The Rainbow Arching over the Fluorescent Thienoviologen Mesophases
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
2.2. Polarizing Optical Microscopy (POM), Differential Scanning Calorimetry (DSC), and X-ray Powder Diffraction (XRD)
2.3. Photophysical Measurements
2.4. Molecular Mechanics Calculation
3. Results
3.1. Thermotropic Behavior
3.2. Bulk Photophysics and Thermofluorochromism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.Y.; Liu, S.; Zhao, Q.; Huang, W. Stimuli–responsive metallopolymers. Coord. Chem. Rev. 2016, 319, 180–195. [Google Scholar] [CrossRef]
- Conesa-Egea, J.; Zamora, F.; Amo-Ochoa, P. Perspectives of the smart Cu-Iodine coordination polymers: A portage to the world of new nanomaterials and composites. Coord. Chem. Rev. 2019, 381, 65–78. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, B.; Zhang, J.; Tan, X.; Wang, L.; Chen, J.; Lv, H.; Wen, S.; Li, B.; Ye, L.; et al. Piezochromic Luminescence Based on the Molecular Aggregation of 9,10-Bis((E)-2-(pyrid-2-yl)vinyl)anthracene. Angew. Chem. Int. Ed. 2012, 51, 10782–10785. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.W.; Wang, Y.; Lu, X.F.; Chen, Y.J.; Peng, J.; Zhou, G. Multistimuli-Responsive Luminescence Switching of Pyrazine Derivative Based Donor–Acceptor–Donor Luminophores. Chem. Asian J. 2016, 11, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, Y.; Lei, Y.; Zhou, Y.; Gao, W.; Liu, M.; Huang, X.; Wu, H. Multifunctional properties of a star-shaped triphenylamine-benzene-1,3,5-tricarbohydrazide fluorescent molecule containing multiple flexible chains. Chem. Commun. 2020, 56, 13638–13641. [Google Scholar] [CrossRef]
- Mitani, M.; Ogata, S.; Yamane, S.; Yoshio, M.; Hasegawab, M.; Kato, T. Mechanoresponsive liquid crystals exhibiting reversible luminescent color changes at ambient temperature. J. Mater. Chem. C 2016, 4, 2752–2760. [Google Scholar] [CrossRef]
- Fedorenko, E.V.; Mirochnik, A.G.; Gerasimenko, A.V.; Beloliptsev, A.Y.; Merkulov, E.B. Mechanofluorochromism, thermofluorochromism, solvatochromism, and solid-state luminescence of difluoroboron o-hydroxy-, p-benzoyloxydibenzoylmetanates. Dyes Pigm. 2018, 159, 557–572. [Google Scholar] [CrossRef]
- Bellacanzone, C.; Otaegui, J.R.; Hernando, J.; Ruiz-Molina, D.; Roscini, C. Tunable Thermofluorochromic Sensors Based on Conjugated Polymers. Adv. Opt. Mater. 2022, 10, 2102423. [Google Scholar] [CrossRef]
- Ishchenko, A.A.; Kulinich, A.V.; Bondarev, S.L.; Knyukshto, V.N.; Turban, A.A. Thermochromism and thermofluorochromism of merocyanines with a positive solvatochromism. Opt. Spectrosc. 2006, 101, 90–97. [Google Scholar] [CrossRef]
- Seeboth, A.; Lötzsch, D. Thermochromic Phenomena in Polymers; Smithers Rapra: Shawbury, UK, 2008. [Google Scholar]
- Seeboth, A.; Lötzsch, D.; Ruhmann, R.; Muehling, O. Thermochromic Polymers—Function by Design. Chem. Rev. 2014, 114, 3037–3068. [Google Scholar] [CrossRef]
- Wang, X.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Lin, G.; Clarke, C.; Zhou, J.; Jin, D. Optical Nanomaterials and Enabling Technologies for High-Security-Level Anticounterfeiting. Adv. Mater. 2020, 32, 1901430. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B.; Kalajahi, M.S. Photoluminescent and Chromic Nanomaterials for Anticounterfeiting Technologies: Recent Advances and Future Challenges. ACS Nano 2020, 14, 14417–14492. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Mera, N.A.; Otaegui, J.R.; Sánchez, R.S.; Prats, G.; Guirado, G.; Ruiz-Molina, D.; Roscini, C.; Hernando, J. Color-Tunable White-Light-Emitting Materials Based on Liquid-Filled Capsules and Thermally Responsive Dyes. ACS Appl. Mater. Interfaces 2019, 11, 17751–17758. [Google Scholar] [CrossRef] [PubMed]
- Sol, J.A.H.P.; Dehm, V.; Hecht, R.; Wgrthner, F.; Schenning, A.P.H.J.; Debije, M.G. Temperature-Responsive Luminescent Solar Concentrators: Tuning Energy Transfer in a Liquid Crystalline Matrix. Angew. Chem. Int. Ed. 2018, 57, 1030–1033. [Google Scholar] [CrossRef]
- Otaegui, J.R.; Ruiz-Molina, D.; Latterini, L.; Hernando, J.; Roscini, C. Thermoresponsive multicolor-emissive materials based on solid lipid nanoparticles. Mater. Horiz. 2021, 8, 3043–3054. [Google Scholar] [CrossRef]
- Liao, R.; Gu, S.; Wang, X.; Zhang, X.; Xie, X.; Sun, H.; Huang, W. Approaching an adjustable organic thermochromic luminophore library via the synergistic effects between structure-related molecular dynamics and aggregation-related luminescence. J. Mater. Chem. C 2020, 8, 8430–8439. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Li, X.; Wan, W.; Li, X.S.; Fu, K.; Wu, Y.; Li, A.D.Q. Cooperatively assembled liquid crystals enable temperature-controlled Förster resonance energy transfer. Chem. Sci. 2021, 12, 3146–3151. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, G.; Guo, H.; Yang, F. Tetraphenylethylene-rufigallol-tetraphenylethylene trimers: Novel fluorescence liquid crystals in aggregated states. J. Mol. Struct. 2022, 1249, 131643. [Google Scholar] [CrossRef]
- O’Neill, M.; Kelly, S.M. Liquid Crystals for Charge Transport, Luminescence, and Photonics. Adv. Mater. 2003, 15, 1135–1146. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.K.; Abraham, S.; Akiyama, H.; Furumi, S.; Tamaoki, N.; Das, S. Photoresponsive Glass-Forming Butadiene-Based Chiral Liquid Crystals with Circularly Polarized Photoluminescence. Adv. Funct. Mater. 2008, 18, 2510–2517. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Ding, A.; Yuan, M.; Zhang, G.; Xu, W.; Zhang, G.; Wang, X.; Qiu, L.; Yang, J. A luminescent liquid crystal with multistimuli tunable emission colors based on different molecular packing structures. New J. Chem. 2014, 38, 3429–3433. [Google Scholar] [CrossRef]
- Liao, R.; Wang, X.; Peng, L.; Sun, H.; Huang, W. Achieving Organic Smart Fluorophores by Controlling the Balance between Intermolecular Interactions and External Stimuli. ACS Appl. Mater. Interfaces 2021, 13, 27491–27499. [Google Scholar] [CrossRef] [PubMed]
- Goossen, K.; Lava, K.; Bielewski, C.W.; Binnemans, K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Axenov, K.V.; Laschat, S. Thermotropic Ionic Liquid Crystals. Materials 2011, 4, 206–259. [Google Scholar] [CrossRef]
- Mansueto, M.; Laschat, S. Ionic Liquid Crystals. In Handbook of Liquid Crystals, 2nd ed.; Nanostructured and Amphiphilic Liquid Crystals; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 6, p. 231. [Google Scholar]
- Uchida, J.; Soberats, S.; Gupta, G.; Kato, T. Advanced Functional Liquid Crystals. Adv. Mater. 2022, 34, 2109063. [Google Scholar] [CrossRef] [PubMed]
- Veltri, L.; Maltese, V.; Auriemma, F.; Santillo, C.; Cospito, S.; La Deda, M.; Chidichimo, G.; Gabriele, B.; De Rosa, C.; Beneduci, A. Mesophase Tuning in Discotic Dimers π-Conjugated Ionic Liquid Crystals through Supramolecular Interactions and the Thermal History. Cryst. Growth Des. 2016, 16, 5646–5656. [Google Scholar] [CrossRef]
- Yazaki, S.; Funahashi, M.; Kato, T. An Electrochromic Nanostructured Liquid Crystal Consisting of π-Conjugated and Ionic Moieties. J. Am. Chem. Soc. 2008, 130, 13206–13207. [Google Scholar] [CrossRef]
- Yazaki, S.; Funahashi, M.; Kagimoto, J.; Ohno, H.; Kato, T. Nanostructured Liquid Crystals Combining Ionic and Electronic Functions. J. Am. Chem. Soc. 2010, 132, 7702–7708. [Google Scholar] [CrossRef]
- Beneduci, A.; Cospito, S.; Crispini, A.; Gabriele, B.; Nicoletta, F.P.; Veltri, L.; Chidichimo, G. Switching from columnar to calamitic mesophases in a new class of rod-like thienoviologens. J. Mater. Chem. C 2013, 1, 2233–2240. [Google Scholar] [CrossRef]
- Cospito, S.; Beneduci, A.; Veltri, L.; Salamonczyk, M.; Chidichimo, G. Mesomorphism and electrochemistry of thienoviologen liquid crystals. Phys. Chem. Chem. Phys. 2015, 17, 17670–17678. [Google Scholar] [CrossRef] [PubMed]
- Beneduci, A.; Cospito, S.; Imbardelli, D.; De Simone, B.C.; Chidichimo, G. n-Type Columnar Liquid Crystal Combining Ionic and Electronic Functions. Mol. Cryst. Liq. Cryst. 2015, 610, 108–115. [Google Scholar] [CrossRef]
- Pibiri, I.; Beneduci, A.; Carraro, M.; Causin, V.; Casella, G.; Corrente, G.A.; Chidichimo, G.; Pace, A.; Riccobono, A.; Saielli, G. Mesomorphic and electrooptical properties of viologens based on non-symmetric alkyl/polyfluoroalkyl functionalization and on an oxadiazolyl-extended bent core. J. Mater. Chem. C 2019, 7, 7974–7983. [Google Scholar] [CrossRef]
- Veltri, L.; Cavallo, G.; Beneduci, A.; Metrangolo, P.; Corrente, G.A.; Ursini, M.; Romeo, R.; Terraneo, G.; Gabriele, B. Synthesis and thermotropic properties of new green electrochromic ionic liquid crystals. New J. Chem. 2019, 43, 18285–18293. [Google Scholar] [CrossRef]
- Beneduci, A.; Corrente, G.A.; Chidichimo, G. Electrochromic and Electrofluorescence Liquid Crystals. In Electrochromic Smart Materials: Fabrication and Applications; Xu, J.W., Chua, M.H., Shah, K.W., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 261–292. [Google Scholar]
- Chidichimo, G.; De Simone, B.C.; Imbardelli, D.; De Benedittis, M.; Barberio, M.; Ricciardi, L.; Beneduci, A. Influence of oxygen impurities on the electrochromic response of viologen-based plastic films. J. Phys. Chem. C 2014, 118, 13484–13492. [Google Scholar] [CrossRef]
- Cospito, S.; De Simone, B.C.; Beneduci, A.; Imbardelli, D.; Chidichimo, G. Novel electrochromic gel with high optical contrast in the visible and near-infrared. Mater. Chem. Phys. 2013, 140, 431–434. [Google Scholar] [CrossRef]
- Beneduci, A.; Cospito, S.; La Deda, M.; Chidichimo, G. Highly Fluorescent Thienoviologen-Based Polymer Gels for Single Layer Electrofluorochromic Devices. Adv. Funct. Mater. 2015, 25, 1240–1247. [Google Scholar] [CrossRef]
- Beneduci, A.; Cospito, S.; La Deda, M.; Veltri, L.; Chidichimo, G. Electrofluorochromism in π-conjugated ionic liquid crystals. Nat. Commun. 2014, 5, 3105. [Google Scholar] [CrossRef]
- de Mello, J.C.; Wittmann, H.F.; Friend, R.H. An improved experimental determination of external photoluminescence quantum efficiency. Adv. Mater. 1997, 9, 230–232. [Google Scholar] [CrossRef]
- Palsson, L.O.; Monkman, A.P. Measurements of Solid-State Photoluminescence Quantum Yields of Films Using a Fluorimeter. Adv. Mater. 2002, 14, 757–758. [Google Scholar] [CrossRef]
- Porres, L.; Holland, A.; Palsson, L.O.; Monkman, A.P.; Kemp, C.; Beeby, A. Absolute Measurements of Photoluminescence Quantum Yields of Solutions Using an Integrating Sphere. J. Fluoresc. 2006, 16, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Akkermans, R.L.C.; Spenley, N.A.; Robertson, S.H. COMPASS III: Automated fitting workflows and extension to ionic liquids. Mol. Simul. 2021, 47, 540–551. [Google Scholar] [CrossRef]
- Cornil, J.; Beljonne, D.; Calbert, J.P.; Brédas, J.L. Interchain Interactions in Organic π-Conjugated Materials: Impact on Electronic Structure, Optical Response, and Charge Transport. Adv. Mater. 2001, 13, 1053–1067. [Google Scholar] [CrossRef]
- Corrente, G.A.; Beneduci, A. Overview on the Recent Progress on Electrofluorochromic Materials and Devices: A Critical Synopsis. Adv. Opt. Mater. 2020, 8, 2000887. [Google Scholar] [CrossRef]
- Reeves, B.D.; Unur, E.; Ananthakrishnan, N.; Reynolds, J.R. Defunctionalization of ester-substituted electrochromic dioxythiophene polymers. Macromolecules 2007, 40, 5344–5352. [Google Scholar] [CrossRef]
- Chen, G.; Li, W.; Zhou, T.; Peng, Q.; Zhai, D.; Li, H.; Yuan, W.Z.; Zhang, Y.; Tang, B.Z. Conjugation-induced rigidity in twisting molecules: Filling the gap between aggregation-caused quenching and aggregation-induced emission. Adv. Mater. 2015, 27, 4496–4501. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Wu, J.; Shi, Y.; Zheng, J.; Xu, C. Electrochromic polymer achieving synchronous electrofluorochromic switching for optoelectronic application. Org. Electron. 2017, 51, 295–303. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Lu, P.; Chen, S.; Lam, J.W.Y.; Wang, Z.; Liu, Y.; Kwok, H.S.; Ma, Y.; Tang, B.Z. Changing the Behavior of Chromophores from Aggregation-Caused Quenching to Aggregation-Induced Emission: Development of Highly Efficient Light Emitters in the Solid State. Adv. Mater. 2010, 22, 2159–2163. [Google Scholar] [CrossRef]
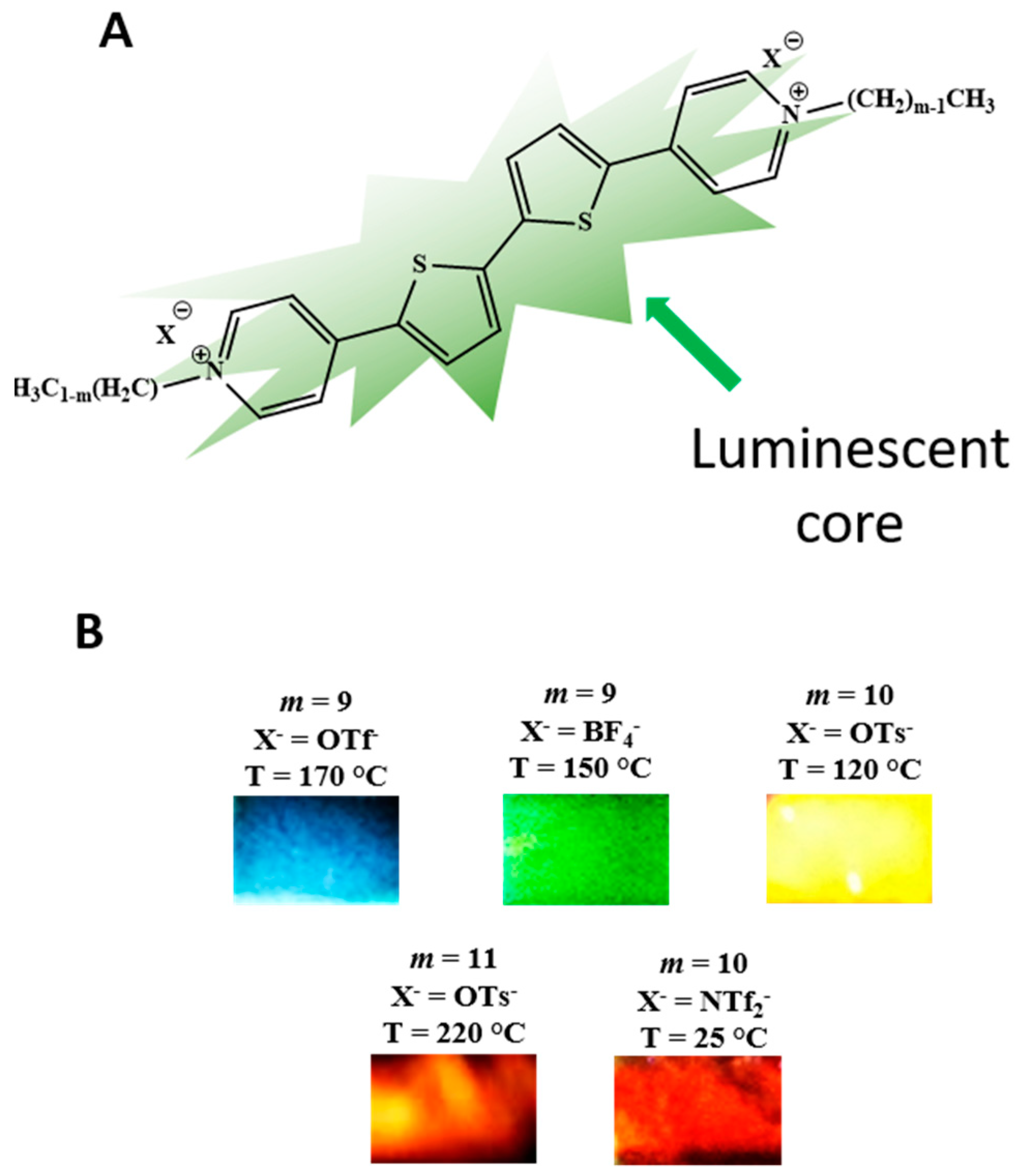
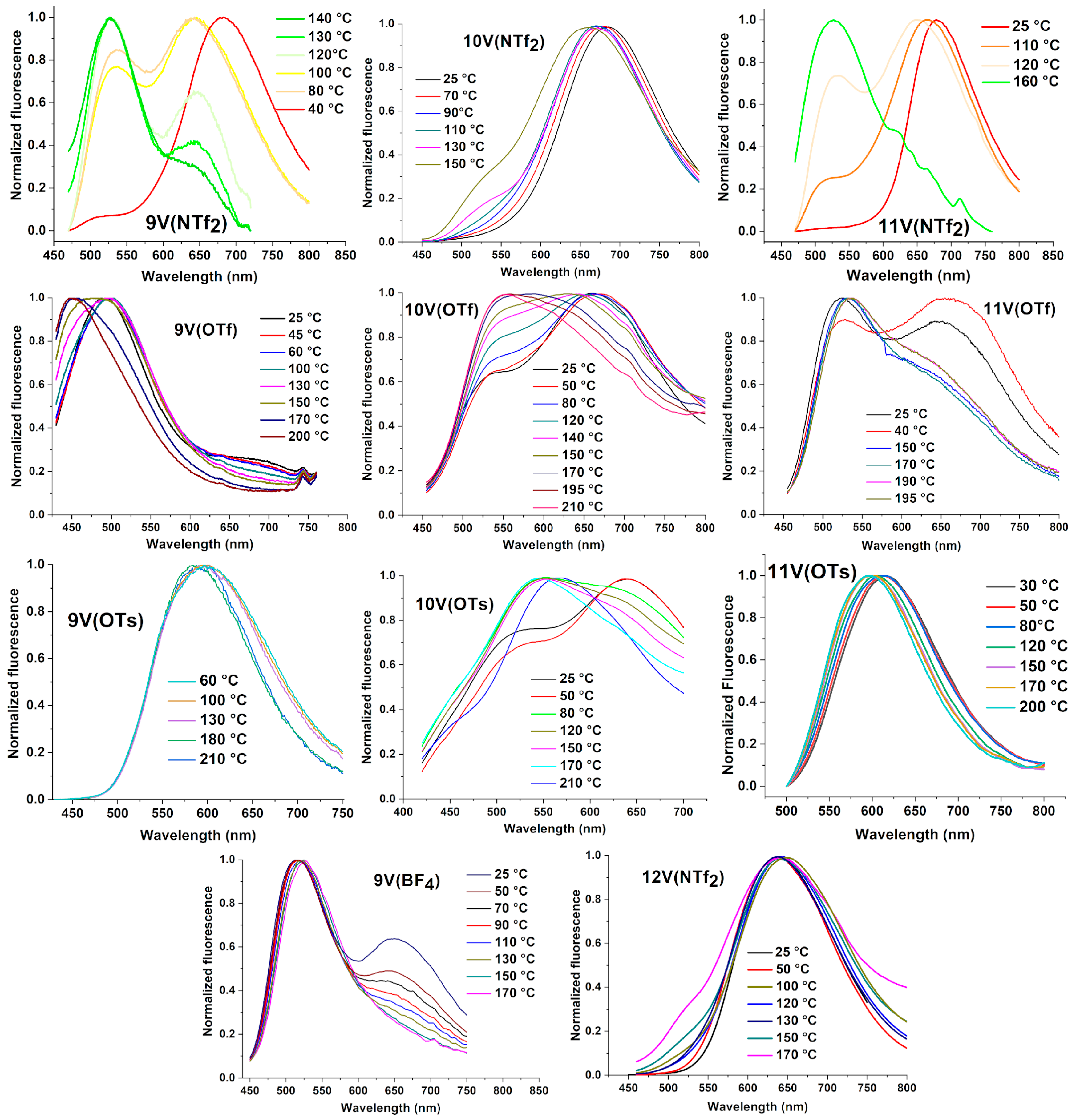
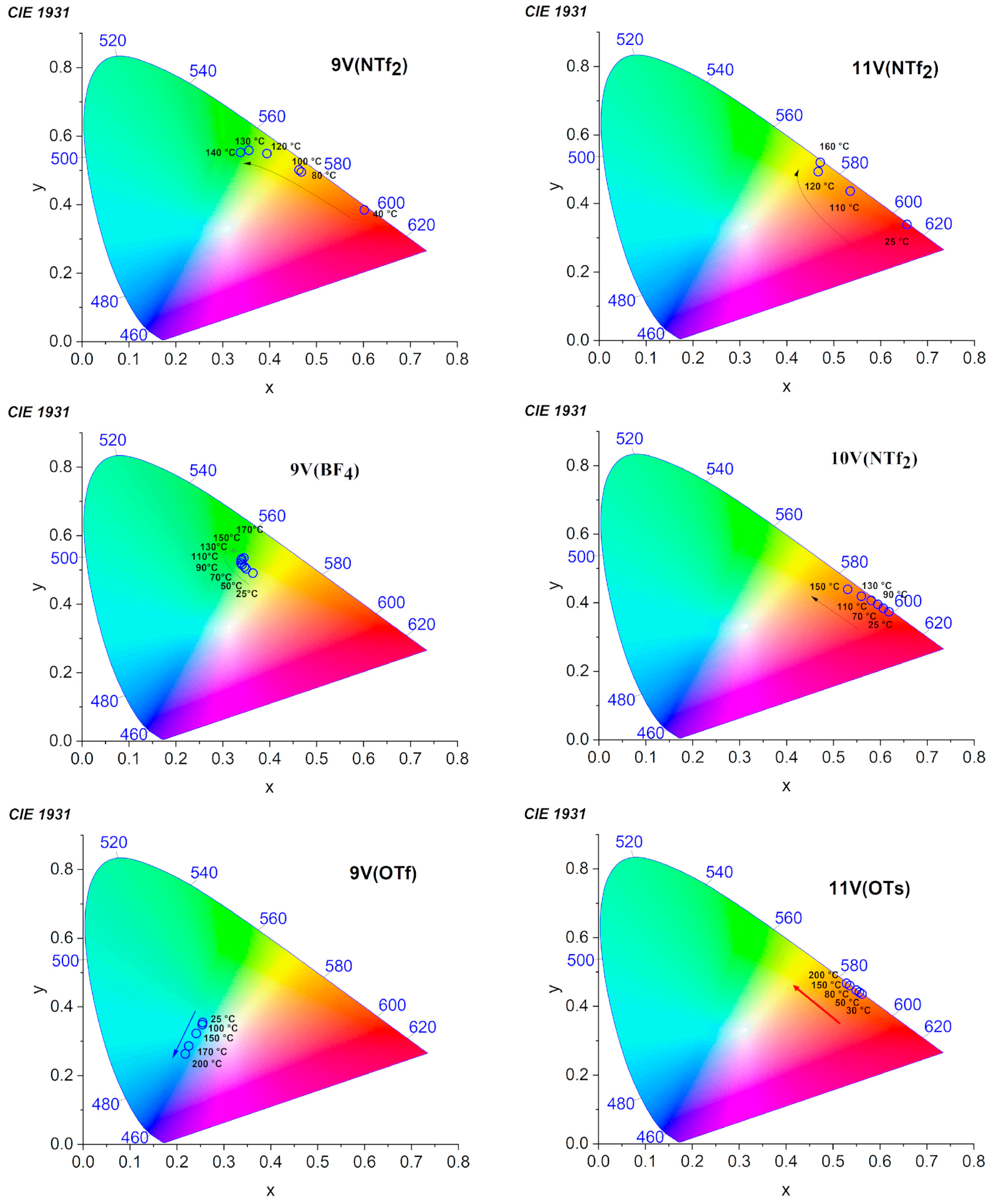

| Compounds mVX | Transition | Temperature (°C) | [Enthalpy] (J/g) |
|---|---|---|---|
| 9V(OTf) 2 | IL→SmC SmC→ColL ColL→K(Colro) | ≈180–170 157.5 131.9 | [n.d.] 3 [13.3] [15.3] |
| 10V(OTf) 2 | IL→Colro1 Colro1→K(Colro2) | 170.1 147.0, 139.1 (double peak) | [13.1] [12.9] |
| 11V(OTf) 2 | IL→NC NC→K(ColL) | 182.9 147.5 | [9.3] [8.0] |
| 9V(OTs) 2 | SmC→ColL/Colrd ColL/Colrd→K(Colro2) | 222.1, 209.4 194.1 | [15.9] [13.0] |
| 10V(OTs) 2 | ND/NC→Colrd2 Colrd2→Colrd1 Colrd1→K(Colro) | 229.7 158.7 113.5 | [8.1] [3.8] [5.8] |
| 11V(OTs) 2 | IL→ND/NC ND/NC→K(ND/NC) | 233.0 194.3 | [5.9] [15.2] |
| 9V(BF4) 4 | IL→SmA SmA→Glassy SmA | 203.0 144.9 | [12.0] [9.9] |
| 9V(NTf2) 5 | IL→Colro1 Colro1→Colro2 | 128.8 109.2 | [13.2] [5.2] |
| 10V(NTf2) 5 | IL→SmA SmA→ColL | 134.2 115.1 | [5.8] [0.9] |
| 11V(NTf2) 5 | IL→SmA SmA→Glassy SmA | 146.0 114.3 | [5.8] [8.8] |
| 12V(NTf2) 6 | IL→SmA SmA→Glassy SmA Glassy SmA→K | 190 123.5 108 | [n.d.] [6.7] [6.5] |
| Compound | λem [nm] | Φ [%] | τ1 [ns] | τ 2 [ns] | τ 3 [ns] |
|---|---|---|---|---|---|
| 9V(OTs) | 610 | 0.50 | 0.70 (10%) | 3.40 (76%) | 6.90 (14%) |
| 10V(OTs) | 535 | 0.58 | 0.50 (40%) | 2.10 (44%) | 5.90 (16%) |
| 635 | 0.30 (23%) | 1.60 (60%) | 4.34 (17%) | ||
| 11V(OTs) | 630 | 0.54 | 1.00 (20%) | 3.90 (80%) | |
| 9V(OTf) | 480 | 0.64 | 0.26 (87%) | 0.90 (10%) | 2.50 (3%) |
| 620 | 0.45 (45%) | 1.49 (50%) | 5.33 (5%) | ||
| 10V(OTf) | 530 | 0.77 | 0.60 (27%) | 1.90 (61%) | 6.44 (12%) |
| 660 | 0.40 (16%) | 1.70 (71%) | 5.10 (13%) | ||
| 11V(OTf) | 525 | 0.84 | 0.46 (20%) | 2.20 (56%) | 5.70 (24%) |
| 640 | 0.45 (53%) | 1.70 (42%) | 7.00 (5%) | ||
| 9V(BF4) | 520 | 0.46 | 0.60 (56%) | 2.8 (44%) | |
| 648 | 1.00 (43%) | 3.1 (57%) | |||
| 9V(NTf2) 1 | 630 | 51.0 | 0.54 (30%) | 1.80 (70%) | |
| 10V(NTf2) | 650 | 15.0 | 1.00 (47%) | 3.00 (53%) | |
| 11V(NTf2) 1 | 670 | 48.0 | 0.81 (37%) | 2.67 (63%) | |
| 12V(NTf2) | 647 | 28.0 | 1.00 (33%) | 3.10 (67%) |
| X- | m | Phase | CIE Coordinates [T °C] | Coloration |
|---|---|---|---|---|
| OTf- | 9 | IL | (0.218; 0.263) [200] |  |
| SmC | (0.241; 0.323) [160] |  | ||
| ColL | (0.248; 0.339) [130] |  | ||
| K(Colro) | (0.254; 0.348) [25] |  | ||
| 10 | IL | (0.44; 0.489) [195] |  | |
| Colro1 | (0.453; 0.481) [150] |  | ||
| K(Colro2) | (0.46; 0.462) [25] |  | ||
| 11 | IL | (0.412; 0.506) [190] |  | |
| NC | (0.399; 0.509) [150] |  | ||
| K(ColL) | (0.432; 0.481) [40] |  | ||
| OTs- | 9 | SmC | (0.509; 0.479) [210] |  |
| ColL/Colrd | (0.513; 0.475) [200] |  | ||
| K(Colro2) | (0.512; 0.475) [100] |  | ||
| 10 | Colrd2 | (0.399; 0.434) [210] |  | |
| Colrd1 | (0.385; 0.420) [120] |  | ||
| K(Colro) | (0.413; 0.41) [50] |  | ||
| 11 | ND/NC | (0.529; 0.467) [200] |  | |
| K(ND/NC) | (0.561; 0.436) [30] |  | ||
| BF4- | 9 | SmA | (0.345; 0.535) [170] |  |
| Glassy SmA | (0.364; 0.491) [25] |  | ||
| NTf2- | 9 | IL | (0.338; 0.553) [140] |  |
| Colro1 | (0.394; 0.549) [120] |  | ||
| Colro2 | (0.602; 0.385) [40] |  | ||
| 10 | IL | (0.53; 0.439) [150] |  | |
| SmA | (0.559; 0.419) [130] |  | ||
| ColL | (0.618; 0.343) [25] |  | ||
| 11 | IL | (0.471; 0.52) [160] |  | |
| SmA | (0.535; 0.437) [110] |  | ||
| Glassy SmA | (0.656; 0.339) [25] |  | ||
| 12 | SmA | (0.523; 0.442) [170] |  | |
| Glassy SmA | (0.585; 0.407) [120] |  | ||
| K | (0.608; 0.389) [25] |  |
| Space Group Symmetry Changes | |||||||
|---|---|---|---|---|---|---|---|
| Phases | Compound | Space Group Symmetry 1 | Number of Salt Units/Cross Section (Z) | Intracolumnar Correlation Distance 2 D (Å) | ar (Å) | br (Å) | Disk Basal Area (Å) 2 |
| Columnar | 9V(OTf) | p2mg/p2mg | 4/4 | 4.45/4.45 | 42.40/33.30 | 30.74/33.34 | 326/278 |
| 10V(OTf) | p2mm/p2mg | 2/4 | 4.47/4.47 | 24.07/40.00 | 26.37/33.34 | 317/333 | |
| 11V(OTf) | p2mm/p2mg | 2/4 | 4.47/4.47 | 25.14/41.38 | 27.18/36.06 | 342/373 | |
| 9V(OTs) | p2mm/p2mg | 2/4 | 4.39/4.36 | 25.79/39.36 | 25.61/32.13 | 330/316 | |
| 10V(OTs) | p2mg/p2mg | 4/4 | 4.39/4.18 | 39.36/35.20 | 32.13/33.34 | 316/293 | |
| 11V(OTs) | p2mm/p2mg/P1 | 2/4 | 4.41/4.36 | 17.85/35.06 | 36.06/32.65 | 322/286 | |
| 9V(NTf2) | p21/a | 4/4 | 4.50 | 55.8/54.2 | 22.6/22.0 | 315/298 | |
| 10V(NTf2) | SmA/ColL | 4 (ColL) | 4.50 (ColL) | 4.70 (ColL) | 20.3 (ColL) | ||
| Smectic | Interplanar distance (Å) | ||||||
| 11V(NTf2) | |||||||
| 12V(NTf2) | 35/34 | ||||||
| 9V(BF4) | 20.3 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrente, G.A.; Di Maio, G.; La Deda, M.; Ruiz de Ballesteros, O.; Gabriele, B.; Veltri, L.; Auriemma, F.; Beneduci, A. The Rainbow Arching over the Fluorescent Thienoviologen Mesophases. Nanomaterials 2022, 12, 4284. https://doi.org/10.3390/nano12234284
Corrente GA, Di Maio G, La Deda M, Ruiz de Ballesteros O, Gabriele B, Veltri L, Auriemma F, Beneduci A. The Rainbow Arching over the Fluorescent Thienoviologen Mesophases. Nanomaterials. 2022; 12(23):4284. https://doi.org/10.3390/nano12234284
Chicago/Turabian StyleCorrente, Giuseppina Anna, Giuseppe Di Maio, Massimo La Deda, Odda Ruiz de Ballesteros, Bartolo Gabriele, Lucia Veltri, Finizia Auriemma, and Amerigo Beneduci. 2022. "The Rainbow Arching over the Fluorescent Thienoviologen Mesophases" Nanomaterials 12, no. 23: 4284. https://doi.org/10.3390/nano12234284
APA StyleCorrente, G. A., Di Maio, G., La Deda, M., Ruiz de Ballesteros, O., Gabriele, B., Veltri, L., Auriemma, F., & Beneduci, A. (2022). The Rainbow Arching over the Fluorescent Thienoviologen Mesophases. Nanomaterials, 12(23), 4284. https://doi.org/10.3390/nano12234284
















