Hybrid G/BN@2H-MoS2 Nanomaterial Composites: Structural, Electronic and Molecular Adsorption Properties
Abstract
1. Introduction
2. Computational Methods
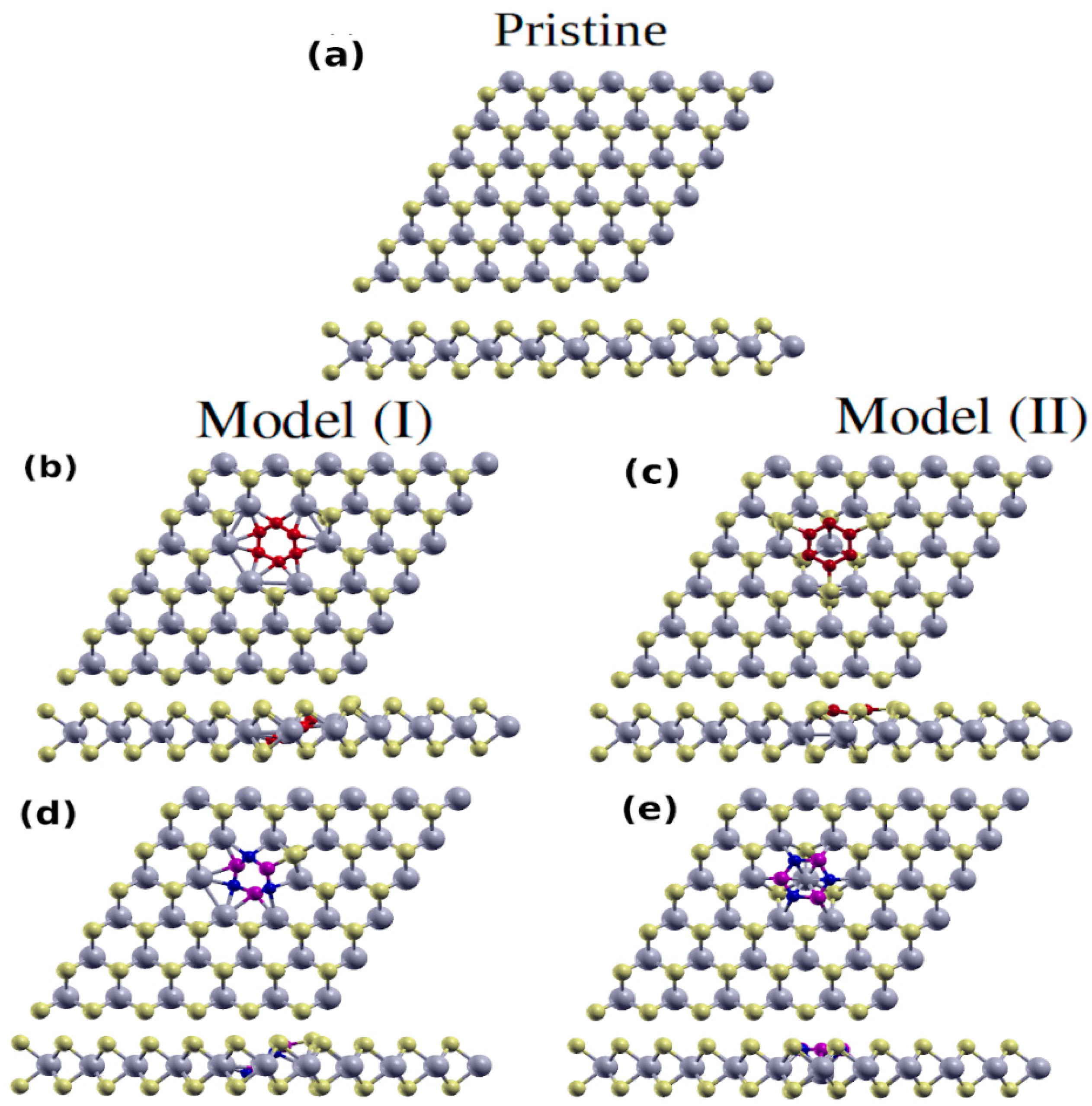
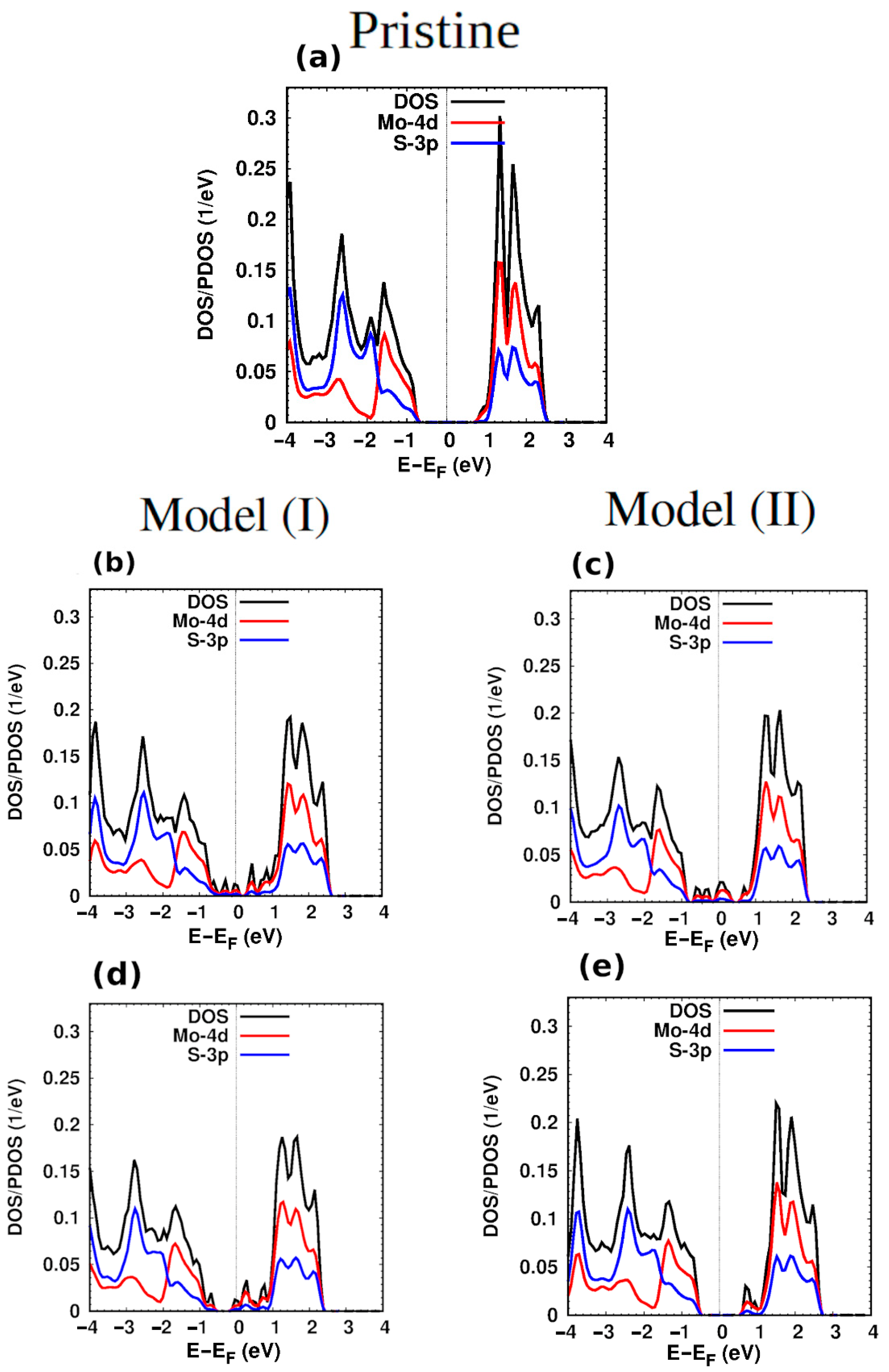
3. Structural and Electronic Properties of Hybrid Monolayers
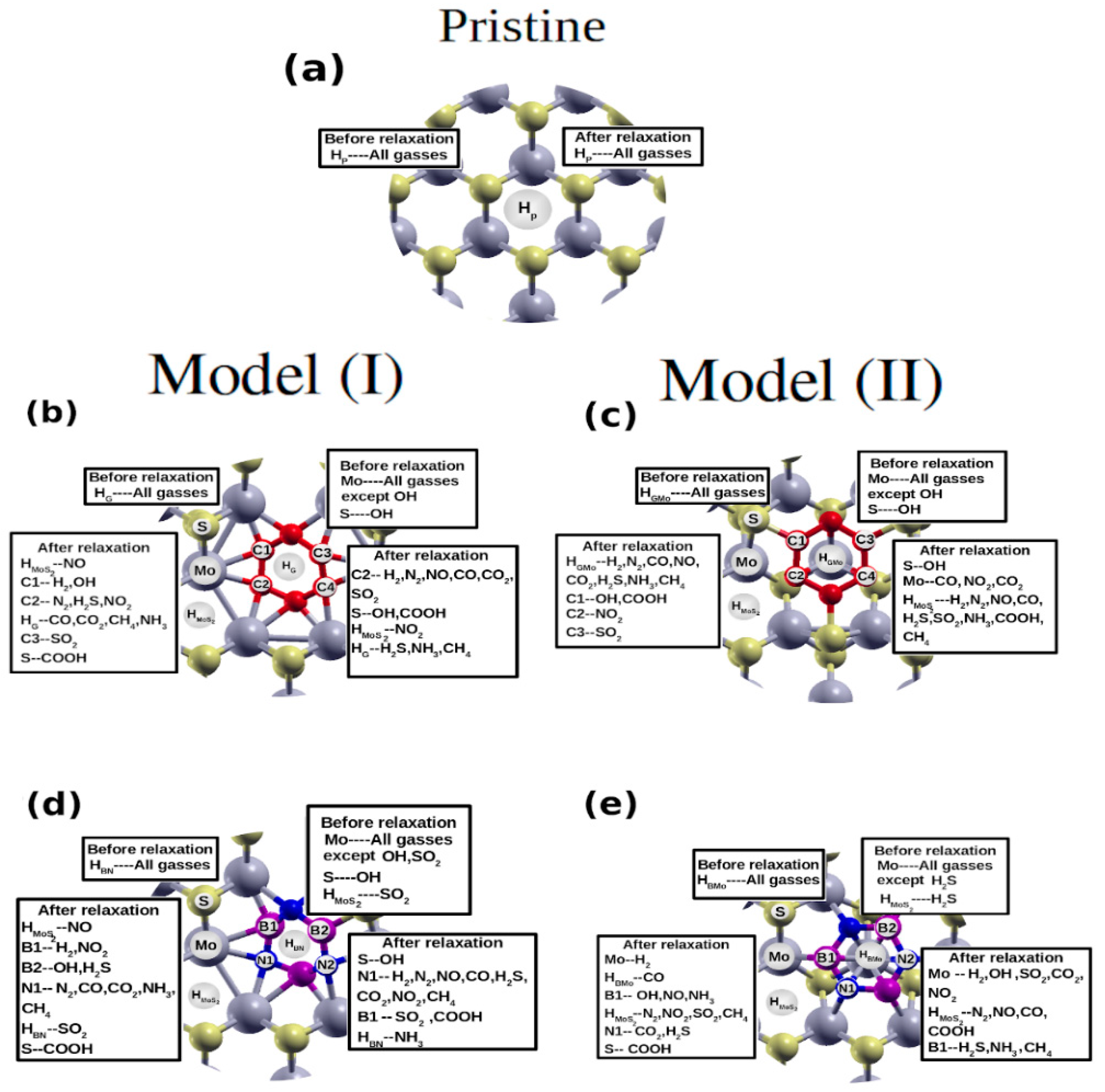
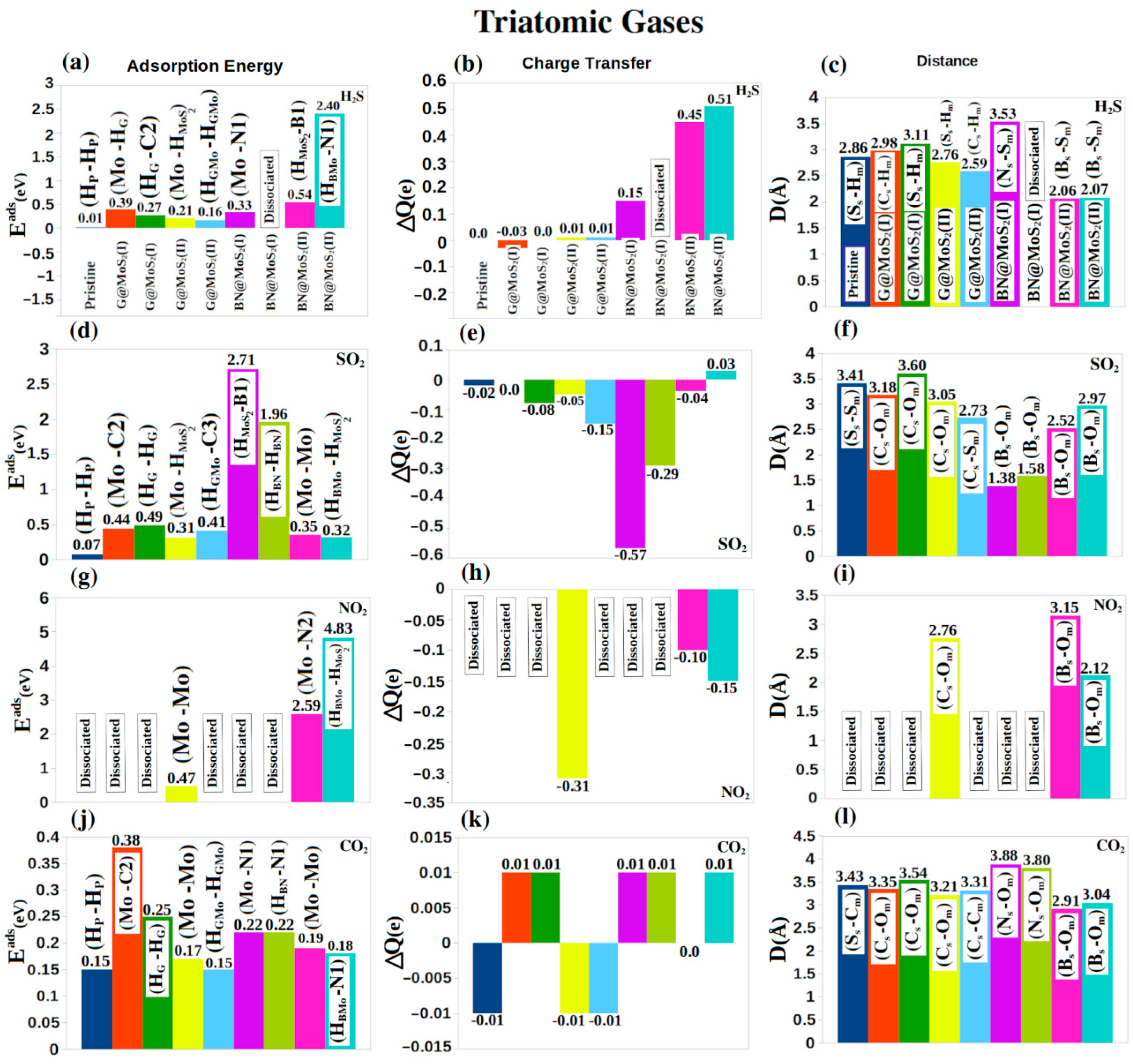
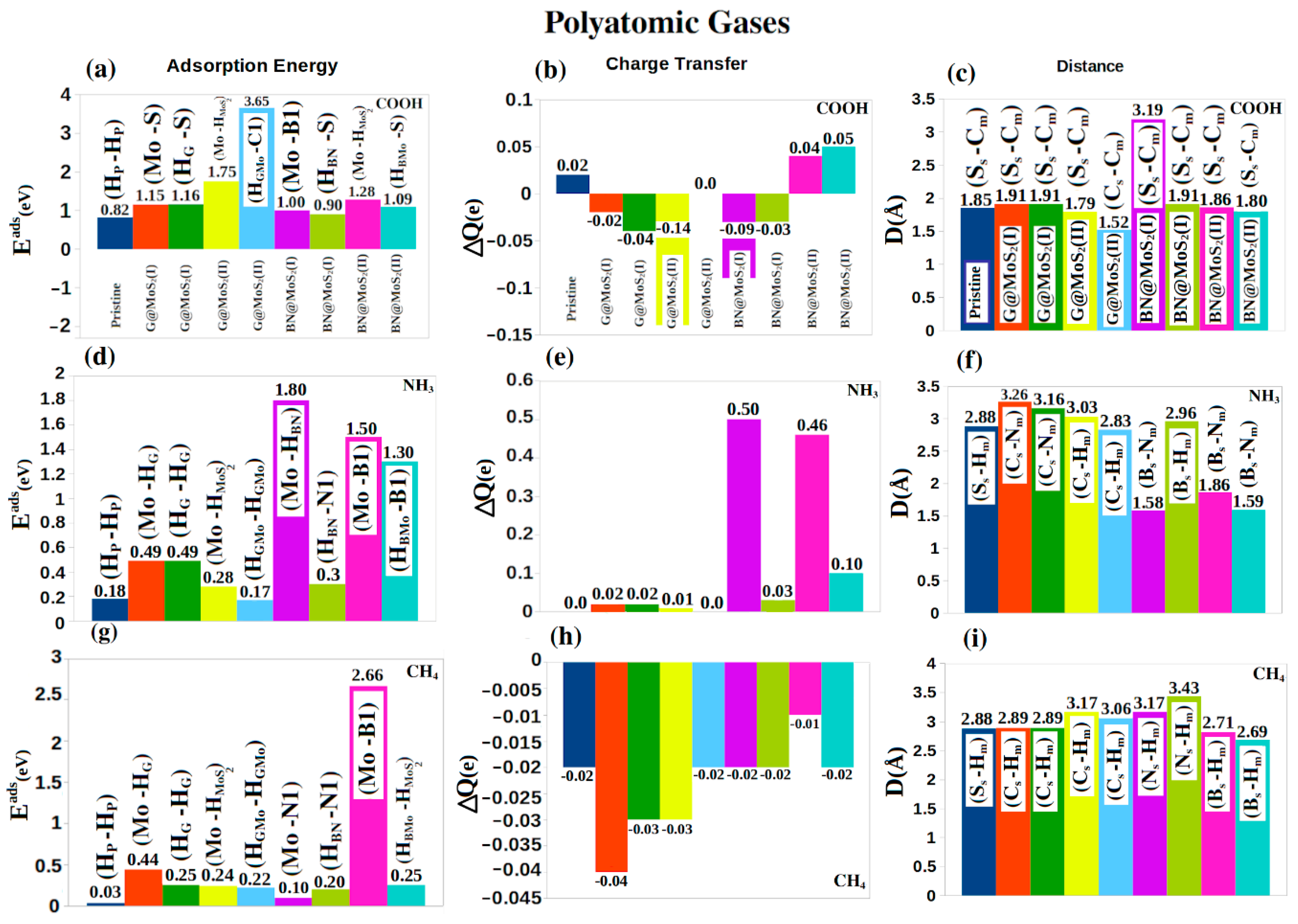
4. Molecular Adsorptions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451. [Google Scholar] [CrossRef] [PubMed]
- Osada, M.; Sasaki, T. 2D Inorganic Nanosheets: Two-Dimensional Dielectric Nanosheets: Novel Nanoelectronics from Nanocrystal Building Block. Adv. Mater. 2012, 24, 209. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Darwish, A.A.; Fadlallah, M.M.; Maarouf, A.A. Adsorption of Sugars on Al- and Ga-doped Boron nitride Surfaces: A Computational Study. App. Surf. Sci. 2016, 377, 9. [Google Scholar] [CrossRef]
- Bafekry, A.; Faraji, M.; Hieu, N.N.; Khatibani, A.B.; Fadlallah, M.M.; Gogova, D.; Ghergherehchi, M. Tunable electronic properties of porous graphitic carbon nitride (C6N7) monolayer by atomic doping and embedding: A first-principle study. Appl. Surf. Sci. 2022, 583, 152270. [Google Scholar] [CrossRef]
- Bafekry, A.; Faraji, M.; Fadlallah, M.M.; Sarsari, A.; Jappor, H.R.; Fazeli, S.; Ghergherehchi, M. Two-dimensional porous graphitic carbon nitride C6 N7 monolayer: First-principles calculations. App. Phys. Lett. 2021, 119, 142102. [Google Scholar] [CrossRef]
- Das, S.; Zhang, W.; Demarteau, M.; Hoffmann, A.; Dubey, M.; Roelofs, A. Tunable transport gap in phosphorene. Nano Lett. 2014, 14, 5733. [Google Scholar] [CrossRef]
- Bafekry, A.; Faraji, M.; Fadlallah, M.M.; Khatibani, A.B.; Ziabari, A.A.; Ghergherehchi, M.; Gogova, D. Tunable electronic and magnetic properties of MoSi2 N4 monolayer via vacancy defects, atomic adsorption and atomic doping. Appl. Surf. Sci. 2021, 559, 149862. [Google Scholar] [CrossRef]
- Abdelati, M.A.; Maarouf, A.A.; Fadlallah, M.M. Substitutional transition metal doping in MoSi2 N4 monolayer: Structural, electronic and magnetic properties. Phys. Chem. Chem. Phys. 2022, 24, 3035. [Google Scholar] [CrossRef]
- Bafekry, A.; Fadlallah, M.M.; Faraji, M.; Shafique, A.; Jappor, H.R.; Sarsari, I.A.; Ang, Y.S.; Ghergherehchi, M. Two-dimensional penta-like PdPSe with a puckered pentagonal structure: A first-principles study. Phys. Chem. Chem. Phys. 2022, 24, 9990. [Google Scholar] [CrossRef] [PubMed]
- Gillen, R.; Robertson, J.; Maultzsch, J. Indirect doping effects from impurities in MoS2 /h-BN heterostructures. Phys. Rev. B 2014, 90, 075437. [Google Scholar] [CrossRef]
- Helal, M.A.; El-Sayed, H.M.; Maarouf, A.A.; Fadlallah, M.M. Metal dichalcogenide nanomeshes: Structural, electronic and magnetic properties. Phys. Chem. Chem. Phys. 2021, 23, 21183. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.R.; Wu, W. Emerging devices based on two-dimensional monolayer materials for energy harvesting. Research 2019, 2019, 7367828. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Dumcenco, D.O.; Komsa, H.P.; Niimi, Y.; Krasheninnikov, A.V.; Huang, Y.S.; Suenaga, K. Properties of individual dopant atoms in single-layer MoS2: Atomic structure, migration, and enhanced reactivity. Adv. Mater. 2014, 26, 2857. [Google Scholar] [CrossRef]
- Liu, H.; Grasseschi, D.; Dodda, A.; Fujisawa, K.; Olson, D.; Kahn, E.; Terrones, M. Spontaneous chemical functionalization via coordination of Au single atoms on monolayer MoS2. Sci. Adv. 2020, 6, eabc9308. [Google Scholar] [CrossRef]
- Ghorbani-Asl, M.; Kretschmer, S.; Spearot, D.E.; Krasheninnikov, A.V. Two-dimensional MoS2 under ion irradiation: From controlled defect production to electronic structure engineering. 2D Mater. 2017, 4, 025078. [Google Scholar] [CrossRef]
- Komsa, H.P.; Krasheninnikov, A.V. Native defects in bulk and monolayer MoS2 from first principles. Phys. Rev. B 2015, 91, 125304. [Google Scholar] [CrossRef]
- Dolui, K.; Rungger, I.; Pemmaraju, C.D.; Sanvito, S. Possible doping strategies for MoS2 monolayers: An ab initio study. Phys. Rev. B 2013, 88, 075420. [Google Scholar] [CrossRef]
- Sukhanova, E.V.; Kvashnin, D.G.; Popov, Z.I. Induced spin polarization in graphene via interactions with halogen doped MoS2 and MoSe2 monolayers by DFT calculations. Nanoscale 2020, 12, 23248. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Yi, J. Electronic and magnetic properties of Co doped MoS2 monolayer. Sci. Rep. 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Kang, K.; Shayan, K.; Yoshimura, A.; Dadras, S.; Wang, X.; Yang, E.H. Enabling room temperature ferromagnetism in monolayer MoS2 via in situ iron-doping. Nat. Commun. 2020, 11, 2034. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, Z.; Lupini, A.R.; Shi, G.; Lin, J.; Najmaei, S.; Lin, Z.; Elías, A.L.; Berkdemir, A.; You, G.; et al. Band Gap Engineering and Layer-by-Layer Mapping of Selenium-Doped Molybdenum Disulfide. Nano Lett. 2014, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, M.; Zhang, Y.; Liu, Z. Hexagonal Boron Nitride–Graphene Heterostructures: Synthesis and Interfacial Properties. Small 2016, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, S.-S.; Jeon, J.; Yoon, S.I.; Hong, S.; Cho, Y.J.; Misra, A.; Ozdemir, S.; Yin, J.; Ghazaryan, D.; et al. Planar and van der Waals heterostructures for vertical tunnelling single electron transistors. Nat. Commun. 2019, 10, 230. [Google Scholar] [CrossRef]
- Wei, W.; Pan, J.; Euaruksakul, C.; Yang, Y.; Cui, Y.; Fu, Q.; Bao, X. Dynamic observation of in-plane h-BN/graphene heterostructures growth on Ni(111). Nano Res. 2020, 13, 1789. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W. L-cysteine-assisted synthesis of layered MoS2/graphene composites with excellent electrochemical performances for lithium ion batteries. ACS Nano 2011, 5, 4720. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, J.; Wang, X.; Shi, G.; Lei, S.; Lin, Z.; Ajayan, P.M. Vertical and in-plane heterostructures from WS2 /MoS2 monolayers. Nat. Mater. 2014, 13, 1135. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, Y.; Larson, D.T.; Zhu, Z.; Fang, S.; Kaxiras, E.; Huang, S. Spectroscopic Signatures of Interlayer Coupling in Janus MoSSe/MoS2 Heterostructures. ACS Nano 2021, 15, 14394. [Google Scholar] [CrossRef]
- Cheng, R.; Li, D.; Zhou, H.; Wang, C.; Yin, A.; Jiang, S.; Duan, X. Electroluminescence and photocurrent generation from atomically sharp WSe2 /MoS2 heterojunction p–n diodes. Nano Lett. 2014, 14, 5590. [Google Scholar] [CrossRef]
- Zhai, X.; Xu, X.; Peng, J.; Jing, F.; Zhang, Q.; Liu, H.; Hu, Z. Enhanced optoelectronic performance of CVD-grown metal–semiconductor NiTe2 /MoS2 heterostructures. ACS Appl. Mater. Interfaces 2020, 12, 24093. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, Z.; Huang, W.; Jing, Q.; Liu, N. Construction of N-doped TiO2 /MoS2 heterojunction with synergistic effect for enhanced visible photodegradation activity. Mater. Res. Bull. 2018, 105, 126. [Google Scholar] [CrossRef]
- Le, K.; Zhang, X.; Zhao, Q.; Liu, Y.; Yi, P.; Xu, S.; Liu, W. Controllably doping nitrogen into 1T/2H MoS2 heterostructure nanosheets for enhanced supercapacitive and electrocatalytic performance by low-power N2 plasma. ACS Appl. Mater. Interfaces 2021, 13, 44427. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.M.; Metwalli, O.I.; Saber, M.R.; Khabiri, G.; Ali, M.E.; Hassen, A.; Khalil, M.M.H.; Maarouf, A.A.; Khalil, A.S.G. Revealing the role of the 1T phase on the adsorption of organic dyes on MoS2 nanosheets. RSC Adv. 2019, 9, 28345. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.; Yan, J.; Liu, Y.; Jiang, H.; Zhang, H.; Tang, J.; Liu, Q. Research progresses on the application of perovskite in adsorption and photocatalytic removal of water pollutants. J. Hazard. Mater. 2022, 442, 130024. [Google Scholar] [CrossRef] [PubMed]
- Bafekry, A.; Faraji, M.; Ziabari, A.; Fadlallah, M.M.; Nguyen, C.V.; Ghergherehchi, M.; Feghhi, S.A.H.; van der Waals, A. Heterostructure of MoS2 /MoSi2N4: A first-principles study. New J. Chem. 2021, 45, 8291. [Google Scholar] [CrossRef]
- Liu, C.; Hong, H.; Wang, Q.; Liu, P.; Zuo, Y.; Liang, J.; Liu, K. Strong-coupled hybrid structure of carbon nanotube and MoS2 monolayer with ultrafast interfacial charge transfer. Nanoscale 2019, 11, 17195. [Google Scholar] [CrossRef]
- Bhanu, U.; Islam, M.R.; Tetard, L.; Khondaker, S.I. Photoluminescence quenching in gold-MoS2 hybrid nanoflakes. Sci. Rep. 2014, 4, 1. [Google Scholar] [CrossRef]
- Chang, X.; Qiao, X.; Li, K.; Wang, P.; Xiong, Y.; Li, X.; Xue, Q. UV assisted ppb-level acetone detection based on hollow ZnO/MoS2 nanosheets core/shell heterostructures at low temperature. Sens. Actuators B Chem. 2020, 317, 128208. [Google Scholar] [CrossRef]
- Alhajri, F.; Fadlallah, M.M.; Alkhaldi, A.; Maarouf, A.A. Hybrid MXene-Graphene/Hexagonal Boron Nitride Structures: Electronic and Molecular Adsorption Properties. Nanomaterials 2022, 12, 2739. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter. 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D.C.; Lundqvist, B.I. Van Der Waals Density Functional for General Geometries. Phys. Rev. Lett. 2004, 92, 246401. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A.; Yoffe, A.D. The Transition Metal Dichalcogenides Discussion and Interpretation of the Observed Optical, Electrical and Structural Properties. Adv. Phys. 1969, 18, 193. [Google Scholar] [CrossRef]
- Liu, X.; Gao, J.; Zhang, G.; Zhang, Y.W. MoS2-graphene in-plane contact for high interfacial thermal conduction. Nano Res. 2017, 10, 2944. [Google Scholar] [CrossRef]
- Liu, F.; Ziffer, M.E.; Hansen, K.R.; Wang, J.; Zhu, X. Direct determination of band-gap renormalization in the photoexcited monolayer MoS2. Phys. Rev. Lett. 2019, 122, 246803. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, Z.; Chen, Q.; Hu, J.; Wang, J. Band Structure Engineering of Monolayer MoS2 by Surface Ligand Functionalization for Enhanced Photoelectrochemical Hydrogen Production Activity. Nanoscale 2014, 6, 13565. [Google Scholar] [CrossRef]
- Lu, S.C.; Leburton, J.P. Electronic structures of defects and magnetic impurities in MoS2 monolayers. Nanoscale Res. Lett. 2014, 9, 1. [Google Scholar] [CrossRef]
- Zhao, S.; Xue, J.; Kang, W. Gas adsorption on MoS2 monolayer from first-principles calculations. Chem. Phys. Lett. 2014, 595, 35. [Google Scholar] [CrossRef]
- Ferreira, F.; Carvalho, A.; Moura, Í.J.; Coutinho, J.; Ribeiro, R.M. Adsorption of H2, O2, H2O, OH and H on monolayer MoS2. J. Condens. Matter Phys. 2017, 30, 035003. [Google Scholar] [CrossRef]
- Demaison, J.; Herman, M.; Liévin, J. The equilibrium OH bond length. Int. Rev. Phys. 2007, 26, 391. [Google Scholar] [CrossRef]
- Demaison, J.; Császár, A.G. Equilibrium CO bond lengths. J. Mol. Struct. 2012, 1023, 7. [Google Scholar] [CrossRef]
- Yue, Q.; Shao, Z.; Chang, S.; Li, J. Adsorption of gas molecules on monolayer MoS2 and effect of applied electric field. Nanoscale Res. Lett. 2013, 8, 1. [Google Scholar] [CrossRef]
- Enriquez, J.I.G.; Al Rey, C.V. Hydrogen adsorption on pristine, defected, and 3d-block transition metal-doped penta-Graphene. Int. J. Hydrog. Energy 2016, 41, 12157. [Google Scholar] [CrossRef]
- Krishnan, S.; Vadapoo, R.; Riley, K.E.; Velev, J.P. Dispersion-corrected density functional theory comparison of hydrogen adsorption on boron-nitride and carbon nanotubes. Phys. Rev. B 2011, 84, 165408. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, G.; Choi, J.H. Adsorption Behavior of the Hydroxyl Radical and Its Effects on Monolayer MoS2. ACS Omega 2020, 5, 1982. [Google Scholar] [CrossRef] [PubMed]
- Carey, F.A.; Sundberg, R.J. Chemical bonding and molecular structure. In Advanced Organic Chemistry; Springer: Berlin, Germany, 2007; pp. 1–117. [Google Scholar]
- Li, H.; Huang, M.; Cao, G. Markedly different adsorption behaviors of gas molecules on defective monolayer MoS2: A first-principles study. Phys. Chem. Chem. Phys. 2016, 18, 15110. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Ren, Y.; Wang, M.; Zhang, Z.; Zhao, W.; Yun, J. NO gas adsorption properties of MoS2 from monolayer to trilayer: A first-principles study. Mater. Res. Express 2021, 8, 015024. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Kim, G.; Jung, S.C.; Han, Y.K. Selectively Strong Molecular Adsorption on Boron Nitride Monolayer Induced by Transition Metal Substrate. Curr. Appl. Phys. 2013, 13, 2059. [Google Scholar] [CrossRef]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98. [Google Scholar] [CrossRef]
- Hellmann, R.; Bich, E.; Vogel, E.; Vesovic, V. Ab Initio Intermolecular Potential Energy Surface and Thermophysical Properties of Hydrogen Sulfide. Phys. Chem. Chem. Phys. 2011, 13, 13749. [Google Scholar] [CrossRef] [PubMed]
- Grabowsky, S.; Luger, P.; Buschmann, J.; Schneider, T.; Schirmeister, T.; Sobolev, A.N.; Jayatilaka, D. The Significance of Ionic Bonding in Sulfur Dioxide: Bond Orders from X-ray Diffraction Data. Angew. Chem. Int. Ed. 2012, 51, 6776. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, I.; Bentley, J. Comparison of the Magnetic Properties and Harmonic Force Fields of Nitrogen Dioxide and Carbon Dioxide (1-)(CO2-) by ab initio calculation. J. Phys. Chem. 1985, 89, 2951. [Google Scholar] [CrossRef]
- Aguilar, N.; Aparicio, S. Theoretical insights into CO2 adsorption by MoS2 nanomaterials. J. Phys. Chem. C 2019, 123, 26338. [Google Scholar] [CrossRef]
- Ignatchenko, A.V. Density functional theory study of carboxylic acids adsorption and enolization on monoclinic zirconia surfaces. J. Phys. Chem. C 2011, 115, 16012. [Google Scholar] [CrossRef]
- Ullah, H.; Ayub, K.; Ullah, Z.; Hanif, M.; Nawaz, R.; Bilal, S.; Ali Shah, U.H. A Theoretical Insight of Polypyrrole Ammonia Gas Sensor. Synth. Met. 2013, 172, 14. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khaldi, A.; Fadlallah, M.M.; Alhajri, F.; Maarouf, A.A. Hybrid G/BN@2H-MoS2 Nanomaterial Composites: Structural, Electronic and Molecular Adsorption Properties. Nanomaterials 2022, 12, 4351. https://doi.org/10.3390/nano12244351
Al-Khaldi A, Fadlallah MM, Alhajri F, Maarouf AA. Hybrid G/BN@2H-MoS2 Nanomaterial Composites: Structural, Electronic and Molecular Adsorption Properties. Nanomaterials. 2022; 12(24):4351. https://doi.org/10.3390/nano12244351
Chicago/Turabian StyleAl-Khaldi, Amal, Mohamed M. Fadlallah, Fawziah Alhajri, and Ahmed A. Maarouf. 2022. "Hybrid G/BN@2H-MoS2 Nanomaterial Composites: Structural, Electronic and Molecular Adsorption Properties" Nanomaterials 12, no. 24: 4351. https://doi.org/10.3390/nano12244351
APA StyleAl-Khaldi, A., Fadlallah, M. M., Alhajri, F., & Maarouf, A. A. (2022). Hybrid G/BN@2H-MoS2 Nanomaterial Composites: Structural, Electronic and Molecular Adsorption Properties. Nanomaterials, 12(24), 4351. https://doi.org/10.3390/nano12244351





