X-ray Absorption Spectroscopy Study of Thickness Effects on the Structural and Magnetic Properties of Pr2−δNi1−xMn1+xO6−y Double Perovskite Thin Films
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
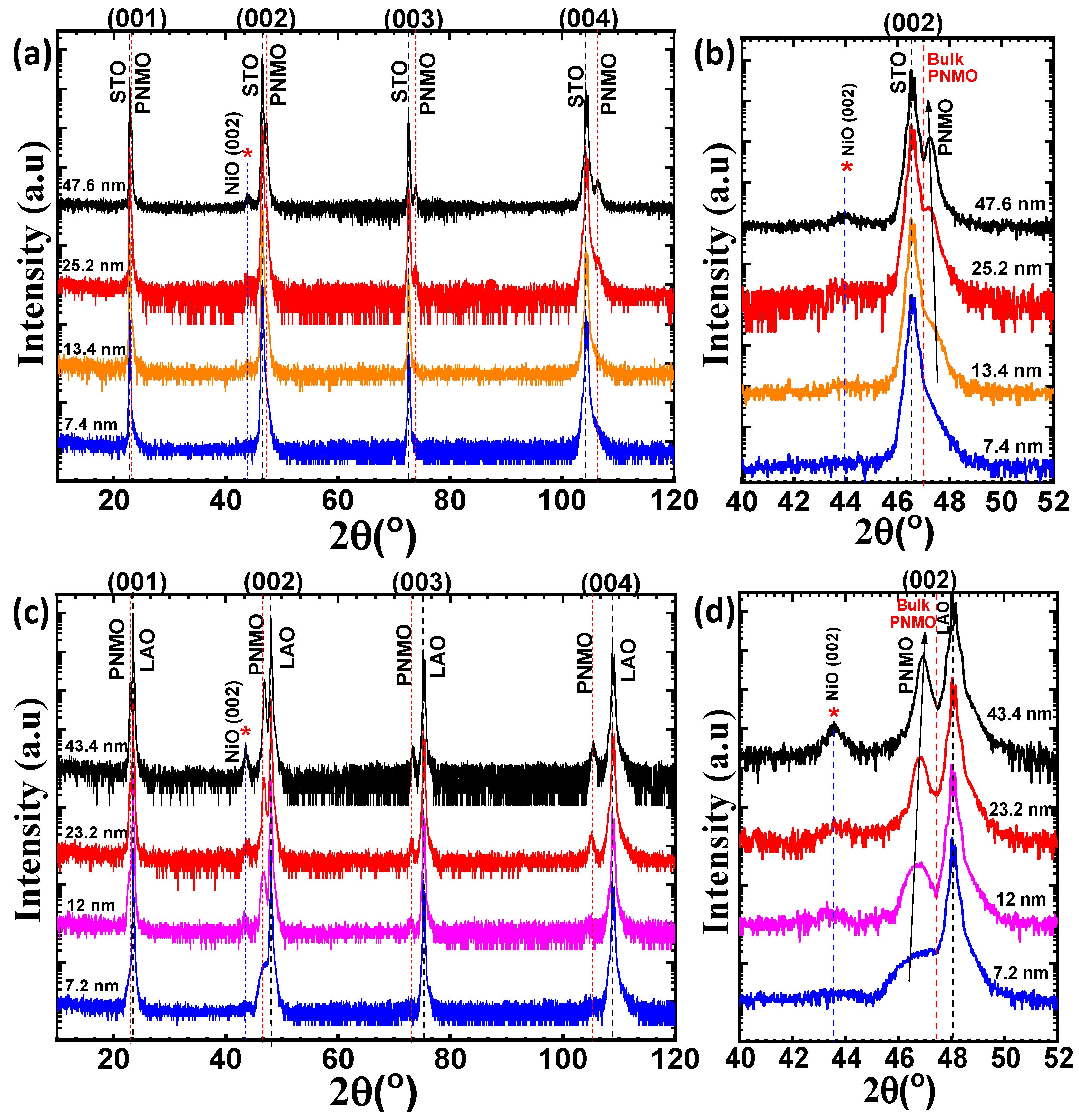
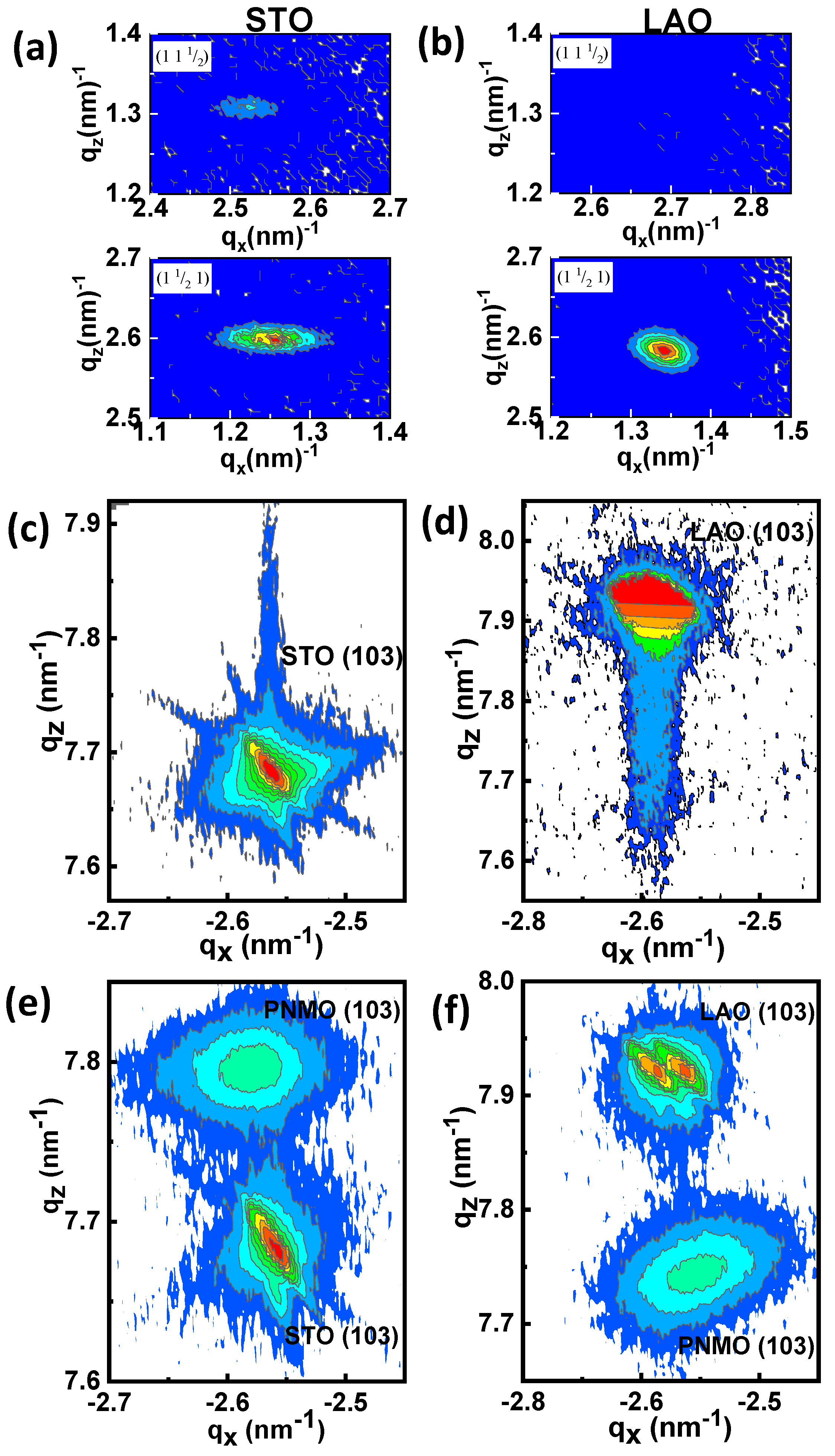
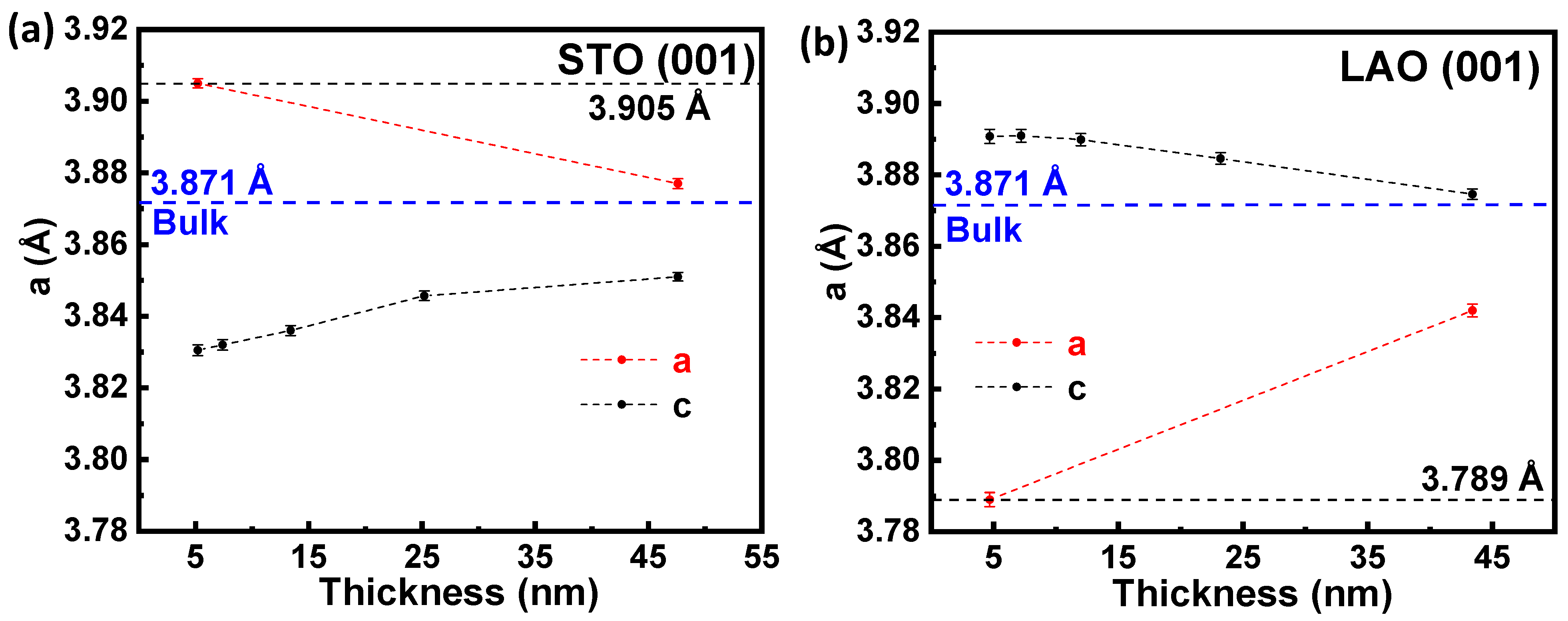
3.1. Structural Properties
3.2. Magnetic Properties
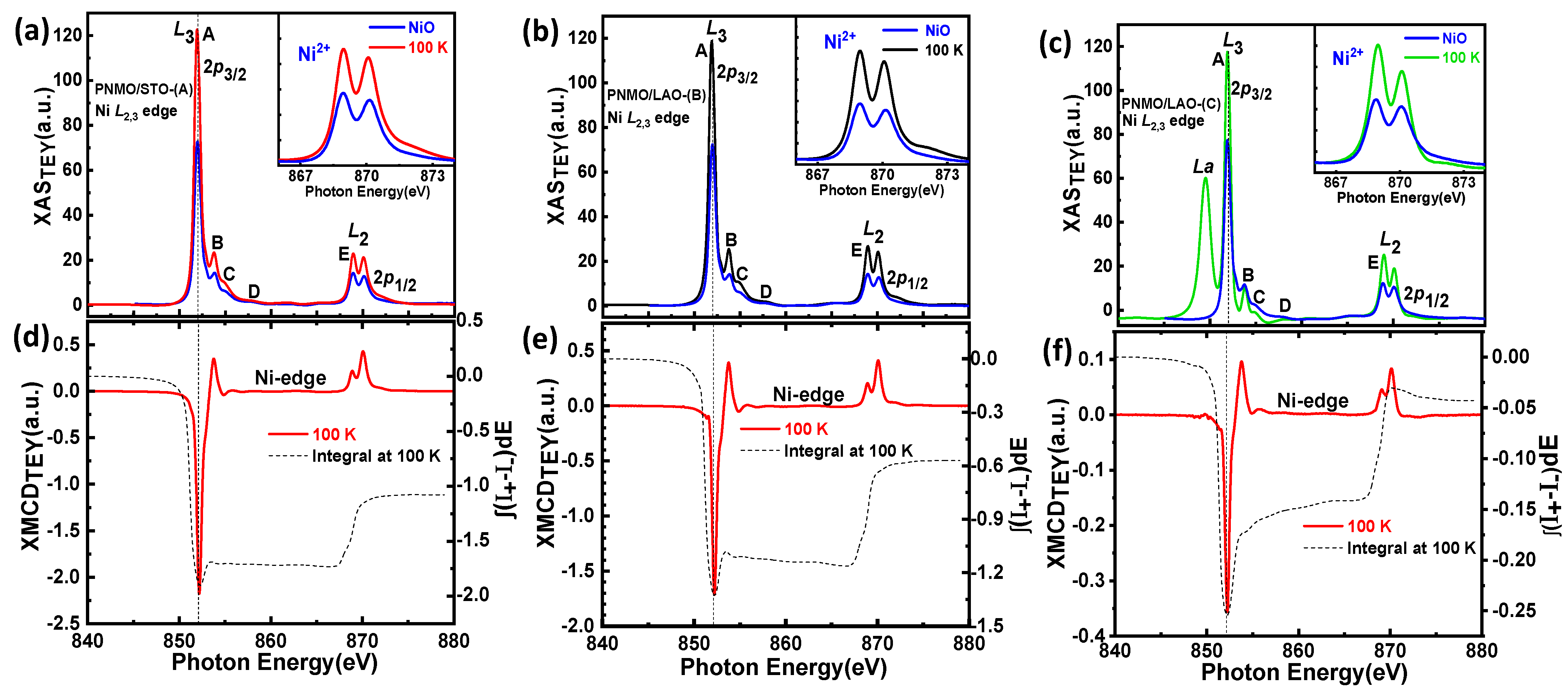
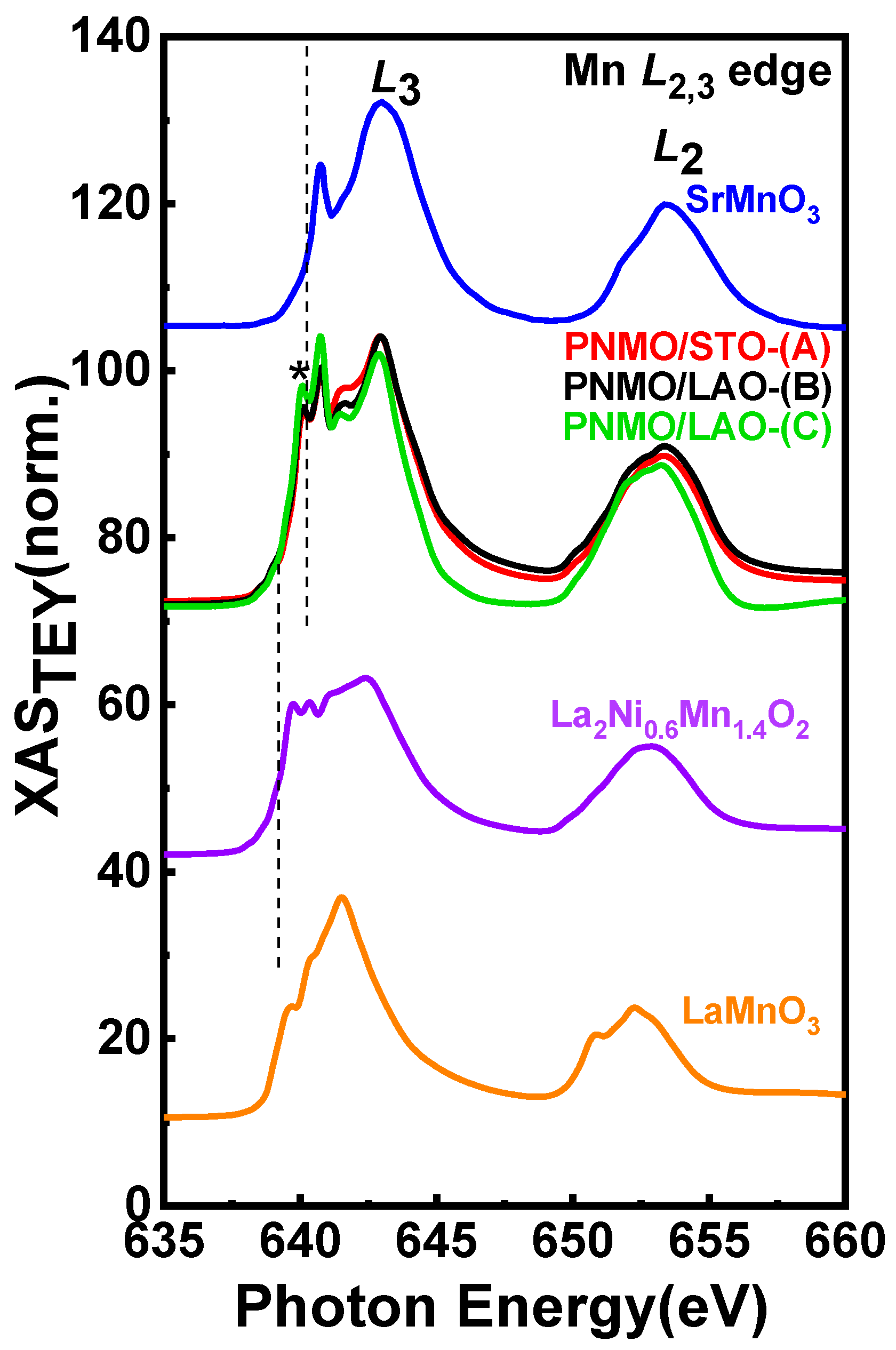
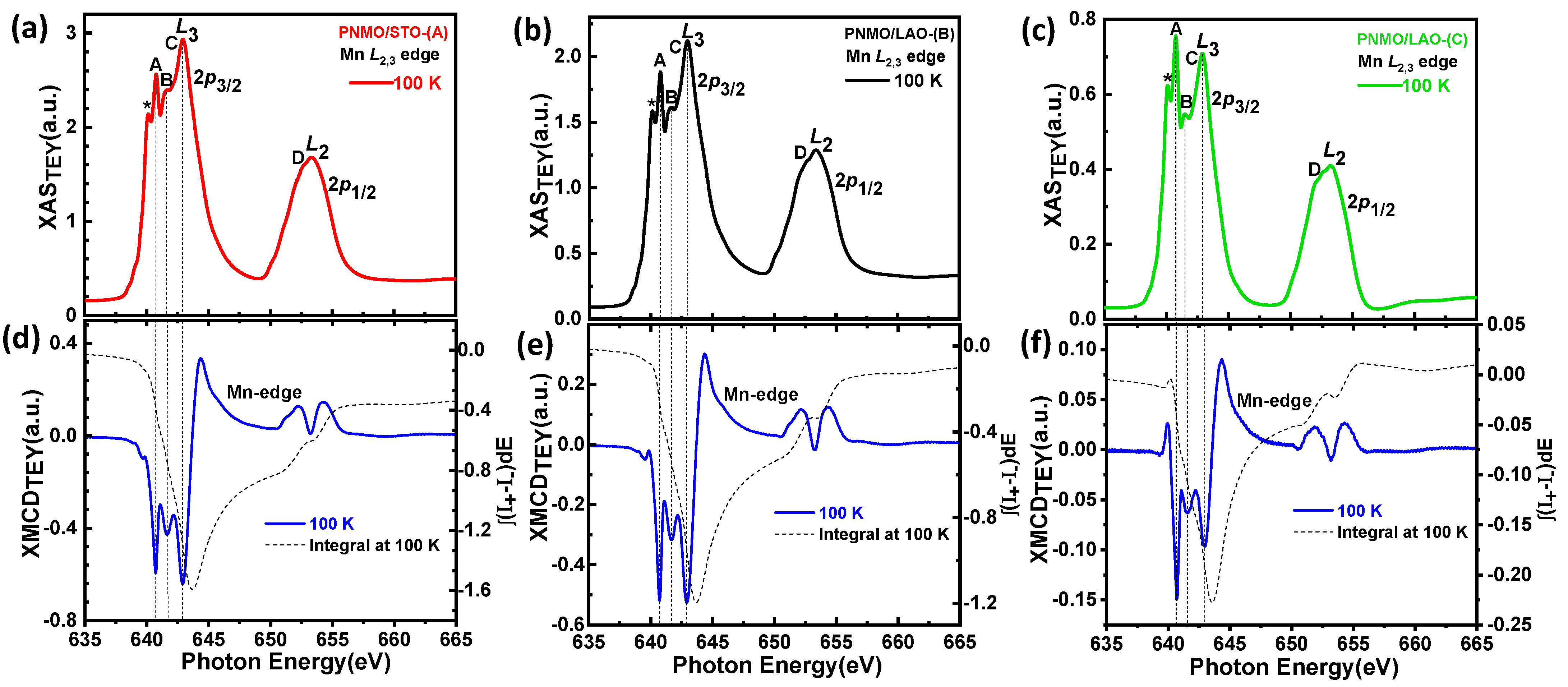
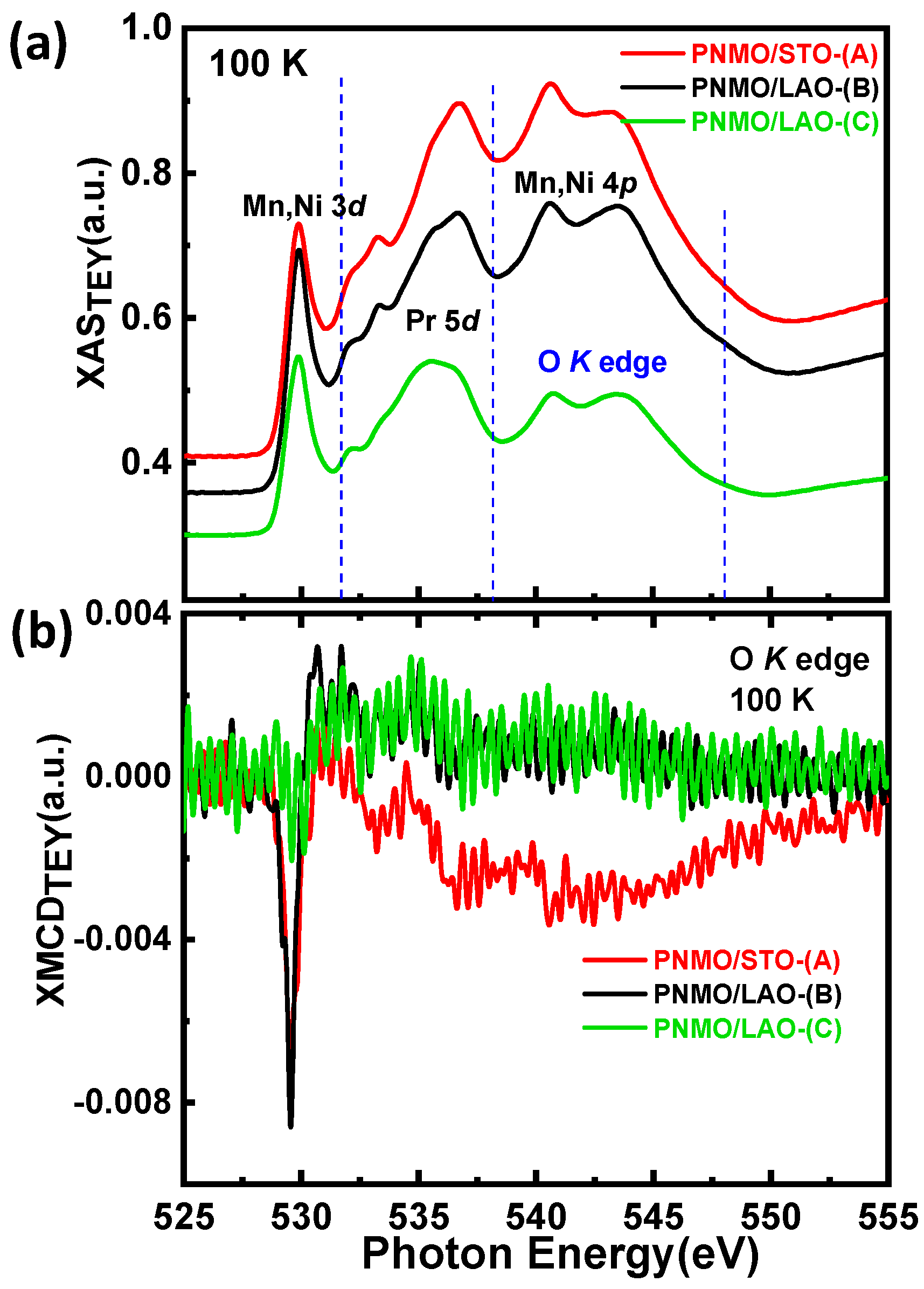
3.3. XAS and XMCD
3.3.1. Ni and Mn L2,3 Edges
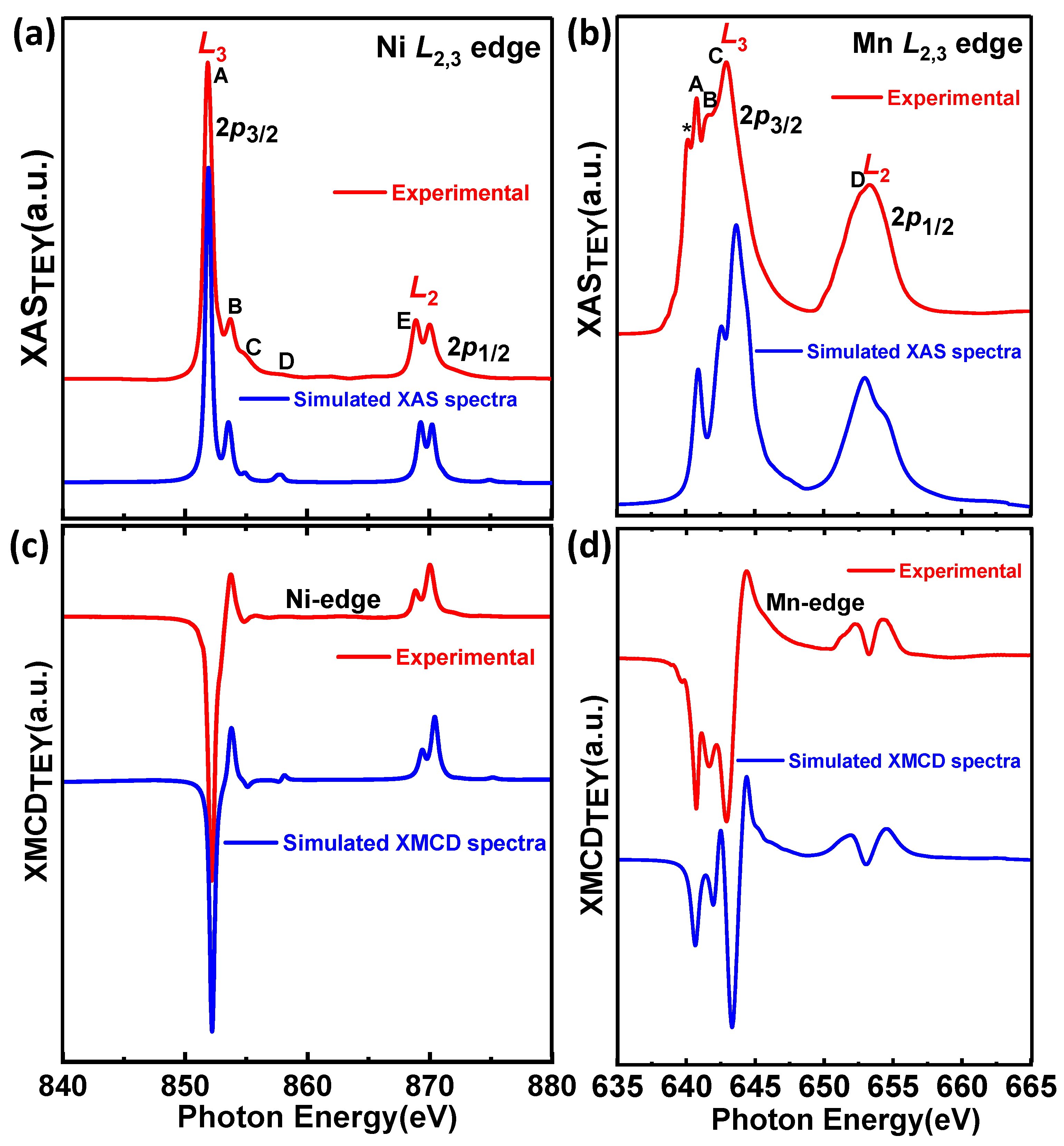
3.3.2. Numerical Simulation of XAS and XMCD Spectra of Ni2+ and Mn4+ Edges
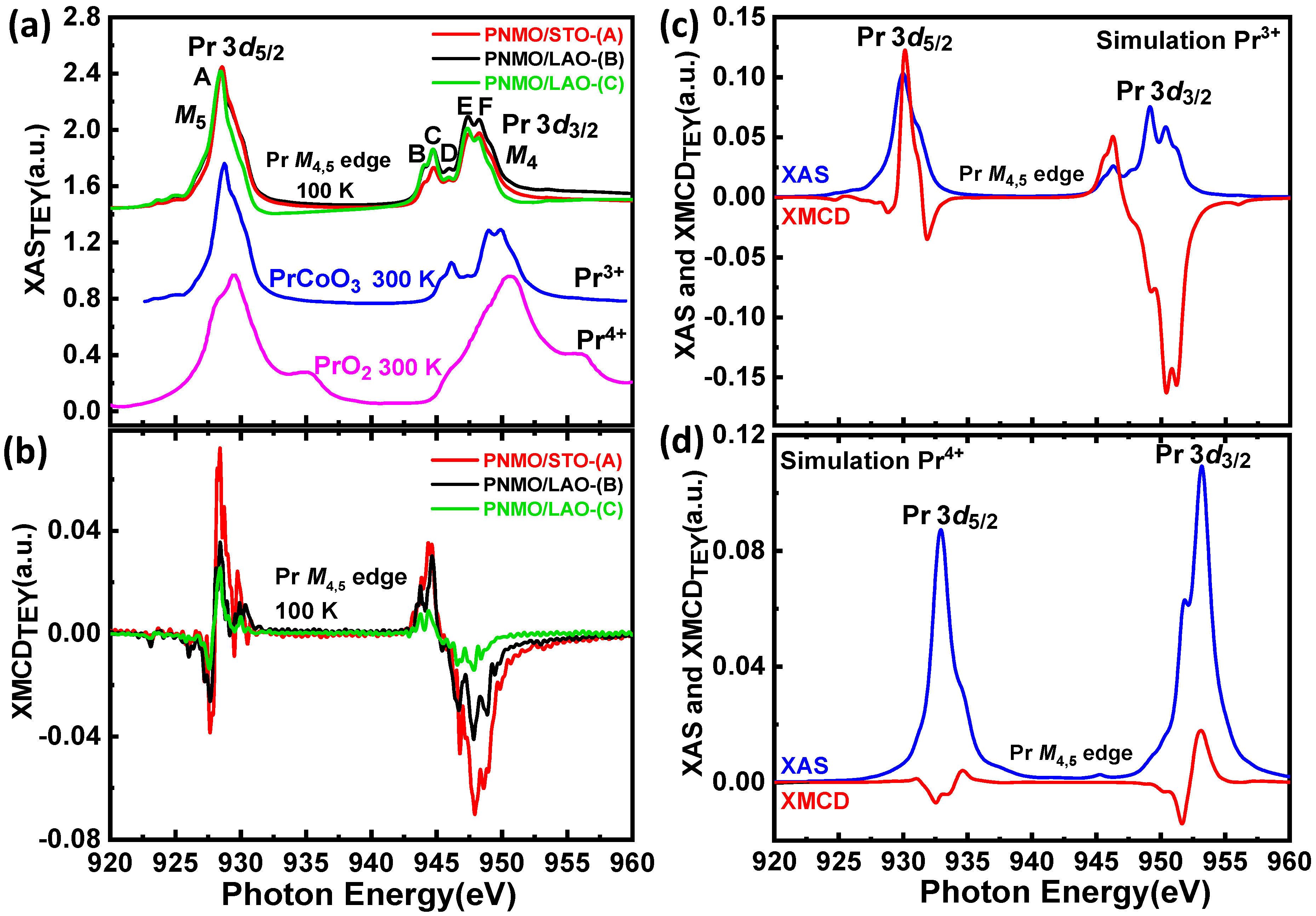
3.3.3. Pr M4,5 Edges
3.3.4. O K Edge
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogado, N.S.; Li, J.; Sleight, A.W.; Subramanian, M.A. Magnetocapacitance and magnetoresistance near room temperature in a ferromagnetic semiconductor: La2NiMnO6. Adv. Mater. 2005, 17, 2225–2227. [Google Scholar] [CrossRef]
- Hashisaka, M.; Kan, D.; Masuno, A.; Takano, M.; Shimakawa, Y. Epitaxial growth of ferromagnetic with ordered double-perovskite structure. Appl. Phys. Lett. 2006, 89, 032504. [Google Scholar] [CrossRef]
- Serrate, D.; de Teresa, J.M.; Ibarra, M.R. Double perovskites with ferromagnetism above room temperature. J. Phys. Condens. Matter 2007, 19, 023201. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, L.; Zhou, S.; Zhao, J.; Liu, W. Near room-temperature magnetoresistance effect in double perovskite La2NiMnO6. Appl. Phys. Lett. 2013, 102, 222401. [Google Scholar] [CrossRef]
- Hashisaka, M.; Kan, D.; Masuno, A.; Terashima, T.; Takano, M.; Mibu, K. Spin-filtering effect of ferromagnetic semiconductor La2NiMnO6. J. Magn. Magn. Mater. 2007, 310, 1975–1977. [Google Scholar] [CrossRef]
- Dass, R.I.; Yan, J.-Q.; Goodenough, J.B. Oxygen stoichiometry, ferromagnetism and transport properties of La2−xNiMnO6+δ. Phys. Rev. B 2003, 68, 064415. [Google Scholar] [CrossRef]
- Singh, M.P.; Truong, K.D.; Jandl, S.; Fournier, P. Magnetic properties and phonon behavior of Pr2NiMnO6 thin films. Appl. Phys. Lett. 2011, 98, 162506. [Google Scholar] [CrossRef]
- Booth, R.J.; Fillman, R.; Whitaker, H.; Nag, A.; Tiwari, A.M.; Ramanujachary, K.V.; Gopalakrishnan, J.; Lofland, S.E. An investigation of structural, magnetic and dielectric properties of R2NiMnO6 (R = rare earth, Y). Mater. Res. Bull. 2009, 44, 1559. [Google Scholar] [CrossRef]
- Moodera, J.S.; Hao, X.; Gibson, G.A.; Meservey, R. Electron-Spin Polarization in Tunnel Junctions in Zero Applied Field with Ferromagnetic EuS Barriers. Phys. Rev. Lett. 1988, 61, 637–640. [Google Scholar] [CrossRef]
- LeClair, P.; Ha, J.K.; Swagten, H.J.M.; van de Vin, C.H.; Kohlhepp, J.T.; de Jonge, W.J.M. Large magnetoresistance using hybrid spin filter devices. Appl. Phys. Lett. 2002, 80, 625–627. [Google Scholar] [CrossRef]
- Fuh, H.R.; Liu, Y.P.; Xiao, Z.R.; Wang, Y.K. New type of ferromagnetic insulator: Double perovskite La2NiMO6 (M= Mn, Tc, Re, Ti, Zr, and Hf). J. Magn. Magn. Mater. 2014, 357, 7–12. [Google Scholar] [CrossRef]
- Iliev, M.N.; Guo, H.; Gupta, A. Raman spectroscopy evidence of strong spin-phonon coupling in epitaxial thin films of the double perovskite La2NiMnO6. Appl. Phys. Lett. 2007, 90, 151914. [Google Scholar] [CrossRef]
- Chandrasekhar, K.D.; Das, A.K.; Mitra, C.; Venimadhav, A. The extrinsic origin of the magnetodielectric effect in the double perovskite La2NiMnO6. J. Phys. Cond. Matter 2012, 24, 495901. [Google Scholar] [CrossRef]
- Sun, H.; Song, S.; Xu, X.; Dai, J.; Yu, J.; Zhou, W.; Shao, Z.; Jung, W. Recent Progress on Structurally Ordered Materials for Electrocatalysis. Adv. Energy Mater. 2021, 11, 2101937. [Google Scholar] [CrossRef]
- Goodenough, J.B. Theory of the role of covalence in the perovskite-type manganites [La,M(II)]MnO3. Phys. Rev. 1955, 100, 564–573. [Google Scholar] [CrossRef]
- Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 1959, 10, 87–98. [Google Scholar] [CrossRef]
- Majumder, S.; Tripathi, M.; Raghunathan, R.; Rajput, P.; Jha, S.N.; de Souza, D.O.; Olivi, L.; Choudhary, R.J.; Phase, D.M. Tailoring the magnetic states by controlling octahedral cation arrangement in double perovskite. arXiv 2020, arXiv:2009.06030v2. [Google Scholar] [CrossRef]
- Nasir, M.; Kumar, S.; Patra, N.; Bhattacharya, D.; Jha, S.N.; Basaula, D.R.; Bhatt, S.; Khan, M.; Liu, S.-W.; Biring, S.; et al. Role of Antisite Disorder, Rare-Earth Size, and Superexchange Angle on Band Gap, Curie Temperature and Magnetization of R2NiMnO6 Double Perovskites. ACS Appl. Electron. Mater. 2019, 1, 141–153. [Google Scholar] [CrossRef]
- Guo, H.Z.; Burgess, J.; Ada, E.; Street, S.; Gupta, A.; Iliev, M.N.; Kellock, A.J.; Magen, C.; Varela, M.; Pennycook, S.J. Influence of defects on structural and magnetic properties of multifunctional La2NiMnO6 thin films. Phys. Rev. B 2008, 77, 174423. [Google Scholar] [CrossRef]
- Truong, K.D.; Singh, M.P.; Jandl, S.; Fournier, P. Influence of Ni/Mn cation order on the spin-phonon coupling in multifunctional La2NiMnO6 epitaxial films by polarized Raman spectroscopy. Phys. Rev. B 2009, 80, 134424. [Google Scholar] [CrossRef]
- Zhao, S.; Shi, L.; Zhou, S.; Zhao, J.; Yang, H.; Guo, Y. Size-dependent magnetic properties and Raman spectra of La2NiMnO6 nanoparticles. J. Appl. Phys. 2009, 106, 123901. [Google Scholar] [CrossRef]
- Zhao, H.J.; Liu, X.Q.; Chen, X.M.; Bellaiche, L. Effects of chemical and hydrostatic pressures on structural, magnetic, and electronic properties of R2NiMnO6 (R = rare−earth ion) double perovskites. Phys. Rev. B 2014, 90, 195147. [Google Scholar] [CrossRef]
- Retuerto, M.; Muñoz, A.; Martínetz-Lope, M.J.; Alonso, J.A.; Mompeán, F.J.; Fernández-Díaz, M.T.; Sánchez-Benítez, J. Magnetic Interactions in the Double Perovskites R2NiMnO6 (R = Tb, Ho, Er, Tm) Investigated by Neutron Diffraction. Inorg. Chem. 2015, 54, 10890–10900. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Bandyopadhyay, P.; Roy, S. An overview of double perovskites A2BB’O6 with small ions at A site: Synthesis, structure and magnetic properties. J. Alloys Compd. 2018, 740, 414–427. [Google Scholar] [CrossRef]
- Lacorre, P.; Torrance, J.B.; Pannetier, J.S.A.I.; Nazzal, A.I.; Wang, P.W.; Huang, T.C. Synthesis, crystal structure, and properties of metallic PrNiO3: Comparison with metallic NdNiO3 and semiconducting SmNiO3. J. Solid State Chem. 1991, 91, 225–237. [Google Scholar] [CrossRef]
- Toulemonde, O.; Millange, F.; Studer, F.; Raveau, B.; Park, J.H.; Chen, C.T. Changes in the Jahn-Teller distortion at the metal-insulator transition in CMR manganites. J. Phys. Cond. Matter 1999, 11, 109–120. [Google Scholar] [CrossRef]
- Truong, K.D.; Singh, M.P.; Jandl, S.; Fournier, P. Investigation of phonon behavior in Pr2NiMnO6 by micro-Raman spectroscopy. J. Phys. Condens. Matter 2011, 23, 052202. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Joshi, S.R.; Yadav, R.; de Groot, F.M.F.; Singh, A.K.; Ray, A.; Gupta, M.; Singh, A.; Maurya, S.; Elizabeth, S.; et al. Electronic structure of Pr2MnNiO6 from X-ray photoemission, absorption and density functional theory. J. Phys. Condens. Matter 2018, 30, 435603. [Google Scholar] [CrossRef]
- Bernal-Salamanca, M.; Balcells, L.; Konstantinović, Z.; Pomar, A.; Martínez, B.; Frontera, C. Optimization of the Growth Process of Double Perovskite Pr2−δNi1−xMn1+xO6−y Epitaxial Thin Films by RF Sputtering. Materials 2022, 15, 5046. [Google Scholar] [CrossRef]
- Liu, J.; Kim, J.K.; Wang, Y.; Kim, H.; Belotti, A.; Koo, B.; Wang, Z.; Jung, W.C.; Ciucci, F. Understanding and mitigating A-site surface enrichment in Ba-containing perovskites: A combined computational and experimental study of BaFeO3. Energy Environ. Sci. 2022, 15, 4069. [Google Scholar] [CrossRef]
- Wei, Z.; Liedienov, N.A.; Li, Q.; Pashchenko, A.V.; Xu, W.; Turchenko, V.A.; Yuan, M.; Fesych, I.V.; Levchenko, G.G. Influence of post-annealing, defect chemistry and high pressure on the magnetocaloric effect of non-stoichiometric La0.8−xK0.2Mn1+xO3 compounds. Ceram. Int. 2021, 47, 24553–24563. [Google Scholar] [CrossRef]
- Pashchenko, A.V.; Liedienov, N.A.; Pashchenko, V.P.; Prokopenko, V.K.; Burhovetskii, V.V.; Voznyak, A.V.; Fesych, I.V.; Tatarchuk, D.D.; Didenko, Y.V.; Gudymenko, A.I.; et al. Modification of multifunctional properties of the magnetoresistive La0.6Sr0.15Bi0.15Mn1.1−xBxO3−δ ceramics when replacing manganese with 3d-ions of Cr, Fe, Co, Ni. J. Alloys Compd. 2018, 767, 1117. [Google Scholar] [CrossRef]
- Iwahori, K.; Watanabe, S.; Kawaia, M.; Mizuno, K.; Sasaki, K.; Yoshimoto, M. Nanoscale composition analysis of atomically flat SrTiO3 (001) by friction force microscopy. J. Appl. Phys. 2000, 88, 7099–7103. [Google Scholar] [CrossRef]
- van der Torren, A.J.H.; van der Molen, S.J.; Aarts, J. Formation of a mixed ordered termination on the surface of LaAlO3 (001). Phys. Rev. B 2015, 91, 245426. [Google Scholar] [CrossRef]
- Barla, A.; Nicolás, J.; Cocco, D.; Valvidares, S.M.; Herrero-Martín, J.; Gargiani, P.; Moldes, J.; Ruget, C.; Pellegrin, E.; Ferrer, S. Design and performance of BOREAS, the Beamline fOr REsonant X-ray Absorption and Scattering experiments at the ALBA Synchrotron Light Source. J. Synchrotron Radiat. 2016, 23, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Mouallem-Bahout, M.; Roisnel, T.; Bourée, F.; André, G.; Moure, C.; Peña, O. Neutron diffraction evidence for a cationic order in the REMn0.5Ni0.5O3 (RE = La, Nd) and YMn0.5Co0.5O3 perovskites. Prog. Solid State Chem. 2007, 35, 257–264. [Google Scholar] [CrossRef]
- Chen, X.J.; Habermeier, H.-U.; Zhang, H.; Gu, G.; Varela, M.; Santamaria, J.; Almasan, C.C. Metal-insulator transition above room temperature in maximum colossal magnetoresistance manganite thin films. Phys. Rev. B 2005, 72, 104403. [Google Scholar] [CrossRef]
- Sakurai, Y.; Ohkubo, I.; Matsumoto, Y.; Koinuma, H.; Oshima, M. Influence of substrates on epitaxial growth of B-site-ordered perovskite La2NiMnO6 thin films. J. Appl. Phys. 2011, 110, 063913. [Google Scholar] [CrossRef]
- Liao, Z.; Li, F.; Gao, P.; Li, L.; Guo, J.; Pan, X.; Jin, R.; Plummer, E.W.; Zhalng, J. Origin of the metal-insulator transition in ultrathin films of La2/3Sr1/3MnO3. Phys. Rev. B 2015, 92, 125123. [Google Scholar] [CrossRef]
- Huijben, M.; Martin, L.W.; Chu, Y.-H.; Holcomb, M.B.; Yu, P.; Rijnders, G.; Blank, D.H.A.; Ramesh, R. Critical thickness and orbital ordering in ultrathin La0.7Sr0.3MnO3 films. Phys. Rev. B 2008, 78, 094413. [Google Scholar] [CrossRef]
- Cui, B.; Song, C.; Wang, G.Y.; Mao, H.J.; Zeng, F.; Pan, F. Strain engineering induced interfacial self-assembly and intrinsic exchange bias in a manganite perovskite film. Sci. Rep. 2013, 3, 2542. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Z.; Abraham, D.W.; Rao, R.A.; Eom, C.B. Thickness-dependent magnetotransport in ultrathin manganite films. Appl. Phys. Lett. 1999, 74, 3017–3019. [Google Scholar] [CrossRef]
- Stavitski, E.; de Groot FM, F. The CTM4XAS Program for EELS and XAS spectral shape analysis of transition metal L edges. Micron 2010, 41, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.quanty.org (accessed on 16 November 2022).
- Available online: http://esrf.fr/computing/scientific/crispy (accessed on 16 November 2022).
- Guo, H.; Gupta, A.; Varela, M.; Pennycook, S.; Zhang, J. Local valence and magnetic characteristics of La2NiMnO6. Phys. Rev. B 2009, 79, 172402. [Google Scholar] [CrossRef]
- Choudhury, D.; Mandal, P.; Mathieu, R.; Hazarika, A.; Rajan, S.; Sundaresan, A.; Waghmare, U.V.; Knut, R.; Karis, O.; Nordblad, P.; et al. Near-room-temperature colossal magnetodielectricity and multiglass properties in partially disordered La2NiMnO6. Phys. Rev. Lett. 2012, 108, 127201. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Jana, S.; Govinda, S.; Pal, B.; Mukherjee, S.; Keshavarz, S.; Thonig, D.; Kvashnin, Y.; Pereiro, M.; Mathieu, R.; et al. Peculiar magnetic states in the double perovskite Nd2NiMnO6. Phys. Rev. B 2019, 100, 045122. [Google Scholar] [CrossRef]
- Van Der Laan, G.; Zaanen, J.; Sawatzky, G.A.; Karnatak, R.; Esteva, J.M. Comparison of X-ray absorption with X-ray photoemission of nickel dihalides and NiO. Phys. Rev. B 1986, 33, 4253–4263. [Google Scholar] [CrossRef]
- Medarde, M.; Fontaine, A.; Garcia-Munoz, J.L.; Rodriguez-Carvajal, J.; De Santis, M.; Sacchi, M.; Rossi, G.; Lacorre, P. RNiO3 perovskites (R = Pr,Nd): Nickel valence and the metal-insulator transition investigated by X-ray-absorption spectroscopy. Phys. Rev. B 1992, 46, 14975–14984. [Google Scholar] [CrossRef]
- Bernal-Salamanca, M.; Konstantinovic, Z.; Balcells, L.; Pannunzio-Miner, E.; Andiumenge, F.; Lopez, L.; Bozzo, B.; rrero-Martin, J.H.; Pomar, A.; Frontera, C.; et al. Nonstoichiometry driven ferromagnetism in double perovskite La2Ni1−xMn1+xO6 insulating thin films. Cryst. Growth Des. 2019, 19, 2765–2771. [Google Scholar] [CrossRef]
- Kang, J.-S.; Lee, H.J.; Kim, D.H.; Kolesnik, S.; Dabrowski, B.; Świerczek, K.; Lee, J.; Kim, B.; Min, B.I. Valence and spin states, and the metal-insulator transition in ferromagnetic La2−xSrxMnNiO6 (x = 0,0.2). Phys. Rev. B 2009, 80, 045115. [Google Scholar] [CrossRef]
- Kang, J.-S.; Lee, S.M.; Kim, D.H.; Kolesnik, S.; Dabrowski, B.; Park, B.-G.; Kim, J.-Y.; Lee, J.; Kim, B.; Min, B.I. Temperature-dependent magnetic circular dichroism study of ferromagnetic double perovskite La2MnNiO6. J. Appl. Phys. 2010, 107, 09D721. [Google Scholar] [CrossRef]
- Blasco, J.; García, J.; Sánchez, M.C.; Campo, J.; Subías, G.; Pérez-Cacho, J. Magnetic properties of LaNi1−x MnxO3+δ perovskites. Eur. Phys. J. B 2002, 30, 469–479. [Google Scholar] [CrossRef]
- Burnus, T.; Hu, Z.; Hsieh, H.H.; Joly, V.L.J.; Joy, P.A.; Haverkort, M.W.; Wu, H.; Tanaka, A.; Lin, H.-J.; Chen, C.T.; et al. Local electronic structure and magnetic properties of LaMn0.5Co0.5O3 studied by X-ray absorption and magnetic circular dichroism spectroscopy. Phys. Rev. B 2008, 77, 125124. [Google Scholar] [CrossRef]
- Wu, H.; Burnus, T.; Hu, Z.; Martin, C.; Maignan, A.; Cezar, J.C.; Tanaka, A.; Brookes, N.B.; Khomskii, D.I.; Tjeng, L.H. Ising magnetism and ferroelectricity in Ca3CoMnO6. Phys. Rev. Lett. 2009, 102, 026404. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.L.; Tonner, B.P.; Harp, G.R.; Parkin, S.S.P. J Experimental investigation of dichroism sum rules for V, Cr, Mn, Fe, Co, and Ni: Influence of diffuse magnetism. Appl. Phys. 1994, 76, 6462. [Google Scholar] [CrossRef]
- Dunn, J.H.; Arvanitis, D.; Mårtensson, N.; Tischer, M.; May, F.; Russo, M.; Baberschke, K. An angle-dependent magnetic circular X-ray dichroism study of Co/Cu (100): Experiment versus theory. J. Phys. Condens. Matter 1995, 7, 1111–1119. [Google Scholar] [CrossRef]
- Heald, S.M.; Stern, E.A. Anisotropic X-ray absorption in layered compounds. Phys. Rev. B 1977, 16, 5549–5559. [Google Scholar] [CrossRef]
- Stern, E.; Kim, K. Thickness effect on the extended-X-ray-absorption-fine-structure amplitude. Phys. Rev. B 1981, 23, 3781–3787. [Google Scholar] [CrossRef]
- Nakajima, R.; Stöhr, J.; Idzerda, Y.U. Electron-yield saturation effects in L-edge X-ray magnetic circular dichroism spectra of Fe, Co, and Ni. Phys. Rev. B 1999, 59, 6421–6429. [Google Scholar] [CrossRef]
- Ruosi, A.; Raisch, C.; Verna, A.; Werner, R.; Davidson, B.A.; Fujii, J.; Kleiner, R.; Koelle, D. Electron sampling depth and saturation effects in perovskite films investigated by soft X-ray absorption spectroscopy. Phys. Rev. B 2014, 90, 125120. [Google Scholar] [CrossRef]
- Carra, P.; Thole, B.T.; Altarelli, M.; Wang, X. X-ray Circular Dichroism and Local Magnetic Fields. Phys. Rev. Lett. 1993, 70, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Thole, B.T.; Carra, P.; Sette, F.; van der Laan, G. X-ray Circular Dichroism as a Probe of Orbital Magnetization. Phys. Rev. Lett. 1992, 68, 1943–1946. [Google Scholar] [CrossRef] [PubMed]
- Piamonteze, C.; Miedema, P.; de Groot, F.M.F. Accuracy of the spin sum rule in XMCD for the transition-metal L deges from mangense to copper. Phys. Rev. B 2009, 80, 184410. [Google Scholar] [CrossRef]
- Kuepper, K.; Raekers, M.; Taubitz, C.; Uhlarz, M.; Piamonteze, C.; de Groot, F.M.F.; Arenholz, E.; Galakhov, V.R.; Mukovskii, Y.M.; Neumann, M. The X-ray magnetic circular dichroism spin sum rule for 3d4 systems: Mn3+ ions in colossal magnetoresistance manganites. J. Phys. Condens. Matter 2012, 24, 435602. [Google Scholar] [CrossRef] [PubMed]
- Teramura, Y.; Tanaka, A.; Jo, T. Effect of Coulomb Interaction on the X-ray Magnetic Circular Dichroism Spin Sum Rule in 3d Transition Elements. J. Phys. Soc. Jpn. 1996, 65, 1053–1055. [Google Scholar] [CrossRef]
- Ghiasi, M.; Delgado-Jaime, M.U.; Malekzadeh, A.; Wang, R.; Miedema, P.S.; Beye, M.; de Groot, F.M.F. Mn and Co Charge and Spin Evolutions in LaMn1−xCoxO3 Nanoparticles. J. Phys. Chem. C 2016, 120, 8167–8174. [Google Scholar] [CrossRef]
- Ikeno, H.; de Groot, F.M.F.; Stavitski, E.; Tanaka, I. Multiplet calculations of L2,3 X-ray absorption near edge structures for 3d transition-metal compounds. J. Phys. Condens. Matter 2009, 21, 104208. [Google Scholar] [CrossRef][Green Version]
- de Groot, F.M.F.; Fuggle, J.C.; Thole, B.T.; Sawatzky, G.A. 2p X-ray absorption of 3d transition-metal compounds: An atomic multiplet description including the crystal field. Phys. Rev. B 1990, 42, 5459–5468. [Google Scholar] [CrossRef]
- Mizokawa, T.; Namatame, H.; Fujimori, A.; Akeyama, K.; Kondoh, H.; Kuroda, H.; Kosugi, N. Origin of the Band Gap in the Negative Charge-Transfer-Energy Compound NaCuO2. Phys. Rev. Lett. 1993, 70, 1565. [Google Scholar] [CrossRef]
- Cowan, R.D. The Theory of Atomic Structure and Spectra; University of California Press: Berkeley, CA, USA, 1981. [Google Scholar] [CrossRef]
- Wang, H.; Frontera, C.; Herrero-Martin, J.; Pomar, A.; Roura, P.; Martinez, B.; Mestres, N. Aqueous Chemical Solution Deposition of Functional Double Perovskite Epitaxial Thin Films. Chem. A Eur. J. 2020, 26, 9338–9347. [Google Scholar] [CrossRef]
- Padilla-Pantoja, J.; Herrero-Martín, J.; Gargiani, P.; Valvidares, S.M.; Cuartero, V.; Kummer, K.; Watson, O.; Brookes, N.B.; García-Muñoz, J.L. Stability of the Cationic Oxidation States in Pr0.50Sr0.50CoO3 across the Magnetostructural Transition by X-ray Absorption Spectroscopy. Inorg. Chem. 2014, 53, 8854–8858. [Google Scholar] [CrossRef]
- Ogasarawa, A.; Kotani, K. Okada, and B.T. Thole. Theory of X-ray-absorption spectra in PrO2 and some other rare-earth compounds. Phys. Rev. B 1991, 43, 854–859. [Google Scholar] [CrossRef]
- Karnatak, R.C.; Esteva, J.-M.; Dexpert, H.; Gasgnier, M.; Caro, P.E.; Albert, L. X-ray absorption studies of CeO2, PrO2, and TbO2. I. Manifestation of localized and extended f states in the 3d absorption spectra. Phys. Rev. B 1987, 36, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Thole, B.T.; van der Laan, G.; Fuggle, J.C.; Sawatzky, G.A.; Karnatak, R.C.; Esteva, J.M. 3d X-ray-absorption Lines and the 3d94fn+1 Multiplets of the Lanthanides. Phys. Rev. B 1985, 32, 5107–5118. [Google Scholar] [CrossRef] [PubMed]
- de Groot, F.M.F.; Spectrosc, J.E. X-ray absorption and dichroism of transition metals and their compounds. J. Electron Spectrosc. Relat. Phenom. 1994, 67, 529. [Google Scholar] [CrossRef]
- Sugar, J. Interpretation of Photoabsorption in the Vicinity of the 3d Edges in La, Er, and Tm. Phys. Rev. A 1972, 6, 1764–1767. [Google Scholar] [CrossRef]
- Abbate, M.; Pen, H.; Czyzyk, M.T.; de Groot, F.M.F.; Fuggle, J.C.; Ma, Y.J.; Chen, C.T.; Sette, F.; Fujimori, A.; Ueda, Y.; et al. Soft X-ray absorption spectroscopy of vanadium oxides. J. Electron Spectrosc. Relat. Phenom. 1993, 62, 185–195. [Google Scholar] [CrossRef]
- Abbate, M.; de Groot, F.M.F.; Fuggle, J.C.; Fujimori, A.; Strebel, O.; Lopez, F.; Domke, M.; Kaindl, G.; Sawatzky, G.A.; Takano, M.; et al. Controlled-valence properties of La1−xSrxFeO3 and La1−xSrxMnO3 studied by soft-X-ray absorption spectroscopy. Phys. Rev. B 1992, 46, 4511–4519. [Google Scholar] [CrossRef]
- Abbate, M.; Fuggle, J.C.; Fujimori, A.; Tjeng, L.H.; Chen, C.T.; Potze, R.; Sawatzky, G.A.; Eisaki, H.; Uchida, S. Electronic structure and spin-state transition of LaCoO3. Phys. Rev. B 1993, 47, 16124–16130. [Google Scholar] [CrossRef] [PubMed]
- Abbate, M.; Zampieri, G.; Prado, F.; Caneiro, A.; Gonzalez-Calbet, J.M.; Vallet-Regi, M. Electronic structure and metal-insulator transition in LaNiO3−δ. Phys. Rev. B 2002, 65, 155101. [Google Scholar] [CrossRef]
- Gardiner, C.W.; Parkins, A.S. Driving atoms with light of arbitrary statistics. Phys. Rev. A 1994, 50, 1782. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.; Shanthi, N.; Mahadevan, P. Electronic excitation spectra from ab initio band-structure results for LaMO3 (M= Cr, Mn, Fe, Co, Ni). Phys. Rev. B 1996, 54, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Medling, S.; Lee, Y.; Zheng, H.; Mitchell, J.F.; Freeland, J.W.; Harmon, B.N.; Bridges, F. Evolution of magnetic oxygen states in Sr-doped LaCoO3. Phys. Rev. Lett. 2012, 109, 157204. [Google Scholar] [CrossRef] [PubMed]

| M(H) loops | TC(K) | Ms(µB/f.u) | Hc(Oe) | Mr (µB/f.u) |
|---|---|---|---|---|
| 47.6 nm-PNMO/STO film at 10 K | ||||
| ip | 210 | 4.5 | 565 | 2.3 |
| op | 264 | 0.8 | ||
| 43.4 nm-PNMO/LAO film at 10 K | ||||
| ip | 216 | 4.85 | 538 | 2.8 |
| op | 631 | 0.7 | ||
| Parameter | Ni2+ | Mn4+ |
|---|---|---|
| Site symmetry | Oh | Oh |
| Crystal Field (10Dq) (eV) | 1.2 | 2.5 |
| Charge transfer energy (∆) (eV) | 3.0 | 2.5 |
| Udd(eV) | 7.5 | 6.5 |
| Upd(eV) | 7.3 | 8.5 |
| Slater’s integrals reduction (%) (Fdd, Fpd, Gpd) | 0.8 | 0.7 |
| Majority state | d8 | d3 |
| Minority state | d9L | d4L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Salamanca, M.; Herrero-Martín, J.; Konstantinović, Z.; Balcells, L.; Pomar, A.; Martínez, B.; Frontera, C. X-ray Absorption Spectroscopy Study of Thickness Effects on the Structural and Magnetic Properties of Pr2−δNi1−xMn1+xO6−y Double Perovskite Thin Films. Nanomaterials 2022, 12, 4337. https://doi.org/10.3390/nano12234337
Bernal-Salamanca M, Herrero-Martín J, Konstantinović Z, Balcells L, Pomar A, Martínez B, Frontera C. X-ray Absorption Spectroscopy Study of Thickness Effects on the Structural and Magnetic Properties of Pr2−δNi1−xMn1+xO6−y Double Perovskite Thin Films. Nanomaterials. 2022; 12(23):4337. https://doi.org/10.3390/nano12234337
Chicago/Turabian StyleBernal-Salamanca, Mónica, Javier Herrero-Martín, Zorica Konstantinović, Lluis Balcells, Alberto Pomar, Benjamín Martínez, and Carlos Frontera. 2022. "X-ray Absorption Spectroscopy Study of Thickness Effects on the Structural and Magnetic Properties of Pr2−δNi1−xMn1+xO6−y Double Perovskite Thin Films" Nanomaterials 12, no. 23: 4337. https://doi.org/10.3390/nano12234337
APA StyleBernal-Salamanca, M., Herrero-Martín, J., Konstantinović, Z., Balcells, L., Pomar, A., Martínez, B., & Frontera, C. (2022). X-ray Absorption Spectroscopy Study of Thickness Effects on the Structural and Magnetic Properties of Pr2−δNi1−xMn1+xO6−y Double Perovskite Thin Films. Nanomaterials, 12(23), 4337. https://doi.org/10.3390/nano12234337








