Performance of TiO2/UV-LED-Based Processes for Degradation of Pharmaceuticals: Effect of Matrix Composition and Process Variables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Water Matrices
2.3. Experimental Set-Up
2.4. Analytical Determination
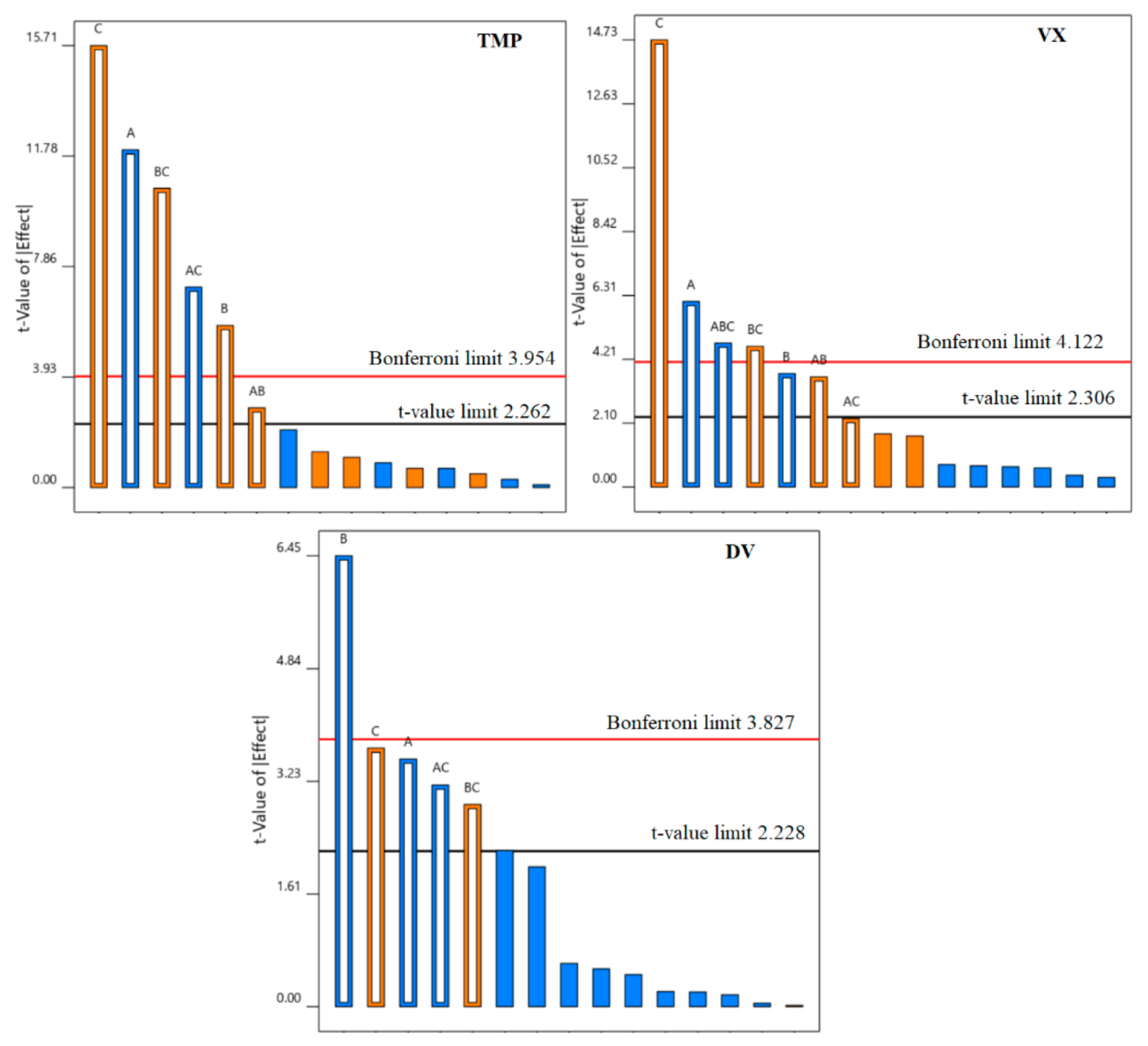
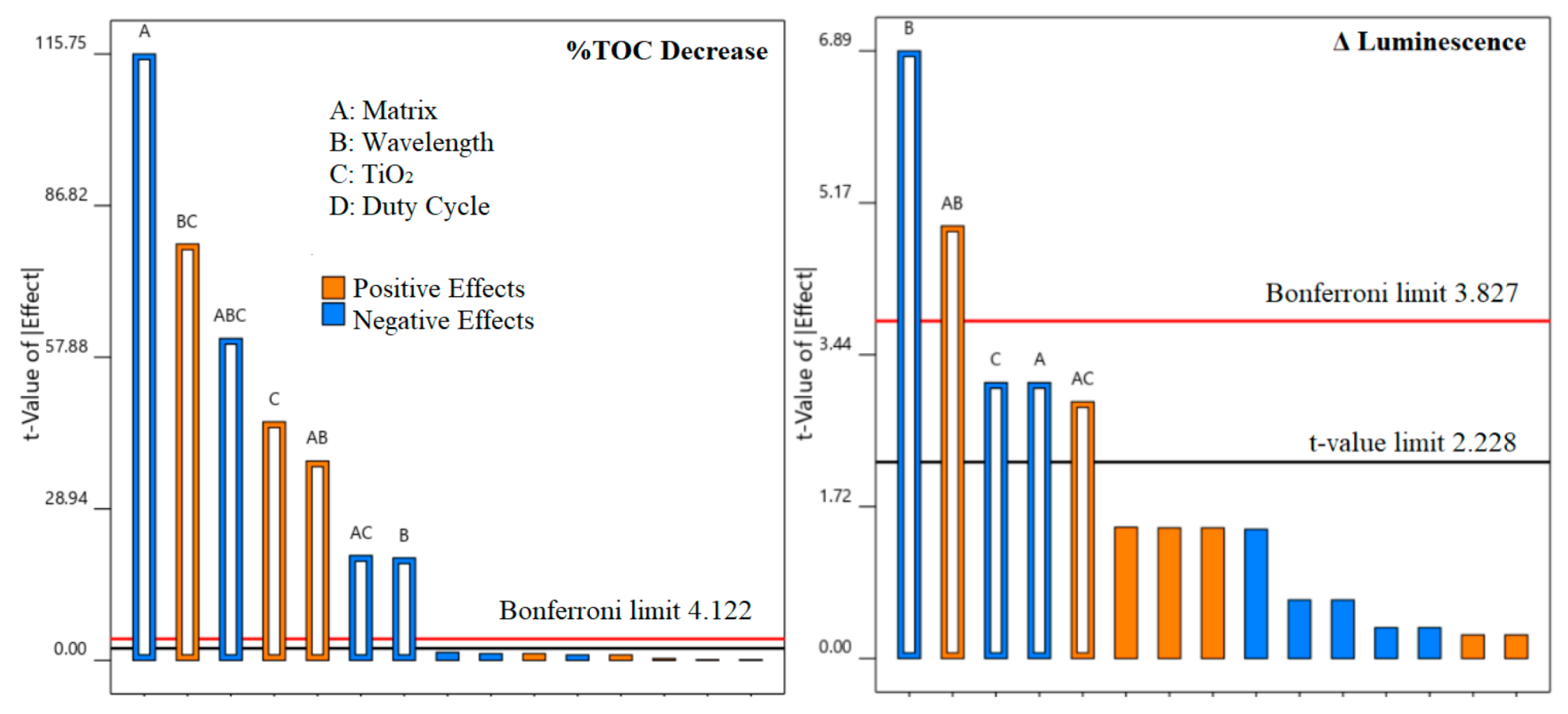
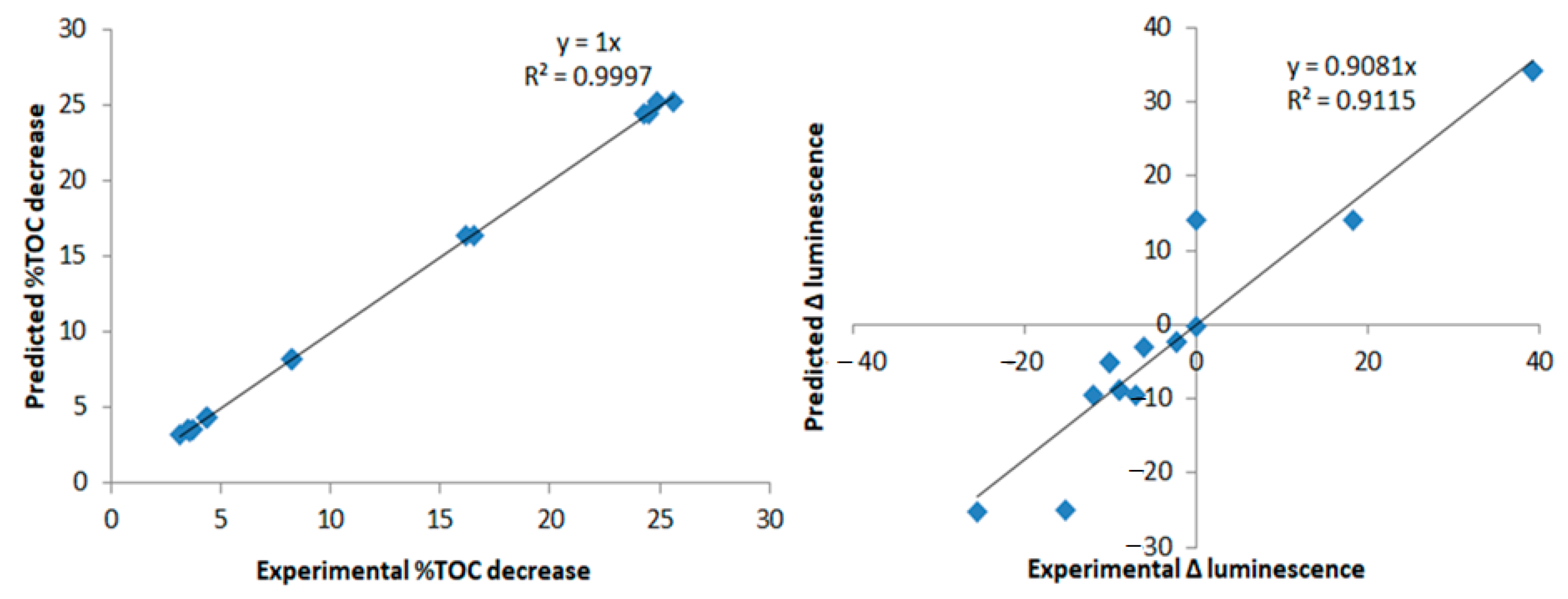
2.5. Design of Experiments
2.6. Electrical Energy per Order (EEO) Analysis
3. Results and Discussion
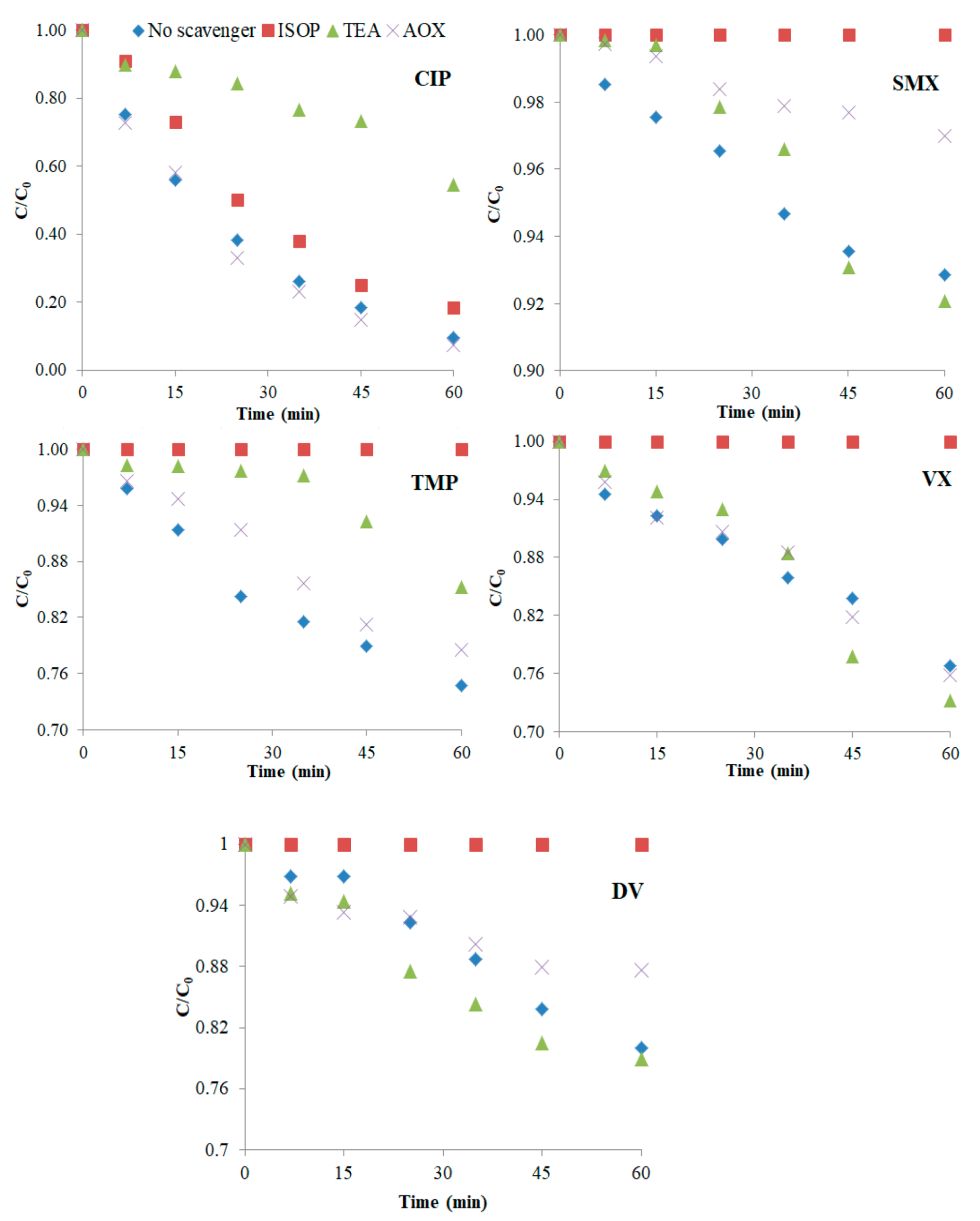
3.1. PhAC Degradation: Individually and in a Mixture
3.2. The Effect of the Catalyst, Light Wavelength, Controlled Periodic Illumination, and Matrix
3.2.1. kapp Analysis
3.2.2. Total Organic Carbon and Toxicity Analysis
3.3. Mechanism of Degradation
3.4. Effect of the Initial pH
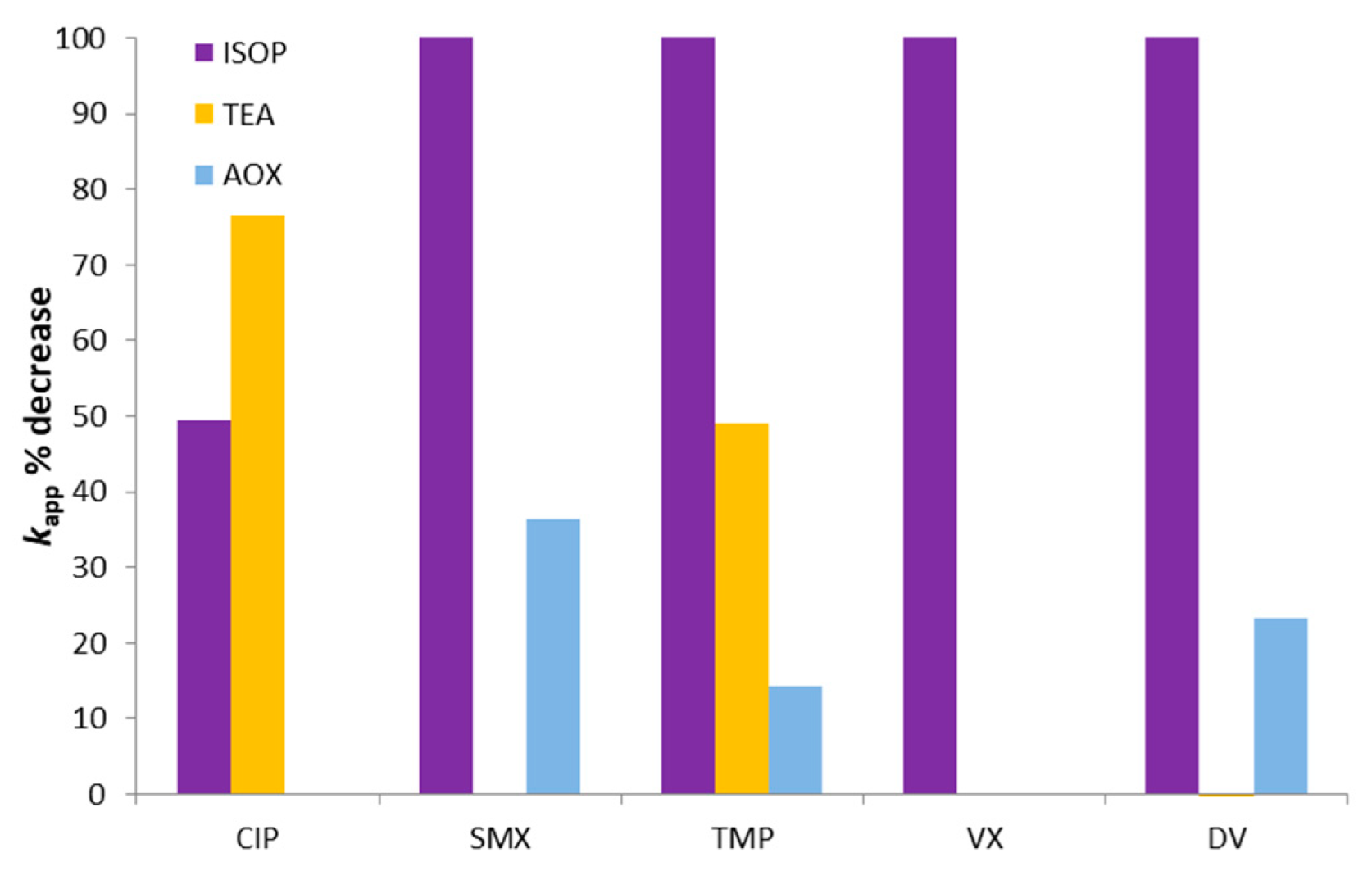
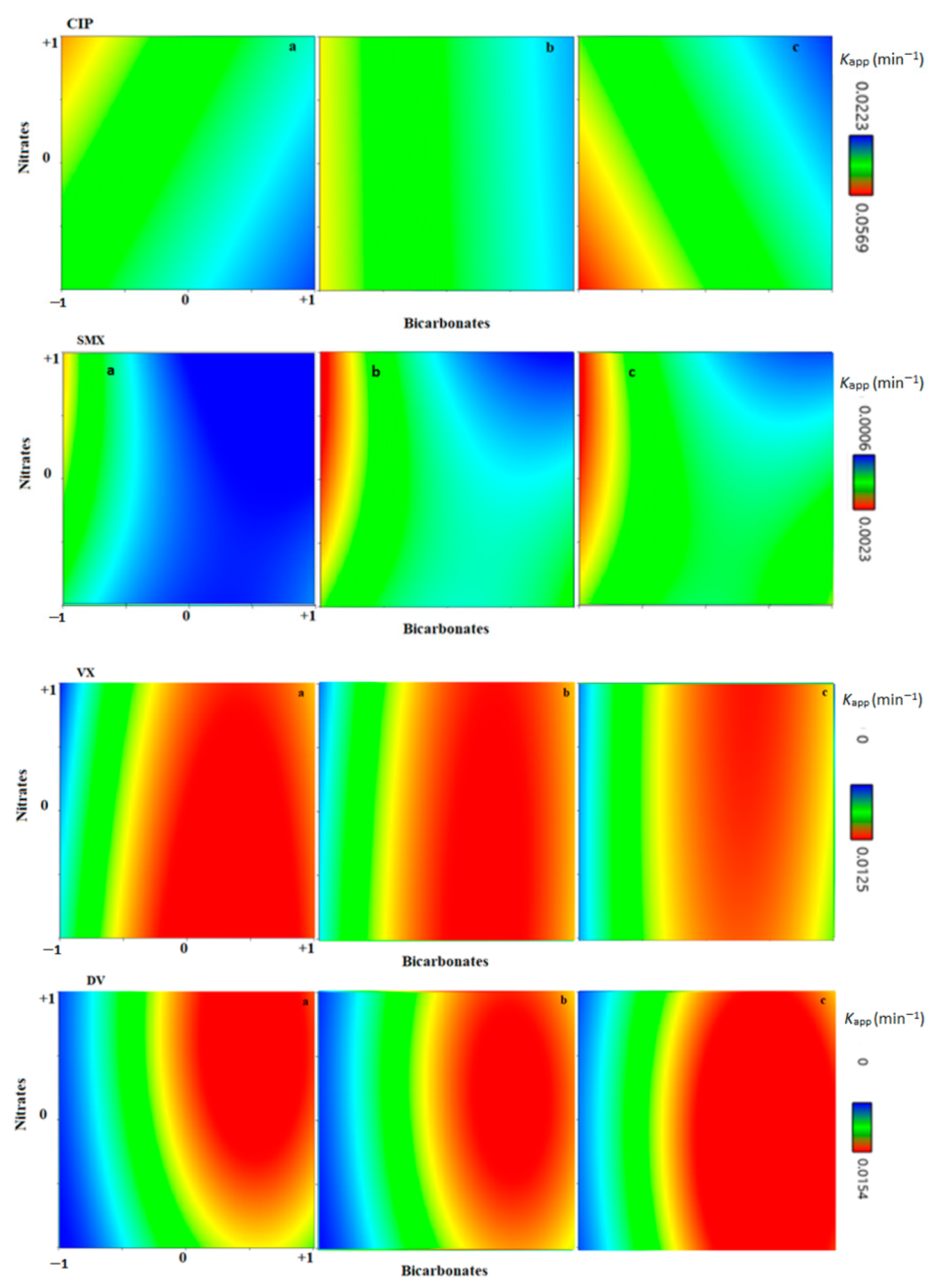
3.5. Effect of the Matrix Constituents: Bicarbonates, Nitrates, and Humic Acids
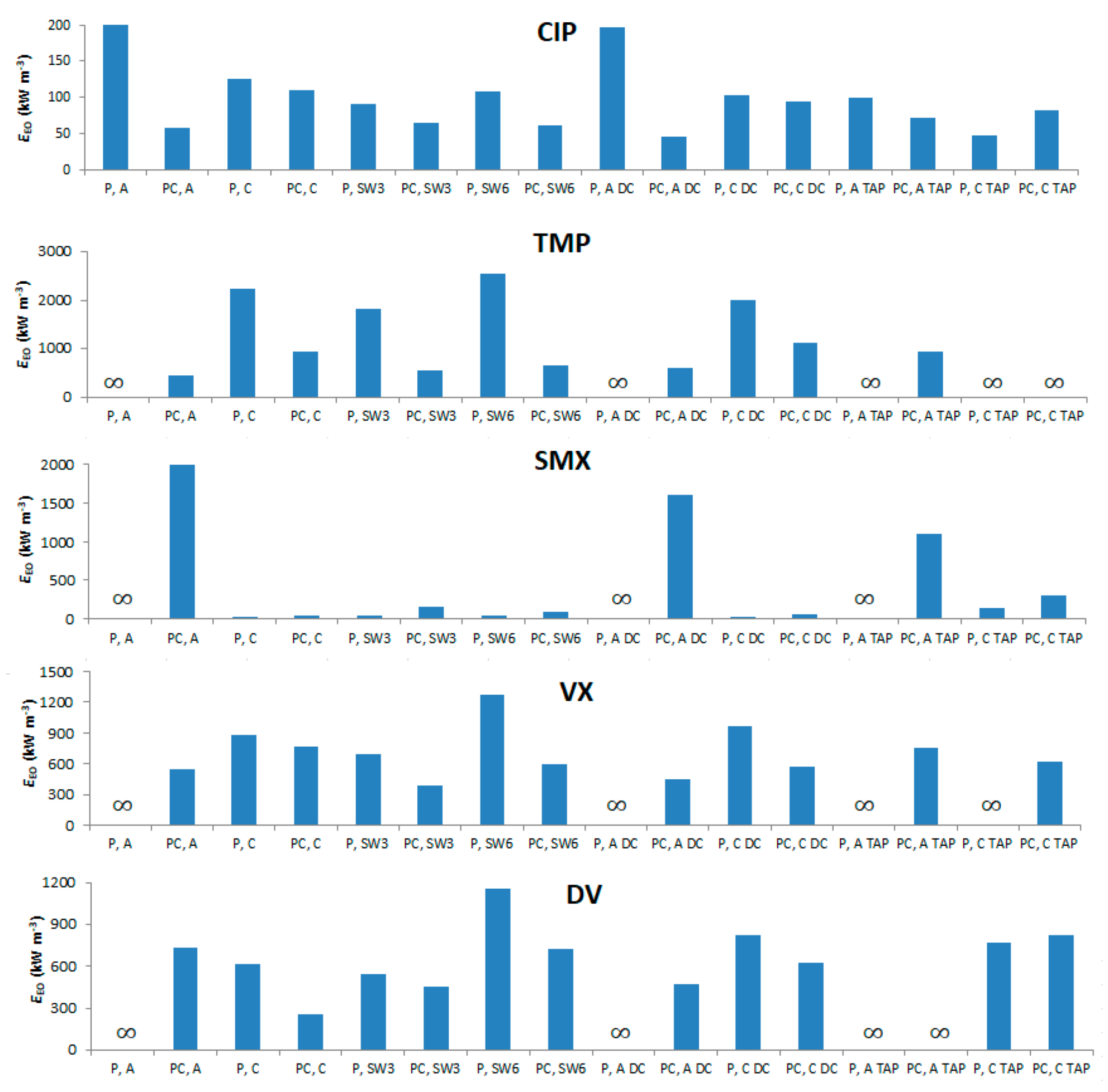
3.6. Electrical Energy per Order (EEO) Analysis
4. Practical Implications of this Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hristov, J.; Barreiro-Hurle, J.; Salputra, G.; Blanco, M.; Witzke, P. Reuse of treated water in European agriculture: Potential to address water scarcity under climate change. Agric. Water Manag. 2021, 251, 106872. [Google Scholar] [CrossRef]
- Kneissl, M.; Seong, T.; Han, J.; Amano, H. The emergence and prospects of deep-ultraviolet light-emitting diode technologies. Nat. Photonics 2019, 13, 233–244. [Google Scholar] [CrossRef]
- Lempart, A.; Kudlek, E.; Dudziak, M. Determination of micropollutants in water samples from swimming pool systems. MDPI Proc. 2018, 2, 177. [Google Scholar] [CrossRef] [Green Version]
- Paíga, P.; Correia, M.; Fernandes, M.; Silva, A.; Carvalho, M.; Vieira, J.; Jorge, S.; Silva, J.; Freire, C.; Delerue-Matos, C. Assessment of 83 Pharmaceuticals in WWTP influent and effluent samples by UHPLC-MS/MS: Hourly variation. Sci. Total Environ. 2019, 648, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, J. Prediction of reaction rate constants of hydroxyl radical with chemicals in water. Water Environ. Res. 2021, 93, 934–939. [Google Scholar] [CrossRef]
- Paredes, L.; Lema, F.; Carballa, M. What happens with organic micropollutants during UV disinfection in WWTPs? A global perspective from laboratory to full-scale. J. Hazard. Mater. 2018, 342, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chen, Z.; Hollert, H.; Zhou, S.; Deutschmann, B.; Seiler, T. Toxicity of 10 organic micropollutants and their mixture: Implications for aquatic risk assessment. Sci. Total Environ. 2019, 666, 1273–1282. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Photocatalytic degradation of organic micropollutants: Inhibition mechanisms by different fractions of natural organic matter. Water Res. 2020, 174, 115643. [Google Scholar] [CrossRef]
- Zeghioud, H.; Khellaf, N.; Djelal, H.; Amrane, A.; Bouhelassa, M. Photocatalytic reactors dedicated to the degradation of hazardous organic pollutants: Kinetics, mechanistic aspects, and design—A review. Chem. Eng. Commun. 2016, 203, 1415–1431. [Google Scholar] [CrossRef]
- Magalhães, P.; Andrade, L.; Nunes, O.; Mendes, A. Titanium dioxide photocatalysis: Fundamentals and application on photoinactivation. Rev. Adv. Mater. Sci. 2017, 51, 91–129. [Google Scholar]
- Teodosiu, C.; Gilca, A.; Barjoveanu, G.; Fiore, S. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Asif, A.; Wang, S.; Sun, H. Hematite-based nanomaterials for photocatalytic degradation of pharmaceuticals and personal care products (PPCPs): A short review. Curr. Opin. Green Sust. Chem. 2021, 28, 100447. [Google Scholar] [CrossRef]
- Miklos, D.; Remy, C.; Jekel, M.; Linden, K.; Drewes, J.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Alvarez, P.; Brame, J.; Cates, E.; Choi, W.; Sedlak, D.; Waite, T.; Westerhoff, P.; Kim, J.; Crittenden, J.; et al. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Foteinis, S.; Borthwick, A.; Frontistis, Z.; Mantzavinos, D.; Chatzisymeon, E. Environmental sustainability of light-driven processes for wastewater treatment applications. J. Clean. Prod. 2018, 182, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Feng, Y.; Bruning, H.; Yntema, D.; Rijnaarts, H. Photocatalytic degradation of metoprolol by TiO2 nanotube arrays and UV-LED: Effects of catalyst properties, operational parameters, commonly present water constituents, and photo-induced reactive species. App. Catal. B Environ. 2018, 220, 171–181. [Google Scholar] [CrossRef]
- Cuervo Lumbaque, E.; Sirtori, C.; Vilar, V. Heterogeneous photocatalytic degradation of pharmaceuticals in synthetic and real matrices using a tube-in-tube membrane reactor with radial addition of H2O2. Sci. Total Environ. 2020, 746, 140629. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, N.; Sayadi, M.; Sobhani, S.; Hajiani, M. Photocatalytic degradation of model pharmaceutical pollutant by novel magnetic TiO2@ZnFe2O4/Pd nanocomposite with enhanced photocatalytic activity and stability under solar light irradiation. J. Environ. Manag. 2020, 271, 110964. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Ao, W.; Xu, H.; Chen, W.; Tao, L.; Shen, Z.; Huihong, L.; Lu, C.; Xie, Z. Facile construction of dual heterojunction CoO@TiO2/MXene hybrid with efficient and stable catalytic activity for phenol degradation with peroxymonosulfate under visible light irradiation. J. Hazardous. Mat. 2021, 420, 126686. [Google Scholar] [CrossRef]
- Deonikar, V.; Mujmule, R.; Patil, D.; Kim, H. Efficient decontamination of toxic phenol pollutant using LaCO3OH nanowires decorated Ag3PO4 hierarchical composites mediated by metallic Ag. Sci. Total. Environ. 2019, 675, 325. [Google Scholar] [CrossRef]
- Silva, A.; Martins, P.; Teixeira, S.; Carabineiro, S.; Kuehn, K.; Cuninberti, G.; Alves, M.; Lanceros-Mendez, S.; Pereira, S. Ciprofloxacin wastewater treated by UVA photocatalysis: Contribution of irradiated TiO2 and ZnO nanoparticles on the final toxicity as assessed by Vibrio Fischeri. RSC Adv. 2016, 6, 95494–95503. [Google Scholar] [CrossRef]
- Escudero, C.; Iglesias, O.; Dominguez, S.; Rivero, M.; Ortiz, I. Performance of electrochemical oxidation and photocatalysis in terms of kinetics and energy consumption. New insights into the p-cresol degradation. J. Environ. Manag. 2017, 195, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, R.; Hu, J. Transformation products formation of ciprofloxacin in UVA/LED and UVA/LED/TiO2 systems: Impact of natural organic matter characteristics. Water Res. 2018, 132, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, N.; Pastrana-Martínez, L.; Ribeiro, A.; Moreira, N.; Faria, J.; Hentati, O.; Silva, A.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Li, Q.; Jia, R.; Shao, J.; He, Y. Photocatalytic degradation of amoxicillin via TiO2 nanoparticle coupling with a novel submerged porous ceramic membrane reactor. J. Clean. Prod. 2019, 209, 755–761. [Google Scholar] [CrossRef]
- Ye, Y.; Bruning, H.; Liu, W.; Rijnaarts, H.; Yntema, D. Effect of dissolved natural organic matter on the photocatalytic micropollutant removal performance of TiO2 nanotube array. J. Photochem. Photobiol. A Chem. 2019, 371, 216–222. [Google Scholar] [CrossRef]
- Verma, M.; Haritash, A. Photocatalytic degradation of amoxicillin in pharmaceutical wastewater: A potential tool to manage residual antibiotics. Environ. Technol. Innov. 2020, 20, 101072. [Google Scholar] [CrossRef]
- Negishi, N.; Miyazaki, Y.; Kato, S.; Yang, Y. Effect of HCO3− concentration in groundwater on TiO2 photocatalytic water purification. App. Catal. B Environ. 2019, 242, 449–459. [Google Scholar] [CrossRef]
- Russell, M.M.; Kempisty, D.; Kanel, S.; Kurwadkar, S.; Brittle, S.; Sizemore, I.; Yaal, L. Destruction of aqueous phase organic pollutants using ultraviolet light-emitting diodes and photocatalysis. Water Air Soil Pollut. 2018, 229, 139. [Google Scholar] [CrossRef]
- Ding, H.; Hu, J. Degradation of ibuprofen by UVA-LED/TiO2/persulfate process: Kinetics, mechanism, water matrix effects, intermediates and energy consumption. Chem. Eng. J. 2020, 397, 125462. [Google Scholar] [CrossRef]
- Ferreira, L.; Fernandes, J.; Rodríguez-Chueca, J.; Peres, J.; Lucas, M.; Tavares, P. Photocatalytic degradation of an agro-industrial wastewater model compound using a UV LEDs system: Kinetic study. J. Environ. Manag. 2020, 269, 110740. [Google Scholar] [CrossRef]
- Tapia-Tlatelpa, T.; Buscio, V.; Trull, J.; Sala, V. Performance analysis and methodology for replacing conventional lamps by optimized LED arrays for photocatalytic processes. Chem. Eng. Res. Des. 2020, 156, 456–468. [Google Scholar] [CrossRef]
- Bertagna Silva, D.; Buttiglieri, G.; Babić, S. State-of-the-art and current challenges for TiO2/UV-LED photocatalytic degradation of emerging organic micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 103–120. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Casado, C.; Marugán, J.; Timmers, R.; Muñoz, M.; van Grieken, R. Comprehensive multiphysics modeling of photocatalytic processes by computational fluid dynamics based on intrinsic kinetic parameters determined in a differential photoreactor. Chem. Eng. J. 2017, 310, 368–380. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Loeb, S.; Kim, J. LED revolution: Fundamentals and prospects for UV disinfection applications. Environ. Sci. Water Res. Technol. 2017, 3, 188–202. [Google Scholar] [CrossRef]
- Sharma, P.; Khan, M.; Choubey, A. LED revolution: Deep UV LED. Int. J. Eng. Technol. 2019, 6, 6486. [Google Scholar]
- Bertagna Silva, D.; Buttiglieri, G.; Babić, T.; Ćurković, L.; Babić, S. Impact of UV-LED photoreactor design on the degradation of contaminants of emerging concern. Process Saf. Environ. Prot. 2021, 153, 94–106. [Google Scholar] [CrossRef]
- Yu, L.; Achari, G.; Langford, C. Design and evaluation of a novel light-emitting diode photocatalytic reactor for water treatment. J. Environ. Eng. 2018, 144, 1–9. [Google Scholar] [CrossRef]
- Casado, C.; Timmers, R.; Sergejevs, A.; Clarke, C.; Allsopp, D.; Bowen, C.; van Grieken, R.; Marugán, J. Design and validation of a LED-based high intensity photocatalytic reactor for quantifying activity measurements. Chem. Eng. J. 2017, 327, 1043–1055. [Google Scholar] [CrossRef]
- Verma, S.; Nakamura, S.; Sillanpää, M. Application of UV-C LED activated PMS for the degradation of anatoxin-A. Chem. Eng. J. 2016, 284, 122–129. [Google Scholar] [CrossRef]
- Neto, J.; Saggioro, S.; Dezotti, M. Investigation of mechanism and kinetics in the TiO2 photocatalytic degradation of indigo carmine dye using radical scavengers. Int. J. Environ. Sci. Technol. 2021, 18, 163–172. [Google Scholar] [CrossRef]
- Lin, P.; Mehrvar, M. Photocatalytic treatment of an actual confectionery wastewater using Ag/TiO2/Fe2O3: Optimization of photocatalytic reactions using surface response methodology. Catalysts 2018, 8, 409. [Google Scholar] [CrossRef] [Green Version]
- Bizi, M.; Bachra, F. Evaluation of the ciprofloxacin adsorption capacity of common industrial minerals and application to tap water treatment. Powder Technol. 2020, 362, 323–333. [Google Scholar] [CrossRef]
- European Commission. EUR-Lex—2020/1161 of 4 August 2020 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2020, 257, 32–35. [Google Scholar]
- Čizmić, M.; Ljubas, D.; Rožman, M.; Ašperger, D.; Ćurković, L.; Babíc, S. Photocatalytic degradation of azithromycin by nanostructured TiO2 film: Kinetics, degradation products, and toxicity. Materials 2019, 12, 873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ćurković, L.; Ljubas, D.; Šegota, S.; Bačić, I. Photocatalytic degradation of Lissamine Green B dye by using nanostructured sol–gel TiO2 films. J. Alloys Compd. 2014, 604, 309–316. [Google Scholar] [CrossRef]
- Waveform Lighting. Real UV LED Strip. 2020. Available online: https://store.waveformlighting.com/collections/led-strips/products/real-uv-led-strip-lights?variant=12527605252198 (accessed on 30 September 2021).
- Waveform Lighting. Clean UV-C Flex Strips Datasheet. 2020. Available online: https://www.waveformlighting.com/datasheets/CS_7026.pdf (accessed on 30 September 2021).
- Arduino. 2020. Available online: https://www.arduino.cc/en/tutorial/PWM. (accessed on 30 September 2021).
- Ljubas, D.; Smoljanić, G.; Juretić, H. Degradation of Methyl Orange and Congo Red dyes by using TiO2 nanoparticles activated by the solar and the solar-like radiation. J. Environ. Manag. 2015, 161, 83–91. [Google Scholar] [CrossRef]
- Council of the European Union. Council Directive 98/83/EC of 3 November 1998 on the Quality of water intended for human consumption (OJ L 330 05.12.1998 p. 32). Doc. Eur. Community Environ. Law 1998, 330, 865–878. [Google Scholar]
- Bolton, J.; Bircher, K.; Tumas, W.; Tolman, C. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems. Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Davididou, K.; Nelson, R.; Monteagudo, J.; Durán, A.; Expósito, A.; Chatzisymeon, E. Photocatalytic degradation of bisphenol-A under UV-LED, blacklight and solar irradiation. J. Clean. Prod. 2018, 203, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, K.; Natarajan, T.; Tayade, H. Photocatalytic reactor based on UV-LED/TiO2 coated quartz tube for degradation of dyes. Chem. Eng. J. 2011, 178, 40–49. [Google Scholar] [CrossRef]
- Liang, R.; Van Leuwen, J.; Bragg, L.; Arlos, M.; Fong, L.C.L.C.; Schneider, O.; Jaciw-Zurakowsky, I.; Fattahi, A.; Rathod, S.; Peng, P.; et al. Utilizing UV-LED pulse width modulation on TiO2 advanced oxidation processes to enhance the decomposition efficiency of pharmaceutical micropollutants. Chem. Eng. J. 2019, 361, 439–449. [Google Scholar] [CrossRef]
- Bianco, A.; Passananti, M.; Perroux, H.; Voyard, G.; Mouchel-Vallon, C.; Chaumerliac, N.; Mailhot, G.; Deguillaume, L.; Brigante, M. A Better Understanding of hydroxyl radical photochemical sources in cloud waters collected at the Puy de Dôme station—Experimental versus modelled formation rates. Atmos. Chem. Phys. 2015, 15, 9191–9202. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, T.; Yu, S. Photolytic degradation of sulfamethoxazole and trimethoprim using UV-A, UV-C and vacuum-UV (VUV). J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2015, 50, 292–300. [Google Scholar] [CrossRef]
- Sarafraz, M.; Sadeghi, M.; Yazdanbakhsh, A.; Amini, M.; Sadani, M.; Eslami, A. Enhanced photocatalytic degradation of ciprofloxacin by black Ti3+/N-TiO2 under visible LED light irradiation: Kinetic, energy consumption, degradation pathway, and toxicity assessment. Process Saf. Environ. Prot. 2020, 137, 261–272. [Google Scholar] [CrossRef]
- Ran, Z.; Fang, Y.; Sun, J.; Ma, C.; Li, S. Photocatalytic oxidative degradation of carbamazepine by TiO2 irradiated by UV light emitting diode. Catalysts 2020, 10, 540. [Google Scholar] [CrossRef]
- Nyangaresi, P.; Qin, Y.; Chen, G.; Zhang, B.; Lu, Y.; Shen, L. Comparison of UV-LED photolytic and UV-LED/TiO2 photocatalytic disinfection for Escherichia coli in water. Catal. Today. 2019, 335, 200–207. [Google Scholar] [CrossRef]
- Nasuhoglu, D.; Yargeau, V.; Berk, D. Photo-removal of sulfamethoxazole (SMX) by photolytic and photocatalytic processes in a batch reactor under UV-C radiation (Λmax = 254 nm). J. Hazard. Mater. 2011, 186, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zrnčić, M.; Babić, S.; Mutavdžic Pavlović, D. Determination of thermodynamic pKa values of pharmaceuticals from five different groups using capillary electrophoresis. J. Sep. Sci. 2015, 38, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Salma, A.; Thoröe-Boveleth, S.; Schmidt, T.; Tuerk, J. Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Zhu, Y.; Zhou, B.; Hu, J. Photocatalytic oxidation of sulfamethoxazole in the presence of TiO2: Effect of matrix in aqueous solution on decomposition mechanisms. Chem. Eng. J. 2019, 359, 1527–1536. [Google Scholar] [CrossRef]
- Giannakis, S.; Hendaoui, I.; Jovic, M.; Grandjean, D.; Alencastro, L.; Girault, H.; Pulgarin, C. Solar photo-Fenton and UV/H2O2 processes against the antidepressant venlafaxine in urban wastewaters and human urine. Intermediates formation and biodegradability assessment. Chem. Eng. J. 2017, 308, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Lajeunesse, A.; Smyth, S.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, Z.; Greaves, J.; Cooper, W.; Song, W. Trimethoprim: Kinetic and mechanistic considerations in photochemical environmental fate and AOP treatment. Water Res. 2012, 46, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Willach, S.; Lutze, H.; Eckey, K.; Löppenberg, K.; Lueling, M.; Wolbert, J.; Kujawinski, D.; Jochmann, M.; Karst, U.; Schmidt, T. Direct photolysis of sulfamethoxazole using various irradiation sources and wavelength ranges—Insights from degradation product analysis and compound-specific stable isotope analysis. Environ. Sci. Technol. 2018, 52, 1225–1233. [Google Scholar] [CrossRef]
- Khan, M.; Shah, J.; Arshad, M.; Halim, S.; Khan, A.; Shaikh, A.; Riaz, N.; Khan, A.J.; Arfan, M.; Shahid, M.; et al. Photocatalytic decolorization and biocidal applications of non-metal doped TiO2: Isotherm, kinetic modeling and in silico molecular docking studies. Molecules 2020, 25, 4468. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Di Capua, G.; Femia, N. Enhanced performances of a photocatalytic reactor for wastewater treatment using controlled modulation of LEDs light. Chem. Eng. Trans. 2017, 57, 553–558. [Google Scholar]
- Tokode, O.; Prabhu, R.; Lawton, L.; Robertson, P. Controlled periodic illumination in semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 2016, 319, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Trinh, T.; Kim, J. Status quo in data availability and predictive models of nano-mixture toxicity. Nanomaterials 2021, 11, 124. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, S.; Qin, W.; Wu, X. Isopropanol-assisted synthesis of highly stable MAPbBr3/p-g-C3N4 intergrowth composite photocatalysts and their interfacial charge carrier dynamics. Nanoscale Adv. 2020, 2, 274–285. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Yin, K.; Liu, C.; Luo, J.; Crittenden, J.; Zhang, W.; Luo, S.; Crittenden, J.; Zhang, W.; Luo, S.; et al. The role of reactive oxygen species and carbonate radical in oxcarbazepine degradation via UV, UV/H2O2: Kinetics, mechanisms and toxicity evaluation. Water Res. 2018, 147, 204–213. [Google Scholar] [CrossRef]
- Alhaji, M.; Sanaullah, K.; Khan, A.; Hamza, A.; Muhammad, A.; Ishola, M.; Rigit, A.; Bhawani, S. Recent developments in immobilizing titanium dioxide on supports for degradation of organic pollutants in wastewater—A review. Int. J. Environ. Sci. Technol. 2017, 14, 2039–2052. [Google Scholar] [CrossRef]
- Ferreira, S.; Bruns, R.; Ferreira, H.; Matos, G.; David, J.; Brandao, G.; da Silva, E.; Portugal, L.; dos Reis, P.; Souza, A.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Analytica Chimica Acta 2007, 597, 179. [Google Scholar] [CrossRef]
- Xiong, P.; Hu, J. Decomposition of acetaminophen (Ace) using TiO2/UVA/LED system. Catal. Today 2017, 282, 48–56. [Google Scholar] [CrossRef]
- Dabić, D.; Babić, S.; Škorić, I. The role of photodegradation in the environmental fate of hydroxychloroquine. Chemosphere 2019, 230, 268–277. [Google Scholar] [CrossRef]
- Vinge, S.; Shaheen, S.L.; Sharpless, C.; Linden, K. Nitrate with benefits: Optimizing radical production during UV water treatment. Environ. Sci. Water Res. Technol. 2020, 6, 1163–1175. [Google Scholar] [CrossRef]
- Doudrick, K.; Yang, T.; Hristovski, K.; Westerhoff, P. Photocatalytic nitrate reduction in water: Managing the hole scavenger and reaction by-product selectivity. Appl. Catal. B Environ. 2013, 136–137, 40–47. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, D.; Wen, D. Use of coupled wavelength ultraviolet light-emitting diodes for inactivation of bacteria in subsea oil-field injection water. Sci. Total Environ. 2018, 640–641, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Chevremont, A.; Farnet, A.; Sergent, M.; Coulomb, B.; Boudenne, J. Multivariate optimization of fecal bioindicator inactivation by coupling UV-A and UV-C LEDs. Desalination 2012, 285, 219–225. [Google Scholar] [CrossRef]
- Konstas, P.; Kosma, C.; Konstantinou, I.; Albanis, T. Photocatalytic Treatment of Pharmaceuticals in Real Hospital Wastewaters for Effluent Quality Amelioration. Water 2019, 11, 2165. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Zhang, J.; Zhang, J.; Niu, J.; Zhao, J.; Wei, Y.; Yao, B. Photocatalytic degradation of ciprofloxacin using Zn-doped Cu2O particles: Analysis of degradation pathways and intermediates. Chem. Eng. J. 2019, 374, 316. [Google Scholar] [CrossRef]
- Li, W.; Zuo, Y.; Jiang, L.; Yao, D.; Chen, Z.; He, G.; Chen, H. Bi2Ti2O7/TiO2/RGO composite for the simulated sunlight-driven photocatalytic degradation of ciprofloxacin. Mater. Chem. Phys. 2020, 256, 123650. [Google Scholar] [CrossRef]
- Biancullo, F.; Moreira, N.; Ribeiro, A.; Manai, C.; Faria, J.; Nunes, O.; Castro-Silva, S.; Silva, A. Heterogeneous photocatalysis using UVA LEDs for the removal of antibiotics and antibiotic resistant bacteria from urban wastewater treatment plant effluents. Chem. Eng. J. 2019, 367, 304. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.; Alalm, M.; Fujii, M.; Ookawara, S.; Ohno, T. Photocatalytic degradation of trimethoprim using S-TiO2 and Ru/WO3/ZrO2 immobilized on reusable fixed plates. J. Water Process. Eng. 2020, 33, 101012. [Google Scholar] [CrossRef]





| Independent Coded Variables | −1 | +1 |
|---|---|---|
| Matrix (A) | MQ water | Tap water |
| LEDs wavelength (B) | 272 nm (UV-C) | 365 nm (UV-A) |
| TiO2 nanofilm presence (C) | No | Yes |
| Duty Cycle (D) | 0.5 | 1.0 (continuous) |
| Independent Coded Variables | −1 | 0 | +1 |
|---|---|---|---|
| Bicarbonates (A) | 0 | 200 | 400 |
| Nitrates (B) | 0 | 15 | 30 |
| Humic acids (C) | 0 | 1.5 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertagna Silva, D.; Buttiglieri, G.; Babić, B.; Ašperger, D.; Babić, S. Performance of TiO2/UV-LED-Based Processes for Degradation of Pharmaceuticals: Effect of Matrix Composition and Process Variables. Nanomaterials 2022, 12, 295. https://doi.org/10.3390/nano12020295
Bertagna Silva D, Buttiglieri G, Babić B, Ašperger D, Babić S. Performance of TiO2/UV-LED-Based Processes for Degradation of Pharmaceuticals: Effect of Matrix Composition and Process Variables. Nanomaterials. 2022; 12(2):295. https://doi.org/10.3390/nano12020295
Chicago/Turabian StyleBertagna Silva, Danilo, Gianluigi Buttiglieri, Bruna Babić, Danijela Ašperger, and Sandra Babić. 2022. "Performance of TiO2/UV-LED-Based Processes for Degradation of Pharmaceuticals: Effect of Matrix Composition and Process Variables" Nanomaterials 12, no. 2: 295. https://doi.org/10.3390/nano12020295
APA StyleBertagna Silva, D., Buttiglieri, G., Babić, B., Ašperger, D., & Babić, S. (2022). Performance of TiO2/UV-LED-Based Processes for Degradation of Pharmaceuticals: Effect of Matrix Composition and Process Variables. Nanomaterials, 12(2), 295. https://doi.org/10.3390/nano12020295







