Removing Lead from Contaminated Sediment Using Indium-Based Perovskite Precursor
Abstract
1. Introduction
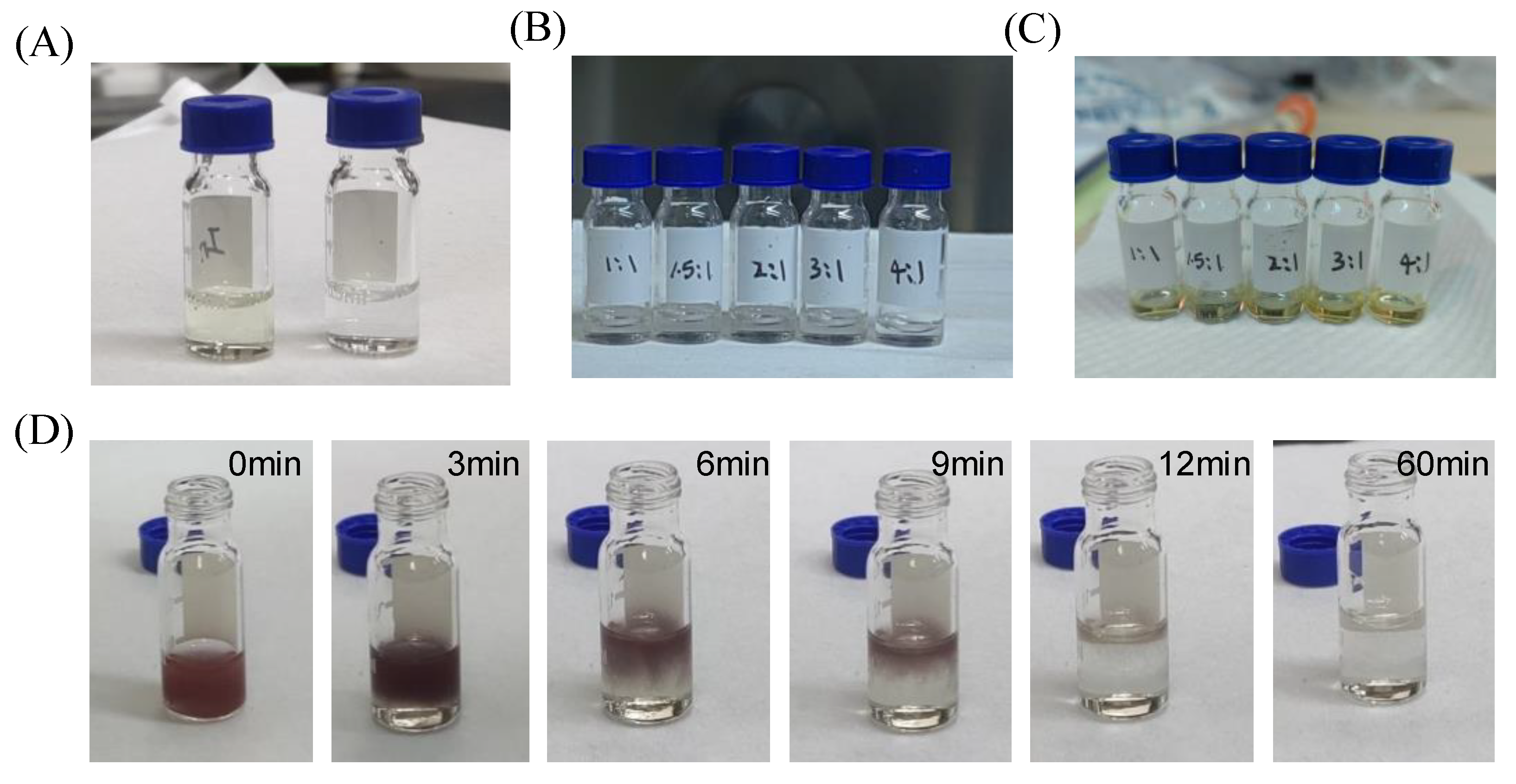
2. Results
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Sample Collection and Analysis
4.3. Solution and Film Preparation
4.4. Characterization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vanthuyne, M.; Maes, A.; Cauwenberg, P. The use of flotation techniques in the remediation of heavy metal contaminated sediments and soils: An overview of controlling factors. Miner. Eng. 2003, 16, 1131–1141. [Google Scholar] [CrossRef]
- Huang, D.; Liu, L.; Zeng, G.; Xu, P.; Huang, C.; Deng, L.; Wang, R.; Wan, J. The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere 2017, 174, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy metal pollution: Source, impact, and remedies. Biomanagement Met.-Contam. Soils 2011, 20, 1–28. [Google Scholar]
- Gong, J.; Xie, P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere 2020, 254, 126790–126800. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Chowdhary, P.; Kaithwas, G.; Bharagava, R. Toxic metals in the environment: Threats on ecosystem and bioremediation approaches. Handb. Met.-Microbe Interact. Bioremediation 2017, 14, 128–141. [Google Scholar]
- Bradl, H. Sources and origins of heavy metals. Interface Sci. Technol. 2005, 6, 1–27. [Google Scholar]
- Sheoran, A.; Sheoran, V. Heavy metal removal mechanism of acid mine drainage in w etlands: A critical review. Miner. Eng. 2006, 19, 105–116. [Google Scholar] [CrossRef]
- Yunus, K.; Zuraidah, M.; John, A.J.E.; Change, C. A review on the accumulation of heavy metals in coastal sediment of Peninsular Malaysia. Ecofeminism Clim. Chang. 2020, 1, 21–35. [Google Scholar] [CrossRef]
- Ayas, Z.; Ekmekci, G.; Yerli, S.V.; Ozmen, M. Heavy metal accumulation in water, sediments and fishes of Nallihan Bird Paradise, Turkey. J. Environ. Biol. 2007, 28, 545–549. [Google Scholar]
- Ke, X.; Bao, Q.; Qi, Y.; Huang, X.; Zhang, H. Toxicity assessment of sediments from the Liaohe River Protected Area (China) under the influence of ammonia nitrogen, heavy metals and organic contaminants. Environ. Toxicol. Pharmacol. 2018, 59, 34–42. [Google Scholar] [CrossRef]
- Costa-Böddeker, S.; Hoelzmann, P.; de Stigter, H.C.; van Gaever, P.; Huy, H.Đ.; Schwalb, A. The hidden threat of heavy metal pollution in high sedimentation and highly dynamic environment: Assessment of metal accumulation rates in the Thi Vai Estuary, Southern Vietnam. Environ. Pollut. 2018, 242, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F.; James, S.; Bennett, H. Metal removal from contaminated soil and sediments by the biosurfactant surfactin. Environ. Sci. Technol. 1999, 33, 3812–3820. [Google Scholar] [CrossRef]
- Sakakibara, M.; Ohmori, Y.; Ha, N.T.H.; Sano, S.; Sera, K. Phytoremediation of heavy metal-contaminated water and sediment by Eleocharis acicularis. Clean Soil Air Water 2011, 39, 735–741. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Lee, C.R. Phytoremediation: Plant-based remediation of contaminated soils and sediments. Bioremediation: Sci. Appl. 1995, 43, 145–156. [Google Scholar]
- Chen, S.Y.; Lin, J.G. Bioleaching of heavy metals from contaminated sediment by indigenous sulfur-oxidizing bacteria in an air-lift bioreactor: Effects of sulfur concentration. Water Res. 2004, 38, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Gougar, M.; Scheetz, B.; Roy, D. Ettringite and C−S−H Portland cement phases for waste ion immobilization: A review. Waste Manag. 1996, 16, 295–303. [Google Scholar] [CrossRef]
- Higgins, T.E.; Halloran, A.R.; Petura, J.C. Traditional and innovative treatment methods for Cr (VI) in soil. J. Soil Contam. 1997, 6, 767–797. [Google Scholar] [CrossRef]
- Wang, F.; Yu, J.; Xiong, W.; Xu, Y.; Chi, R. A two-step leaching method designed based on chemical fraction distribution of the heavy metals for selective leaching of Cd, Zn, Cu, and Pb from metallurgical sludge. Environ. Sci. Pollut. Res. 2018, 25, 1752–1765. [Google Scholar] [CrossRef]
- Thakare, M.; Sarma, H.; Datar, S.; Roy, A.; Pawar, P.; Gupta, K.; Pandit, S.; Prasad, R. Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr. Res. Biotechnol. 2021, 3, 84–98. [Google Scholar] [CrossRef]
- Finkelstein, Y.; Markowitz, M.E.; Rosen, J.F. Low-level lead-induced neurotoxicity in children: An update on central nervous system effects. Brain Res. Rev. 1998, 27, 168–176. [Google Scholar] [CrossRef]
- Pedersen, K.B.; Kirkelund, G.M.; Ottosen, L.M.; Jensen, P.E.; Lejon, T.J. Multivariate methods for evaluating the efficiency of electrodialytic removal of heavy metals from polluted harbour sediments. J. Hazard. Mater. 2015, 283, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Kirkelund, G.M.; Ottosen, L.M.; Villumsen, A. Electrodialytic remediation of harbour sediment in suspension—Evaluation of effects induced by changes in stirring velocity and current density on heavy metal removal and pH. J. Hazard. Mater. 2009, 169, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Dahrazma, B.; Mulligan, C. Investigation of the removal of heavy metals from sediments using rhamnolipid in a continuous flow configuration. Chemosphere 2007, 69, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ahmad, I.; Shah, M.T.; Rehman, S.; Khaliq, A. Use of constructed wetland for the removal of heavy metals from industrial wastewater. J. Environ. Manag. 2009, 90, 3451–3457. [Google Scholar] [CrossRef]
- Wen, J.; Yi, Y.; Zeng, G. Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J. Environ. Manag. 2016, 178, 63–69. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, H.; Feng, S.; Yang, L.; Dong, H.; Wang, J.; Tian, C.; Li, L.; Lu, H.; Jeong, J.; et al. Modulation of perovskite crystallization processes towards highly efficient and stable perovskite solar cells with MXene quantum dot-modified SnO2. Energy Environ. Sci. 2021, 14, 3447–3454. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Y.; Lou, Y.; Li, M.; Igbari, F.; Cao, J.; Chen, J.; Yang, W.; Dong, C.; Li, L.; et al. Smelting recrystallization of CsPbBrI2 perovskites for indoor and outdoor photovoltaics. eScience 2021, 1, 53–59. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, Y.; Zhang, H.; Zhang, T.; Wei, N.; Liu, X.; Chen, Y.; Chen, J.; Zhao, Y. In situ growth of atomic layer perovskitoid to stabilize and passivate MAPbI3 for efficient and stable photovoltaics. eScience 2021, 1, 61–67. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Chen, B.; Li, R.; Ren, N.; Li, Y.; Shi, B.; Huang, Q.; Zhao, Y.; Grätzel, M.; et al. Suppressed recombination for monolithic inorganic perovskite/silicon tandem solar cells with an approximate efficiency of 23%. eScience 2022, 1, 339–346. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Hu, J.; Huang, B.; Sun, M.; Dong, B.; Zheng, G.; Huang, Y.; Chen, Y.; Li, L.J.S. A Eu3+–Eu2+ ion redox shuttle imparts operational durability to Pb-I perovskite solar cells. Science 2019, 363, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.K.; Li, M.; Yang, Y.G.; Hu, Y.; Ma, H.; Gao, X.Y.; Liao, L.S. High efficiency Pb–In binary metal perovskite solar cells. Adv. Mater. 2016, 28, 6695–6703. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yang, Y.; Li, M.; Wang, J.; Cheng, Z.; Li, J.; Ji, G.; Yin, G.; Song, F.; Wang, Z.; et al. High-Performance Perovskite Solar Cells Engineered by an Ammonia Modified Graphene Oxide Interfacial Layer. ACS Appl. Mater. Interfaces 2016, 8, 14503–14512. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Ahlawat, P.; Mishra, A.; Tress, W.R.; Eickemeyer, F.T.; Yang, Y.; Fu, F.; Wang, Z.; Avalos, C. Vapor-assisted deposition of highly efficient, stable black-phase FAPbI3 perovskite solar cells. Science 2020, 370, 6512–6519. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, C.; Liang, Z.; Cheng, L.; Feng, S.; Li, Y.; Yang, Y.; Li, L. Removing Lead from Contaminated Sediment Using Indium-Based Perovskite Precursor. Nanomaterials 2022, 12, 4395. https://doi.org/10.3390/nano12244395
Tian C, Liang Z, Cheng L, Feng S, Li Y, Yang Y, Li L. Removing Lead from Contaminated Sediment Using Indium-Based Perovskite Precursor. Nanomaterials. 2022; 12(24):4395. https://doi.org/10.3390/nano12244395
Chicago/Turabian StyleTian, Chen, Zhenye Liang, Liwei Cheng, Shanglei Feng, Yiwen Li, Yingguo Yang, and Lina Li. 2022. "Removing Lead from Contaminated Sediment Using Indium-Based Perovskite Precursor" Nanomaterials 12, no. 24: 4395. https://doi.org/10.3390/nano12244395
APA StyleTian, C., Liang, Z., Cheng, L., Feng, S., Li, Y., Yang, Y., & Li, L. (2022). Removing Lead from Contaminated Sediment Using Indium-Based Perovskite Precursor. Nanomaterials, 12(24), 4395. https://doi.org/10.3390/nano12244395









