N2O Hydrogenation on Silver Doped Gold Catalysts, a DFT Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reaction Mechanism
2.2. Catalytic Surfaces Modeling
2.3. Computational Details
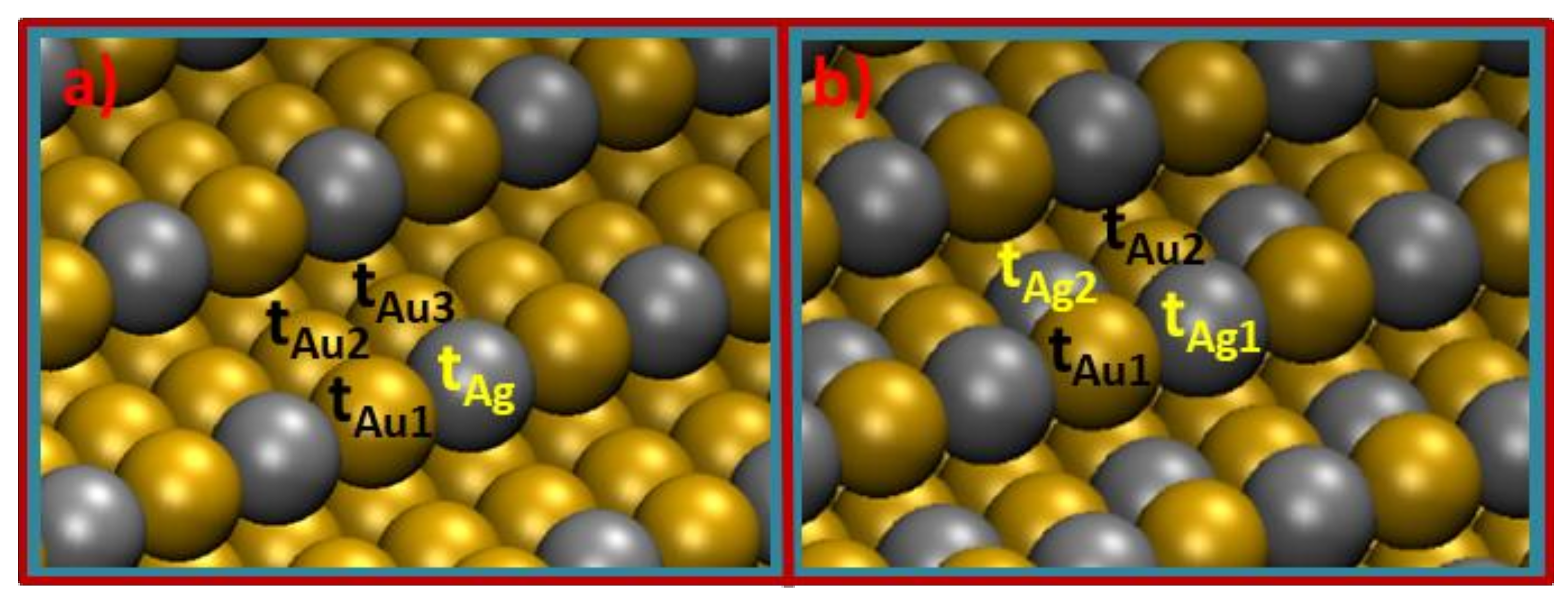
2.4. Experimental Observations and Knowledge Lacks
3. Results
3.1. Adsorption of Reactants and Products
| Ag@Au(210) Surface | |||||
| Species | Adsorption Site a | Vibrational Wavenumbers b | dsuf-mol c | Bond Length d | |
| N2O | tAg | −0.33 | 2340; 1331; 542; 536 | 2.56 (N–Ag) | 1.15 (N–N) 1.19 (N–O) |
| H2O | tAg | −0.50 | 3730; 3442; 1556 | 2.46 (O–Ag) | 0.98 (O–Hb) 0.99 (O–Ha) |
| H2 | tAg | −0.06 | 526 | 2.43 (Ha–Ag)/2.46 (Hb–Ag) | 0.76 (Ha–Hb) |
| N2 | tAg | −0.15 | 2416 | 2.72 (N–Ag) | 1.12 (N–N) |
| OH | bAg-Au3 | −2.29 | 3698; 794 | 2.22 (O–Ag)/2.15 (O–Au3) | 0.98 (O–H) |
| Ag2@Au(210) Surface | |||||
| Species | Adsorption Site a | Vibrational Wavenumbers b | dsuf-mol c | Bond Length d | |
| N2O | bAg1-Ag2 | −0.29 | 2337; 1327; 536; 531 | 2.78 (N–Ag1)/3.34 (O–Ag2) | 1.15 (N–N) 1.20 (N–O) |
| H2O | tAg1 | −0.47 | 3737; 3409; 1560 | 2.47 (O–Ag1) | 0.98 (O–Hb) 0.99 (O–Ha) |
| H2 | tAg1 | −0.03 | 540 | 2.63 (Ha–Ag1)/2.64 (Hb–Ag1) | 0.76 (Ha–Hb) |
| N2 | tAg1 | −0.17 | 2422 | 3.08 (N–Ag1) | 1.12 (N–N) |
| OH | bAg1-Au1 | −2.27 | 3710; 743; 518 | 2.23 (O–Ag1)/2.15 (O–Au1) | 0.98 (O–H) |

3.2. Activation Energy Barriers and Rate Constants for the Steps Involved in the N2O Hydrogenation
| Ag2@Au(210) Surface | |||||
| Species | Adsorption Site a | Vibrational Wavenumbers b | dsuf-mol c | Bond Length d | |
| N2 + O | tAg/hstep | −3.50 | 2426 | 2.53 (N–Ag)/2.67 (O–Ag) /2.28 (O–Au1) /2.13 (O–Au2) /2.19 (O–Au3) | 1.12 (N–N) |
| OH + H | bAg-Au3/bAu1-Au2 | −4.65 | 3691; 1234; 1117; 824 | 2.21 (O–Ag)/1.76 (H–Au1) 2.15 (O–Au3)/1.77 (H–Au2) | 0.98 (O–Ha) |
| H + H | bAg-Au3/bAu1-Au2 | −4.19 | 1566; 1242; 1094; 767 | 1.93 (Ha–Ag)/1.75 (Hb–Au1) 1.68 (Ha–Au3)/1.77 (Hb–Au2) | – |
| O + H | hstep/bAg-Au1 | −5.45 | 1661; 1065 | 2.12 (O–Ag)/1.82 (H–Ag) 2.04 (O–Au1)/1.77 (H–Au1) 2.35 (O–Au2)/ 2.15 (O–Au3)/ | – |
| Ag2@Au(210) Surface | |||||
| Species | Adsorption Site a | Vibrational Wavenumbers b | dsuf-mol c | Bond Length d | |
| N2 + O | tAg/hstep | −3.52 | 2425 | 2.78 (N–Ag1)/2.32 (O–Ag1) /2.19 (O–Au1) /2.26 (O–Au2) /2.31 (O–Ag2) | 1.12 (N–N) |
| OH + H | bAg1-Au2/bAu1-Ag2 | −4.65 | 3681; 1588; 843; 688 | 2.21 (O–Ag1)/1.68 (H–Au1) 2.13 (O–Au2)/1.93 (H–Ag2) | 0.98 (O–Ha) |
| H + H | bAg1-Au1/bAg1-Au2 | −4.25 | 1675; 1569; 665; 600 | 2.01 (Ha–Ag1)/1.97 (Hb–Ag1) 1.66 (Ha–Au1)/1.68 (Hb–Au2) | — |
| O + H | hstep/bAg1-Au1 | −5.48 | 1573; 829 | 2.27 (O–Ag1)/1.88 (H–Ag1) 2.33 (O–Ag2)/1.68 (H–Au1) 2.30 (O–Au1)/ 2.20 (O–Au2)/ | — |

| Ag@Au(210) Surface | ||||||
| Elementary Step | Vibrational Modes a | Bond Length b | k | Imaginary Frequency | ||
| N2O* → N2* + O* | 1858; 554 | 1.58 | 0.84 | 4.8 × 10−10/1.1 × 10−7/9.0 × 10−6/3.3 × 10−4/ 6.8 × 10−3/8.8 × 10−2/8.0 × 10−1/3.0 × 101 | −0.43 | 503 |
| H2* → H* + H* | 1176; 1000; 725; 557 | 1.01 | 0.61 | 2.4 × 10−4/1.1 × 10−2/2.1 × 10−1/2.4 × 100/ 1.8 × 101/9.8 × 101/4.2 × 102/4.3 × 103 | 0.13 | 579 |
| O* + H* → OH* | 1183; 1040 | 1.91 | 0.32 | 4.9 × 104/4.3 × 105/2.5 × 106/1.1 × 107/ 3.5 × 107/9.9 × 107/2.4 × 108/1.0 × 109 | −1.55 | 2.53 |
| OH* + H* → H2O* | 3679; 778; 680; 522 | 1.57 | 0.34 | 8.0 × 103/8.0 × 104/5.1 × 105/2.3 × 106/ 8.3 × 106/2.5 × 107/6.3 × 107/2.9 × 108 | −0.88 | 856 |
| N2* → N2 + * | — | — | 0.15 | 2.0 × 109/5.1 × 109/1.1 × 1010/2.0 × 1010/ 3.2 × 1010/4.9 × 1010/7.0 × 1010/1.2 × 1011 | 0.15 | — |
| H2O* → H2O + * | — | — | 0.50 | 2.5 × 10−1/6.5 × 100/8.7 × 101/7.2 × 102/ 4.2 × 103/1.8 × 104/6.4 × 104/4.8 × 105 | 0.50 | — |
| Ag2@Au(210) Surface | ||||||
| N2O* → N2* + O* | 1825; 565 | 1.56 | 0.69 | 3.3 × 10−7/2.9 × 10−5/1.1 × 10−3/2.1 × 10−2/ 2.5 × 10−1/2.1 × 100/1.3 × 101/2.5 × 102 | −0.62 | 489 |
| H2* → H* + H* | 1476; 925; 679; 503 | 1.05 | 0.57 | 2.2 × 10−3/7.8 × 10−2/1.3 × 100/1.3 × 101/ 9.0 × 101/4.5 × 102/1.8 × 103/1.6 × 104 | 0.07 | 525 |
| O* + H* → OH* | 1674; 524 | 1.93 | 0.29 | 2.4 × 105/1.7 × 106/7.9 × 106/2.9 × 107/ 8.3 × 107/2.1 × 108/4.5 × 108/1.6 × 109 | −1.51 | 314 |
| OH* + H* → H2O* | 3663; 1330; 764 | 1.64 | 0.30 | 6.1 × 104/4.7 × 105/2.4 × 106/9.4 × 106/ 2.9 × 107/7.5 × 107/1.7 × 108/6.6 × 108 | −0.85 | 257 |
| N2* → N2 + * | — | — | 0.17 | 1.7 × 109/5.4 × 109/1.3 × 1010/2.8 × 1010/ 5.3 × 1010/9.1 × 1010/1.4 × 1011/3.0 × 1011 | 0.17 | — |
| H2O* → H2O + * | — | — | 0.47 | 2.5 × 10−1/6.5 × 100/8.7 × 101/7.2 × 102/ 4.2 × 103/1.8 × 104/6.4 × 104/4.8 × 105 | 0.47 | — |
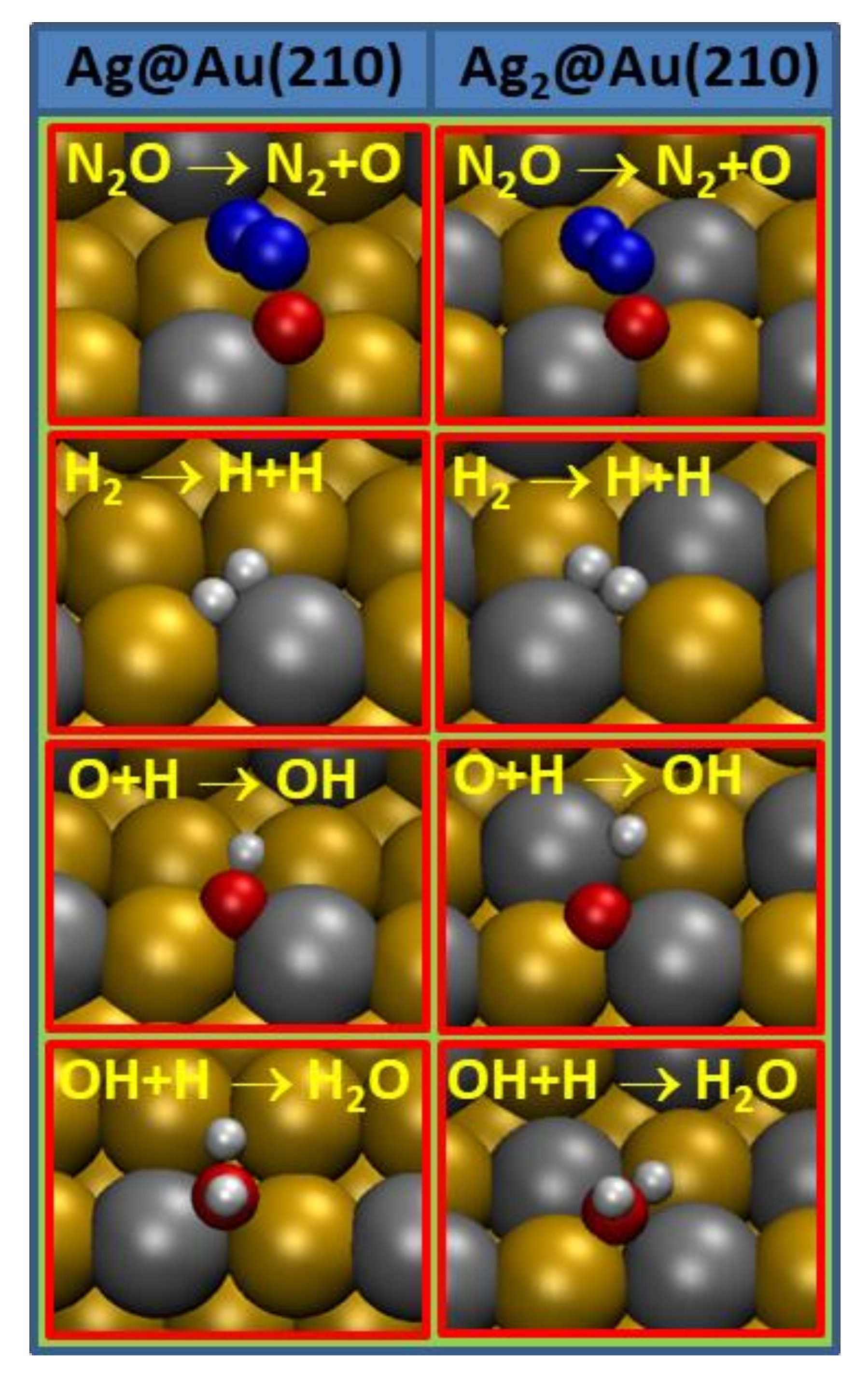
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Inventory of U.S. Greenhouse Gas Emissions and Sinks (1990–2019). Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks (accessed on 11 December 2021).
- Green House Gas Emissions by Industries and Hoseholds—Eurostat. Available online: https://ec.europa.eu/eurostat/statisticsexplained/index.php/Greenhouse_gas_emissions_by_industries_and_households#Greenhouse_gas_emissions (accessed on 11 December 2021).
- Gul, I.; Sayed, M.; Shah, N.S.; Rehman, F.; Khan, J.A.; Gul, S.; Bibi, N.; Iqbal, J. A novel route for catalytic activation of peroxymonosulfate by oxygen vacancies improved bismuth-doped titania for the removal of recalcitrant organic contaminant. Environ. Sci. Pollut. Res. 2021, 28, 23368–23385. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.; Khan, A.; Rauf, S.; Shah, N.S.; Rehman, F.; Al-Kahtani, A.A.; Khan, J.A.; Iqbal, J.; Boczkaj, G.; Gul, I.; et al. Bismuth-doped nano zerovalent iron: A novel catalyst for chloramphenicol degradation and hydrogen production. ACS Omega 2020, 5, 30610–30624. [Google Scholar] [CrossRef] [PubMed]
- Fajín, J.L.C.; Cordeiro, M.N.D.S. Insights into the mechanism of methanol steam reforming for hydrogen production over Ni–Cu-based catalysts. ACS Catal. 2022, 12, 512–526. [Google Scholar] [CrossRef]
- Chang, C.D.; Santiesteban, J.G.; Shihabi, D.S.; Stevenson, S.A. Mobil Oil Corporation. U.S. Patent 5401478, 28 March 1995. [Google Scholar]
- Zhang, R.; Liu, N.; Lei, Z.; Chen, B. Selective transformation of various nitrogen-containing exhaust gases toward N2 over zeolite catalysts. Chem. Rev. 2016, 116, 3658–3721. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, G.; Lu, G.; Tang, Z. Recent progress on establishing structure–activity relationship of catalysts for selective catalytic reduction (SCR) of NOx with NH3. Catal. Surv. Asia 2018, 22, 1–19. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.-Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Miyoshi, N.; Matsumoto, S.; Katoh, K.; Tanaka, T.; Harada, J.; Takahashi, N.; Yokota, K.; Suguira, M.; Kasahara, K. Development of new concept three-way catalyst for automotive lean-burn engines. SAE Tech. Pap. 1995, 104, 950809. [Google Scholar]
- Matsumoto, S.; Ikeda, Y.; Suzuki, H.; Ogai, M.; Miyoshi, N. NOx storage-reduction catalyst for automotive exhaust with improved tolerance against sulfur poisoning. Appl. Catal. B 2000, 25, 115–124. [Google Scholar] [CrossRef]
- Madia, G.; Koebel, M.; Elsener, M.; Wokaun, A. Side reactions in the selective catalytic reduction of NOx with various NO2 fractions. Ind. Eng. Chem. Res. 2002, 41, 4008–4015. [Google Scholar] [CrossRef]
- Ciardelli, C.; Nova, I.; Tronconi, E.; Chatterjee, D.; Bandl-Konrad, B.; Weibel, M.; Krutzsch, B. Reactivity of NO/NO2−NH3 SCR system for diesel exhaust aftertreatment: Identification of the reaction network as a function of temperature and NO2 feed content. Appl. Catal. B 2007, 70, 80–90. [Google Scholar] [CrossRef]
- Zhu, M.; Lai, J.K.; Wachs, I.E. Formation of N2O greenhouse gas during SCR of NO with NH3 by supported vanadium oxide catalysts. Appl. Catal. B 2018, 224, 836–840. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, S.; Liao, Y.; Xiao, X.; Qi, F.; Peng, Y.; Fu, Y.; Shan, W.; Li, J. Mechanism of N2O formation during the low-temperature selective catalytic reduction of NO with NH3 over Mn−Fe spinel. Environ. Sci. Technol. 2014, 48, 10354–10362. [Google Scholar] [CrossRef]
- Xu, J.; Chen, G.; Guo, F.; Xie, J. Development of wide-temperature vanadium-based catalysts for selective catalytic reducing of NOx with ammonia: Review. Chem. Eng. J. 2018, 353, 507–518. [Google Scholar] [CrossRef]
- Arve, K.; Adam, J.; Simakova, O.; Čapek, L.; Eränen, K.; Murzin, D.Y. Selective catalytic reduction of NOx over nano-sized gold catalysts supported on alumina and titania and over bimetallic gold-silver catalysts supported on alumina. Top Catal. 2009, 52, 1762–1765. [Google Scholar] [CrossRef]
- More, P.M.; Nguyen, D.L.; Granger, P.; Dujardin, C.; Dongare, M.K.; Umbarkar, S.B. Activation by pretreatment of Ag–Au/Al2O3 bimetallic catalyst to improve low temperature HC-SCR of NOx for lean burn engine exhaust. Appl. Catal. B 2015, 174–175, 145–156. [Google Scholar] [CrossRef]
- Esteves, P.; Wu, Y.; Dujardin, C.; Dongare, M.; Granger, P. Ceria–zirconia mixed oxides as thermal resistant catalysts for the decomposition of nitrous oxide at high temperature. Catal. Today 2011, 176, 453–457. [Google Scholar] [CrossRef]
- Adamski, A.; Zając, W.; Zasada, F.; Sojka, Z. Copper ionic pairs as possible active sites in N2O decomposition on CuOx/CeO2 catalysts. Catal. Today 2012, 191, 129–133. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, P.; Huang, Z.; Qin, F.; Shen, W.; Xu, H. Preparation of NiCe mixed oxides for catalytic decomposition of N2O. Ind. Eng. Chem. Res. 2013, 52, 4504–4509. [Google Scholar] [CrossRef]
- Perez-Alonso, F.; Melián-Cabrera, I.; Granados, M.L.; Kapteijn, F.; Fierro, J.G. Synergy of FexCe1−xO2 mixed oxides for N2O decomposition. J. Catal. 2006, 239, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, L.; Barroo, C.; Gilis, N.; Lambeets, S.V.; Genty, E.; Visart de Bocarmé, T. Structure reactivity relationships during N2O hydrogenation over Au-Ag alloys: A study by field emission techniques. Appl. Surf. Sci. 2018, 435, 914–919. [Google Scholar] [CrossRef]
- Gilis, N.; Jacobs, L.; Barroo, C.; Visart de Bocarmé, T. Surface segregation in Au–Ag alloys investigated by atom probe tomography. Top. Catal. 2018, 61, 1437–1448. [Google Scholar] [CrossRef]
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Bäumer, M. Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 2010, 327, 319–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittstock, A.; Neumann, B.; Schaefer, A.; Dumbuya, K.; Kübel, C.; Biener, M.M.; Zielasek, V.; Steinrück, H.-P.; Gottfried, J.M.; Biener, J.; et al. Nanoporous Au: An unsupported pure gold catalyst? J. Phys. Chem. C 2009, 113, 5593–5600. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Y.; Wang, H.; Ding, Y. Nanoporous gold as an active low temperature catalyst toward CO oxidation in hydrogen-rich stream. Sci. Rep. 2013, 3, 3015. [Google Scholar] [CrossRef]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. On the theoretical understanding of the unexpected O2 activation by nanoporous gold. Chem. Commun. 2011, 47, 8403–8405. [Google Scholar] [CrossRef]
- Fajín, J.L.C.; Moura, A.S.; Cordeiro, M.N.D.S. First-principles-based kinetic Monte Carlo simulations of CO oxidation on catalytic Au(110) and Ag(110) surfaces. Phys. Chem. Chem. Phys. 2021, 23, 14037–14050. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Liu, J.; Shi, R.; Waterhouse, G.I.N.; Wen, X.-D.; Zhang, T. Titania-supported Ni2P/Ni catalysts for selective solar-driven CO hydrogenation. Adv. Mater. 2021, 33, 2103248. [Google Scholar]
- Zhang, Z.; Chen, X.; Kang, J.; Yu, Z.; Tian, J.; Gong, Z.; Jia, A.; You, R.; Qian, K.; He, S.; et al. The active sites of Cu–ZnO catalysts for water gas shift and CO hydrogenation reactions. Nat. Commun. 2021, 12, 4331. [Google Scholar] [CrossRef]
- Van Santen, R.A.; Neurock, M.; Shetty, S.G. Reactivity theory of transition-metal surfaces: A Brønsted-Evans-Polanyi linear activation energy-free-energy analysis. Chem. Rev. 2010, 110, 2005–2048. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 67, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.R.B.; Fajín, J.L.C.; Cordeiro, M.N.D.S.; Teixeira, C.; Gomes, P.; Pillai, R.S.; Novell-Leruth, G.; Toda, J.; Jorge, M. Density functional treatment of interactions and chemical reactions at interfaces. In Density Functional Theory: Principles, Applications and Analysis; Morin, J., Pelletier, J.M., Eds.; Nova Science Publisher, Inc.: New York, NY, USA, 2013; pp. 1–56. ISBN 978-1-62417-955-6. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, S. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 1999, 111, 7010–7022. [Google Scholar] [CrossRef]
- Laidler, K.J. Chemical Kinetics, 3rd ed.; Harper Collins: New York, NY, USA, 1987; p. 193. [Google Scholar]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. Unraveling the mechanism of the NO reduction by CO on gold based catalysts. J. Catal. 2012, 289, 11–20. [Google Scholar] [CrossRef]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Illas, F.; Gomes, J.R.B. Descriptors controlling the catalytic activity of metallic surfaces toward water splitting. J. Catal. 2010, 276, 92–100. [Google Scholar] [CrossRef]
- Tereshchuk, P.; Da Silva, J.L.F. Ethanol and water adsorption on close-packed 3d, 4d, and 5d transition-metal surfaces: A density functional theory investigation with van der Waals correction. J. Phys. Chem. C 2012, 116, 24695–24705. [Google Scholar] [CrossRef]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. Water dissociation on bimetallic surfaces: General trends. J. Phys. Chem. C 2012, 116, 10120–10128. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajín, J.L.C.; Cordeiro, M.N.D.S. N2O Hydrogenation on Silver Doped Gold Catalysts, a DFT Study. Nanomaterials 2022, 12, 394. https://doi.org/10.3390/nano12030394
Fajín JLC, Cordeiro MNDS. N2O Hydrogenation on Silver Doped Gold Catalysts, a DFT Study. Nanomaterials. 2022; 12(3):394. https://doi.org/10.3390/nano12030394
Chicago/Turabian StyleFajín, José L. C., and Maria Natália D. S. Cordeiro. 2022. "N2O Hydrogenation on Silver Doped Gold Catalysts, a DFT Study" Nanomaterials 12, no. 3: 394. https://doi.org/10.3390/nano12030394
APA StyleFajín, J. L. C., & Cordeiro, M. N. D. S. (2022). N2O Hydrogenation on Silver Doped Gold Catalysts, a DFT Study. Nanomaterials, 12(3), 394. https://doi.org/10.3390/nano12030394







