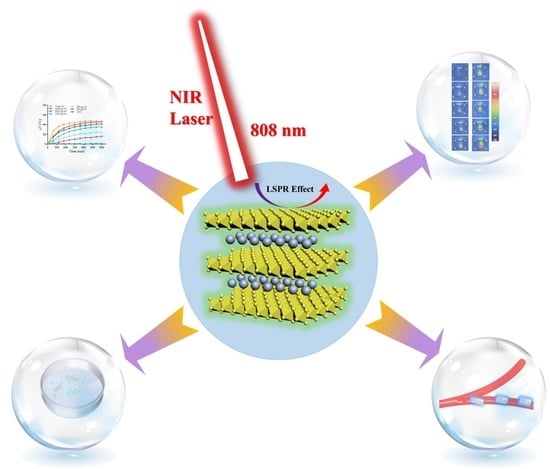
ROS- and pH-Responsive Polydopamine Functionalized Ti3C2Tx MXene-Based Nanoparticles as Drug Delivery Nanocarriers with High Antibacterial Activity
Abstract
1. Introduction
2. Experimental
2.1. Materials
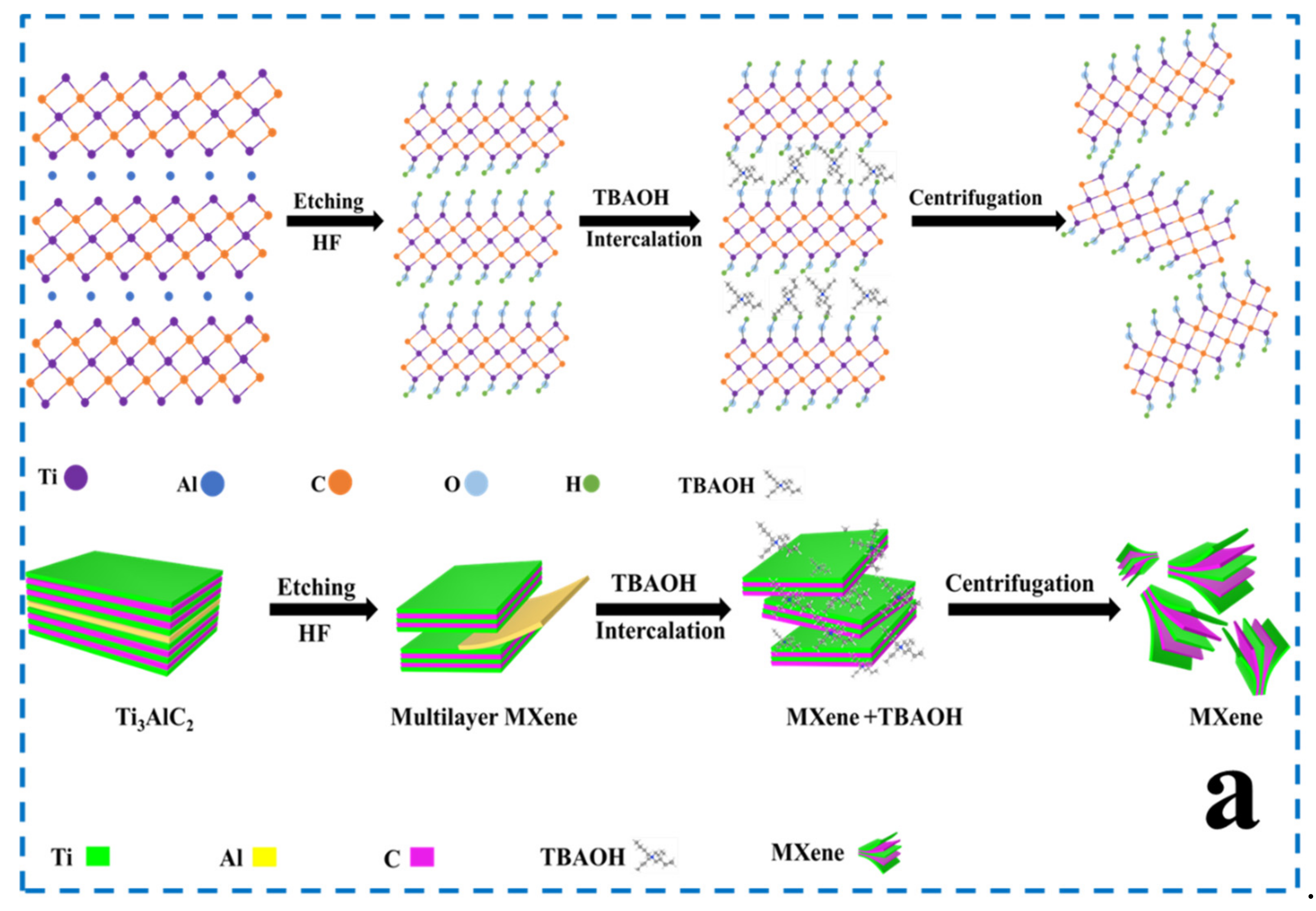
2.2. Synthesis of MXene Nanosheets
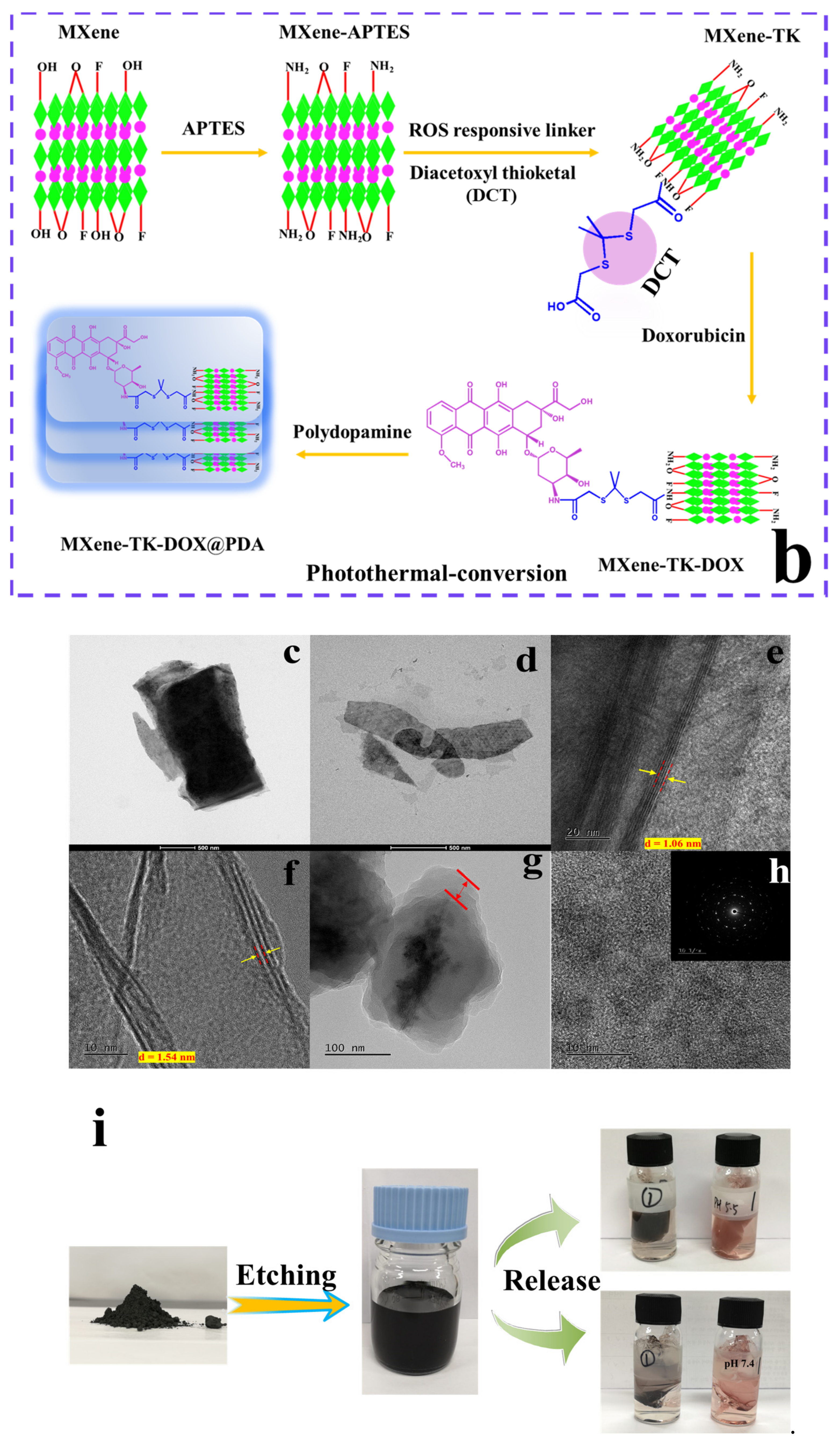
2.3. Synthesis of MXene-NH2 Nanoparticles
2.4. Synthesis of MXene-TK Nanoparticles
2.5. Synthesis of MXene-TK-DOX Nanosized Flakes
2.6. Preparation of MXene-TK-DOX@PDA Nanoparticles
2.7. Synthesis of Diacetoxyl Thioketal (TK) Linker
2.8. Antibacterial Activity
2.9. Loading Capacity and Drug Release Study
2.10. Photothermal Conversion Property
2.11. Characterization
3. Results and Discussion
3.1. Design, Preparation, and Characterization of MXene-Based Drug Delivery Nanoparticles
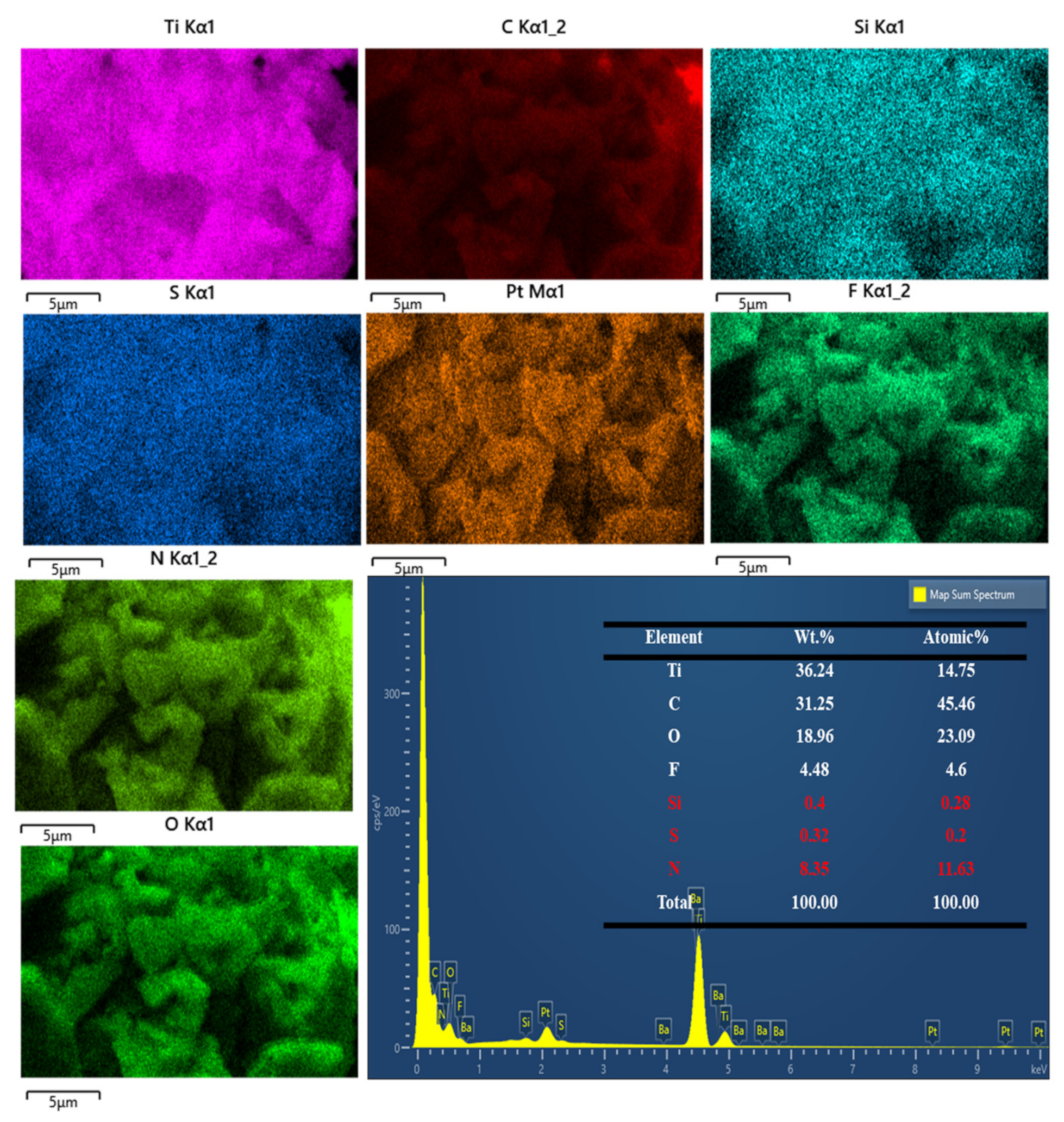
3.2. Physicochemical Analysis
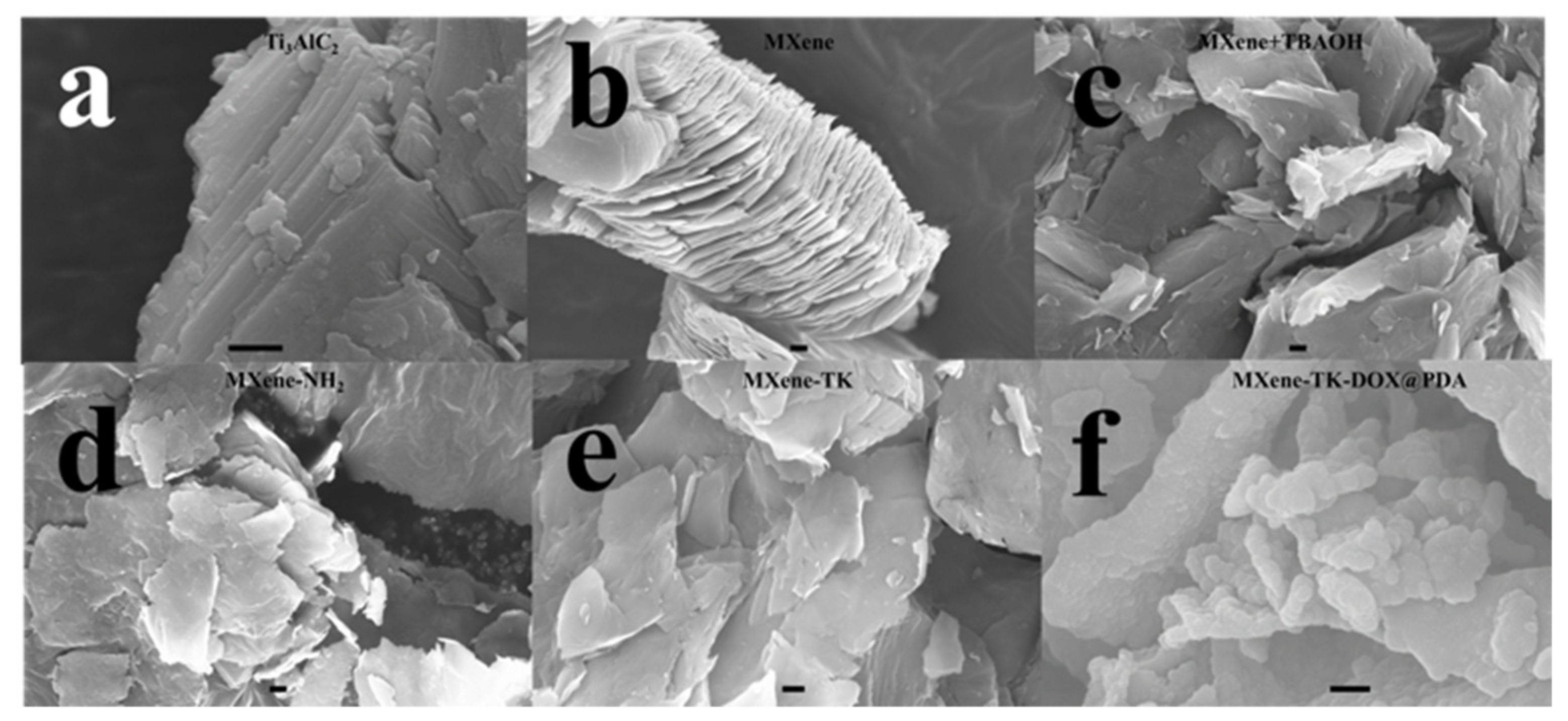
3.3. Morphological Analysis of MXene-Based Nanomaterials
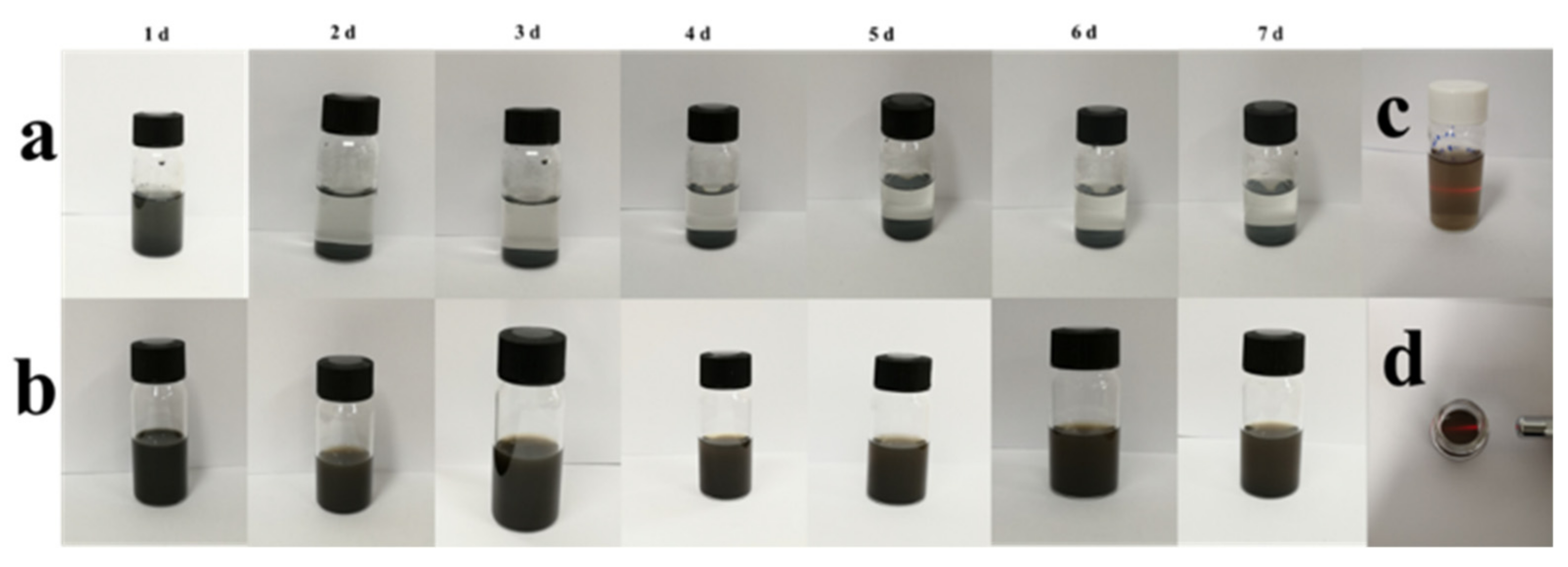
3.4. In Vitro Stability of MXene-Based Nanoparticles
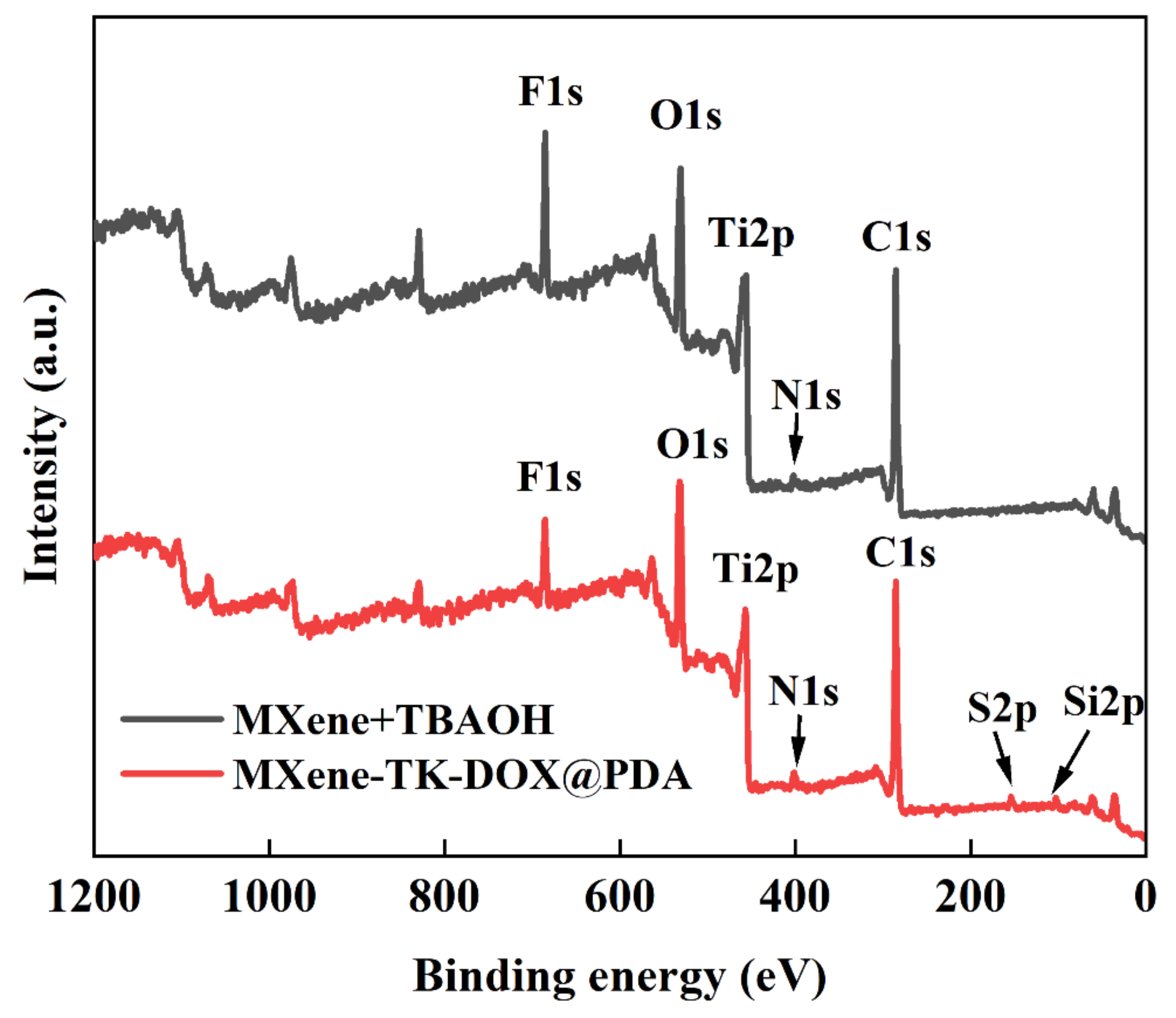
3.5. XPS Analysis
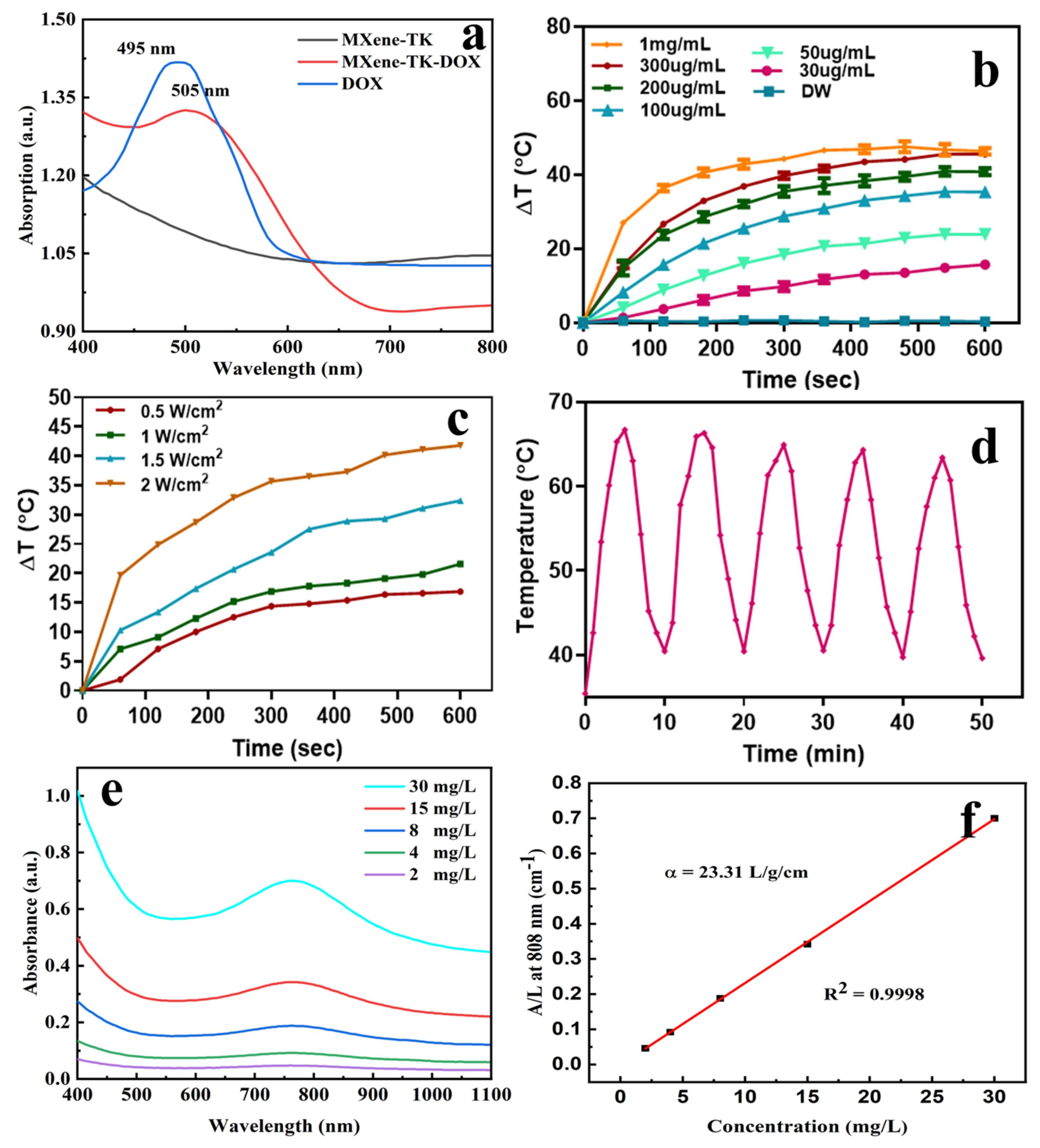
3.6. In Vitro Photothermal Performance of MXene-Based Nanoparticles
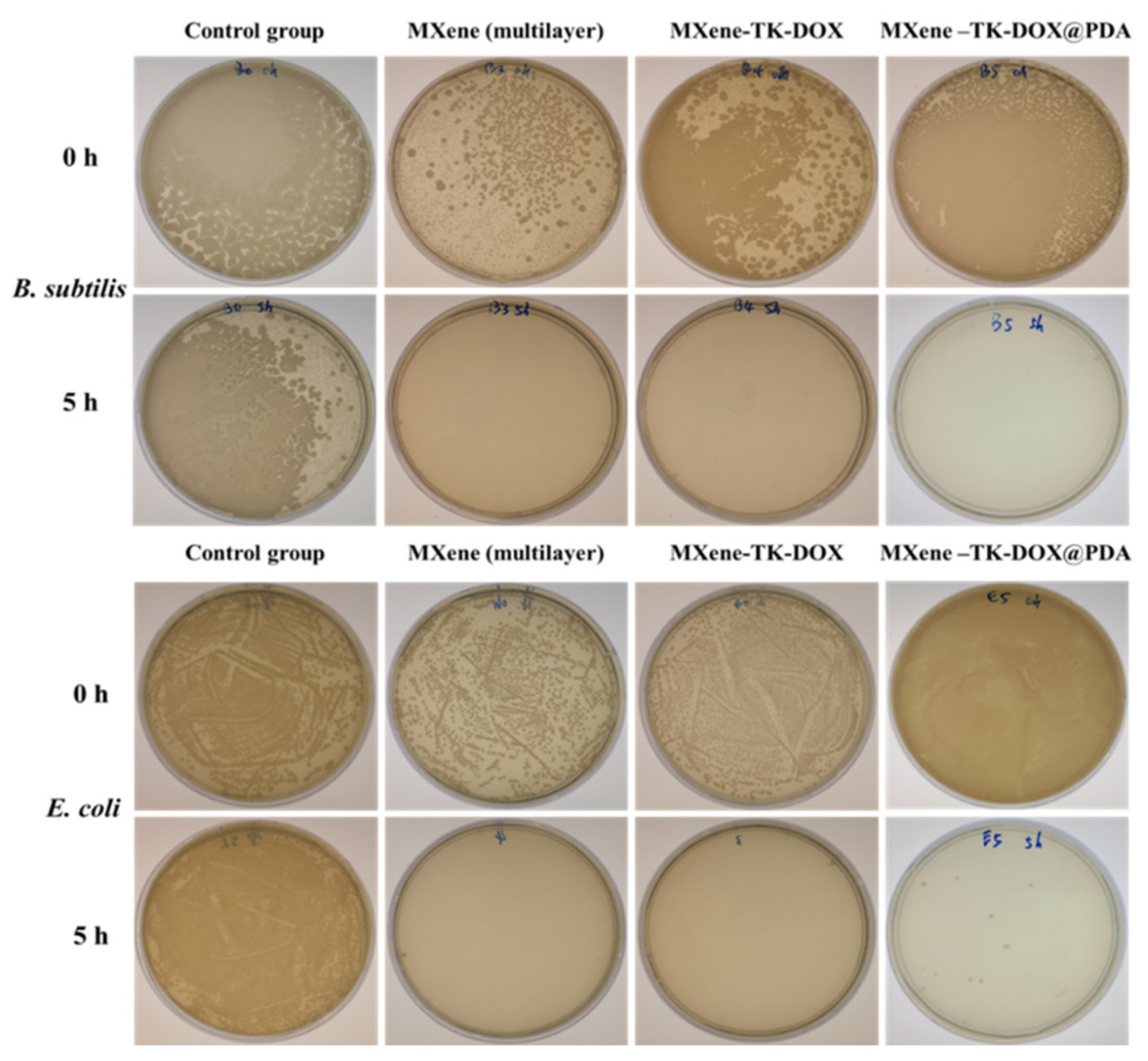
3.7. In Vitro Antibacterial Activity
3.8. In Vitro Drug Release Profile
3.9. Drug Release Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naguib, M.; Gogotsi, Y. Synthesis of Two-Dimensional Materials by Selective Extraction. Acc. Chem. Res. 2015, 48, 128–135. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive Two-Dimensional Titanium Carbide ‘Clay’ with High Volumetric Capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Dong, Y.; Shi, H.; Wu, Z.S. Recent Advances and Promise of MXene-Based Nanostructures for High-Performance Metal Ion Batteries. Adv. Funct. Mater. 2020, 30, 2000706. [Google Scholar] [CrossRef]
- Liu, C.; Xu, W.; Mei, C.; Li, M.-C.; Xu, X.; Wu, Q. Highly Stable H2V3O8/MXene Cathode for Zn-Ion Batteries with Superior Rate Performance and Long Lifespan. Chem. Eng. J. 2021, 405, 126737. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; Miao, J.; Zhang, P.; Wan, P.; He, L.; Xu, B. Flexible 3D Porous MXene Foam for High-Performance Lithium-Ion Batteries. Small 2019, 15, 1904293. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhang, G.; Chen, K.; Zhou, C.; Zhu, X.; Zhang, Y.; Chen, K.; Mi, H.-Y.; Wang, Y.; Liu, C. Mxene/Polylactic Acid Fabric-Based Resonant Cavity for Realizing Simultaneous High-Performance Electromagnetic Interference (EMI) Shielding and Efficient Energy Harvesting. ACS Appl. Mater. Interfaces 2022, 14, 14607–14617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, Z.; Ba, Z.; Li, X.; Dong, J.; Fang, Y.; Zhang, Q.; Zhao, X. 3D MXene-Holey Graphene Hydrogel for Supercapacitor with Superior Energy Storage. J. Energy Storage 2022, 47, 103911. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.C.; Kuo, T.R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831. [Google Scholar]
- Morales-Garcia, A.; Calle-Vallejo, F.; Illas, F. MXenes: New Horizons in Catalysis. ACS Catal. 2020, 10, 13487–13503. [Google Scholar] [CrossRef]
- Wan, Y.; Xiong, P.; Liu, J.; Feng, F.; Xun, X.; Gama, F.M.; Zhang, Q.; Yao, F.; Yang, Z.; Luo, H. Ultrathin, Strong, and Highly Flexible Ti3C2Tx MXene/Bacterial Cellulose Composite Films for High-Performance Electromagnetic Interference Shielding. ACS Nano 2021, 15, 8439–8449. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-Induced Cardiotoxicity: An Update on the Molecular Mechanism and Novel Therapeutic Strategies for Effective Management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, J. MSN Anti-Cancer Nanomedicines: Chemotherapy Enhancement, Overcoming of Drug Resistance, and Metastasis Inhibition. Adv. Mater. 2014, 26, 391–411. [Google Scholar] [CrossRef]
- Yao, Y.; Ding, C.; Gao, J.; Wu, D.; Liu, X.; Qin, Y.; Kong, Y. Construction of Near-Infrared Irradiation-Controlled Drug Delivery System Based on Silica@Polypyrrole@ Mesoporous Silica and PEG-PCL-PEG. Bull. Korean Chem. Soc. 2019, 40, 917–920. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Li, M.-F.; Feng, J. Multifunctional Mesoporous Silica Nanoparticles Based on Charge-Reversal Plug-Gate Nanovalves and Acid-Decomposable ZnO Quantum Dots for Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 26666–26673. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Izzo, L. Dendrimer Biocompatibility and Toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef]
- Katti, D.S.; Lakshmi, S.; Langer, R.; Laurencin, C. Toxicity, Biodegradation and Elimination of Polyanhydrides. Adv. Drug Deliv. Rev. 2002, 54, 933–961. [Google Scholar] [CrossRef]
- Liao, T.; Liu, C.; Ren, J.; Chen, H.; Kuang, Y.; Jiang, B.; Chen, J.; Sun, Z.; Li, C. A Chitosan/Mesoporous Silica Nanoparticle-Based Anticancer Drug Delivery System with A “Tumor-Triggered Targeting” Property. Int. J. Biol. Macromol. 2021, 183, 2017–2029. [Google Scholar] [CrossRef]
- Zhang, X.; van Rijt, S. DNA Modified MSN-Films as Versatile Biointerfaces to Study Stem Cell Adhesion Processes. Colloids Surf. B 2022, 215, 112495. [Google Scholar] [CrossRef]
- Al-Nadaf, A.H.; Dahabiyeh, L.A.; Jawarneh, S.; Bardaweel, S.; Mahmoud, N.N. Folic Acid-Hydrophilic Polymer Coated Mesoporous Silica Nanoparticles Target Doxorubicin Delivery. Pharm. Dev. Technol. 2021, 26, 582–591. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Yan, Y.-Z.; Nagappan, S.; He, S.; Ha, C.-S.; Jin, Y.-S. Dual (Thermo-/pH-) Responsive P (NIPAM-co-AA-co-HEMA) Nanocapsules for Controlled Release of 5-Fluorouracil. J. Macromol. Sci. A 2021, 58, 860–871. [Google Scholar] [CrossRef]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally Delivered Thioketal Nanoparticles Loaded with TNF-α–siRNA Target Inflammation and Inhibit Gene Expression in The Intestines. Nat. Mater. 2010, 9, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, X.; Cheng, L.; Tang, Y.A.; Zhan, G.; Gong, F.; Yang, X. GSH-Depleted PtCu3 Nanocages for Chemodynamic-Enhanced Sonodynamic Cancer Therapy. Adv. Funct. Mater. 2020, 30, 1907954. [Google Scholar] [CrossRef]
- Xu, X.; Saw, P.E.; Tao, W.; Li, Y.; Ji, X.; Bhasin, S.; Farokhzad, O.C. ROS-Responsive Polyprodrug Nanoparticles for Triggered Drug Delivery and Effective Cancer Therapy. Adv. Mater. 2017, 29, 1700141. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Szuplewska, A.; Wojciechowski, T.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Olszyna, A. In Vitro Studies on Cytotoxicity of Delaminated Ti3C2 MXene. J. Hazard. Mater. 2017, 339, 1–8. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Kim, I.-H.; Kim, K.; Choi, Y. ROS-Responsive Activatable Photosensitizing Agent for Imaging and Photodynamic Therapy of Activated Macrophages. Theranostics 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Chu, B.; Qu, Y.; He, X.; Hao, Y.; Yang, C.; Yang, Y.; Hu, D.; Wang, F.; Qian, Z. ROS-Responsive Camptothecin Prodrug Nanoparticles for on-Demand Drug Release and Combination of Chemotherapy and Photodynamic Therapy. Adv. Funct. Mater. 2020, 30, 2005918. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Zhao, R.; Zhou, Y.; Feng, L.; Gai, S.; Yang, P. Pt Decorated Ti3C2Tx MXene with NIR-II Light Amplified Nanozyme Catalytic Activity for Efficient Phototheranostics. ACS Nano 2022, 16, 3105–3118. [Google Scholar] [CrossRef] [PubMed]
- Gholami Derami, H.; Gupta, P.; Weng, K.C.; Seth, A.; Gupta, R.; Silva, J.R.; Raman, B.; Singamaneni, S. Reversible Photothermal Modulation of Electrical Activity of Excitable Cells Using Polydopamine Nanoparticles. Adv. Mater. 2021, 33, 2008809. [Google Scholar] [CrossRef]
- Qi, X.; Huang, Y.; You, S.; Xiang, Y.; Cai, E.; Mao, R.; Pan, W.; Tong, X.; Dong, W.; Ye, F. Engineering Robust Ag-Decorated Polydopamine Nano-Photothermal Platforms to Combat Bacterial Infection and Prompt Wound Healing. Adv. Sci. 2022, 9, 2106015. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; Ogaki, R.; Laursen, A.O.; Lovmand, J.; Sutherland, D.S.; Städler, B. Polydopamine/Liposome Coatings and Their Interaction with Myoblast Cells. ACS Appl. Mater. Interfaces 2011, 3, 2142–2147. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Zheng, W.; Gui, L.; Yang, Y.; Li, A.; Gong, M. Investigating the Biological Effect of Multidimensional Ti3C2 (MXene)-Based Nanomaterials Through a Metabolomics Approach: A Multidimensional-Determined Alteration in Energy Metabolism. Chem. Mater. 2022, 34, 3839–3852. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, T.; Zhang, K.; Meng, X.; Dai, W.; Wang, D.; Zhang, X. Engineered Exosome-Mediated Near-Infrared-II Region V2C Quantum Dot Delivery for Nucleus-Target Low-Temperature Photothermal Therapy. ACS Nano 2019, 13, 1499–1510. [Google Scholar] [CrossRef]
- Unal, M.A.; Bayrakdar, F.; Fusco, L.; Besbinar, O.; Shuck, C.E.; Yalcin, S.; Yilmazer, A. 2D MXenes with Antiviral and Immunomodulatory Properties: A Pilot Study Against SARS-Cov-2. Nano Today 2021, 38, 101136. [Google Scholar]
- Mei, S.; Xu, X.; Priestley, R.D.; Lu, Y. Polydopamine-Based Nanoreactors: Synthesis and Applications in Bioscience and Energy Materials. Chem. Sci. 2020, 11, 12269–12281. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Sun, S.; Sha, X.; Liang, J.; Yang, G.; Hu, X.; He, Z.; Liu, M.; Zhou, N.; Zhang, X.; Wei, Y. Rapid Synthesis of Polyimidazole Functionalized MXene via Microwave-Irradiation Assisted Multi-Component Reaction and Its Iodine Adsorption Performance. J. Hazard. Mater. 2021, 420, 126580. [Google Scholar] [CrossRef]
- Liao, J.-X.; Huang, Q.-F.; Li, Y.-H.; Zhang, D.-W.; Wang, G.-H. Chitosan Derivatives Functionalized Dual ROS-Responsive Nanocarriers to Enhance Synergistic Oxidation-Chemotherapy. Carbohydr. Polym. 2022, 282, 119087. [Google Scholar] [CrossRef]
- Yan, Y.-Z.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Zhai, B.; Shi, Z. Interior Multi-Cavity/Surface Engineering of Alginate Hydrogels with Polyethylenimine for Highly Efficient Chromium Removal in Batch and Continuous Aqueous Systems. J. Mater. Chem. A 2017, 5, 17073–17087. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, L.; Zhu, M.; Wan, G.; Li, C.; Zhang, J.; Wang, Y.; Wang, Y. Reactive Oxygen Species-Responsive Nanoparticles Based on Peglated Prodrug for Targeted Treatment of Oral Tongue Squamous Cell Carcinoma by Combining Photodynamic Therapy and Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 29260–29272. [Google Scholar] [CrossRef]
- Zheng, K.; Li, S.; Jing, L.; Chen, P.Y.; Xie, J. Synergistic Antimicrobial Titanium Carbide (MXene) Conjugated with Gold Nanoclusters. Adv. Healthc. Mater. 2020, 9, 2001007. [Google Scholar] [CrossRef]
- John, J.V.; Uthaman, S.; Augustine, R.; Manickavasagam Lekshmi, K.; Park, I.K.; Kim, I. Biomimetic pH/Redox Dual Stimuli-Responsive Zwitterionic Polymer Block Poly (L-Histidine) Micelles for Intracellular Delivery of Doxorubicin into Tumor Cells. J. Polym. Sci. Polym. Chem. 2017, 55, 2061–2070. [Google Scholar] [CrossRef]
- She, X.; Chen, L.; Velleman, L.; Li, C.; Zhu, H.; He, C.; Wang, T.; Shigdar, S.; Duan, W.; Kong, L. Fabrication of High Specificity Hollow Mesoporous Silica Nanoparticles Assisted by Eudragit for Targeted Drug Delivery. J. Colloid Interface Sci. 2015, 445, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zou, Y.; Li, F.; Wang, J.; Zou, J.; Li, N. Intercalation Engineering of MXenes Towards Highly Efficient Photo (Electrocatalytic) Hydrogen Evolution Reactions. J. Mater. Chem. 2021, 9, 24195–24214. [Google Scholar] [CrossRef]
- Ghidiu, M.; Kota, S.; Halim, J.; Sherwood, A.W.; Nedfors, N.; Rosen, J.; Mochalin, V.N.; Barsoum, M.W. Alkylammonium Cation Intercalation into Ti3C2 (MXene): Effects on Properties and Ion-Exchange Capacity Estimation. Chem. Mater. 2017, 29, 1099–1106. [Google Scholar] [CrossRef]
- Dai, C.; Lin, H.; Xu, G.; Liu, Z.; Wu, R.; Chen, Y. Biocompatible 2D Titanium Carbide (MXenes) Composite Nanosheets for pH-Responsive MRI-Guided Tumor Hyperthermia. Chem. Mater. 2017, 29, 8637–8652. [Google Scholar] [CrossRef]
- Zhang, Q.; Lai, H.; Fan, R.; Ji, P.; Fu, X.; Li, H. High Concentration of Ti3C2Tx MXene in Organic Solvent. ACS Nano 2021, 15, 5249–5262. [Google Scholar] [CrossRef]
- Xue, Y.; Feng, J.; Huo, S.; Song, P.; Yu, B.; Liu, L.; Wang, H. Polyphosphoramide-Intercalated MXene for Simultaneously Enhancing Thermal Stability, Flame Retardancy and Mechanical Properties of Polylactide. Chem. Eng. J. 2020, 397, 125336. [Google Scholar] [CrossRef]
- Rohani, S.; Mohammadi Ziarani, G.; Badiei, A.; Ziarati, A.; Jafari, M.; Shayesteh, A. Palladium-Anchored Multidentate SBA-15/di-Urea Nanoreactor: A Highly Active Catalyst for Suzuki Coupling Reaction. Appl. Organomet. Chem. 2018, 32, e4397. [Google Scholar] [CrossRef]
- Peng, M.; Dong, M.; Wei, W.; Xu, H.; Liu, C.; Shen, C. The Introduction of Amino Termination on Ti3C2 MXene Surface for Its Flexible Film with Excellent Property. Carbon 2021, 179, 400–407. [Google Scholar] [CrossRef]
- Li, Q.; Xu, X.; Guo, J.; Hill, J.P.; Xu, H.; Xiang, L.; Li, C.; Yamauchi, Y.; Mai, Y. Two-Dimensional MXene-Polymer Heterostructure with Ordered in-Plane Mesochannels for High-Performance Capacitive Deionization. Angew. Chem. 2021, 133, 26732–26738. [Google Scholar] [CrossRef]
- Lu, B.; Hu, S.; Wu, D.; Wu, C.; Zhu, Z.; Hu, L.; Zhang, J. Ionic Liquid Exfoliated Ti3C2Tx MXene Nanosheets for Photoacoustic Imaging and Synergistic Photothermal/Chemotherapy of Cancer. J. Mater. Chem. B 2022, 10, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Sarycheva, A.; Gogotsi, Y. Raman Spectroscopy Analysis of the Structure and Surface Chemistry of Ti3C2Tx MXene. Chem. Mater. 2020, 32, 3480–3488. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Lu, B.; Zhu, Z.; Ma, B.; Wang, W.; Zhu, R.; Zhang, J. 2D MXene Nanomaterials for Versatile Biomedical Applications: Current Trends and Future Prospects. Small 2021, 17, 2100946. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Bhargava Reddy, M.S.; Kailasa, S.; Marupalli, B.C.; Sadasivuni, K.K.; Aich, S. A Family of 2D-MXenes: Synthesis, Properties, and Gas Sensing Applications. ACS Sens. 2022, 7, 2132–2163. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent Advances in Transdermal Drug Delivery Systems: A Review. Biomater. Res. 2021, 25, 1–15. [Google Scholar] [CrossRef]
- Mozafari, M.; Soroush, M. Surface Functionalization of MXenes. Mater. Adv. 2021, 2, 7277–7307. [Google Scholar]
- Pazniak, A.; Bazhin, P.; Shplis, N.; Kolesnikov, E.; Shchetinin, I.; Komissarov, A.; Polcak, J.; Stolin, A.; Kuznetsov, D. Ti3C2Tx MXene Characterization Produced from SHS-Ground Ti3AlC2. Mater. Des 2019, 183, 108143. [Google Scholar] [CrossRef]
- Cao, M.; Wang, F.; Wang, L.; Wu, W.; Lv, W.; Zhu, J. Room Temperature Oxidation of Ti3C2 Mxene for Supercapacitor Electrodes. J. Electrochem. Soc. 2017, 164, A3933. [Google Scholar] [CrossRef]
- Mohan, A.; Peter, J.; Rout, L.; Thomas, A.M.; Nagappan, S.; Parambadath, S.; Zhang, W.; Selvaraj, M.; Ha, C.-S. Facile Synthesis of Silver Nanoparticles Stabilized Dual Responsive Silica Nanohybrid: A Highly Active Switchable Catalyst for Oxidation of Alcohols in Aqueous Medium. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125846. [Google Scholar] [CrossRef]
- Shanthi, P.M.; Hanumantha, P.J.; Ramalinga, K.; Gattu, B.; Datta, M.K.; Kumta, P.N. Sulfonic Acid Based Complex Framework Materials (CFM): Nanostructured Polysulfide Immobilization Systems for Rechargeable Lithium–Sulfur Battery. J. Electrochem. Soc. 2019, 166, A1827. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef]
- Dembereldorj, U.; Kim, M.; Kim, S.; Ganbold, E.-O.; Lee, S.Y.; Joo, S.-W. A Spatiotemporal Anticancer Drug Release Platform of Pegylated Graphene Oxide Triggered by Glutathione in vitro and in vivo. J. Mater. Chem. 2012, 22, 23845–23851. [Google Scholar] [CrossRef]
- Hao, Y.; Mao, L.; Zhang, R.; Liao, X.; Yuan, M.; Liao, W. Multifunctional Biodegradable Prussian Blue Analogue for Synergetic Photothermal/Photodynamic/Chemodynamic Therapy and Intrinsic Tumor Metastasis Inhibition. ACS Appl. Bio Mater. 2021, 4, 7081–7093. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, D.; Zhao, P.; Wen, Y.; Fan, M.; Zhou, G.; He, Q. Coordination-Induced Exfoliation to Monolayer Bi-Anchored Mnb 2 Nanosheets for Multimodal Imaging-Guided Photothermal Therapy of Cancer. Theranostics 2020, 10, 1861–1872. [Google Scholar] [CrossRef]
- Chen, W.-H.; Luo, G.-F.; Lei, Q.; Hong, S.; Qiu, W.-X.; Liu, L.-H.; Cheng, S.-X.; Zhang, X.-Z. Overcoming the Heat Endurance of Tumor Cells by Interfering with the Anaerobic Glycolysis Metabolism for Improved Photothermal Therapy. ACS Nano 2017, 11, 1419–1431. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, W.; Chang, J.; Tan, Y.-W.; Li, J.; Chen, M.; Sun, Y.; Li, F. Temperature-Feedback Upconversion Nanocomposite for Accurate Photothermal Therapy at Facile Temperature. Nat. Commun. 2016, 7, 10437. [Google Scholar] [CrossRef]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles Modified by Polydopamine: Working As “Drug” Carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef]
- Liang, C.; Diao, S.; Wang, C.; Gong, H.; Liu, T.; Hong, G.; Shi, X.; Dai, H.; Liu, Z. Tumor Metastasis Inhibition by Imaging-Guided Photothermal Therapy with Single-Walled Carbon Nanotubes. Adv. Mater. 2014, 26, 5646–5652. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, H.; Zhao, M.; Dai, C.; Zhang, S.; Peng, W.; Chen, Y. 2D Superparamagnetic Tantalum Carbide Composite MXenes for Efficient Breast-Cancer Theragnostic. Theranostics 2018, 8, 1648. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Sanchez Casalongue, H.; Vinh, D.; Dai, H. Ultrasmall Reduced Graphene Oxide with High Near-Infrared Absorbance for Photothermal Therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D Tantalum Carbide (MXene). Adv. Mater. 2018, 30, 1703284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jin, L.; Liu, J.; Wang, Y.; Zhang, T.; Liu, Y.; Zhao, Y.; Yin, N.; Niu, R.; Xue, D. Novel FeF2/Fe1–xS Nanoreactor-Mediated Mitochondrial Dysfunction via Oxidative Stress and Fluoride Ions Overloaded for Synergistic Chemodynamic Therapy and Photothermal Therapy. Adv. Funct. Mater. 2022, 32, 2113397. [Google Scholar] [CrossRef]
- Liu, G.; Zou, J.; Tang, Q.; Yang, X.; Zhang, Y.; Zhang, Q.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Surface Modified Ti3C2 MXene Nanosheets For Tumor Targeting Photothermal/Photodynamic/Chemo Synergistic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 40077–40086. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, X.; Yang, P.; Liang, G.; Yang, Y.; Gu, Z.; Li, Y. Regulating the Absorption Spectrum of Polydopamine. Sci. Adv. 2020, 6, eabb4696. [Google Scholar] [CrossRef]
- Yeroslavsky, G.; Richman, M.; Dawidowicz, L.-O.; Rahimipour, S. Sonochemically Produced Polydopamine Nanocapsules with Selective Antimicrobial Activity. Chem. Commun. 2013, 49, 5721–5723. [Google Scholar] [CrossRef]
- Adnan, N.N.M.; Sadrearhami, Z.; Bagheri, A.; Nguyen, T.K.; Wong, E.H.; Ho, K.K.; Lim, M.; Kumar, N.; Boyer, C. Exploiting the Versatility of Polydopamine-Coated Nanoparticles to Deliver Nitric Oxide and Combat Bacterial Biofilm. Macromol. Rapid Commun. 2018, 39, 1800159. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Polega, E.; Thomson, K.; Bhuiyan, M.S.A.; Pinnaratip, R.; Trought, M.; Kendrick, C.; Gao, Y.; Perrine, K.A.; Pan, L. Antibacterial Properties of Mussel-Inspired Polydopamine Coatings Prepared by A Simple Two-Step Shaking-Assisted Method. Front. Chem. 2019, 7, 631. [Google Scholar] [CrossRef]
- Choi, Y.S.; Huh, K.M.; Shim, M.S.; Park, I.S.; Cho, Y.Y.; Lee, J.Y.; Kang, H.C. Disrupting the Redox Balance with a Diselenide Drug Delivery System: Synergistic or Antagonistic? Adv. Funct. Mater. 2021, 31, 2007275. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Zhu, B.; Shi, J.; Liu, C.; Li, J.; Cao, S. In-Situ Self-Assembly of Sandwich-Like Ti3C2 MXene/Gold Nanorods Nanosheets for Synergistically Enhanced Near-Infrared Responsive Drug Delivery. Ceram. Int. 2021, 47, 24252–24261. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Kowalczyk, S.; Plichta, A.; Zgadzaj, A.; Oledzka, E.; Sobczak, M. Synthesis and Characterization of New Biodegradable Injectable Thermosensitive Smart Hydrogels for 5-Fluorouracil Delivery. Int. J. Mol. Sci. 2021, 22, 8330. [Google Scholar] [CrossRef] [PubMed]




| Sample Code | Release Medium | Korsmeyer–Peppas | ||
|---|---|---|---|---|
| n | kp* | R2 | ||
| pH 7.4 | 0.184 | 2.414 | 0.944 | |
| pH 7.4 + H2O2 | 0.481 | 6.686 | 0.991 | |
| MXene-TK-DOX@PDA | pH 5.5 | 0.258 | 7.005 | 0.960 |
| pH 5.5 + H2O2 | 0.416 | 11.56 | 0.985 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.-J.; Li, S.; Vijayan, V.; Lee, J.S.; Park, S.S.; Cui, X.; Chung, I.; Lee, J.; Ahn, S.-k.; Kim, J.R.; et al. ROS- and pH-Responsive Polydopamine Functionalized Ti3C2Tx MXene-Based Nanoparticles as Drug Delivery Nanocarriers with High Antibacterial Activity. Nanomaterials 2022, 12, 4392. https://doi.org/10.3390/nano12244392
Zhang W-J, Li S, Vijayan V, Lee JS, Park SS, Cui X, Chung I, Lee J, Ahn S-k, Kim JR, et al. ROS- and pH-Responsive Polydopamine Functionalized Ti3C2Tx MXene-Based Nanoparticles as Drug Delivery Nanocarriers with High Antibacterial Activity. Nanomaterials. 2022; 12(24):4392. https://doi.org/10.3390/nano12244392
Chicago/Turabian StyleZhang, Wei-Jin, Shuwei Li, Veena Vijayan, Jun Seok Lee, Sung Soo Park, Xiuguo Cui, Ildoo Chung, Jaejun Lee, Suk-kyun Ahn, Jung Rae Kim, and et al. 2022. "ROS- and pH-Responsive Polydopamine Functionalized Ti3C2Tx MXene-Based Nanoparticles as Drug Delivery Nanocarriers with High Antibacterial Activity" Nanomaterials 12, no. 24: 4392. https://doi.org/10.3390/nano12244392
APA StyleZhang, W.-J., Li, S., Vijayan, V., Lee, J. S., Park, S. S., Cui, X., Chung, I., Lee, J., Ahn, S.-k., Kim, J. R., Park, I.-K., & Ha, C.-S. (2022). ROS- and pH-Responsive Polydopamine Functionalized Ti3C2Tx MXene-Based Nanoparticles as Drug Delivery Nanocarriers with High Antibacterial Activity. Nanomaterials, 12(24), 4392. https://doi.org/10.3390/nano12244392