Self-Sustained Three-Dimensional Macroporous TiO2-Graphene Photocatalyst for Sunlight Decolorization of Methyl Orange
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Photocatalyst Synthesis
2.3. Characterization
2.4. Photocatalytic Degradation
3. Results and Discussions
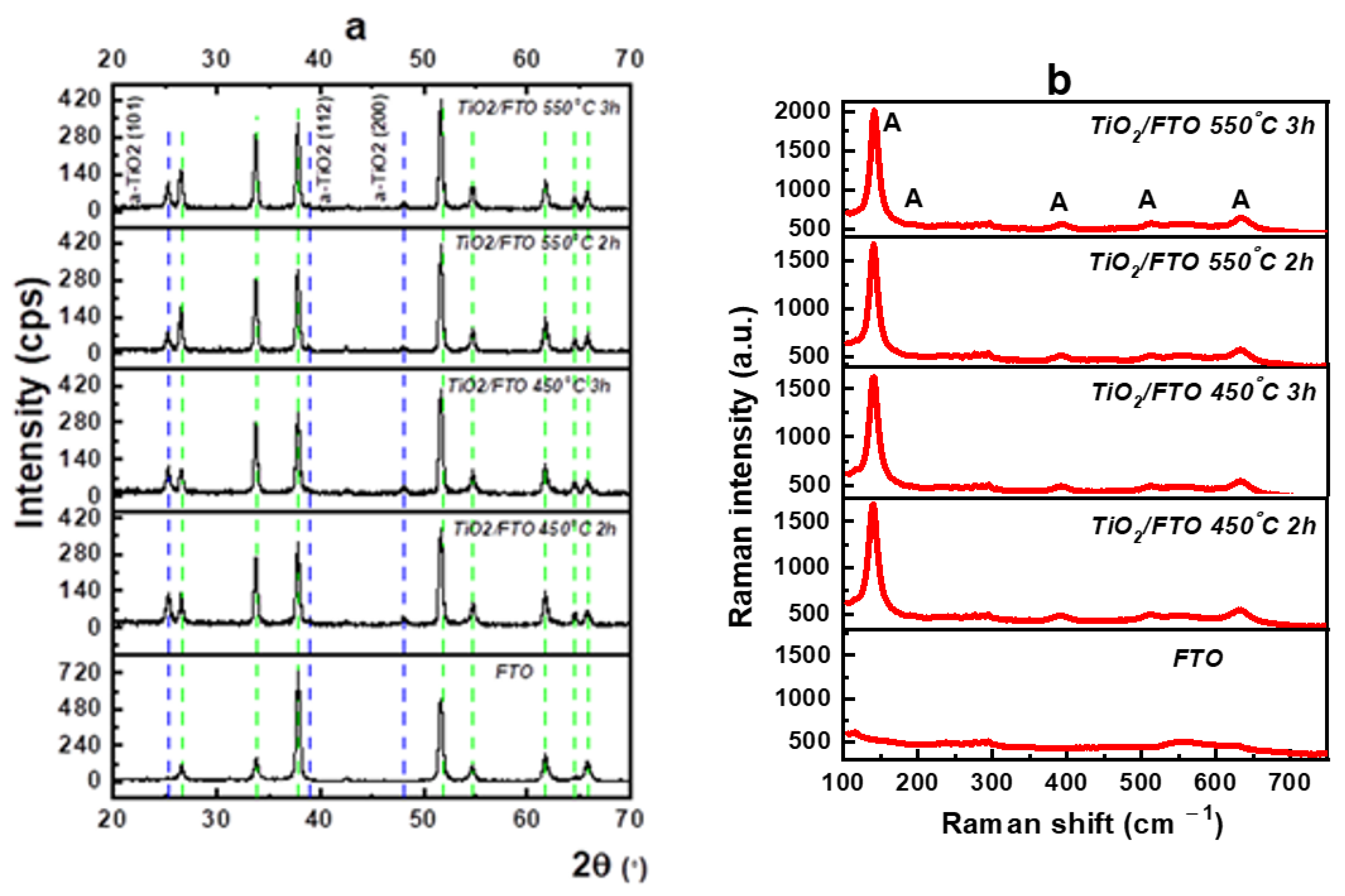
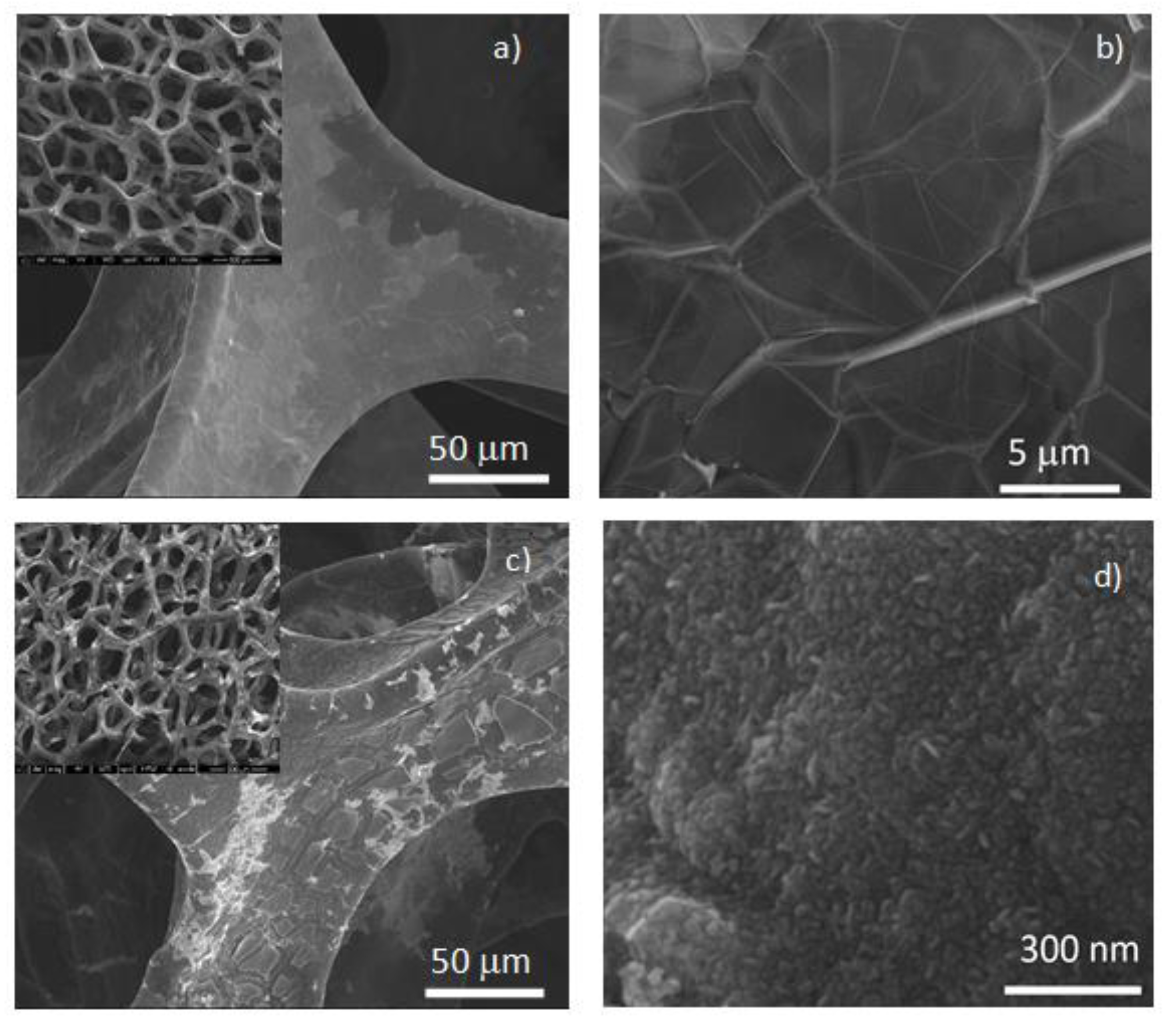
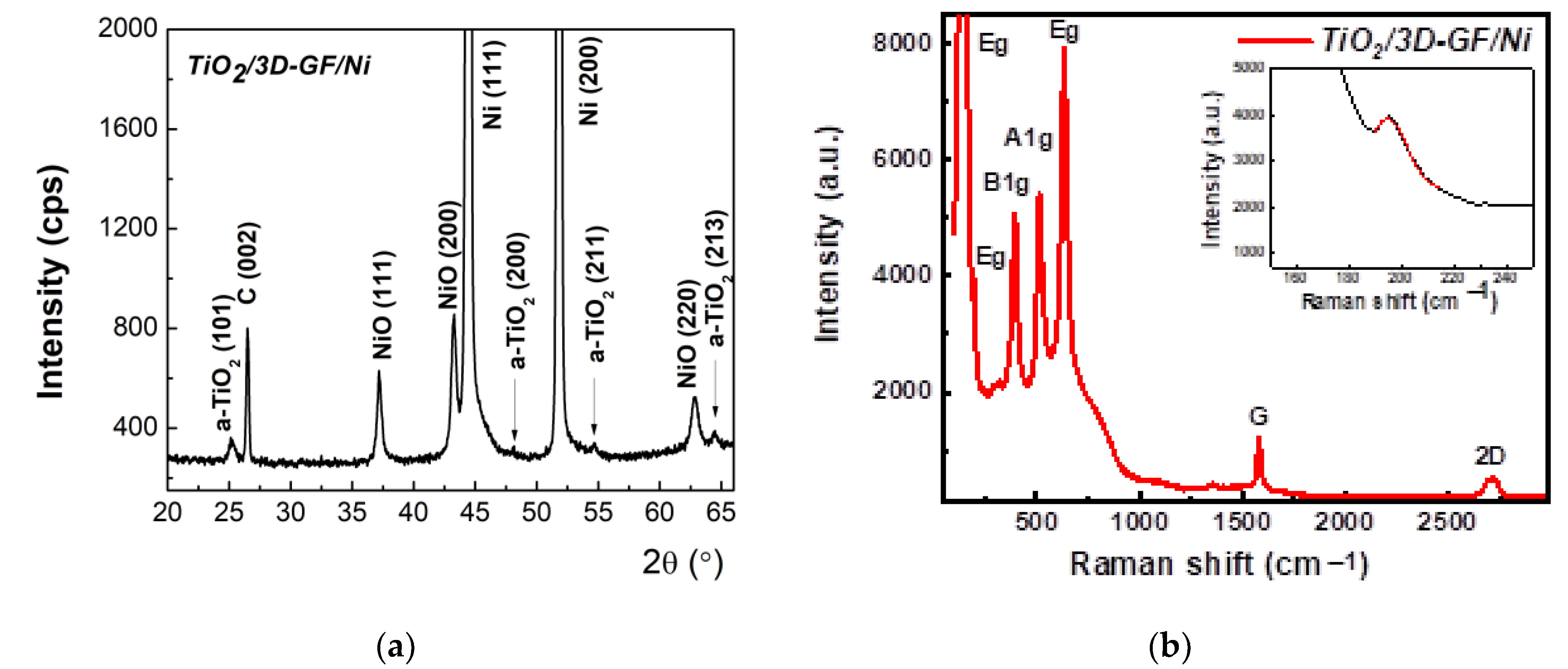
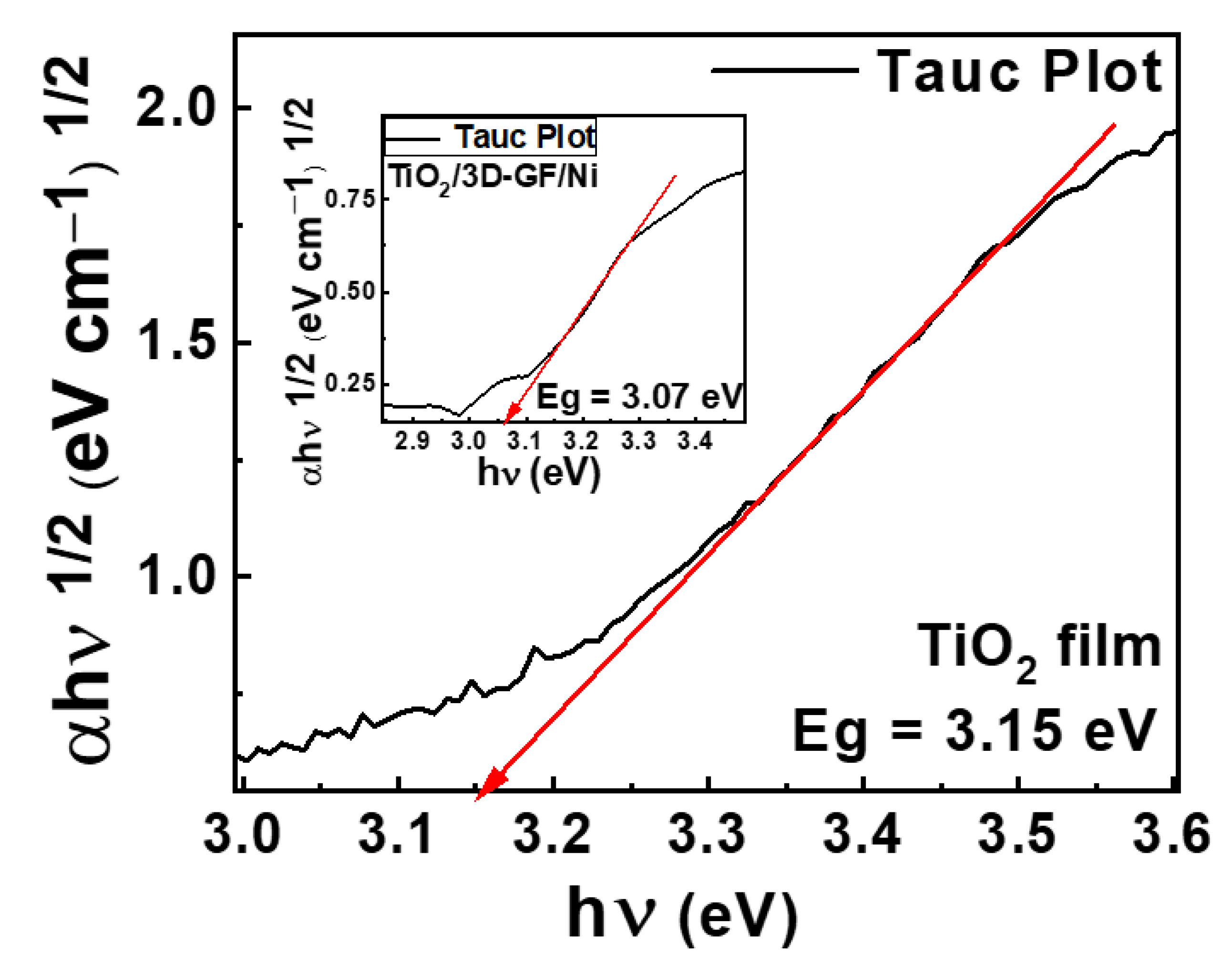
3.1. Characterization of Photocatalysts
3.2. Photocatalytic Activity
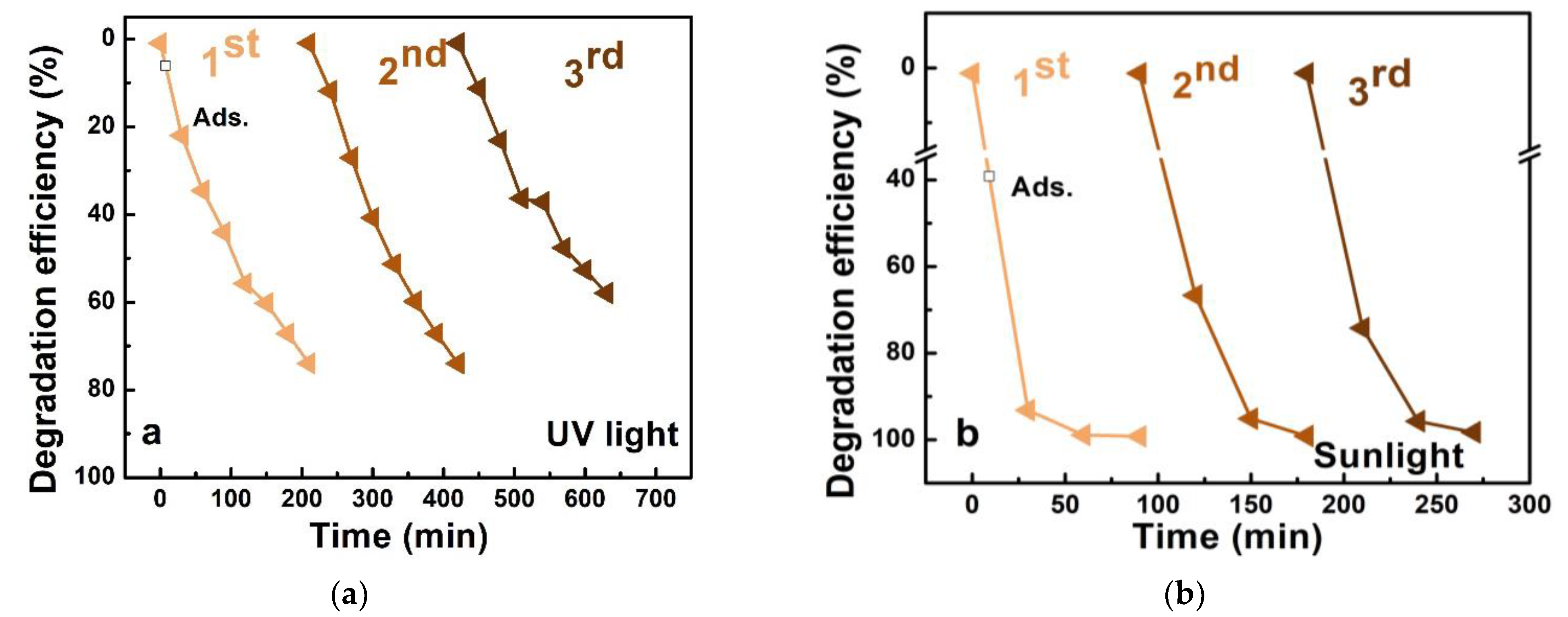
3.3. Photocatalyst Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambigadevi, J.; Kumar, P.S.; Vo, D.-V.N.; Haran, S.H.; Raghavan, T.S. Recent developments in photocatalytic remediation of textile effluent using semiconductor based nanostructured catalyst: A review. J. Environ. Chem. Eng. 2021, 9, 104881. [Google Scholar] [CrossRef]
- Chen, C.; Cai, W.; Long, M.; Zhou, B.; Wu, Y.; Wu, D.; Feng, Y. Synthesis of visible-light responsive graphene oxide/TiO2 composites with p/n heterojunction. ACS Nano. 2010, 4, 6425–6432. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Ng, Y.H.; Bell, N.J.; Zhu, Z.; Amal, R.; Smith, S.C. Hybrid Graphene/Titania Nanocomposite: Interface Charge Transfer, Hole Doping, and Sensitization for Visible Light Response. J. Phys. Chem. Lett. 2011, 2, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. Graphene Transforms Wide Band Gap ZnS to a Visible Light Photocatalyst. The New Role of Graphene as a Macromolecular Photosensitizer. ACS Nano 2012, 6, 9777–9789. [Google Scholar] [CrossRef] [PubMed]
- Ton, N.N.T.; Dao, A.T.N.; Kato, K.; Ikenaga, T.; Trinh, D.X.; Taniike, T. One-pot synthesis of TiO2/graphene nanocomposites for excellent visible light photocatalysis based on chemical exfoliation method. Carbon 2018, 133, 109–117. [Google Scholar] [CrossRef]
- Raliya, R.; Avery, C.; Chakrabarti, S.; Biswas, P. Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl. Nanosci. 2017, 7, 253–259. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene coupled TiO2 photocatalysts for environmental applications: A review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-step fabrication of graphene oxide enhanced magnetic composite gel for highly efficient dye adsorption and catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Alamelu, K.; Raja, V.; Shiamala, L.; Ali, B.J. Biphasic TiO2 nanoparticles decorated graphene nanosheets for visible light driven photocatalytic degradation of organic dyes. Appl. Surf. Sci. 2018, 430, 145–154. [Google Scholar] [CrossRef]
- Brindha, A.; Sivakumar, T. Visible active N, S co-doped TiO2/graphene photocatalysts for the degradation of hazardous dyes. J. Photochem. Photobiol. A Chem. 2017, 340, 146–156. [Google Scholar] [CrossRef]
- Tian, Z.; Yu, N.; Cheng, Y.; Wang, Z.; Chen, Z.; Zhang, L. Hydrothermal synthesis of graphene/TiO2/CdS nanocomposites as efficient visible-lightdriven photocatalysts. Mater Lett. 2017, 194, 172–175. [Google Scholar] [CrossRef]
- Niu, X.; Yan, W.; Zhao, H.; Yang, J. Synthesis of Nb doped TiO2 nanotube/reduced graphene oxide heterostructure photocatalyst with high visible light photocatalytic activity. Appl. Surf. Sci. 2018, 440, 804–813. [Google Scholar] [CrossRef]
- Wang, S.; Cai, J.; Mao, J.; Li, S.; Shen, J.; Gao, S.; Huang, J.; Wang, X.; Parkin, I.P.; Lai, Y. Defective black Ti3+ self-doped TiO2 and reduced graphene oxide composite nanoparticles for boosting visible-light-driven photocatalytic and photoelectrochemical activity. Appl. Surf. Sci. 2018, 467-468, 45–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, F.; Zhou, F.; Yang, X.; Zhang, D.; Chen, Y. Synergistic effect of RGO/TiO2 nanosheets with exposed (0 0 1) facets for boosting visible light photocatalytic activity. Appl. Surf. Sci. 2020, 510, 145451. [Google Scholar] [CrossRef]
- Le, T.-L.T.; Van, K.N.; Van Bui, H.; Le, T.G.; Vo, V. Controlled growth of TiO2 nanoparticles on graphene by hydrothermal method for visible-light photocatalysis. J. Sci. Adv. Mater. Devices 2021, 6, 516–527. [Google Scholar] [CrossRef]
- Chen, L.; Tian, L.; Xie, J.; Zhang, C.; Chen, J.; Wang, Y.; Li, Q.; Lv, K.; Deng, K. One-step solid state synthesis of facet-dependent contact TiO2 hollow nanocubes and reduced graphene oxide hybrids with 3D/2D heterojunctions for enhanced visible photocatalytic activity. Appl. Surf. Sci. 2020, 504, 144353. [Google Scholar] [CrossRef]
- Li, Z.Q.; Wang, H.L.; Zi, L.Y.; Zhang, J.J.; Zhang, Y.S. Preparation and photocatalytic performance of magnetic TiO2–Fe3O4/graphene (RGO) composites under VIS-light irradiation. Ceram. Int. 2015, 41, 10634–10643. [Google Scholar] [CrossRef]
- Liu, S.Q. Magnetic semiconductor nano-photocatalysts for the degradation of organic pollutants. Environ. Chem. Lett. 2012, 10, 209–216. [Google Scholar] [CrossRef]
- Nada, A.A.; Tantawy, H.R.; Elsayed, M.A.; Bechelany, M.; Elmowafy, M.E. Elaboration of nanotitania-magnetic reduced graphene oxide for degradation of tartrazine dye in aqueous solution. Solid State Sci. 2018, 78, 116–125. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, G.; Liu, C.; Luo, S.; Xu, X.; Chen, L.; Wang, B. Magnetic TiO2-graphene composite as a high-performance and recyclable platform for efficient photocatalytic removal of herbicides from water. J. Hazard. Mater. 2013, 252-253, 115–122. [Google Scholar] [CrossRef]
- D’Cruz, B.; Madkour, M.; Amin, M.O.; Al-Hetlani, E. Efficient and recoverable magnetic AC-Fe3O4 nanocomposite for rapid removal of promazine from wastewater. Mater. Chem. Phys. 2019, 240, 122109. [Google Scholar] [CrossRef]
- Ismael, A.M.; El-Shazly, A.N.; Gaber, S.E.; Rashad, M.M.; Kamel, A.H.; Hassan, S.S.M. Novel TiO2/GO/CuFe2O4 nanocomposite: A magnetic, reusable and visible-light-driven photocatalyst for efficient photocatalytic removal of chlorinated pesticides from wastewater. RSC Adv. 2020, 10, 34806–34814. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, F.; Lin, C.; Wang, Z.L. Direct Growth of TiO2 Nanosheet Arrays on Carbon Fibers for Highly Efficient Photocatalytic Degradation of Methyl Orange. Adv. Mater. 2012, 24, 4761–4764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Wu, Y.-H.; Mei, J.-Y.; Zheng, G.-P.; Yan, T.-T.; Zheng, X.-C.; Liu, P.; Guan, X.-X. Synergetic adsorption and photocatalytic degradation of pollutants over 3D TiO2—graphene aerogel composites synthesized via a facile one-pot route. Photochem. Photobiol. Sci. 2016, 15, 1012–1019. [Google Scholar] [CrossRef]
- Wang, B.; Karthikeyan, R.; Lu, X.-Y.; Xuan, J.; Leung, M.K. High photocatalytic activity of immobilized TiO2 nanorods on carbonized cotton fibers. J. Hazard. Mater. 2013, 263, 659–669. [Google Scholar] [CrossRef]
- Bouarioua, A.; Zerdaoui, M. Photocatalytic activities of TiO2 layers immobilized on glass substrates by dip-coating technique toward the decolorization of methyl orange as a model organic pollutant. J. Environ. Chem. Eng. 2017, 5, 1565–1574. [Google Scholar] [CrossRef]
- Veca, L.M.; Nastase, F.; Banciu, C.; Popescu, M.; Romanitan, C.; Lungulescu, M.; Popa, R. Synthesis of macroporous ZnO-graphene hybrid monoliths with potential for functional electrodes. Diam. Relat. Mater. 2018, 87, 70–77. [Google Scholar] [CrossRef]
- Banciu, C.; Lungulescu, M.; Bara, A.; Leonat, L. Teisanu A. 3D graphene network investigation by Raman spectroscopy. Optoelectron. Adv. Mat. 2017, 11, 368–372. [Google Scholar]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Etacheri, V.; Michlits, G.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. A Highly Efficient TiO2–xCx Nano-heterojunction Photocatalyst for Visible Light Induced Antibacterial Applications. ACS Appl. Mater. Interfaces 2013, 5, 1663–1672. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858–2860. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, S.; Sirajudheen, P.; Nabeena, C.P.; Sajna, V.P.; Meenakshi, S. Photocatalytic performance of chitosan tethered magnetic Fe2O3-like (3D/2D) hybrid for the dynamic removal of anionic dyes: Degradation and mechanistic pathways. Int. J. Biol. Macromol. 2021, 183, 2088–2099. [Google Scholar] [CrossRef] [PubMed]
- Kansal, S.K.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Ge, L.; Chen, C.; Li, Y.; Xiao, X.; Zhang, Y.; Guo, L. Novel visible light induced Co3O4-g-C3N4 heterojunction photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2014, 147, 546–553. [Google Scholar] [CrossRef]
- Li, Z.; Yang, S.; Zhou, J.; Li, D.; Zhou, X.; Ge, C.; Fang, Y. Novel mesoporous g-C3N4 and BiPO4 nanorods hybrid architectures and their enhanced visible-light-driven photocatalytic performances. Chem. Eng. J. 2014, 241, 344–351. [Google Scholar] [CrossRef]
- Sun, C.; Li, T.; Wen, W.; Luo, X.; Zhao, L. ZnSe/CdSe core–shell nanoribbon arrays for photocatalytic applications. CrystEngComm 2019, 22, 895–904. [Google Scholar] [CrossRef]
- Qiao, F.; Liang, Q.; Hou, X.; Yang, J.; Xu, Q. Two-Step Preparation of ZnO/ZnSe Heterostructure with Remarkable Photocatalytic Activity by Ultrasonic and Hydrothermal Approach. J. Electron. Mater. 2019, 48, 4418–4423. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Shi, Z.; Zhu, B.; Chen, X.; Yang, J. Synthesis of porous ZnS/ZnSe nanosheets for enhanced visible light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 11605–11612. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, B.; Shi, J.; Li, M.; He, Z. Rapid Photocatalytic Decolorization of Methyl Orange under Visible Light Using VS4/Carbon Powder Nanocomposites. ACS Sustain. Chem. Eng. 2017, 5, 7690–7699. [Google Scholar] [CrossRef]
- Shi, W.; Shi, J.; Yu, S.; Liu, P. Ion-exchange synthesis and enhanced visible-light photocatalytic activities of CuSe-ZnSe flower-like nanocomposites. Appl. Catal. B Environ. 2013, 138–139, 184–190. [Google Scholar] [CrossRef]
- Wang, H.-J.; Sun, Y.-Y.; Wang, C.-F.; Cao, Y. Controlled synthesis, cytotoxicity and photocatalytic comparison of ZnO films photocatalysts supported on aluminum matrix. Chem. Eng. J. 2012, 198–199, 154–162. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, B.; Niu, J.; Huang, X.; Zhu, Y. Solvothermal synthesis of BiOBr thin film and its photocatalytic performance. Appl. Surf. Sci. 2014, 288, 369–372. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, J.; Ke, Y. A novel approach of preparing TiO2 films at low temperature and its application in photocatalytic degradation of methyl orange. J. Hazard. Mater. 2010, 177, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Arconada, N.; Castro, Y.; Durán, A. Photocatalytic properties in aqueous solution of porous TiO2-anatase films prepared by sol–gel process. Appl. Catal. A Gen. 2010, 385, 101–107. [Google Scholar] [CrossRef]
- Shah, A.P.; Jain, S.; Mokale, V.J.; Shimpi, N.G. High performance visible light photocatalysis of electrospun PAN/ZnO hybrid nanofibers. J. Ind. Eng. Chem. 2019, 77, 154–163. [Google Scholar] [CrossRef]
- Xiong, L.; Lan, D.; Liang, H.; Chen, L.; Wang, Q. Grafting TiO2 nanoparticles onto carbon fiber via “thiol-ene” click chemistry and its photodegradation performance for methyl orange. Mater. Lett. 2018, 211, 296–299. [Google Scholar] [CrossRef]
- Naikwade, A.G.; Jagadale, M.B.; Kale, D.P.; Gophane, A.D.; Garadkar, K.M.; Rashinkar, G.S. Photocatalytic Degradation of Methyl Orange by Magnetically Retrievable Supported Ionic Liquid Phase Photocatalyst. ACS Omega 2020, 5, 131–144. [Google Scholar] [CrossRef] [PubMed]

| No. | Photocatalyst Generation | Photocatalyst | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|
| 1. | First—single component | ZnO | UV | 120 | 98 | [31] |
| Fe2O3@chitosan | Visible | 60 | 89.2 | [32] | ||
| ZnO | Visible | 90 | 98.92 | [33] | ||
| 2. | Second—multiple components/heterojunction | Co3O4-g-C3N4 | Visible | 180 | 99 | [34] |
| BiPO4/C3N4 | Visible | 120 | 96 | [35] | ||
| ZnSe/CdSe | Visible | 120 | 30 | [36] | ||
| ZnO/ZnSe | Visible | 540 | 94.3 | [37] | ||
| ZnS/ZnSe | Visible | 60 | 95.8 | [38] | ||
| VS4/CP | UV | 30 | 67 | [39] | ||
| Visible/sunlight | 30 | 98/70.1 | ||||
| CuSe/ZnSe | Visible | 90 | 100 | [40] | ||
| 3. | Third—photocatalyst immobilized on different substrates | Al/ZnO | UV | 180 | 98 | [41] |
| FTO/BiOBr | Visible | 300 | 48 | [42] | ||
| Ni/TiO2 | UV | 120 | 85 | [43] | ||
| TiO2 film | UV | 900 | 99 | [44] | ||
| ZnO/PAN | Visible | 280 | 99 | [45] | ||
| TiO2/CF | UV | 50 | ~ 99 | [23] | ||
| CF-TiO2 | UV | 150 | 89 | [46] | ||
| [FemIL@SiO2@Mag]2MoO4 | UV | 90 | 75 | [47] | ||
| TiO2/3D-GF/Ni | UV | 90 (180) | 93 (99) | This work | ||
| Simulated sunlight | 30 (90) | 94 (99.5) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, E.M.; Mihalache, I.; Istrate, A.-I.; Banciu, C.A.; Romanitan, C.; Brincoveanu, O.; Tanasa, E.; Banu, A.; Veca, L.M. Self-Sustained Three-Dimensional Macroporous TiO2-Graphene Photocatalyst for Sunlight Decolorization of Methyl Orange. Nanomaterials 2022, 12, 4393. https://doi.org/10.3390/nano12244393
Mihai EM, Mihalache I, Istrate A-I, Banciu CA, Romanitan C, Brincoveanu O, Tanasa E, Banu A, Veca LM. Self-Sustained Three-Dimensional Macroporous TiO2-Graphene Photocatalyst for Sunlight Decolorization of Methyl Orange. Nanomaterials. 2022; 12(24):4393. https://doi.org/10.3390/nano12244393
Chicago/Turabian StyleMihai, Elena Madalina, Iuliana Mihalache, Anca-Ionela Istrate, Cristina Antonela Banciu, Cosmin Romanitan, Oana Brincoveanu, Eugenia Tanasa, Alexandra Banu, and Lucia Monica Veca. 2022. "Self-Sustained Three-Dimensional Macroporous TiO2-Graphene Photocatalyst for Sunlight Decolorization of Methyl Orange" Nanomaterials 12, no. 24: 4393. https://doi.org/10.3390/nano12244393
APA StyleMihai, E. M., Mihalache, I., Istrate, A.-I., Banciu, C. A., Romanitan, C., Brincoveanu, O., Tanasa, E., Banu, A., & Veca, L. M. (2022). Self-Sustained Three-Dimensional Macroporous TiO2-Graphene Photocatalyst for Sunlight Decolorization of Methyl Orange. Nanomaterials, 12(24), 4393. https://doi.org/10.3390/nano12244393








