Synthesis and Application of Cobalt Oxide (Co3O4)-Impregnated Olive Stones Biochar for the Removal of Rifampicin and Tigecycline: Multivariate Controlled Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Equipment, and Software
2.2. Preparation of Olive Stone Biochar (OSBC)
2.3. Synthesis of Co-Olive Stone Biochar (Co-OSBC)
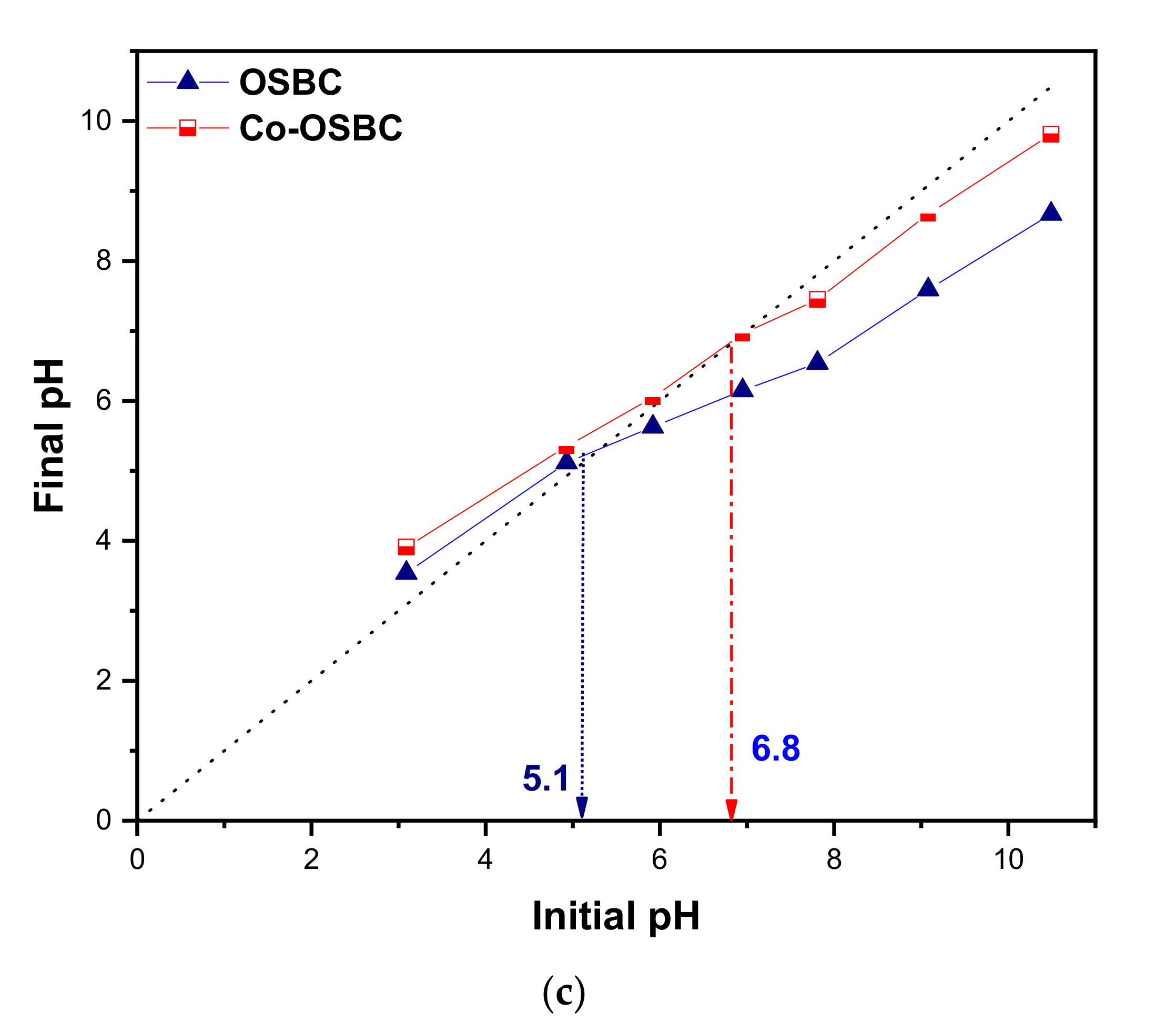
2.4. Point-of-Zero-Charge (pHPZC)
2.5. Investigation of Variables Affecting the Sorption Efficiency of Co-OSBC Using Full Factorial Design (FFD)
2.6. Equilibrium and Kinetic Studies
3. Results and Discussion
3.1. Adsorbents’ Characterization and Surface Chemistry
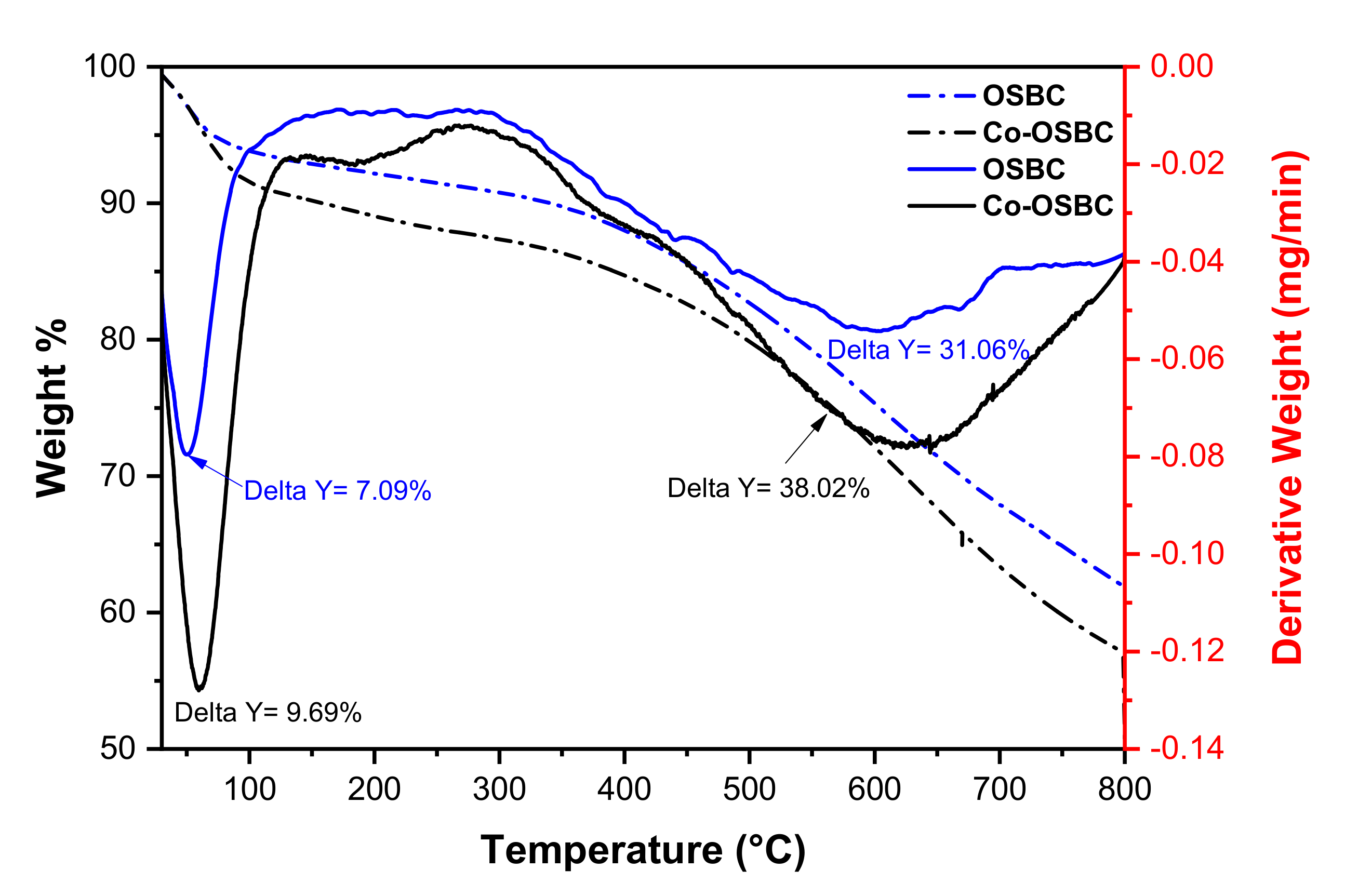
3.1.1. Thermogravimetric Analysis (TGA)
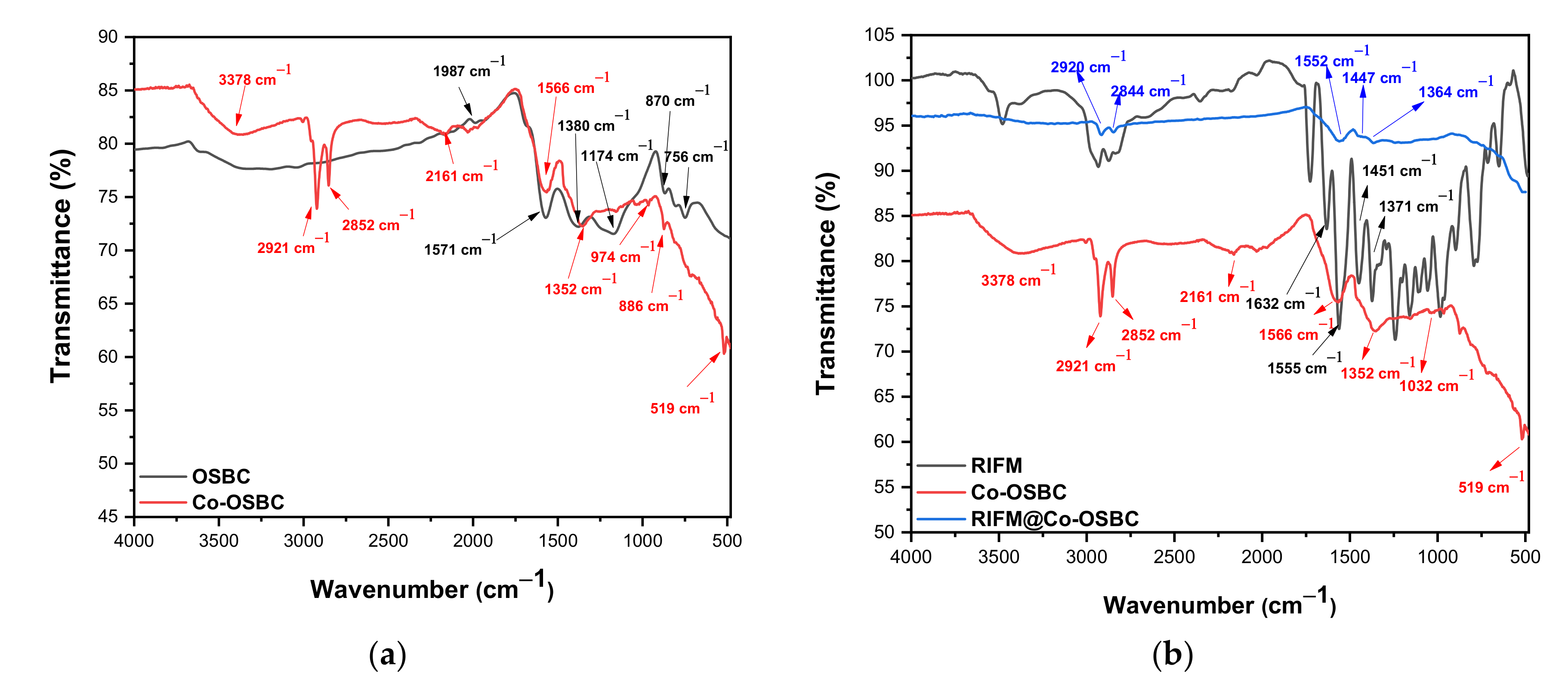
3.1.2. FT-IR Analysis and Point-of-Zero-Charge (pHPZC)
3.1.3. Raman Spectroscopy
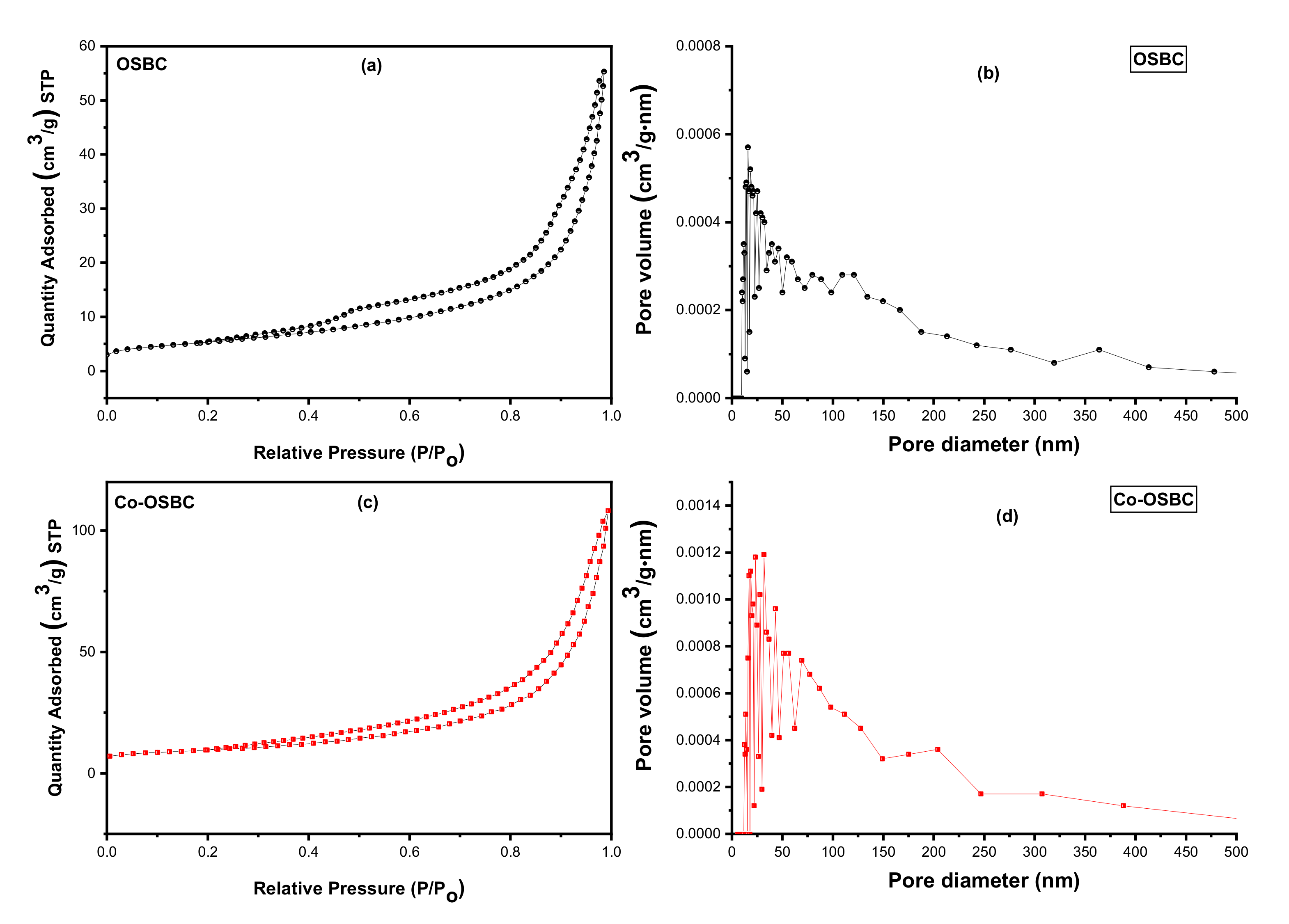
3.1.4. Textural Features
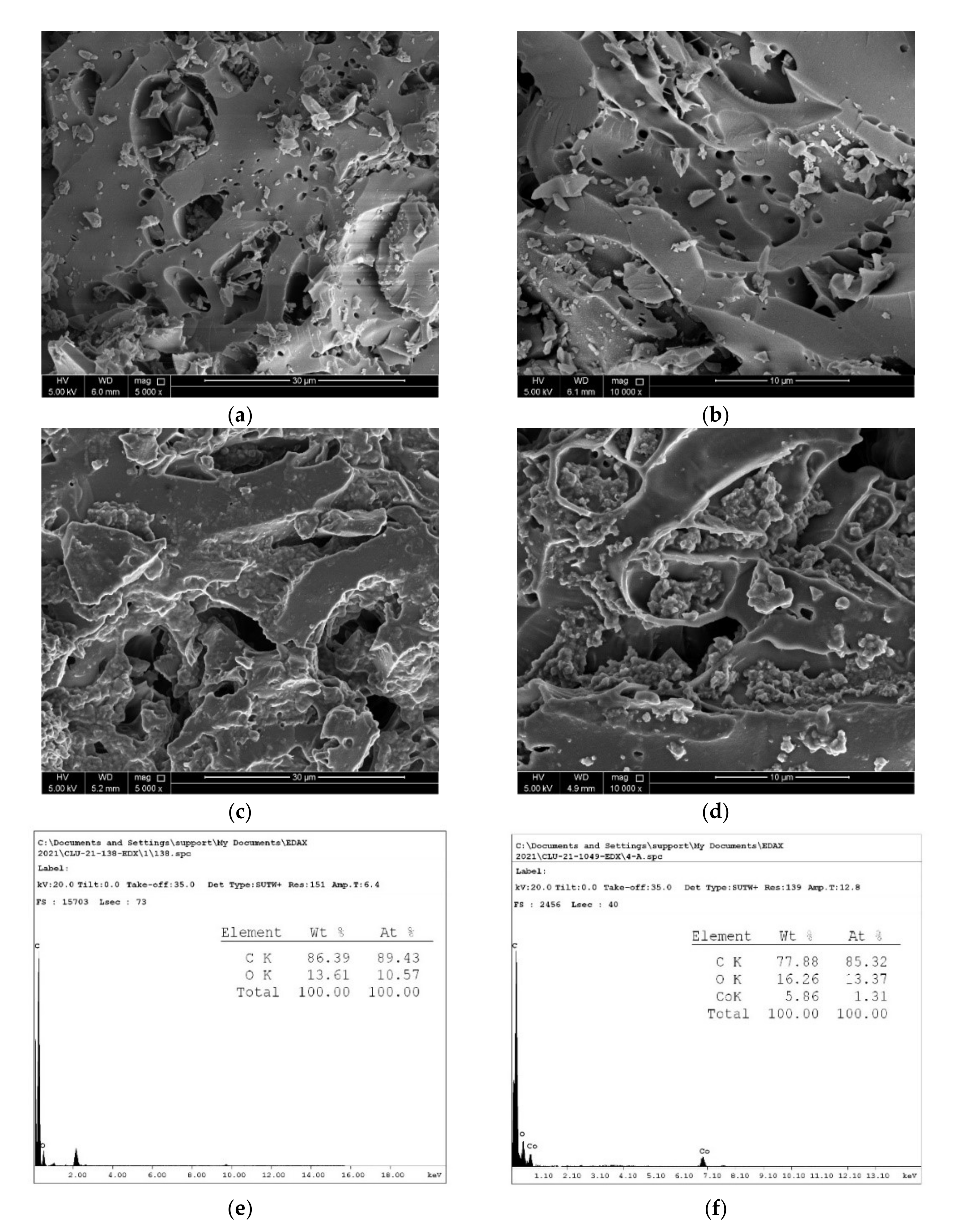
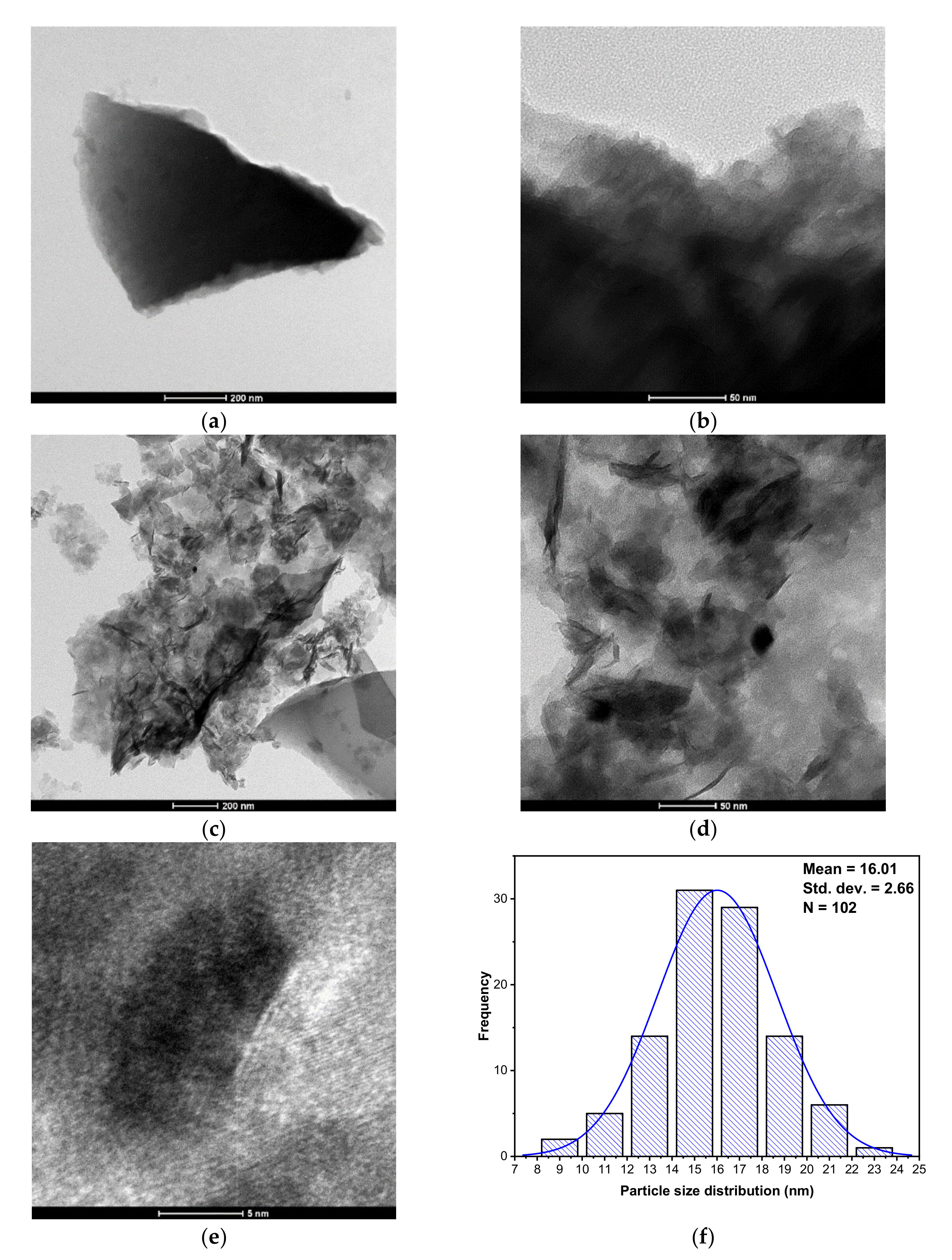
3.1.5. Morphological Features of OSBC and Co-OSBC: SEM, EDX, and TEM Analyses
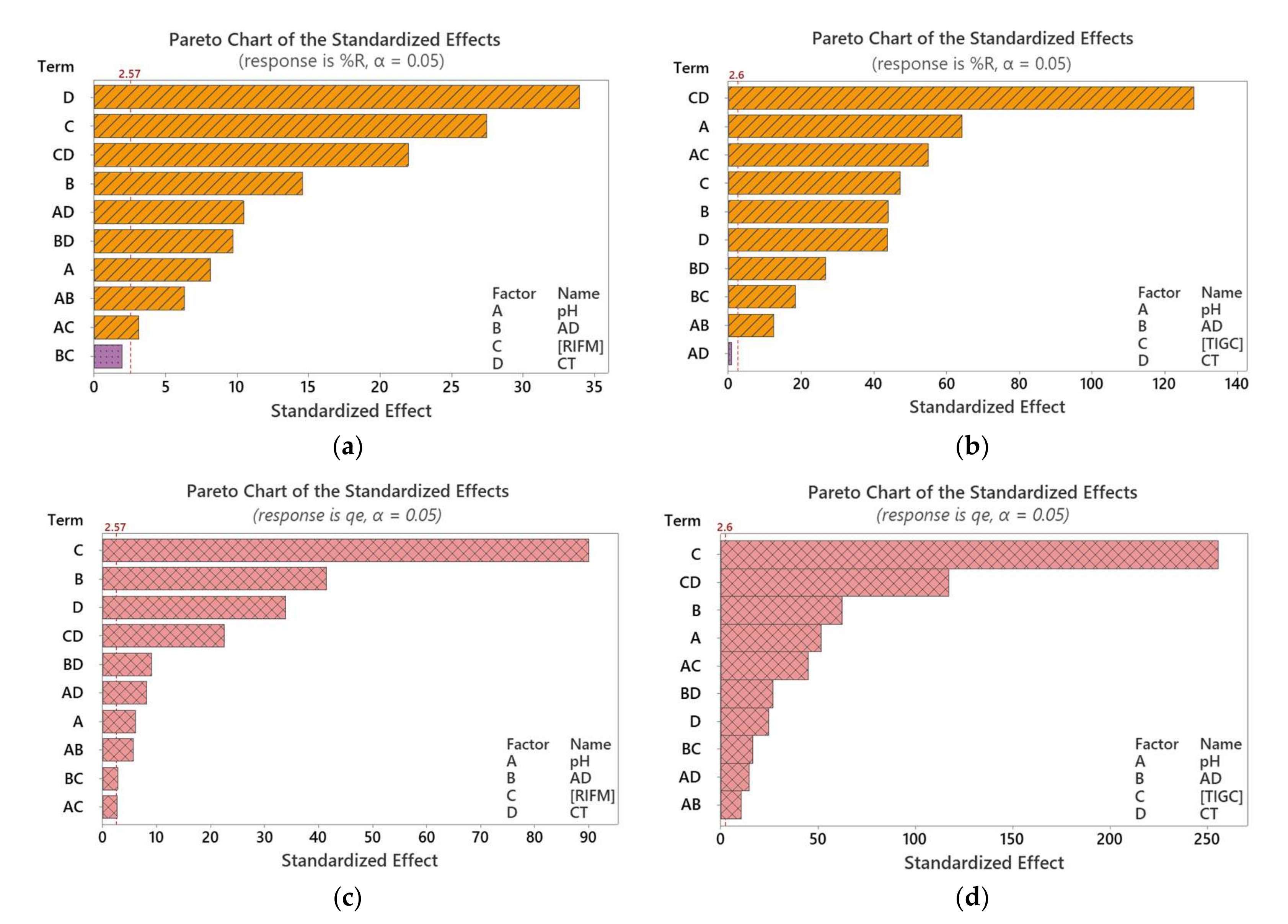
3.2. Full Factorial Design (FFD)
3.2.1. Screening Phase
3.2.2. Development of the Model Equations: Analysis of Variance (ANOVA)
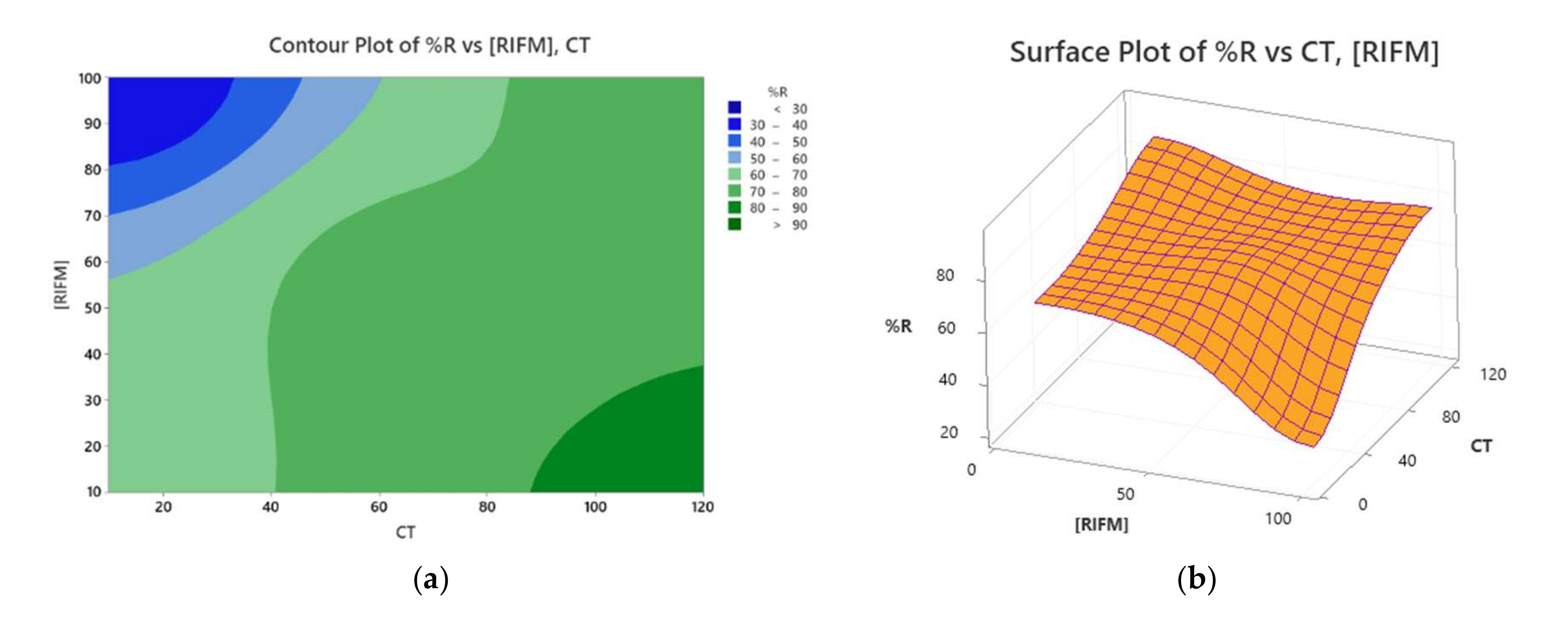
3.2.3. Optimization Phase
3.3. Adsorption Isotherms and Kinetic Studies
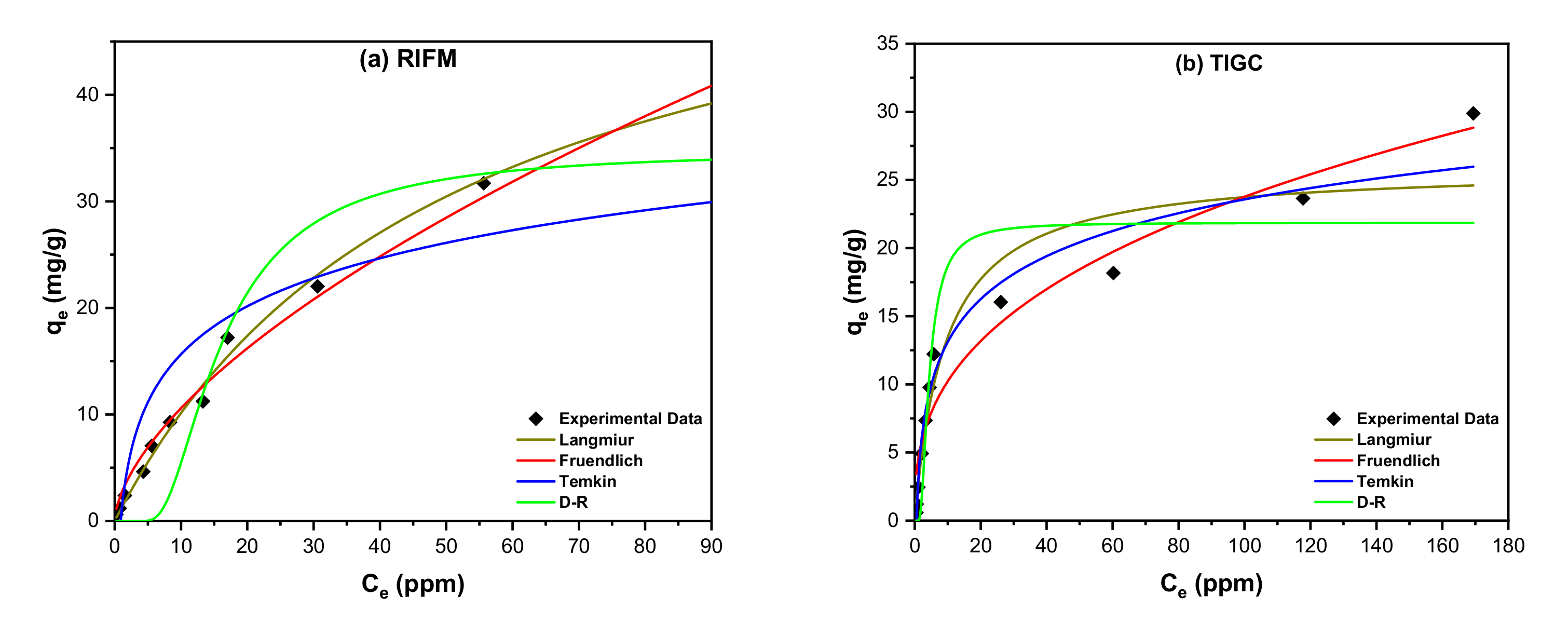
3.3.1. Adsorption Isotherms
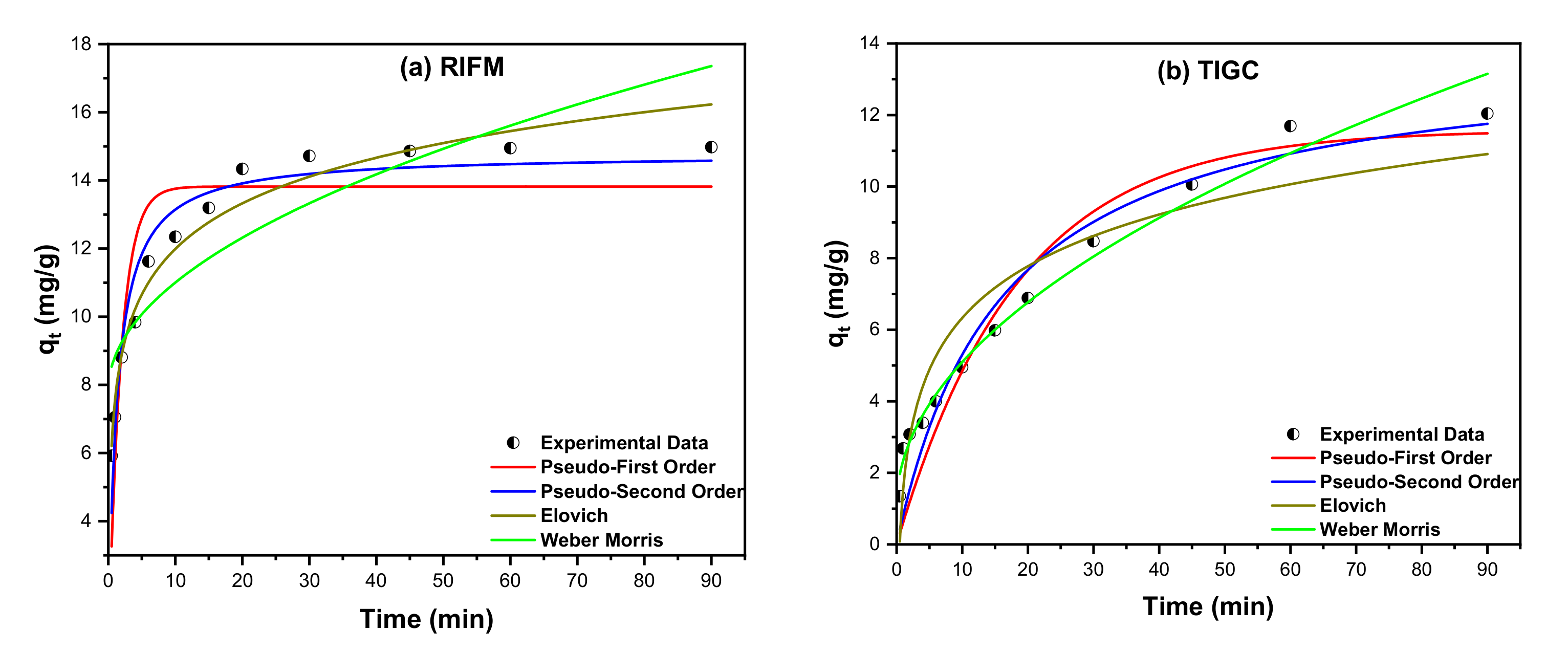
3.3.2. Kinetic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, W.; Duan, L.; Zhang, Y.; Zhou, Y.; Wang, B.; Yu, G. Pollution characterization of pharmaceutically active compounds (PhACs) in the northwest of Tai Lake Basin, China: Occurrence, temporal changes, riverine flux and risk assessment. J. Hazard. Mater. 2022, 422, 126889. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and Consequences of Groundwater Contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jayasiri, H.B.; Purushothaman, C.S. Pharmaceutically Active Compounds (PhACs): A Threat for Aquatic Environment? J. Mar. Sci. Res. Dev. 2013, 4, e122. [Google Scholar] [CrossRef]
- El-Gendy, A.; El-Shafie, A.S.; Issa, A.; Al-Meer, S.; Al-Saad, K.; El-Azazy, M. Carbon-Based Materials (CBMs) for Determination and Remediation of Antimicrobials in Different Substrates: Wastewater and Infant Foods as Examples; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Gin, K.Y.-H. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Wise, R. Antimicrobial resistance: Priorities for action. J. Antimicrob. Chemother. 2002, 49, 585–586. [Google Scholar] [CrossRef]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use–present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Al-Meer, S.; Al-Saad, K.A. Eco-Structured Adsorptive Removal of Tigecycline from Wastewater: Date Pits’ Biochar versus the Magnetic Biochar. Nanomaterials 2021, 11, 30. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, R.; Zhang, H.; Yang, M.; Zhang, Y. Developmental dynamics of antibiotic resistome in aerobic biofilm microbiota treating wastewater under stepwise increasing tigecycline concentrations. Environ. Int. 2019, 131, 105008. [Google Scholar] [CrossRef] [PubMed]
- Kais, H.; Yeddou Mezenner, N.; Trari, M. Biosorption of rifampicin from wastewater using cocoa shells product. Sep. Sci. Technol. 2020, 55, 1984–1993. [Google Scholar] [CrossRef]
- Xu, G.; Liu, H.; Jia, X.; Wang, X.; Xu, P. Mechanisms and detection methods of Mycobacterium tuberculosis rifampicin resistance: The phenomenon of drug resistance is complex. Tuberculosis 2021, 128, 102083. [Google Scholar] [CrossRef]
- Rico, A.; Jacobs, R.; Van den Brink, P.J.; Tello, A. A probabilistic approach to assess antibiotic resistance development risks in environmental compartments and its application to an intensive aquaculture production scenario. Environ. Pollut. 2017, 231, 918–928. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, M.J.; Smith, A.; Heckelman, P.E.; Budavari, S. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals; Merck and Co. Inc.: Whitehouse Station, NJ, USA, 2001; Volume 767, p. 1474. [Google Scholar]
- Dorn, C.; Kratzer, A.; Liebchen, U.; Schleibinger, M.; Murschhauser, A.; Schlossmann, J.; Kees, F.; Simon, P.; Kees, M.G. Impact of experimental variables on the protein binding of tigecycline in human plasma as determined by ultrafiltration. J. Pharm. Sci. 2018, 107, 739–744. [Google Scholar] [CrossRef]
- FDA. Drug Approval Package: Tygacil (Tigecycline) NDA#021821. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21-821_Tygacil.cfm (accessed on 16 December 2021).
- Cai, W.; Weng, X.; Chen, Z. Highly efficient removal of antibiotic rifampicin from aqueous solution using green synthesis of recyclable nano-Fe3O4. Environ. Pollut. 2019, 247, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Van Ingen, J.; Aarnoutse, R.E.; Donald, P.R.; Diacon, A.H.; Dawson, R.; Plemper van Balen, G.; Gillespie, S.H.; Boeree, M.J. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin. Infect. Dis. 2011, 52, e194–e199. [Google Scholar] [CrossRef] [Green Version]
- Soni, H.; Gautam, D.; Sharma, S.; Malik, J. Rifampicin as potent inhibitor of COVID-19 main protease: In-silico docking approach. Saudi J. Med. Pharm. Sci. 2020, 6, 588–593. [Google Scholar] [CrossRef]
- Shafaati, M.; Miralinaghi, M.; Shirazi, R.H.S.M.; Moniri, E. The use of chitosan/Fe3O4 grafted graphene oxide for effective adsorption of rifampicin from water samples. Res. Chem. Intermed. 2020, 46, 5231–5254. [Google Scholar] [CrossRef]
- Marathe, N.P.; Svanevik, C.S.; Ghavidel, F.Z.; Grevskott, D.H. First report of mobile tigecycline resistance gene tet (X4)-harbouring multidrug-resistant Escherichia coli from wastewater in Norway. J. Glob. Antimicrob. Resist. 2021, 27, 37–40. [Google Scholar] [CrossRef]
- Greer, N.D. Tigecycline (Tygacil): The first in the glycylcycline class of antibiotics. Proc. Bayl. Univ. Med. Cent. Proc. 2006, 19, 155–161. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Drug Safety Communication: Increased Risk of Death with Tygacil (Tigecycline) Compared to Other Antibiotics Used to Treat Similar Infections. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-increased-risk-death-tygacil-tigecycline-compared-other-antibiotics (accessed on 18 December 2021).
- Hu, Q.; Jung, J.; Chen, D.; Leong, K.; Song, S.; Li, F.; Mohan, B.C.; Yao, Z.; Prabhakar, A.K.; Lin, X.H.; et al. Biochar industry to circular economy. Sci. Total Environ. 2021, 757, 143820. [Google Scholar] [CrossRef]
- Varma, R.S. Biomass-Derived Renewable Carbonaceous Materials for Sustainable Chemical and Environmental Applications. ACS Sustain. Chem. Eng. 2019, 7, 6458–6470. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.S.; El-Shafie, A.S.; Zaher, N.; El-Azazy, M. Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD). Molecules 2020, 25, 3752. [Google Scholar] [CrossRef]
- El-Shafie, A.S.; Hassan, S.S.; Akther, N.; El-Azazy, M. Watermelon rinds as cost-efficient adsorbent for acridine orange: A response surface methodological approach. Environ. Sci. Pollut. Res. 2021, in press. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Chai, C.; Cao, S.; Bai, X.; Ma, K.; Jin, X.; Shi, X.; Jin, P. Adsorption of micropollutants from wastewater using iron and nitrogen co-doped biochar: Performance, kinetics and mechanism studies. J. Hazard. Mater. 2022, 424, 127606. [Google Scholar] [CrossRef] [PubMed]
- El-Azazy, M.; El-Shafie, A.S.; El-Gendy, A.; Issa, A.; Al-Meer, S.; Al-Saad, K. A comparison between different agro-wastes and carbon nanotubes for Removal of sarafloxacin from wastewater: Kinetics and equilibrium studies. Molecules 2020, 25, 5429. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Juela, D.M. Promising adsorptive materials derived from agricultural and industrial wastes for antibiotic removal: A comprehensive review. Sep. Purif. Technol. 2021, 284, 120286. [Google Scholar] [CrossRef]
- Shen, W.; Guo, Q.; Zhang, Y.; Liu, Y.; Zheng, J.; Cheng, J.; Fan, J. The effect of activated carbon fiber structure and loaded copper, cobalt, silver on the adsorption of dichloroethylene. Colloids Surf. A Physicochem. Eng. Asp. 2006, 273, 147–153. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Liu, M.; Wang, X.; Wu, Z.; Yang, L.; Xia, S.; Zhao, J. Cr (VI) removal from water using cobalt-coated bamboo charcoal prepared with microwave heating. Ind. Crops Prod. 2012, 39, 81–88. [Google Scholar] [CrossRef]
- Gul, E.; Al Bkoor Alrawashdeh, K.; Masek, O.; Skreiberg, Ø.; Corona, A.; Zampilli, M.; Wang, L.; Samaras, P.; Yang, Q.; Zhou, H.; et al. Production and use of biochar from lignin and lignin-rich residues (such as digestate and olive stones) for wastewater treatment. J. Anal. Appl. Pyrolysis 2021, 158, 105263. [Google Scholar] [CrossRef]
- Arrebola, J.C.; Rodríguez-Fernández, N.; Caballero, Á. Decontamination of Wastewater Using Activated Biochar from Agricultural Waste: A Practical Experiment for Environmental Sciences Students. J. Chem. Educ. 2020, 97, 4137–4144. [Google Scholar] [CrossRef]
- El-Azazy, M.; Nabil, I.; Hassan, S.S.; El-Shafie, A.S. Adsorption Characteristics of Pristine and Magnetic Olive Stones Biochar with Respect to Clofazimine. Nanomaterials 2021, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A review of waste management options in olive oil production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Elazazy, M.S.; Ganesh, K.; Sivakumar, V.; Huessein, Y.H.A. Interaction of p-synephrine with p-chloranil: Experimental design and multiple response optimization. RSC Adv. 2016, 6, 64967–64976. [Google Scholar] [CrossRef]
- Benhabiles, S.; Rida, K. Production of efficient activated carbon from sawdust for the removal of dyes in single and binary systems–a full factorial design. Part. Sci. Technol. 2021, 39, 237–251. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Zhang, Y.; Lin, T.; Zeng, G.; Zhang, S.; Wang, Y.; He, W.; Zhang, M.; Long, H. An efficient adsorbent: Simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption. Bioresour. Technol. 2019, 288, 121511. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.e.k.; Bouhfid, R. Functional CoFe2O4-modified biochar derived from banana pseudostem as an efficient adsorbent for the removal of amoxicillin from water. Sep. Purif. Technol. 2021, 266, 118592. [Google Scholar] [CrossRef]
- Hu, B.; Tang, Y.; Wang, X.; Wu, L.; Nong, J.; Yang, X.; Guo, J. Cobalt-gadolinium modified biochar as an adsorbent for antibiotics in single and binary systems. Microchem. J. 2021, 166, 106235. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Q.; Lu, P.; Chen, M.; Yang, H. Degradation mechanisms of cefotaxime using biochar supported Co/Fe bimetallic nanoparticles. Environ. Sci. Water Res. Technol. 2018, 4, 964–975. [Google Scholar] [CrossRef]
- Jafari, K.; Heidari, M.; Rahmanian, O. Wastewater treatment for Amoxicillin removal using magnetic adsorbent synthesized by ultrasound process. Ultrason. Sonochem. 2018, 45, 248–256. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, Z.; Zhou, Y.; Wei, Y.; Long, X.; He, Y.; Zhi, D.; Yang, J.; Luo, L. A sustainable ferromanganese biochar adsorbent for effective levofloxacin removal from aqueous medium. Chemosphere 2019, 237, 124464. [Google Scholar] [CrossRef] [PubMed]
- El-Azazy, M.; El-Shafie, A.S.; Morsy, H. Biochar of Spent Coffee Grounds as Per Se and Impregnated with TiO2: Promising Waste-Derived Adsorbents for Balofloxacin. Molecules 2021, 26, 2295. [Google Scholar] [CrossRef] [PubMed]
- Bumajdad, A.; Zaki, M.I.; Eastoe, J.; Pasupulety, L. Microemulsion-based synthesis of CeO2 powders with high surface area and high-temperature stabilities. Langmuir 2004, 20, 11223–11233. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xu, Z.; Wei, Y.; Zhou, Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.; Luo, L.; Zhou, Z. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef]
- Yang, Y.-p.; Liu, R.-s.; HUANG, K.-l.; Zhang, P. Preparation and characterization of Co3O4 by thermal decomposition from Co2(OH)2CO3. J. Cent. South Univ. Technol. 2008, 39, 108–111. [Google Scholar]
- Abdelhadi, S.O.; Dosoretz, C.G.; Rytwo, G.; Gerchman, Y.; Azaizeh, H. Production of biochar from olive mill solid waste for heavy metal removal. Bioresour. Technol. 2017, 244, 759–767. [Google Scholar] [CrossRef]
- Sharma, S.; Rahman, N.; Azmi, S.N.H. Determination of thorium (IV) with rifampicin in synthetic mixture and soil samples by spectrophotometry. Arab. J. Chem. 2016, 9, S1163–S1169. [Google Scholar]
- Ivashchenko, O.; Tomila, T.; Ulyanchich, N.; Yarmola, T.; Uvarova, I. Fourier-Transform Infrared Spectroscopy of antibiotic loaded Ag-free and Ag-doped hydroxyapatites. Adv. Sci. Eng. Med. 2014, 6, 193–202. [Google Scholar] [CrossRef]
- Moubarik, A.; Grimi, N. Valorization of olive stone and sugar cane bagasse by-products as biosorbents for the removal of cadmium from aqueous solution. Food Res. Int. 2015, 73, 169–175. [Google Scholar] [CrossRef]
- Na, C.W.; Woo, H.-S.; Kim, H.-J.; Jeong, U.; Chung, J.-H.; Lee, J.-H. Controlled transformation of ZnO nanobelts into CoO/Co3O4 nanowires. Cryst. Eng. Comm. 2012, 14, 3737–3741. [Google Scholar] [CrossRef] [Green Version]
- Chlebda, D.K.; Jodłowski, P.J.; Jędrzejczyk, R.J.; Łojewska, J. 2D-COS of in situ μ-Raman and in situ IR spectra for structure evolution characterisation of NEP-deposited cobalt oxide catalyst during n-nonane combustion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 186, 44–51. [Google Scholar] [CrossRef]
- Gao, X.; Huang, C.J.; Zhang, N.; Li, J.; Weng, W.Z.; Wan, H. Partial oxidation of methane to synthesis gas over Co/Ca/Al2O3 catalysts. Catal. Today 2008, 131, 211–218. [Google Scholar] [CrossRef]
- Rashad, M.; Rüsing, M.; Berth, G.; Lischka, K.; Pawlis, A. CuO and Co3O4 nanoparticles: Synthesis, characterizations, and Raman spectroscopy. J. Nanomater. 2013, 2013, 82. [Google Scholar] [CrossRef] [Green Version]
- Buttersack, C. Modeling of type IV and V sigmoidal adsorption isotherms. Phys. Chem. Chem. Phys. 2019, 21, 5614–5626. [Google Scholar] [CrossRef] [Green Version]
- Box, G.E.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wang, J. Comparison of linearization methods for modeling the Langmuir adsorption isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Araújo, C.S.; Almeida, I.L.; Rezende, H.C.; Marcionilio, S.M.; Léon, J.J.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb (II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Moussavi, G.; Barikbin, B. Biosorption of chromium (VI) from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
- Sarma, G.K.; Khan, A.; El-Toni, A.M.; Rashid, M.H. Shape-tunable CuO-Nd(OH)3 nanocomposites with excellent adsorption capacity in organic dye removal and regeneration of spent adsorbent to reduce secondary waste. J. Hazard. Mater. 2019, 380, 120838. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yuan, Y.; Li, H.; Li, X.; Zhang, C.; Guo, F.; Liu, X.; Wang, K.; Zhao, X. Waste-cellulose-derived porous carbon adsorbents for methyl orange removal. Chem. Eng. J. 2019, 371, 55–63. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]


| Feedstock | Nanosorbent | Antibiotic(s) | Method of Nanoparticles Preparation | Parameters Optimization | Surface Area (m2/g) | qe (mg/g) | %R | Ref |
|---|---|---|---|---|---|---|---|---|
| Olive Stones | Cobalt Oxide (Co3O4)-Olive Stone Biochar (Co-OSBC) | Rifampicin (RIFM) Tigecycline (TIGC) | Microemulsion Method | Full Factorial Design (FFD) | 39.85 | 61.1025.94 | 95.18 75.48 | Current Study |
| Camphor Leaf | ZnO Nanoparticle Modified Magnetic Biochar | Ciprofloxacin | Ultrasound Assisted Wet-Chemical Method | Univariate Analysis | 950 | 449.40 * | ** NS | [41] |
| Banana Pseudostem (BP) Fibers | CoFe2O4-Modified Biochar (BP350 and BP650) | Amoxicillin | Co-Precipitation | Univariate Analysis | BP350:100.9 BP650:190.5 | 99.99 | ** NS | [42] |
| Camellia oleifera Shells | Cobalt-Gadolinium Modified Biochar | Ciprofloxacin Tetracycline | Mixing of Co(II) and Gd(III) with the Biochar, Shaking, Drying then Pyrolysis | Univariate Analysis | 370.3737 | 44.44 119.05 | System-Dependent | [43] |
| Sawdust | Modified Biochar Supported Co/Fe Nanoparticles (Co/Fe/MB) | Cefotaxime | Liquid Phase Reduction Method under Anaerobic Conditions | Univariate Analysis | 262 | 30.07 | 99.23 | [44] |
| Olive Kernel | Magnetic Adsorbent Prepared from Olive Kernel | Amoxicillin | Ultrasonic Process | Univariate Analysis | 2188.0 | 238.1 | ** NS | [45] |
| Vinasse Wastes | Ferromanganese Modified Biochar (Fe/Mn-BC) | Levofloxacin | Co-Precipitation | Univariate Analysis | 93.4 | 181 | ** NS | [46] |
| Spent Coffee Grounds | Spent Coffee Grounds Biochar Impregnated with Titanium Oxide (TiO2) | Balofloxacin | Hydrothermal Method with Modifications | Plackett-Burman Design | 50.54 | 196.73 | 91.78 | [47] |
| Factors and Units | −1 | 0 | +1 |
|---|---|---|---|
| pH (A, pH Unit) | 5.0 | 7.0 | 9.0 |
| Sorbent Dosage (AD, B, mg/13 mL) | 50.0 | 100.0 | 150.0 |
| Initial Antibiotic Concentration ([Antibiotic], C, ppm) | 10.0 | 55.0 | 100.0 |
| Contact Time (CT, D, min) | 10.0 | 65.0 | 120.0 |
| Response Variables | Percentage Removal (%R) | ||
| Adsorption Capacity (qe, mg/g) | |||
| Run Number | Block | Variables | RIFM | TIGC | |||||
|---|---|---|---|---|---|---|---|---|---|
| pH | AD | [Antibiotic] | CT | %R | qe | %R | qe | ||
| 01 | 2 | 9 (+) | 150 (+) | 100 (+) | 10 (−) | 38.80 | 3.35 | 26.24 | 2.27 |
| 02 | 2 | 5 (−) | 50 (−) | 10 (−) | 10 (−) | 42.34 | 1.07 | 2.64 | 0.07 |
| 03 | 2 | 9 (+) | 50 (−) | 10 (−) | 120 (+) | 82.59 | 2.15 | 32.37 | 0.84 |
| 04 | 2 | 7 (0) | 100 (0) | 55 (0) | 65 (0) | 76.00 | 5.43 | 2.96 | 0.21 |
| 05 | 2 | 5 (−) | 150 (+) | 100 (+) | 120 (+) | 87.08 | 7.55 | 14.47 | 1.25 |
| 06 | 1 | 9 (+) | 50 (−) | 100 (+) | 120 (+) | 69.98 | 18.20 | 11.48 | 2.98 |
| 07 | 1 | 9 (+) | 150 (+) | 10 (−) | 10 (−) | 83.93 | 0.73 | 10.66 | 0.09 |
| 08 | 1 | 5 (−) | 50 (−) | 100 (+) | 10 (−) | 19.86 | 5.10 | 29.05 | 7.39 |
| 09 | 1 | 5 (−) | 150 (+) | 10 (−) | 120 (+) | 95.18 | 0.82 | 27.89 | 0.24 |
| 10 | 1 | 7 (0) | 100 (0) | 55 (0) | 65 (0) | 73.43 | 5.25 | 2.85 | 0.20 |
| 11 | 4 | 7 (0) | 100 (0) | 55 (0) | 65 (0) | 73.90 | 5.28 | 3.86 | 0.28 |
| 12 | 4 | 5 (−) | 150 (+) | 100 (+) | 10 (−) | 32.74 | 2.84 | 39.08 | 3.39 |
| 13 | 4 | 9 (+) | 150 (+) | 100 (+) | 120 (+) | 83.84 | 7.27 | 19.80 | 1.72 |
| 14 | 4 | 9 (+) | 50 (−) | 10 (−) | 10 (−) | 71.04 | 1.85 | 10.32 | 0.27 |
| 15 | 4 | 5 (−) | 50 (−) | 10 (−) | 120 (+) | 91.45 | 2.38 | 11.46 | 0.30 |
| 16 | 3 | 5 (−) | 150 (+) | 10 (−) | 10 (−) | 76.52 | 0.66 | 3.55 | 0.03 |
| 17 | 3 | 9 (+) | 50 (−) | 100 (+) | 10 (−) | 29.38 | 7.64 | 26.31 | 6.84 |
| 18 | 3 | 7 (0) | 100 (0) | 55 (0) | 65 (0) | 74.20 | 5.31 | 2.35 | 0.17 |
| 19 | 3 | 9 (+) | 150 (+) | 10 (−) | 120 (+) | 77.59 | 0.67 | 75.46 | 0.65 |
| 20 | 3 | 5 (−) | 50 (−) | 100 (+) | 120 (+) | 64.19 | 16.69 | 7.31 | 1.90 |
| Parameters | OSBC | Co-OSBC |
|---|---|---|
| Langmuir Surface Area (m2/g) | 22.20 | 39.85 |
| Total Pore Volume (cm3/g) | 0.086 | 0.168 |
| Average Pore Radius (Å) | 86.1 | 93.0 |
| Contaminant | Response | R2% | R2–adj% | R2–pred% | Optimum Conditions and Desirability (d) Values |
|---|---|---|---|---|---|
| RIFM | %R | 99.84 | 99.39 | 98.28 | pH = 5.0, AD = 150 mg, [RIFM] = 10 ppm, CT = 120 min, (d = 1.0000, %R = 100%) |
| qe | 99.96 | 99.85 | 99.45 | pH = 9.0, AD = 50 mg, [RIFM] = 100 ppm, CT = 120 min, (d = 1.0000, qe = 18.30 mg/g) | |
| TIGC | %R | 99.82 | 99.31 | 96.06 | pH = 9.0, AD = 150 mg, [TIGC] = 10 ppm, CT = 120 min, (d = 1.0000, %R = 82.37%) |
| qe | 99.99 | 99.97 | 99.87 | pH = 9.0, AD = 50 mg, [TIGC] = 100 ppm, CT = 10 min, (d = 1.0000, qe = 7.83 mg/g) |
| Isotherm | Equations (Nonlinear Forms) | Parameters | Value | |
|---|---|---|---|---|
| RIFM | TIGC | |||
| Langmuir | qm (mg/g) | 61.10 | 25.94 | |
| KL (L·mol−1) | 0.019 | 0.108 | ||
| R2 | 0.9748 | 0.9299 | ||
| Freundlich | 1/n | 0.61 | 0.37 | |
| KF (mol/g) (L/mol)1/n | 2.57 | 4.39 | ||
| R2 | 0.9894 | 0.9481 | ||
| Temkin | bT (J/mol) | 381.28 | 544.71 | |
| AT (L/mol) | 1.112 | 1.783 | ||
| R2 | 0.8091 | 0.9629 | ||
| D–R | ) | 6.63 × 10−8 | 5.67 × 10−9 | |
| E (kJ/mol) | 2.746 | 9.391 | ||
| qm (mg/g) | 34.76 | 21.87 | ||
| R2 | 0.8839 | 0.8568 | ||
| Models | Parameter | Value | |
|---|---|---|---|
| RIFM | TIGC | ||
| = k1(qe−qt) | K1 (min−1) | 0.539 | 0.0543 |
| qe (mg/g) | 13.82 | 11.58 | |
| R2 | 0.7907 | 0.9085 | |
| = k2(qe−qt)2 | K2 (g·mg−1·min−1) | 0.054 | 0.004 |
| qe (mg/g) | 14.78 | 13.86 | |
| R2 | 0.9356 | 0.9346 | |
| α | 96.225 | 4.339 | |
| β | 0.518 | 0.4796 | |
| R2 | 0.9624 | 0.9033 | |
| KI | 1.004 | 1.273 | |
| C | 7.826 | 1.071 | |
| R2 | 0.7582 | 0.9802 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shafie, A.S.; Ahsan, I.; Radhwani, M.; Al-Khangi, M.A.; El-Azazy, M. Synthesis and Application of Cobalt Oxide (Co3O4)-Impregnated Olive Stones Biochar for the Removal of Rifampicin and Tigecycline: Multivariate Controlled Performance. Nanomaterials 2022, 12, 379. https://doi.org/10.3390/nano12030379
El-Shafie AS, Ahsan I, Radhwani M, Al-Khangi MA, El-Azazy M. Synthesis and Application of Cobalt Oxide (Co3O4)-Impregnated Olive Stones Biochar for the Removal of Rifampicin and Tigecycline: Multivariate Controlled Performance. Nanomaterials. 2022; 12(3):379. https://doi.org/10.3390/nano12030379
Chicago/Turabian StyleEl-Shafie, Ahmed S., Insharah Ahsan, Mohamed Radhwani, Mohammed Ali Al-Khangi, and Marwa El-Azazy. 2022. "Synthesis and Application of Cobalt Oxide (Co3O4)-Impregnated Olive Stones Biochar for the Removal of Rifampicin and Tigecycline: Multivariate Controlled Performance" Nanomaterials 12, no. 3: 379. https://doi.org/10.3390/nano12030379
APA StyleEl-Shafie, A. S., Ahsan, I., Radhwani, M., Al-Khangi, M. A., & El-Azazy, M. (2022). Synthesis and Application of Cobalt Oxide (Co3O4)-Impregnated Olive Stones Biochar for the Removal of Rifampicin and Tigecycline: Multivariate Controlled Performance. Nanomaterials, 12(3), 379. https://doi.org/10.3390/nano12030379









