Influence of Pre-Dispersion Media on the Batch Reactor Dissolution Behavior of Al2O3 Coated TiO2 (NM-104) and Two ZnO (NM-110 and NM-111) Nanomaterials in Biologically Relevant Test Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Pre-Dispersion of Nanomaterials
2.3. Evaluation of the Dispersion Quality and Stability
2.4. Albumin Adsorption
2.5. Physiological Simulant Test Fluids
2.6. Dissolution Testing in Stirred Batch Reactors
2.7. Dissolved Ionic Fraction
2.8. Determination of Initial Dissolution Rates
2.9. Statistics
3. Results and Discussion
3.1. Albumin Adsorption
3.2. Dispersion Quality
3.3. Effect of Pre-Dispersion Medium and Quality on Dissolution Behavior
3.4. Pro et Con Analysis Regarding the Selection of Pre-Dispersion Media for Dissolution Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fröhlich, E.; Salar-Behzadi, S. Toxicological assessment of inhaled nanoparticles: Role of in vivo, ex vivo, in vitro, and in Silico Studies. Int. J. Mol. Sci. 2014, 15, 4795–4822. [Google Scholar] [CrossRef] [PubMed]
- Avramescu, M.L.; Rasmussen, P.E.; Chénier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behaviour of engineered nanomaterials. Environ. Sci. Pollut. Res. 2017, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Verleysen, E.; Wagner, T.; Lipinski, H.G.; Kägi, R.; Koeber, R.; Boix-Sanfeliu, A.; De Temmerman, P.J.; Mast, J. Evaluation of a TEM based Approach for Size Measurement of Particulate (Nano)materials. Materials 2019, 12, 2274. [Google Scholar] [CrossRef] [Green Version]
- Holmfred, E.; Loeschner, K.; Sloth, J.J.; Jensen, K.A. Validation and demonstration of an Atmosphere-Temperature-pH-controlled stirred batch reactor system for determination of (nano)material solubility and dissolution kinetics in physiological simulant lung fluids. Nanomaterials 2022, 12, 517. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Jensen, K.A.; Baun, A.; Rasmussen, K.; Rauscher, H.; Tantra, R.; Cupi, D.; Gilliland, D.; Pianella, F.; Riego Sintes, J.M. Techniques and Protocols for Dispersing Nanoparticle Powders in Aqueous Media—Is there a Rationale for Harmonization? J. Toxicol. Environ. Health Part B 2015, 18, 299–326. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.A.; Pojana, G.; Bilanicova, D. Characterization of Manufactured Nanomaterials, Dispersion, and Exposure for Toxicological Testing, 2nd ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2014; pp. 44–73. [Google Scholar]
- Wu, F.; Harper, B.J.; Harper, S.L. Comparative dissolution, uptake, and toxicity of zinc oxide particles in individual aquatic species and mixed populations. Environ. Toxicol. Chem. 2019, 38, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.A.; Kembouche, Y.; Christiansen, E.; Jacobsen, N.R.; Wallin, H.; Guiot, C.; Spalla, O.; Witschger, O. Towards a Method for Detecting the Potential Genotoxicity of Nanomaterials. Deliverable 3. Final Protocol for Producing Suitable Manufactured Nanomaterial Exposure Media. The Generic NANOGENOTOX Dispersion Protocol. Standard Operating Procedure (SOP) and Background Documentation. 2011, pp. 1–33. Available online: https://www.anses.fr/en/system/files/nanogenotox_deliverable_5.pdf (accessed on 6 September 2021).
- Hadrup, N.; Bengtson, S.; Jacobsen, N.R.; Jackson, P.; Nocun, M.; Saber, A.T.; Jensen, K.A.; Wallin, H.; Vogel, U. Influence of dispersion medium on nanomaterial-induced pulmonary inflammation and DNA strand breaks: Investigation of carbon black, carbon nanotubes and three titanium dioxide nanoparticles. Mutagenesis 2017, 32, 581–597. [Google Scholar] [CrossRef] [Green Version]
- Sauer, U.G.; Aumann, A.; Ma-Hock, L.; Landsiedel, R.; Wohlleben, W. Influence of dispersive agent on nanomaterial agglomeration and implications for biological effects in vivo or in vitro. Toxicol. In Vitro 2015, 29, 182–186. [Google Scholar] [CrossRef]
- Utembe, W.; Potgieter, K.; Stefaniak, A.B.; Gulumian, M. Dissolution and biodurability: Important parameters needed for risk assessment of nanomaterials. Part. Fibre Toxicol. 2015, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD—Organisation for Economic Co-Operation and Development Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2012)40&docLanguage=En (accessed on 6 September 2021).
- Keller, J.G.; Peijnenburg, W.; Werle, K.; Landsiedel, R.; Wohlleben, W. Understanding dissolution rates via continuous flow systems with physiologically relevant metal ion saturation in lysosome. Nanomaterials 2020, 10, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, C.A.; Galceran, J.; Rey-Castro, C.; Puy, J.; Companys, E.; Salvador, J.; Monné, J.; Wallace, R.; Vakourov, A. Dissolution kinetics and solubility of ZnO nanoparticles followed by AGNES. J. Phys. Chem. C 2012, 116, 11758–11767. [Google Scholar] [CrossRef]
- Da Silva, E.; Kembouche, Y.; Tegner, U.; Baun, A.; Jensen, K.A. Interaction of biologically relevant proteins with ZnO nanomaterials: A confounding factor for in vitro toxicity endpoints. Toxicol. In Vitro 2019, 56, 41–51. [Google Scholar] [CrossRef]
- Da Silva, E.; Kembouche, Y.; Tegner, U.; Baun, A.; Jensen, K.A. Data supporting the investigation of interaction of biologically relevant proteins with ZnO nanomaterials: A confounding factor for in vitro toxicity endpoints. Data Br. 2019, 23, 103795. [Google Scholar] [CrossRef]
- Guiot, C.; Spalla, O. Stabilization of TiO2 nanoparticles in complex medium through a pH adjustment protocol. Environ. Sci. Technol. 2013, 47, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Clausen, P.A.; Kofoed-Sørensen, V.; Nørgaard, A.W.; Sahlgren, N.M.; Jensen, K.A. Thermogravimetry and mass spectrometry of extractable organics from manufactured nanomaterials for identification of potential coating components. Materials 2019, 12, 3657. [Google Scholar] [CrossRef] [Green Version]
- Guldberg, M.; Madsen, A.L.; Sebastian, K.; Fellmann, J.; Potter, R.; Bauer, J.; Searl, A.; Maquin, B.; Jubb, G. In-vitro dissolution of vitreous silicate fibres according to EURIMA test guideline—Results of two Round Robins. Glass Sci. Technol. 2003, 76, 199–205. [Google Scholar]
- Stefaniak, A.B.; Guilmette, R.A.; Day, G.A.; Hoover, M.D.; Breysse, P.N.; Scripsick, R.C. Characterization of phagolysosomal simulant fluid for study of beryllium aerosol particle dissolution. Toxicol. In Vitro 2005, 19, 123–134. [Google Scholar] [CrossRef]
- De Temmerman, P.-J.; Mast, J.; Guiot, C.; Spalla, O.; Rousset, D.; Shivachev, B.; Tarassov, M.; Jensen, K.A. Towards A Method for Detecting the Potential Genotoxicity of Nanomaterials. Nanogenotox Deliverable 4.2: Transmission Electron Microscopic Characterization of NANOGENOTOX Nanomaterials. Available online: http://www.nanogenotox.eu/index.php?option=com_content&view=article&id=136&Itemid=158 (accessed on 6 September 2021).
- OECD—Organisation for Economic Co-operation and Development Dossier on Zinc Oxide. Series on the Safety of Manufactured Nanomaterials. No. 52. Available online: http://www.oecd.org/officialdocuments/displaydocumentpdf?cote=env/jm/mono(2010)46&doclanguage=en (accessed on 6 September 2021).
- Llewellyn, S.V.; Conway, G.E.; Zanoni, I.; Jørgensen, A.K.; Shah, U.K.; Seleci, D.A.; Keller, J.G.; Kim, J.W.; Wohlleben, W.; Jensen, K.A.; et al. Understanding the impact of more realistic low-dose, prolonged engineered nanomaterial exposure on genotoxicity using 3D models of the human liver. J. Nanobiotechnol. 2021, 19, 1–24. [Google Scholar] [CrossRef]
- Rasmussen, K.; Mast, J.; De Temmerman, P.-J.; Verleysen, E.; Waegeneers, N.; Van Steen, F.; Pizzolon, J.C.; De Temmerman, L.; Van Doren, E.; Jensen, K.A.; et al. Titanium Dioxide, NM-100, NM-101, NM-102, NM-103, NM-104, NM-105: CharacterisaCtion and Physico- Chemical Properties. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC86291 (accessed on 6 September 2021).
- Singh, C.; Friedrichs, S.; Levin, M.; Birkedal, R.; Jensen, K.A.; Pojana, G.; Wohlleben, W.; Schulte, S.; Wiench, K.; Turney, T.; et al. Zinc Oxide NM-110, NM-111, NM-112, NM-113 Characterisation and Test Item Preparation, NM-Series of Representative Manufactured Nanomaterials. Eur. Sci. Tech. Res. Rep. 2011, 1–141. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC64075 (accessed on 1 February 2022).
- Oberdörster, G.; Kuhlbusch, T.A.J. In vivo effects: Methodologies and biokinetics of inhaled nanomaterials. NanoImpact 2018, 10, 38–60. [Google Scholar] [CrossRef]
- Keller, J.G.; Graham, U.M.; Koltermann-jülly, J.; Gelein, R.; Lan, M.; Landsiedel, R.; Wiemann, M.; Oberdörster, G.; Elder, A. Predicting dissolution and transformation of inhaled nanoparticles in the lung using abiotic flow cells: The case of barium sulfate. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 3rd ed.; Pearson Education Limited: Hongkong, China, 1999; ISBN 9780135317167. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 9781420039870. [Google Scholar]
- Mejia, J.; Unamur, S.L.; Booth, A.; Sintef, J.F.; Sabella, S.; Bove, P.; Iit, M.M.; Jensen, K.A.; Kembouche, Y.; Da Silva, E.; et al. NANoREG—Deliverable 2.08: Protocols for Exposure-Fate Characterization in Ecotoxicity and In Vitro Studies. 2016, pp. 1–82. Available online: https://www.rivm.nl/en/documenten/nanoreg-d208-fs-protocols-for-exposure-fate-characterization-in-ecotoxicity-and-in-vitro (accessed on 3 February 2022).
- European Union Observatory for Nanomaterials ENM eNanomapper. Available online: http://www.enanomapper.net/ (accessed on 8 September 2021).
- Shahnawaz Khan, M.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine serum albumin interactions with metal complexes. Clujul Med. 2014, 87, 5. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Tabrez, S.; Rehman, M.T.; Alokail, M.S. Al (III) metal augment thermal aggregation and fibrillation in protein: Role of metal toxicity in neurological diseases. Saudi J. Biol. Sci. 2020, 27, 2221–2226. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T. All About Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press an Imprint of Elsevier: Amsterdam, The Netherlands, 1995; ISBN 9780125521109. [Google Scholar]
- Vergaro, V.; Carlucci, C.; Cascione, M.; Lorusso, C.; Conciauro, F.; Scremin, B.F.; Congedo, P.M.; Cannazza, G.; Citti, C.; Ciccarella, G. Interaction between human serum albumin and different anatase TiO2 nanoparticles: A nano-bio interface study. Nanomater. Nanotechnol. 2015, 5, 30. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | NM-104 | NM-110 | NM-111 |
|---|---|---|---|
| Primary particle size ECD * (nm) | 25.0 ± 1.7 a | 75.4 ± 58.4 b | 40.6 c |
| Average aggregate size ECD * (nm) | 58.5 ± 46.3 a | 114 ± 97 d | 106 ± 69 d |
| Crystallite size by XRD (nm) | 27 e | 42 f | 24–42 f |
| Specific surface area (m2/g) | 56.8 ± 0.5 e | 12.4 ± 0.6 d | 15.1 ± 0.6 d |
| Material | TiO2—Rutile e | ZnO—Zincite f | ZnO—Zincite e |
| Inorganic coating (wt.%) | 6.08 Al2O3 g | None f | None e |
| Organic coating (wt.%) | 3.17 ± 0.07 h glycerine h | ND f | 2.1 ± 0.31 g triethoxycaprylsilane h |
| Moisture content (wt.%) | 1.50 ± 0.10 g | 0.28 ± 0.11 g | ND g |
| Simulated Lung Fluid | Component | Chemical Formula | Concentration [mg/L] |
|---|---|---|---|
| Phagolysosomal simulant fluid (PSF) | Sodium phosphate dibasic anhydrous | Na2HPO4 | 142 |
| Sodium chloride | NaCl | 6650 | |
| Sodium sulphate anhydrous | Na2SO4 | 71 | |
| Calcium chloride dihydrate | CaCl2·2H2O | 29 | |
| Glycine | H2NCH2CO2H | 450 | |
| Potassium hydrogen phthalate | (1-(HO2C)-2-(CO2K)-C6H4) | 4085 | |
| Alkylbenzyldimethylammonium chloride | - | 50 | |
| Low-calcium Gamble’s solution | Sodium chloride | NaCl | 6600 |
| Sodium bicarbonate | NaHCO3 | 2703 | |
| Calcium chloride | CaCl2 | 22 | |
| Sodium phosphate dibasic dodecahydrate | Na2HPO4·12H2O | 358 | |
| Sodium sulphate anhydrous | Na2SO4 | 79 | |
| Magnesium chloride hexahydrate | MgCl·6H2O | 212 | |
| Glycine | H2NCH2CO2H | 118 | |
| Sodium citrate dihydrate | Na3C6H5O7 ·2H2O | 153 | |
| Sodium tartrate dihydrate | Na2C4H4O6 ·2H2O | 180 | |
| Sodium pyruvate | C3H3NaO3 | 172 | |
| Sodium lactate | C3H5NaO3 | 175 |
| Parameters (Unit) | TiO2 (NM-104) Dissolution in PSF | TiO2 (NM-104) Dissolution in Gamble’s | ZnO (NM-110 and NM-111) Dissolution in PSF | ZnO (NM-110 and NM-111) Dissolution Gamble’s |
|---|---|---|---|---|
| Nebulizer gas flow rate (L/min) | 0.89 | 1.06 | 1.02 | 1.04 |
| Auxiliary gas flow rate (L/min) | 0.80 | 0.80 | 0.80 | 0.80 |
| Helium cell gas flow rate (L/min) | 4.35 | 4.58 | No cell gas | No cell gas |
| Monitored isotopes (m/z) | 27Al and 48Ti | 27Al and 48Ti | 64Zn | 64Zn |
| Internal standard (m/z) | 103Rh | 103Rh | 103Rh | 103Rh |
| Limit of detection | Al: 19 µg/L, Ti: 3.2 µg/L | Al: 22 µg/L, Ti: 1.2 µg/L | 10 µg/L | 5.4 µg/L |
| Dilution factor for ICP-MS analysis | ×10 | ×4 | ×1000 | ×200 |
| Particle Concentration (mg/mL) | Sample | µg BSA/mg NM | Percent Adsorbed of the BSA Added | Literature Values µg BSA/mg NM | Literature Values Percent Adsorbed of the BSA Added |
|---|---|---|---|---|---|
| 2.56 | TiO2 (NM-104) | 131.8 ± 22.0 | 69 | 143.7 ± 12.9 # | 66 # |
| 2.56 | ZnO (NM-110) | 138.3 ± 18.4 | 67 | 151.8 ± 27.0 ⱡ | 69 ⱡ |
| 2.56 | ZnO (NM-111) | 88.3 ± 27.1 | 43 | 292.8 ± 41.5 ⱡ | 40 ⱡ |
| Test Medium | Nanomaterial | Dispersion Medium | Zave [nm] | PDI | Zpot [mV] | Zave,benchmark | Zave/Daggr | Zave/Zave,benchmark |
|---|---|---|---|---|---|---|---|---|
| Low-calcium Gamble’s solution | TiO2 (NM-104) | Water | 157.8 ± 2.3 | 0.24 ± 0.01 | 14.9 ± 0.7 | 234 ± 4 a | 2.7 | 0.7 |
| GS | 2827 ± 1895 | 0.88 ± 0.3 | 14.9 + 0.7 | 48.3 | 12.1 | |||
| 0.05% BSA | 724.0 ± 160.2 | 0.74 ± 0.1 | −0.8 ± 1.0 | 12.4 | 3.1 | |||
| ZnO (NM-110) | Water | 225.3 ± 2.2 | 0.15 ± 0.02 | 30.7 ± 0.6 | 233.1 ± 7.3 b | 2.0 | 1.0 | |
| GS | 1824 ± 343 | 1.0 ± 0.0 | −16.2 ± 0.6 | 16.0 | 7.8 | |||
| 0.05% BSA | 250.6 ± 1.1 | 0.14 ± 0.02 | −13.4 ± 0.3 | 2.2 | 1.1 | |||
| ZnO (NM-111) | Water | 735.1 ± 97.0 | 0.37 ± 0.07 | 12.5 ± 0.5 | 247.4 ± 4.9 b | 6.9 | 3.0 | |
| GS | 3578 ± 303 | 0.31 ± 0.1 | −13.1 ± 1.0 | 33.8 | 14.5 | |||
| 0.05% BSA | 278.9 ± 2.9 | 0.16 ± 0.02 | −14.5 ± 0.5 | 2.6 | 1.1 | |||
| PSF | TiO2 (NM-104) | Water | 162.9 ± 1.9 | 0.24 ± 0.01 | 13.4 ± 0.5 | 234 ± 4 a | 2.8 | 0.7 |
| PSF | 3448 ± 2283 | 0.93 ± 0.2 | 0.6 ± 0.8 | 58.9 | 14.7 | |||
| 0.05% BSA | 366.7 ± 153.7 | 0.30 ± 0.09 | −1.3 ± 0.4 | 6.3 | 1.6 | |||
| ZnO (NM-110) | Water | 257.0 ± 1.6 | 0.16 ± 0.02 | 24.6 ± 0.6 | 233.1 ± 7.3 b | 2.3 | 1.1 | |
| PSF | 3989 ± 820 | 1.0 ± 0.0 | −15.0 ± 2.6 | 35.0 | 17.1 | |||
| 0.05% BSA | 247.1 ± 2.5 | 0.30 ± 0.09 | −14.6 ± 0.6 | 2.2 | 1.1 | |||
| ZnO (NM-111) | Water | 868.1 ± 118.5 | 0.35 ± 0.07 | 12.0 ± 0.3 | 247.4 ± 4.9 b | 8.2 | 3.5 | |
| PSF | 5410 ± 680 | 0.61 ± 0.06 | −13.4 ± 2.3 | 51.0 | 21.9 | |||
| 0.05% BSA | 275.9 ± 2.6 | 0.15 ± 0.02 | -16.7 ± 0.8 | 2.6 | 1.1 |
| Interaction | TiO2 (NM-104) | ZnO (NM-110) | ZnO (NM-111) |
|---|---|---|---|
| Gamble’s solution vs. water | 0.5233 | 0.3792 | 0.2858 |
| Gamble’s solution vs. 0.05% BSA | 0.0168 | 0.3534 | <0.0001 |
| Water vs. 0.05% BSA | 0.0040 | 0.3909 | 0.0711 |
| PSF vs. Water | 0.6167 | 0.5987 | 0.0081 |
| PSF vs. 0.05% BSA | <0.0001 | 0.0267 | 0.0023 |
| Water vs. 0.05% BSA | <0.0001 | 0.0141 | 0.0011 |
| Test Medium: Gamble’s Solution | Test Medium: PSF | ||||||
|---|---|---|---|---|---|---|---|
| Dispersion Medium | Dispersion Medium | ||||||
| Nanomaterial | Initial Concentration and Dissolution Rates | Gamble’s Solution | Water | 0.05% BSA | PSF | Water | 0.05% BSA |
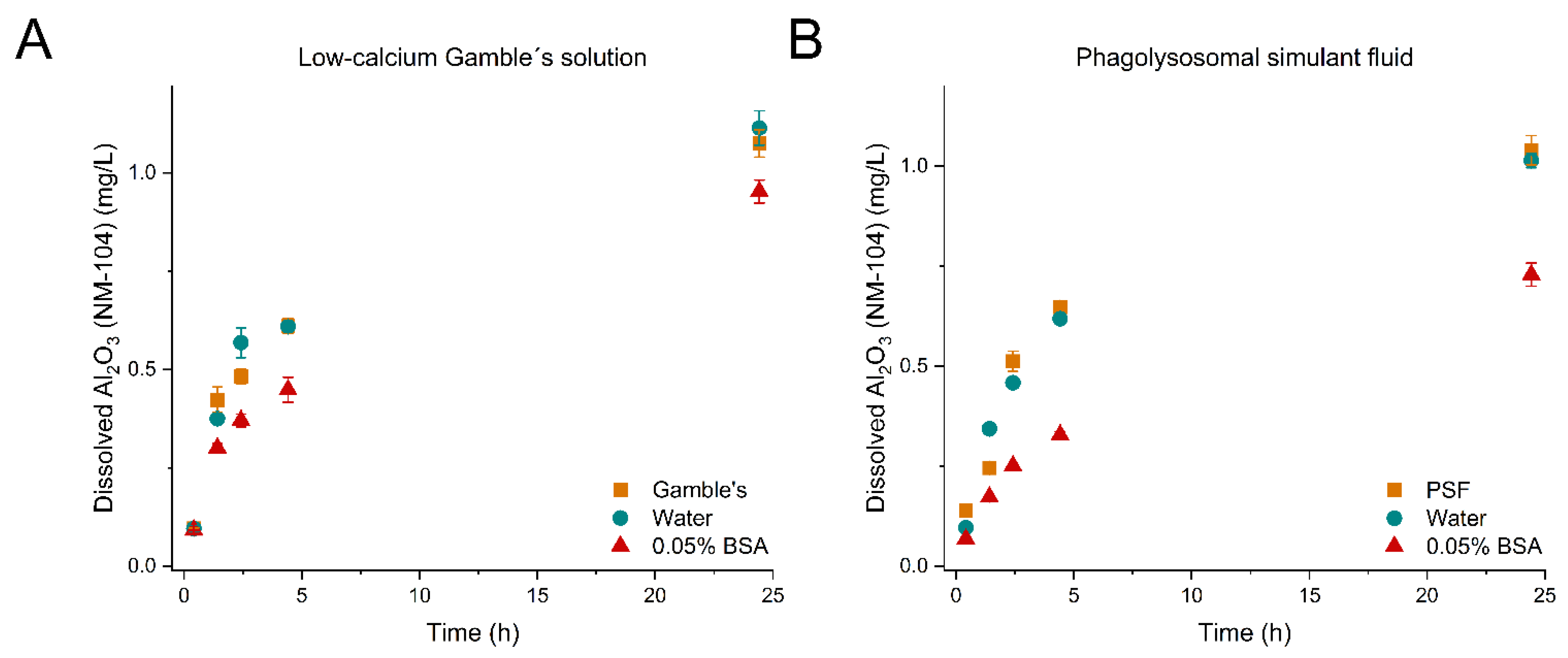
| TiO2 (NM-104), Al2O3 coating | S@ti (mg/L) | 0.097 ± 2.04 × 10−3 | 0.096 ± 7.94 × 10−3 | 0.092 ± 1.65 × 10−3 | 0.139 ± 2.39 × 10−3 | 0.0965 ± 2.91 × 10−3 | 0.069 ± 4.0 × 10−3 |
| , (mg/L/h) | 0.171 ± 0.064 | 0.126 ± 0.018 | 0.160 ± 0.038 | 0.230 ± 0.031 | 0.220 ± 0.014 | 0.096 ± 0.002 | |
| , (cm2/L/sec) | 0.027 ± 0.010 | 0.020 ± 2.8 × 10−3 | 0.025 ± 5.92 × 10−3 | 0.036 ± 4.8 × 10−3 | 0.034 ± 2.26 × 10−3 | 0.015 ± 2.73 × 10−4 | |
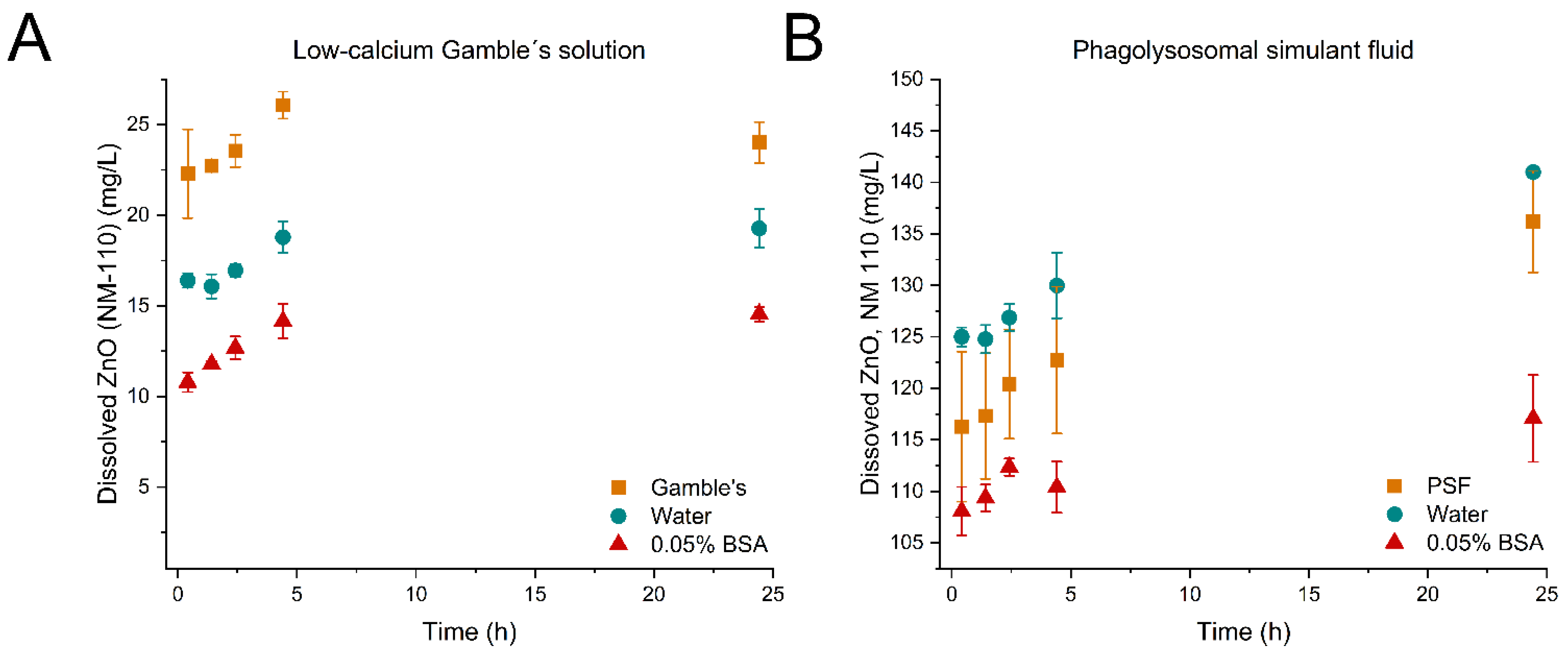
| ZnO (NM-110) | S@ti (mg/L) | 22.3 ± 2.5 | 16.4 ± 0.4 | 10.7 ± 0.5 | 116.3 ± 7.3 | 125.0 ± 0.9 | 108.1 ± 2.3 |
| , (mg/L/h) | 2.72 ± 2.59 | 0.720 ± 0.191 | 2.04 ± 0.22 | ND | ND | ND | |
| , (cm2/L/sec) | 0.094 ± 0.089 | 0.025± 6.6 × 10−3 | 0.070 ± 7.74 × 10−3 | ND | ND | ND | |
| ZnO (NM-111) | S@ti (mg/L) | 4.83 ± 0.27 | 5.76 ± 0.23 | 4.10 ± 0.61 | 112.1 ± 0.8 | 104.5 ± 3.8 | 74.67 ± 4.52 |
| , (mg/L/h) | 0.604 ± 0.582 | 2.09 ± 1.71 | 1.95 ± 0.26 | ND | ND | ND | |
| (cm2/L/sec) | 0.025 ± 0.024 | 0.088± 0.072 | 0.082 ± 0.011 | ND | ND | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmfred, E.; Sloth, J.J.; Loeschner, K.; Jensen, K.A. Influence of Pre-Dispersion Media on the Batch Reactor Dissolution Behavior of Al2O3 Coated TiO2 (NM-104) and Two ZnO (NM-110 and NM-111) Nanomaterials in Biologically Relevant Test Media. Nanomaterials 2022, 12, 566. https://doi.org/10.3390/nano12030566
Holmfred E, Sloth JJ, Loeschner K, Jensen KA. Influence of Pre-Dispersion Media on the Batch Reactor Dissolution Behavior of Al2O3 Coated TiO2 (NM-104) and Two ZnO (NM-110 and NM-111) Nanomaterials in Biologically Relevant Test Media. Nanomaterials. 2022; 12(3):566. https://doi.org/10.3390/nano12030566
Chicago/Turabian StyleHolmfred, Else, Jens J. Sloth, Katrin Loeschner, and Keld Alstrup Jensen. 2022. "Influence of Pre-Dispersion Media on the Batch Reactor Dissolution Behavior of Al2O3 Coated TiO2 (NM-104) and Two ZnO (NM-110 and NM-111) Nanomaterials in Biologically Relevant Test Media" Nanomaterials 12, no. 3: 566. https://doi.org/10.3390/nano12030566
APA StyleHolmfred, E., Sloth, J. J., Loeschner, K., & Jensen, K. A. (2022). Influence of Pre-Dispersion Media on the Batch Reactor Dissolution Behavior of Al2O3 Coated TiO2 (NM-104) and Two ZnO (NM-110 and NM-111) Nanomaterials in Biologically Relevant Test Media. Nanomaterials, 12(3), 566. https://doi.org/10.3390/nano12030566







