the TiO
2N experiment and 94% for the AgHEC experiment, i.e., the modelled concentration was slightly overestimated in both cases.
FF concentration is caused by mixing concentrations from NF to FF. The average geometric mean FF concentration caused by NF1 was 4 μg/m3 (GSD 1.452) for TiO2N experiment and 1 μg/m3 (GSD 1.444) for AgHEC experiment. Due to the spray chamber symmetry, it is assumed that the entrance increases the FF concentration in a similar amount, while the FF concentration is doubled. This leads to average FF concentrations of 8 and 2 μg/m3 for TiO2N and AgHEC experiments, respectively. It is good to note that this assumption violates mass balance principles, as noted earlier. However, the effect is not significant. For example, if the whole spray chamber would be enclosed with NF volume having a triangular 3, 6, and 16 m3 as minimum, mode and maximum volumes and emissions from both sides would be the same (i.e., the generation rate to the NF volume would be doubled), the average FF concentration in TiO2N experiments would be 8 μg/m3. Thus, the assumption to double the FF concentration when calculated from single spray chamber side emissions is reasonable.
Mixing of concentrations in the NF volume is one critical uncertainty factor, i.e., how homogenously the concentrations are dispersed in the NF. This can be measured using multiple on-line measurements, e.g., diffusion chargers. Another critical uncertainty factor is the flow balance between the spray chamber and NF. It was not known if the chamber was under negative or positive pressure compared to NF. Assuming that the operational conditions can lead to a flow from the spray chamber to the NF, the emission rate increases according to the chamber concentration and volume flow rate. Here, the volume flow rate was below 1.34 m3/min and the average chamber concentrations were 1180 and 178 μg/m3, respectively, for TiO2N and AgHEC processes. On average, this would correspond to a total emission rate of 1.6 and 0.24 mg/min, respectively, for TiO2N and AgHEC processes, or, respectively, 60% and 16% of the average emission rate. It is also unclear if emissions from the spray chamber entrance to NF2 can impact on the NF1 concentrations at significant level.
Based on a qualitative emission rate uncertainty assessment, a reasonable range for the emission rate was set as a linear distribution, where the lower limit is the quantified emission rate and the upper limit is twice the emission rate, i.e., ≤ G ≤ . This represents a precautionary parametrization to avoid exposure underestimation in NF and FF.
3.7. Transfer Efficiency and Environmental Emissions
Sprayed suspension volumes, mass flows of TiO
2N and AgHEC NPs to LEV exhaust and room air, calculated transfer efficiencies and emission factors to room and environment are given in
Table S6, Supplementary Material S2. The chamber particle number and mass concentrations were too high for optical counting due to particles coincidence counting (data not shown; [
42]), which is the reason why the task specific mass flows and transfer efficiencies cannot be specified. Assuming that the concentrations are fully mixed inside the spray chamber, the mass flow of NPs by LEV exhaust was on average 37.3 mg-TiO
2/min and 0.7 mg-Ag/min when calculated from the LEV volume flow and the spray chamber mass concentration measured by the PM sampler. According to Equation (2), the transfer efficiencies were on average 97.6% and 98.9% for TiO
2N and AgHEC spraying processes, respectively. Here, the transfer efficiency includes chamber wall deposit, which was assumed to be insignificant.
3.8. Quantifying CoU
Current REL values given as respirable fraction are 300 for TiO
2 [
25] and 0.9 μg/m
3 for Ag [
26] as an 8-h TWA. The predicted exposure risk is considered highly-controlled when a 95th percentile of the exposure concentration distribution is below 10% of the occupational exposure limit value [
21,
43]. Here, the REL values were used because legally binding exposure limit values do not exist for nanosized TiO
2 or Ag particles.
The worker is rarely at the NF because the process is automated and controlled from FF. Under RWC conditions, the worker is expected to spend, during the 8-h work shift, 10% at the NF and 90% at FF. It is assumed that the RWC process time in the 8-h work shift is 6-h, divided into two 3-h continuous spray events. The RWC exposure in this worker time use is called weighted exposure. Precautionary emission rates were used assuming linear distribution between ≤ G ≤ , i.e., the upper limit for the emission rate is twice the emission rate from the spray chamber to the NF.
Under experimental conditions (
Table 2), the NF NP concentrations were up to 80 TiO
2-μg/m
3 and 0.78 Ag-μg/m
3 when assuming that 44% and 1.1% of the total concentrations contain TiO
2 and Ag, respectively.
Under RWC conditions, the NF 95th percentile NP concentrations were up to 924 TiO
2-μg/m
3 and 10.6 Ag-μg/m
3, which is ca. 12 and 14 times higher than during the experiments (
Table 3 and
Table S7.1, Supplementary Material S2). Under RWC conditions, the weighted 95th percentile exposure levels range from 26 to 171 TiO
2-μg/m
3 and from 0.6 to 1.9 Ag-μg/m
3 during an 8-h work shift process.
Under experimental conditions, the NF NP concentrations were up to 27% and 78% of the respective REL for TiO
2 and Ag, respectively. Under RWC conditions, the NF NP concentrations exceeded up to ca. 3 and 10 times the RELs for TiO
2 and Ag, respectively. The RWC weighted exposure was up to ca. 0.6 and 1.9 times the REL for TiO
2 and Ag, respectively. Note that the RWC concentrations were simulated using the total fractions while REL values are given as respirable fractions. Here, the mass fraction of particles below 4 μm was ca. 90% from the measured particle mass size distribution (
Figure 5A).
3.8.1. Sensitivity Analysis
Test 12 RWC conditions were varied to see the effect of general ventilation, room volume, NF volume, and the air mixing flow rate between NF and FF (
β) to the NF and FF concentration levels and the weighted exposure (
Table S7.2, Supplementary Material S2). Halving the general ventilation rate, room volume or NF volume increases the NF concentration only up to 5%. However, halving the general ventilation rate or room volume, doubles the FF concentration, thus increasing the weighted exposure by 40%. Halving the
β, doubles the NF concentration but the FF concentration remains at similar level, increasing the weighted exposure by factor of 1.5. Under RWC conditions, where room volume, NF volume, general ventilation, and
β are halved (
Text S7, Supplementary Material S1), the NF and FF concentrations increase by a factor of 2.0 times and 4.0, respectively, which increases weighted exposure by a factor of 2.9.
3.8.2. CoU under RWC Conditions
To meet the condition of 0.1 times REL value of the 95th percentile of the exposure distribution for highly controlled exposure [
21], the exposure under RWC conditions should be reduced by a factor of 19 times to achieve compliance with AgHEC coating process. If LEV exhaust flow rate increased by 20 m
3/min, it would extract the replacement air from the spray chamber inlet and exit at 10 m
3/min each. This would act as an LEV for the NF. Assuming that NF LEV would reduce the emissions from the spray chamber to room by 80%, the NF and FF 95th percentile concentrations under test 12 continuous spraying process would be 0.56 and 0.032 μg-Ag/m
3, respectively (
Table S7.3, Supplementary Material S2). The weighted 95th percentile exposure would be 0.084 μg-Ag/m
3, which is below 0.1 time the Ag-REL value. Additional measurements should be conducted to ensure 80% reduction in emissions to the room.
3.9. Process Parameters Effect on the NF Concentration and Emissions
3.10. Modeled Deposited Dose in Respiratory Tract in NF Conditions
Deposition fractions and dose rates during inhalation were calculated for each test (
Table S9.1, Supplementary Material S2). Total average deposition fraction was 0.30 (0.08) for TiO
2N experiments and 0.44 (0.14) for AgHEC experiments, where brackets show standard deviation. The deposited dose rates were 0.20 (0.15) for TiO
2N and 0.16 (0.12) μg/min for AgHEC experiments. In TiO
2N experiments, the particles were deposited 10% (6%) in head airways, 7.6% (0.6%) tracheo-bronchial, and 13% (1.5%) pulmonary regions. In AgHEC experiments, the particles were deposited 17% (15%) in head airways, 10% (1%) tracheo-bronchial, and 16% (2%) pulmonary regions. For the same particles, the variation in deposition fractions between the experiments was caused by the differences in the particle size values approximated using a log-Normal distribution. Deposition rates were calculated for individual tests, which showed also variation between the tests.
Under RWC conditions, the total weighted deposited mass (µg/day) in each of the airway regions was also calculated for each test. (
Table S9.2, Supplementary Material S2). Deposited mass in the pulmonary region was 9.0 (µg/day) (7.3) for TiO
2N experiments and 6.1 (µg/day) (2.7) for the AgHEC experiments.
4. Discussion
Setting CoU for an industrial process relies on regulatory limit values such as occupational exposure limit values, which are not available for NMs. Current proposed occupational exposure levels vary for nano-TiO
2 from 0.8 to 5000 μg/m
3 and for nano-Ag from 0.098 to 10 μg/m
3 when given in different size fractions and specified under different experimental conditions [
44,
45,
46]. In this exercise, it was decided to use the NIOSH’s RELs because they are well recognized and provide confidence for use. Since there are no legally binding limit values for NMs, it is not possible to specify regulatory accepted CoU for this process.
Understanding environmental and occupational risks associated to the spray coating process requires quantification of exposure determinants such as transfer efficiency, and emission rates from chamber to the room and outdoors via LEV system. Understanding workers’ exposure and risk requires additional information about the dispersion of particles in work atmosphere and worker exposure times. This can be obtained by simulating exposures in RWC exposure scenarios or under realistic conditions when the exposure determinants and process specific emissions are known.
In the framework of the Directive 98/24/EC on chemical agents at work, worker exposure by inhalation is traditionally quantified by measuring personal breathing zone concentration across the work shift [
31]. However, it is not always obvious if the measurement scenario is representing RWC exposure scenario. Moreover, low exposure limit values bring additional challenges, such as the Ag-REL of 0.9 μg/m
3. Probabilistic exposure models can be used to predict exposures in all work shift combinations and RWC conditions typically without issues with limit of quantification related to personal sampling [
3]. In this case, the measured scenario would be classified
well controlled for TiO
2 coating process (95th percentile of the exposure distribution is <0.5 × REL; [
21]) and
controlled for Ag coating process (95th percentile of the exposure distribution is ≤REL; [
21]) based on NF concentrations. However, in a continuous coating process using four nozzles, the situation changes to
poorly controlled because of higher material uses per work shift and due to environmental conditions favoring higher concentration levels (
Table 2 and
Table 3).
As a precautionary assumption, all particles can be assumed to consist of NPs. Here, it was found that total NF concentration during TiO2N and AgHEC spraying processes contains 44% and 1.1% TiO2 and Ag, respectively. Thus, the elemental analysis of collected particles significantly improve the exposure estimates.
Transfer efficiency can be used to calculate environmental emissions according to the material use. For example, TiO
2N mass flow in coating process is 1.65 g-TiO
2N/min per nozzle and the transfer efficiency is ca. 97.6%. Thus, emissions to air are ca. 40 mg/min where ca. 6% is released to workplace air from spray chamber inlet and exit and 94% are exhausted via the LEV. The LEV filter filtration efficiency was on average 50%. Thus, the environmental emissions via LEV exhaust are ca. 20 mg/min when assuming LEV exhaust tube losses insignificant. In a six-hour coating process with one nozzle, would be emitted ca. 3600 mg-TiO
2 particles outdoor air and filter TiO
2 loading would increase a similar amount. As another example, assuming that factory is consuming 10 kg TiO
2N NPs per year by using this coating machine, the environmental TiO
2 emission to air would be ca. 110 g when calculated using an emission factor of 22.6 mg-TiO
2/g-TiO
2N and 50% filtration efficiency in LEV. This concept can be coupled directly with environmental exposure models (e.g., [
47]).
Process emissions from the spray chamber to the NF can be quantified according to the process parametrizations. In our case, these are substrate (Textile/PMMA), coating suspension type, AgHEC concentration, and number of nozzles (also suspension flow rate). Changing the flow rate from 200 to 400 mL/min increases the NF concentration on average 1.7 times (SD = 0.3) and for TiO
2N coating process PMMA substrate caused 1.4-, 1.2-, and 1.1-times higher NF mass concentrations than textile at flow rates of 200, 400, and 800 mL/min, respectively (
Table 2 and
Table S8, Supplementary Material S2).
Thinking about a future sustainable smart manufacturing, process parameter specific emissions and concentration levels can be used by digital technologies, such as Digital Twins, as input data to predict in real time environmental emissions and workers’ exposures, and to optimize the sustainability of the manufacturing process itself [
48].
4.1. Methodological Uncertainties Related to Effective Density
The particle effective density defined from the gravimetric samples and particle number size distributions include several assumptions: (i) spherical particles, (ii) identical mobility and optical size, (iii) constant agglomerates density over the measured particle size range, (iv) pre-process background concentration remains the same during the process. Spherical particle assumption is an intrinsic property of the effective density.
Mobility and optical size of TiO
2 and Ag particles are not the same because the optical size also depends on the particle refractive index and shape [
49,
50,
51]. The refractive index and extinction coefficient at 632.8 nm are for TiO
2 (rutile) 2.8736 + 0
i and for elemental Ag 0.056 + 4.27
i, respectively. These vary significantly from the coefficient used by the OPC. For example, for highly agglomerated TiO
2 NPs [
52], the optical diameter was 90 nm + 0.14 times the mobility diameter (
Supplementary Material S3). However, here, TiO
2 particles were spherical, doped with nitrate and the relation cannot be used in this case. Agglomerate density reduces with increasing agglomerate size when the primary particle size remains the same that is shown, e.g., for soot agglomerates [
53,
54]. This assumption overestimates the large particles mass fraction and underestimates small particles mass fractions. The background concentration assumption is expected to cause <10% error on the process particle mass concentration calculation.
4.2. Model Uncertainties
Major uncertainties in the model are related to air flow balance between the spray chamber and the NF (
QNF,SC), how well the concentration measurements represent the NF average concentrations level, and the air mixing flow rate between NF and FF. Here, the particles were assumed to diffuse from the spray chamber, i.e.,
QNF,SC = 0 m
3/min, but if the LEV flows are not in balance it will change the emissions proportional to the
QNF,SC. Concentration gradients in the NF volume can cause high under- or overestimation in emission rates. Concentration mapping requires simultaneous measurements with multiple instruments that were not available in this measurement campaign. Here, concentration measurements were expected to be representative for the mean NF concentration level because there were many small openings for particles to leak to the NF forming a local fugitive emission source rather than point source. Air mixing flow rate between NF and FF dilutes the NF concentrations. If dilution is overestimated, i.e.,
β is higher than true value, then the emission rate is overestimated. Here, the measured random air flow speed was up to 1.8 m/min (lower detection limit), which is below the typical random air speed at schools and offices of 2.5 m/min [
55]. Modelled concentrations showed small GSD because the parametrization was made according to the best knowledge and it does not account all identified uncertainties, which were included subjectively by increasing the emission rate range.
4.3. Deposited Dose
In occupational settings, deposition in the airways depend on the aerosol properties (particle size distribution, particle concentration, density, etc.) that are highly determined by the exposure scenario conditions, such as spraying time and worker location. If the particle size distribution shape and breathing conditions are the same during the work shift, deposition fractions can be used to estimate the deposited dose in airways under different exposure conditions when concentration and exposure duration, i.e., volume of inhaled air, are known.
The clearance mechanism present in the different parts of the airway will determine the final fate and uptake of deposited particles in the organism [
56,
57]. In the head and the tracheobronchial regions, most of the materials will be relatively rapidly eliminated either by expulsion or by translocation into the gastrointestinal tract (head region) or cleared by the mucocyliary system and translocated to the gastrointestinal tract (TB region; [
56]). In both cases, a small fraction would be subjected to a more prolonged retention.
In the pulmonary region, higher retention rates may be assumed than for the head and tracheobronchial region. However, this will depend among other factors on the clearance mechanism in the alveolar region (e.g., alveolar macrophage clearance) or transformation (e.g., dissolution in the lung lining fluid) and translocation mechanisms.
Process parameter specific emissions, aerosol concentration levels and calculated effective densities can be used as direct inputs in respiratory dosimetry models as the MPPD model to predict deposition of nanoparticles in the human lungs. These predictions can be of high relevance for risk estimation or risk prioritization purposes, including Safe(r)-by-design strategies. For instance, differences in calculated depositions were found between the different processes for the same NM. Under RWC TiO
2N coating process, the predicted daily deposited mass in the pulmonary region was 9.0 µg/day (7.3) for TiO
2N (
Table S9.2, Supplementary Material S2), which is below the
no significant dose level for particle overload, chronic inflammation and cell proliferation (300 µg/day) and tumor incidence (44 µg/day) in lungs suggested for TiO
2 (respirable fraction including nano-TiO
2) by Thompson et al. [
41] for a lifetime exposure. This outcome suggest that processes are well controlled for TiO
2 coating processes even at RWC where the worker spends the workday at the NF.