Engineered Faceted Cerium Oxide Nanoparticles for Therapeutic miRNA Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Methods
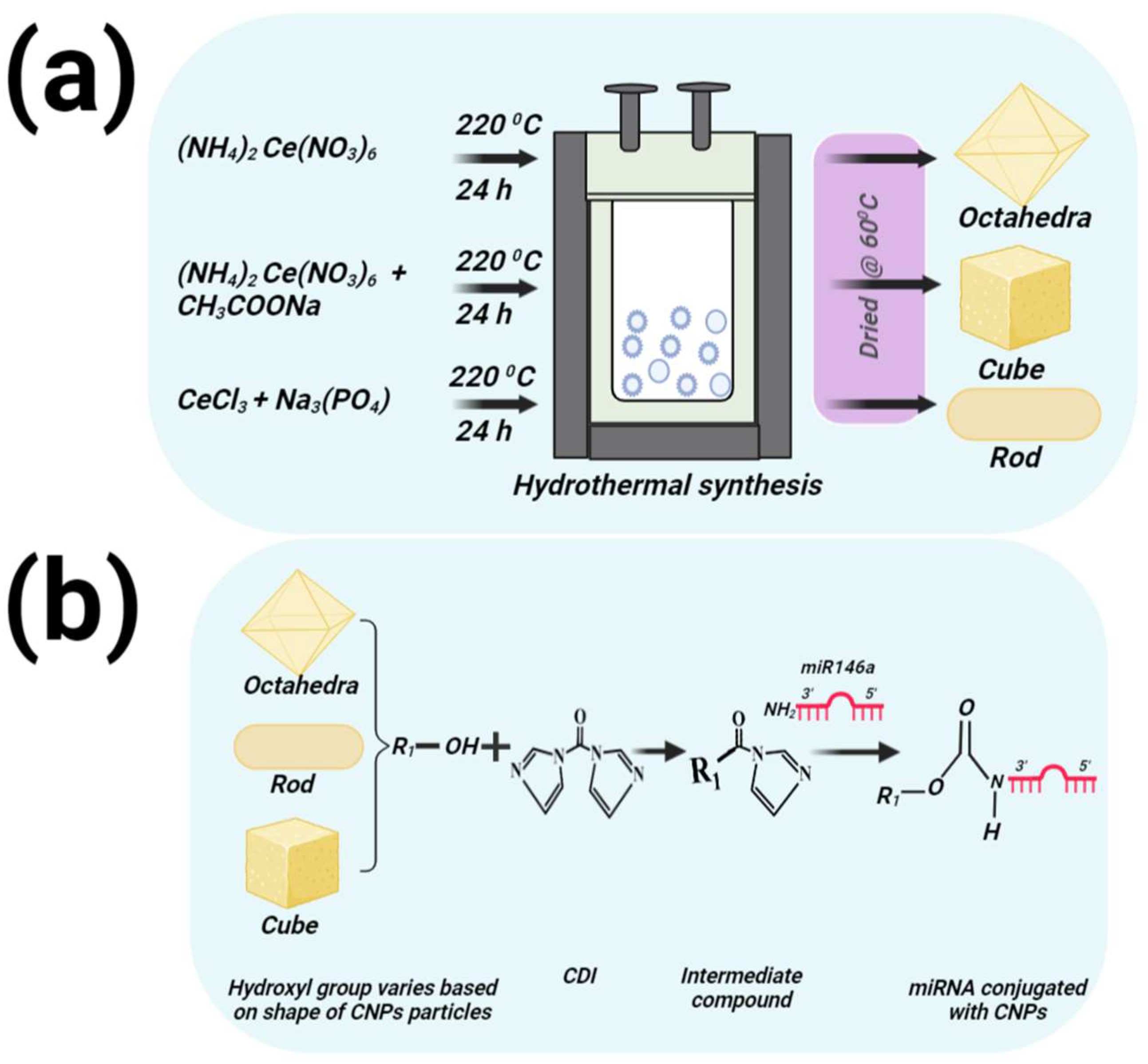
2.2. Different Shapes of CNPs Preparation
2.3. MiR146a Conjugation with Different Shapes of CNPs
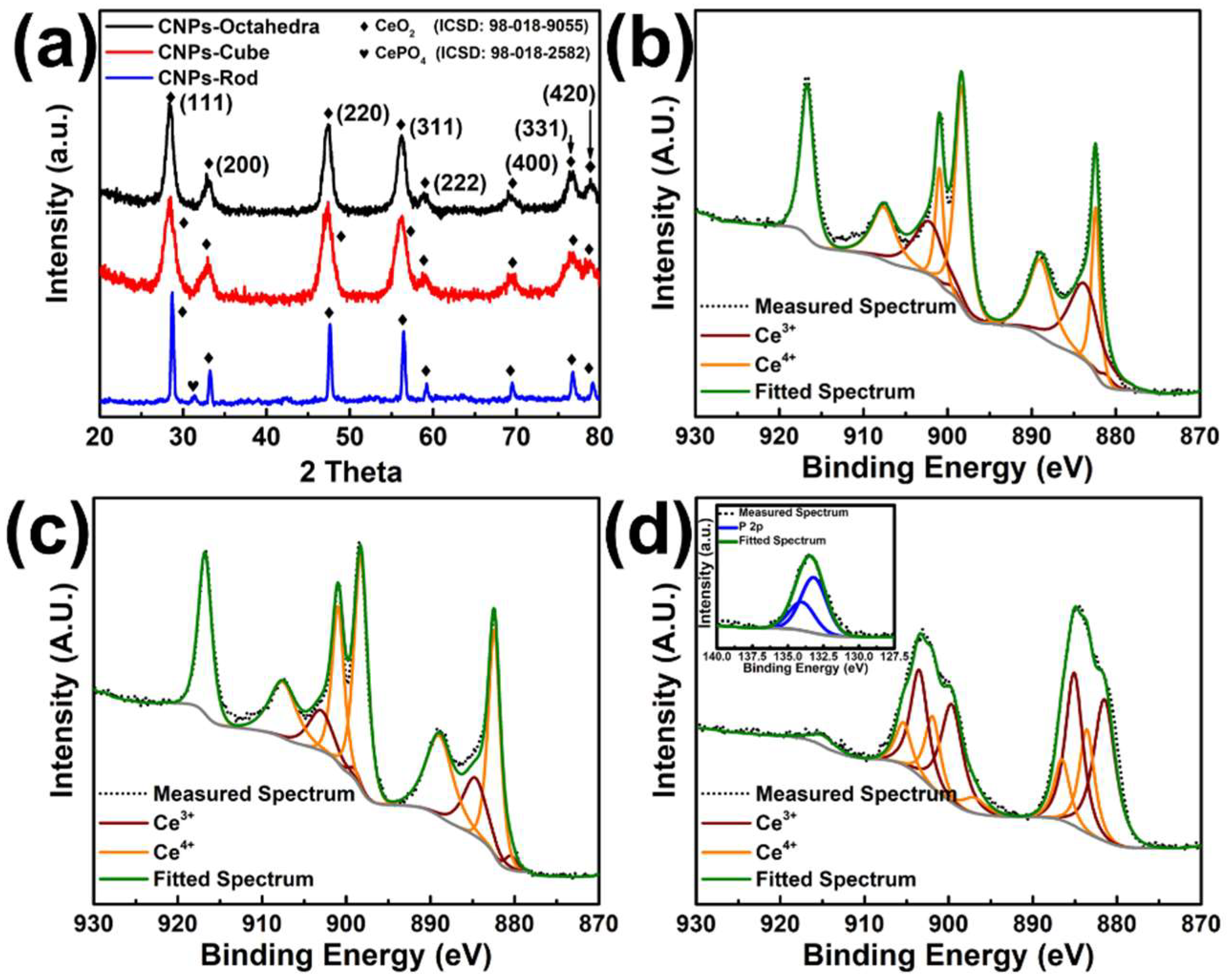
2.4. Characterization
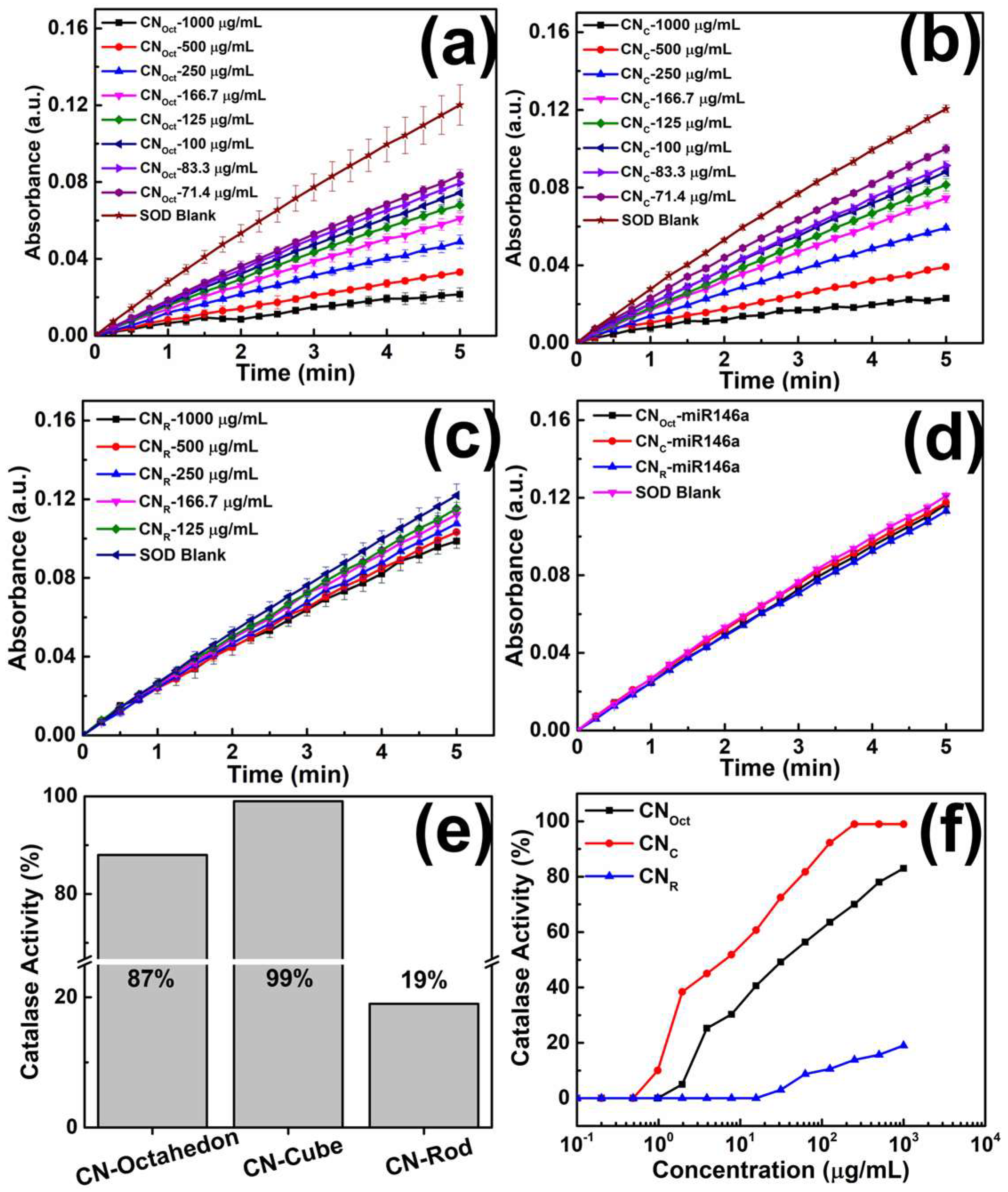
2.4.1. Catalase-Mimetic Activity (CAT)
2.4.2. Superoxide Dismutase Mimetic Activity (SOD)
SOD blank-slope of absorbance change of specimen)/slope of absorbance change of SOD blank
× 100%
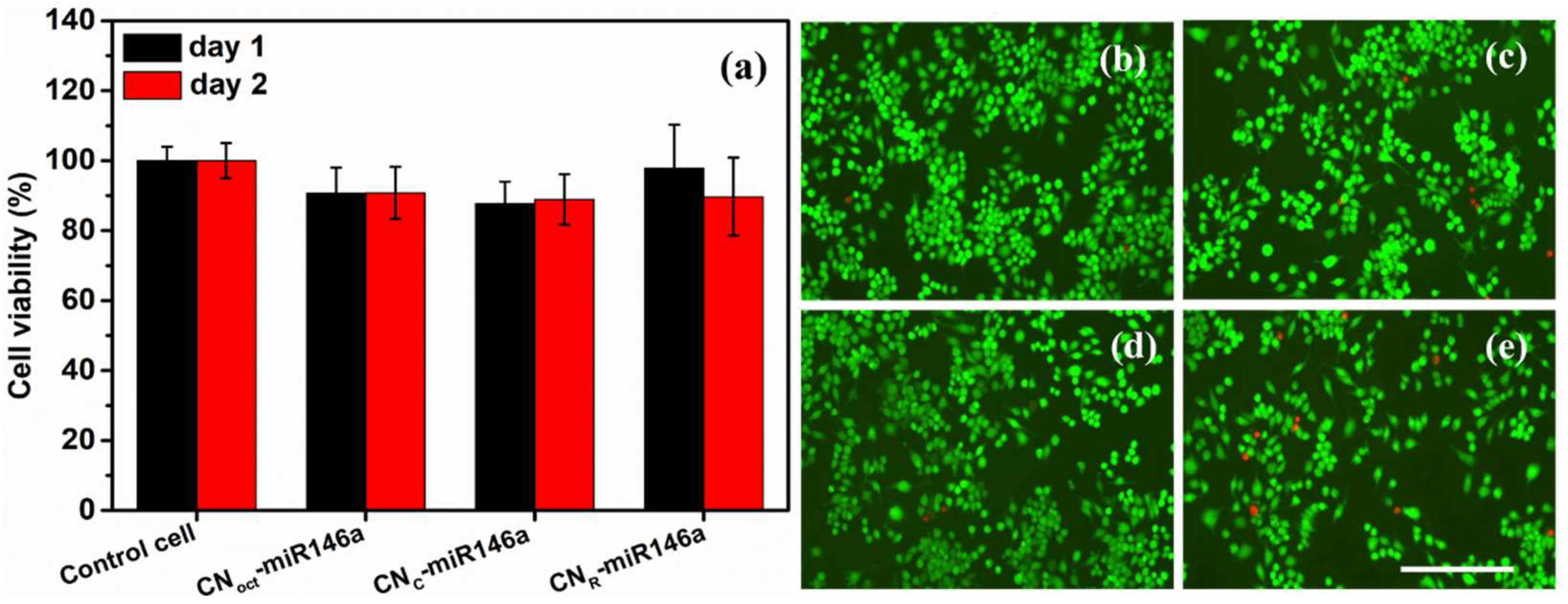
2.4.3. Cytotoxicity Studies on miR146a Conjugated Nanoparticles
2.4.4. microRNA Delivery and Real-Time qPCR
3. Results and Discussion
3.1. Structural and Compositional Analysis of Synthesized Nanoparticles
3.2. Size and Morphology Analysis of Synthesized Nanoparticles
3.3. Formulation Colloidal Stability and Influence of microRNA Conjugation
3.4. Enzymatic Activity Assay (SOD and CAT)
3.5. Biological Studies
3.5.1. Biocompatibility Studies
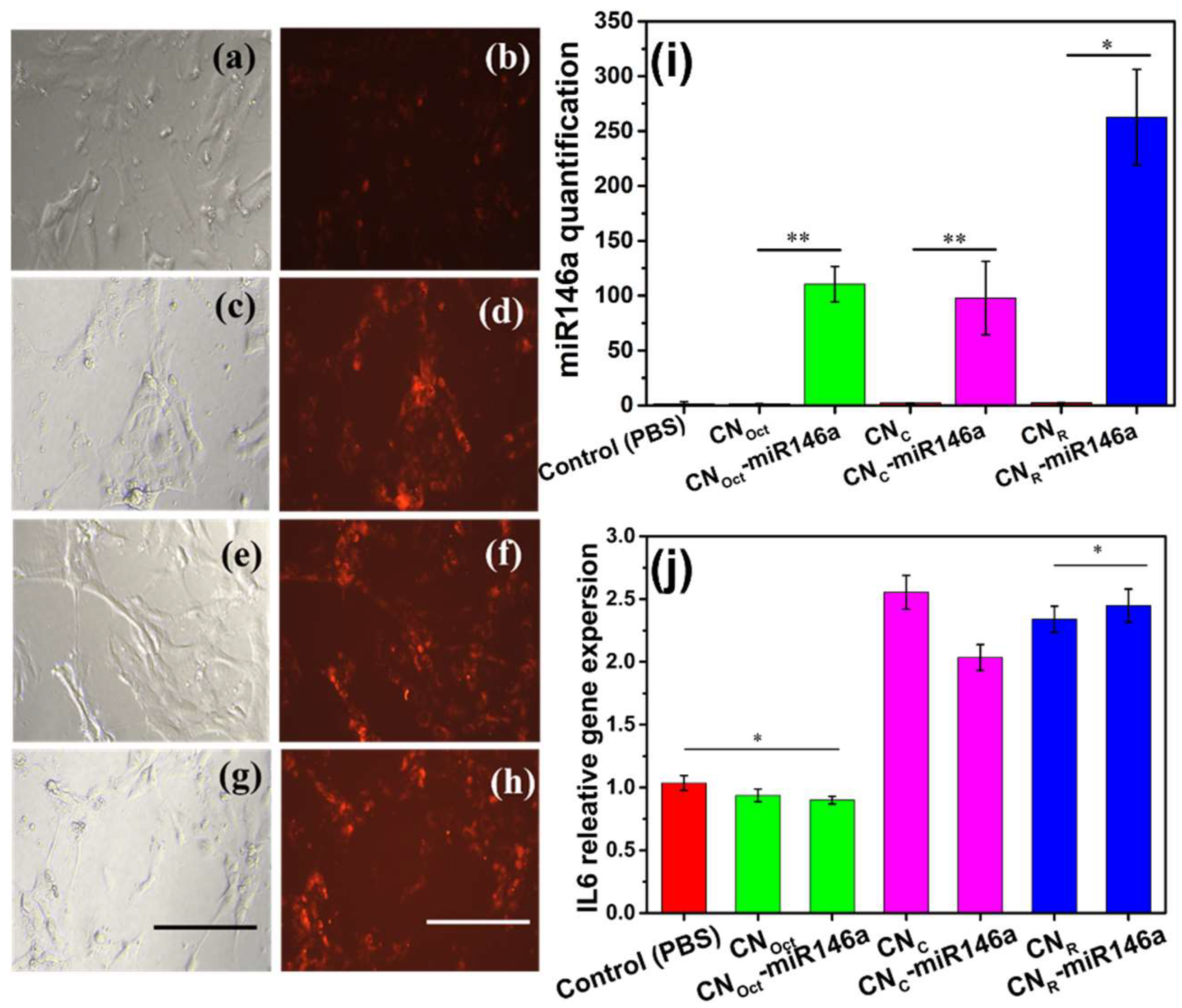
3.5.2. microRNA Delivery and Real-Time qPCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Ozdemir, D.; Feinberg, M.W. MicroRNAs in diabetic wound healing: Pathophysiology and therapeutic opportunities. Trends Cardiovasc. Med. 2019, 29, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Børsheim, E.; Carvalho, E. The role of micrornas in diabetic complications—Special emphasis on wound healing. Genes 2014, 5, 926–956. [Google Scholar] [CrossRef] [PubMed]
- Dewberry, L.C.; Niemiec, S.M.; Hilton, S.A.; Louiselle, A.E.; Singh, S.; Sakthivel, T.S.; Hu, J.; Seal, S.; Liechty, K.W.; Zgheib, C. Cerium oxide nanoparticle conjugation to microRNA-146a mechanism of correction for impaired diabetic wound healing. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102483. [Google Scholar] [CrossRef]
- Kolanthai, E.; Fu, Y.; Kumar, U.; Babu, B.; Venkatesan, A.K.; Liechty, K.W.; Seal, S. Nanoparticle mediated RNA delivery for wound healing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1741. [Google Scholar] [CrossRef] [PubMed]
- El Ghzaoui, C.; Neal, C.J.; Kolanthai, E.; Fu, Y.; Kumar, U.; Hu, J.; Zgheib, C.; Liechty, K.W.; Seal, S. Assessing the Bio-stability of microRNA-146a conjugated Nanoparticles via Electroanalysis. Nanoscale Adv. 2022. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. 2009, 121, 2344–2348. [Google Scholar] [CrossRef]
- Dhall, A.; Burns, A.; Dowding, J.; Das, S.; Seal, S.; Self, W. Characterizing the phosphatase mimetic activity of cerium oxide nanoparticles and distinguishing its active site from that for catalase mimetic activity using anionic inhibitors. Environ. Sci. Nano 2017, 4, 1742–1749. [Google Scholar] [CrossRef]
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Singh, S. SOD mimetic cerium oxide nanorods protect human hepatocytes from oxidative stress. Emergent Mater. 2021, 4, 1305–1317. [Google Scholar] [CrossRef]
- Forest, V.; Leclerc, L.; Hochepied, J.-F.; Trouvé, A.; Sarry, G.; Pourchez, J. Impact of cerium oxide nanoparticles shape on their in vitro cellular toxicity. Toxicol. Vitr. 2017, 38, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.; Fromm, K.M. Toxicity and protective effects of cerium oxide nanoparticles (nanoceria) depending on their preparation method, particle size, cell type, and exposure route. Eur. J. Inorg. Chem. 2015, 2015, 4510–4517. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef]
- Singh, S.; Ly, A.; Das, S.; Sakthivel, T.S.; Barkam, S.; Seal, S. Cerium oxide nanoparticles at the nano-bio interface: Size-dependent cellular uptake. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S956–S963. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, X.; Zhang, H.; Lin, S.; Meng, H.; Sun, B.; George, S.; Xia, T.; Nel, A.E.; Zink, J.I. Designed synthesis of CeO2 nanorods and nanowires for studying toxicological effects of high aspect ratio nanomaterials. ACS Nano 2012, 6, 5366–5380. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. Assay of superoxide dismutase activity in a plate assay using WST-1. Free Radic. Biol. Med. 2017, 103, 188–191. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. [Google Scholar]
- Vinothkumar, G.; Lalitha, A.I.; Suresh Babu, K. Cerium phosphate–cerium oxide heterogeneous composite Nanozymes with enhanced peroxidase-like biomimetic activity for glucose and hydrogen peroxide sensing. Inorg. Chem. 2018, 58, 349–358. [Google Scholar] [CrossRef]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 1056–1058. [Google Scholar] [CrossRef]
- Amaldoss, M.J.N.; Mehmood, R.; Yang, J.-L.; Koshy, P.; Kumar, N.; Unnikrishnan, A.; Sorrell, C.C. Anticancer therapeutic effect of cerium-based nanoparticles: Known and unknown molecular mechanisms. Biomater. Sci. 2022, 10, 3671–3694. [Google Scholar] [CrossRef]
- Singh, S.; Dosani, T.; Karakoti, A.S.; Kumar, A.; Seal, S.; Self, W.T. A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties. Biomaterials 2011, 32, 6745–6753. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhai, Y.; Zhou, K.; Wang, L.; Tan, H.; Luan, Q.; Yao, X. The vital role of buffer anions in the antioxidant activity of CeO2 nanoparticles. Chem.—Eur. J. 2012, 18, 11115–11122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Yin, J.J.; Ma, Y.; Zhang, P.; Li, J.; Ding, Y.; Zhang, J.; Zhao, Y.; Chai, Z. Acquired superoxide-scavenging ability of ceria nanoparticles. Angew. Chem. 2015, 127, 1852–1855. [Google Scholar] [CrossRef]
- Naganuma, T. Shape design of cerium oxide nanoparticles for enhancement of enzyme mimetic activity in therapeutic applications. Nano Res. 2017, 10, 199–217. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, Z.; Huang, W.; Liu, L.; Li, J.; Li, J.; Wu, Q. Redox enzyme-mimicking activities of CeO2 nanostructures: Intrinsic influence of exposed facets. Sci. Rep. 2016, 6, 35344. [Google Scholar] [CrossRef] [PubMed]
- Turovsky, E.A.; Varlamova, E.G. Mechanism of Ca2+-dependent pro-apoptotic action of selenium nanoparticles, mediated by activation of Cx43 hemichannels. Biology 2021, 10, 743. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, G.; Chen, Y.; Wang, C.; Guo, M.; Xu, T.; Zhao, M.; Zhou, Y. Tumor-targeted delivery of siRNA to silence Sox2 gene expression enhances therapeutic response in hepatocellular carcinoma. Bioact. Mater. 2021, 6, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, A.; Karakoti, A.; Seal, S.; Self, W.T. Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol. BioSyst. 2010, 6, 1813–1820. [Google Scholar] [CrossRef]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. [Google Scholar] [CrossRef]
- Agarwal, R.; Singh, V.; Jurney, P.; Shi, L.; Sreenivasan, S.; Roy, K. Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc. Natl. Acad. Sci. USA 2013, 110, 17247–17252. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form follows function: Nanoparticle shape and its implications for nanomedicine. Chem. Rev. 2017, 117, 11476–11521. [Google Scholar] [CrossRef]
- Xu, A.; Yao, M.; Xu, G.; Ying, J.; Ma, W.; Li, B.; Jin, Y. A physical model for the size-dependent cellular uptake of nanoparticles modified with cationic surfactants. Int. J. Nanomed. 2012, 7, 3547. [Google Scholar]
- Zhang, P.; Li, B.; Du, J.; Wang, Y. Regulation the morphology of cationized gold nanoparticles for effective gene delivery. Colloids Surf. B Biointerfaces 2017, 157, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Nangia, S.; Sureshkumar, R. Effects of nanoparticle charge and shape anisotropy on translocation through cell membranes. Langmuir 2012, 28, 17666–17671. [Google Scholar] [CrossRef] [PubMed]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Tyrsted, C.; Jensen, K.M.; Bøjesen, E.D.; Lock, N.; Christensen, M.; Billinge, S.J.L.; Iversen, B.B. Understanding the Formation and Evolution of Ceria Nanoparticles Under Hydrothermal Conditions. Angew. Chem. Int. Ed. 2012, 51, 9030–9033. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Li, C.-S. Inactivation of Viruses on Surfaces by Ultraviolet Germicidal Irradiation. J. Occup. Environ. Hyg. 2007, 4, 400–405. [Google Scholar] [CrossRef]
- Shoko, E.; Smith, M.F.; McKenzie, R. Charge distribution near bulk oxygen vacancies in cerium oxides. J. Phys. Condens. Matter 2010, 22, 223201. [Google Scholar] [CrossRef]
- Ganduglia-Pirovano, M.V.; Da Silva, J.L.; Sauer, J. Density-Functional Calculations of the Structure of near-Surface Oxygen Vacancies and Electron Localization on Ceo2 (111). Phys. Rev. Lett. 2009, 102, 026101. [Google Scholar] [CrossRef] [PubMed]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef]
- Zhao, Y.; Teng, B.-T.; Wen, X.-D.; Zhao, Y.; Chen, Q.-P.; Zhao, L.-H.; Luo, M.-F. Superoxide and Peroxide Species on Ceo2 (111), and Their Oxidation Roles. J. Phys. Chem. C 2012, 116, 15986–15991. [Google Scholar] [CrossRef]
- Nolan, M. Healing of oxygen vacancies on reduced surfaces of gold-doped ceria. J. Chem. Phys. 2009, 130, 144702. [Google Scholar] [CrossRef] [PubMed]
- Namai, Y.; Fukui, K.-I.; Iwasawa, Y. Atom-resolved noncontact atomic force microscopic and scanning tunneling microscopic observations of the structure and dynamic behavior of CeO2(111) surfaces. Catal. Today 2003, 85, 79–91. [Google Scholar] [CrossRef]
- Choi, Y.; Abernathy, H.; Chen, H.T.; Lin, M.C.; Liu, M. Characterization of O2–Ceo2 Interactions Using in Situ Raman Spectroscopy and First-Principle Calculations. ChemPhysChem 2006, 7, 1957–1963. [Google Scholar] [CrossRef]

| Sample | Particles Size (nm) from TEM | Crystalline Size (nm) from Scherrer’s Equation | Hydrodynamic Diameter (nm) | Zeta Potential (mV) | Specific Surface Area (m2/g) | Loaded miR146a (Strand/Particles) |
|---|---|---|---|---|---|---|
| CNOct | 5.8 ± 0.85 | 7.8 ± 0.5 | 221.3 ± 52.0 | +30.2 ± 1.1 | 98.7 ± 0.15 | (-) |
| CNC | 6.13 ± 0.74 | 5.5 ± 0.2 | 237.6 ± 32.2 | +26.9 ± 1.0 | 117.0 ± 0.61 | (-) |
| CNR | 21.6 ± 4.5 | 26.5 ± 1.5 | 307.7 ± 76.8 | +32.5 ± 1.4 | 63.3 ± 0.01 | (-) |
| CNOct-miR146a | (-) | (-) | 245.5 ± 3.3 | −25.5 ± 8.8 | (-) | ~100 |
| CNC-miR146a | (-) | (-) | 447.2 ± 28.0 | −33.0 ± 3.6 | (-) | ~85 |
| CNR-miR146a | (-) | (-) | 553.7 ± 23.5 | −20.0 ± 3.1 | (-) | ~1200 |
| Concentration of Nanoparticles Used in the Assay | % SOD Activity | ||
|---|---|---|---|
| Bare NPs | CNOct | CNC | CNR |
| 71.4 µg/mL | 31.6 | 17.4 | (-) |
| 83.3 µg/mL | 34.8 | 25.4 | (-) |
| 100 µg/mL | 39.3 | 27.4 | (-) |
| 125 µg/mL | 43.9 | 33.0 | 3.1 |
| 166.7 µg/mL | 50.6 | 38.7 | 7.2 |
| 250 µg/mL | 59.5 | 50.9 | 11.4 |
| 500 µg/mL | 73.0 | 67.5 | 14.1 |
| 1000 µg/mL | 82.0 | 77.2 | 16.5 |
| Conjugated NPs | CNOct-miR146a | CNC-miR146a | CNR-miR146a |
| 1–6 µg/mL * | 3 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Kolanthai, E.; Neal, C.J.; Kumar, U.; Zgheib, C.; Liechty, K.W.; Seal, S. Engineered Faceted Cerium Oxide Nanoparticles for Therapeutic miRNA Delivery. Nanomaterials 2022, 12, 4389. https://doi.org/10.3390/nano12244389
Fu Y, Kolanthai E, Neal CJ, Kumar U, Zgheib C, Liechty KW, Seal S. Engineered Faceted Cerium Oxide Nanoparticles for Therapeutic miRNA Delivery. Nanomaterials. 2022; 12(24):4389. https://doi.org/10.3390/nano12244389
Chicago/Turabian StyleFu, Yifei, Elayaraja Kolanthai, Craig J. Neal, Udit Kumar, Carlos Zgheib, Kenneth W. Liechty, and Sudipta Seal. 2022. "Engineered Faceted Cerium Oxide Nanoparticles for Therapeutic miRNA Delivery" Nanomaterials 12, no. 24: 4389. https://doi.org/10.3390/nano12244389
APA StyleFu, Y., Kolanthai, E., Neal, C. J., Kumar, U., Zgheib, C., Liechty, K. W., & Seal, S. (2022). Engineered Faceted Cerium Oxide Nanoparticles for Therapeutic miRNA Delivery. Nanomaterials, 12(24), 4389. https://doi.org/10.3390/nano12244389








