Solid Lipid Nanoparticles Based on Monosubstituted Pillar[5]arenes: Chemoselective Synthesis of Macrocycles and Their Supramolecular Self-Assembly
Abstract
1. Introduction
2. Results and Discussion
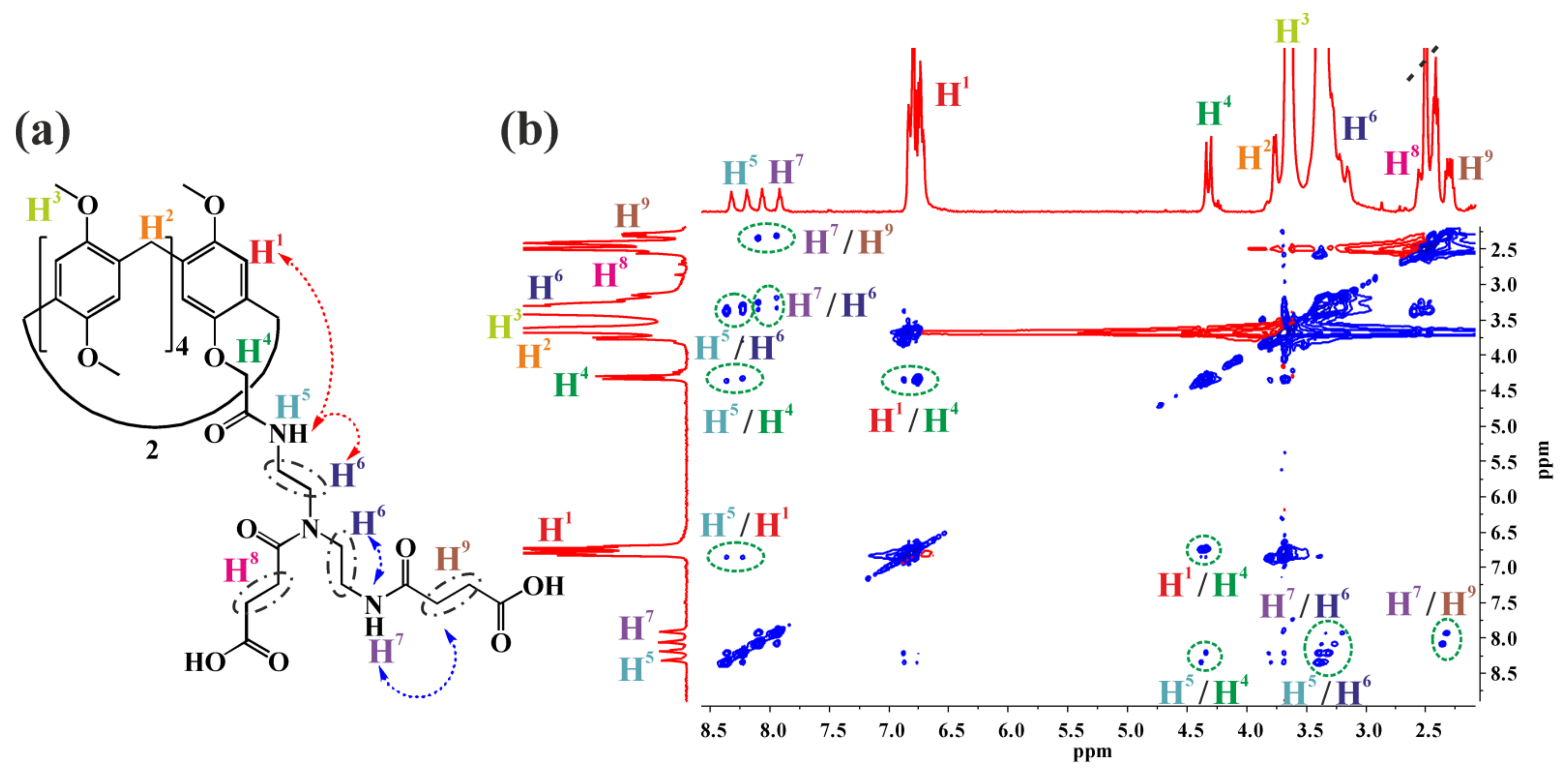
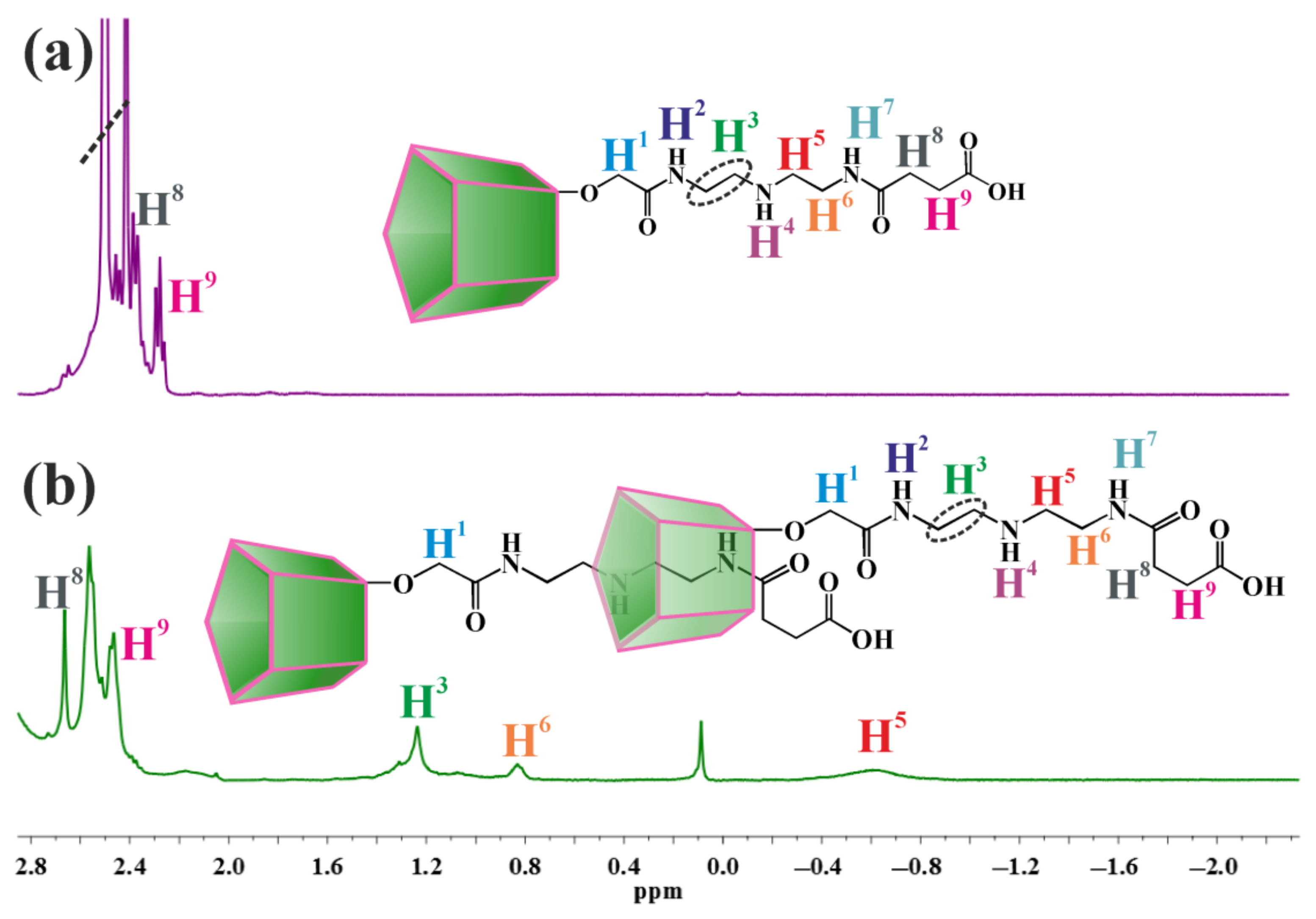
2.1. Synthesis of Monosubstituted Pillar[5]arenes Containing Amide and Carboxyl Groups
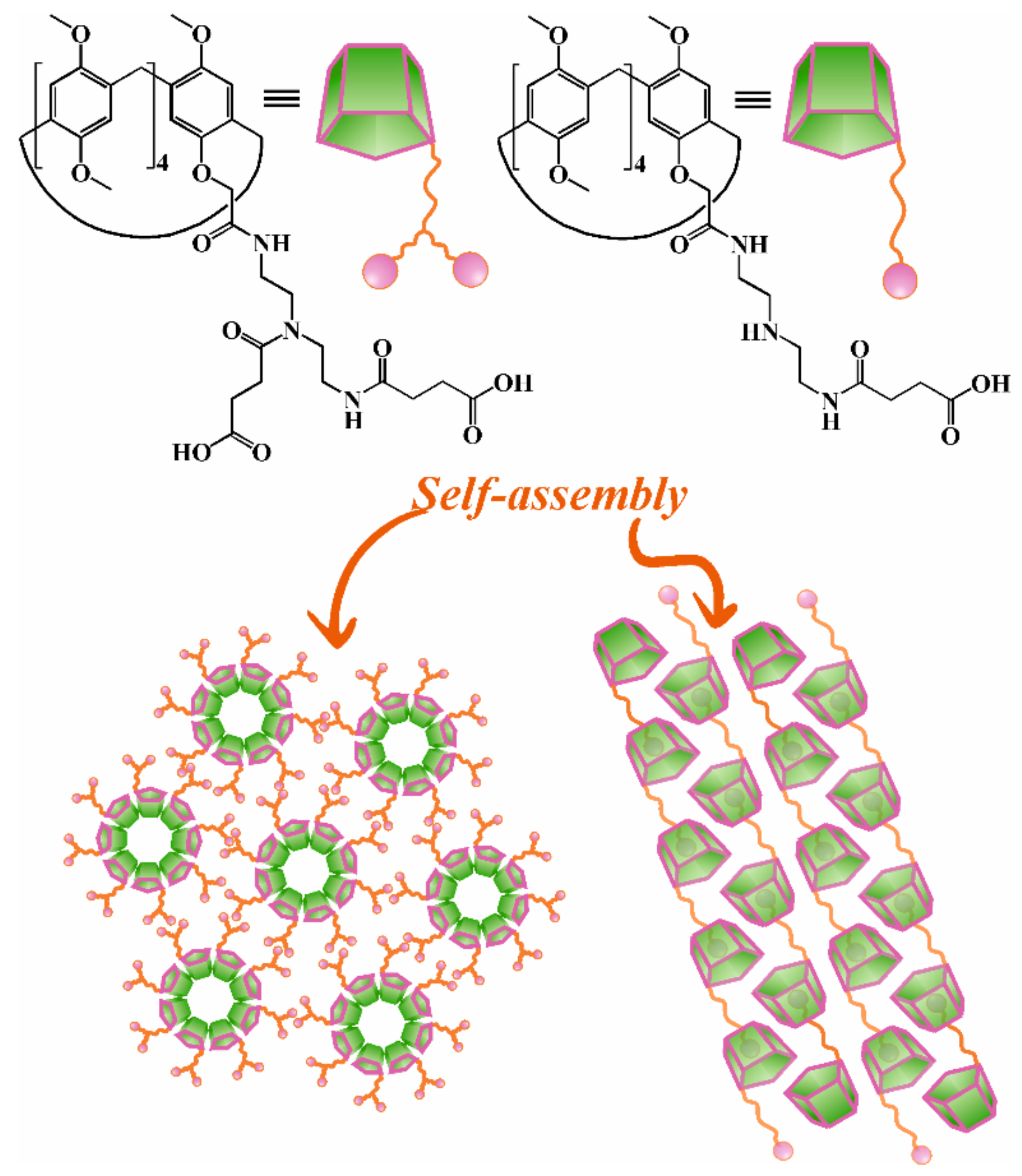
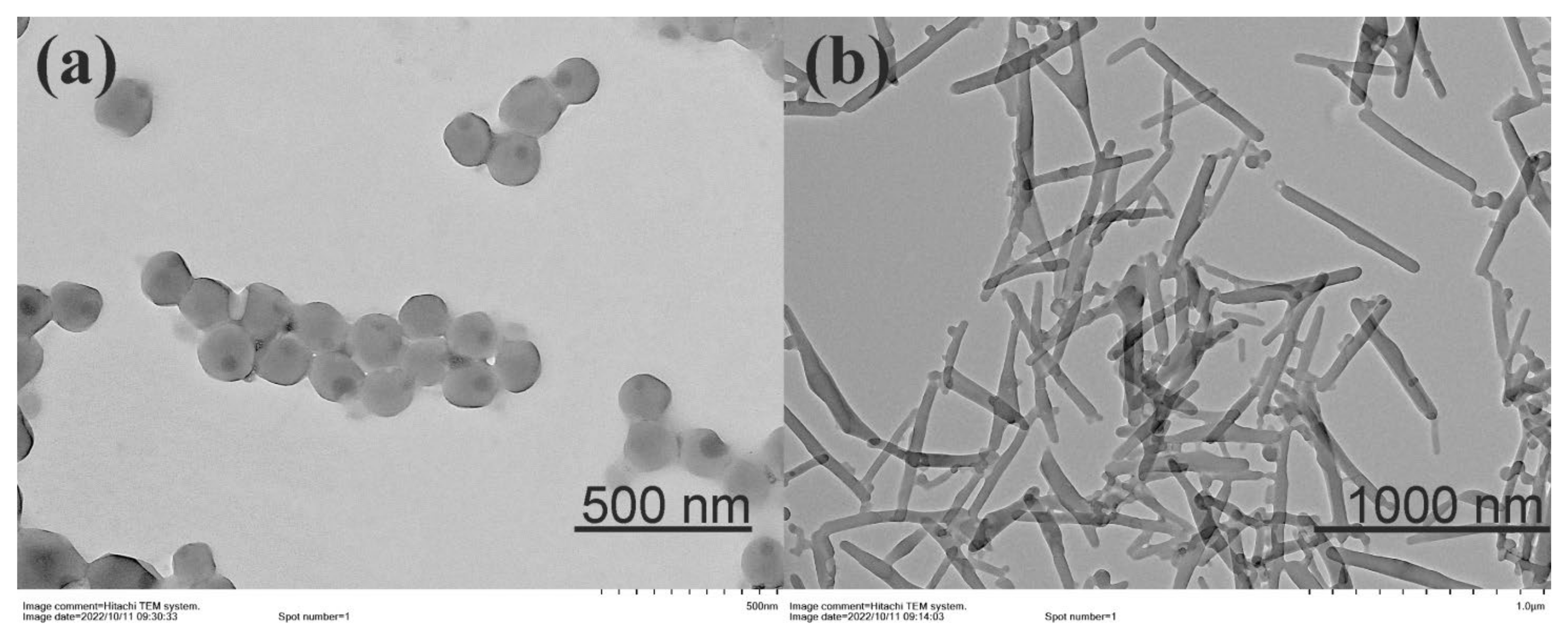
2.2. Aggregation Properties of Monosubstituted Pillar[5]arenes Containing Carboxyl Groups
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortesi, R.; Esposito, E.; Luca, G.; Nastruzzi, C. Production of lipospheres as carriers for bioactive compounds. Biomaterials 2002, 23, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Rai, S.; Vaidya, B.; Khatri, K.; Goyal, A.K.; Mishra, N.; Mehta, A.; Vyas, S.P. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Mirchandani, Y.; Patravale, V.B.; Brijesh, S. Solid lipid nanoparticles for hydrophilic drugs. J. Control. Release 2021, 335, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Andonova, V.; Peneva, P. Characterization methods for solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC). Curr. Pharm. Des. 2017, 23, 6630–6642. [Google Scholar] [CrossRef] [PubMed]
- Elbrink, K.; Van Hees, S.; Chamanza, R.; Roelant, D.; Loomans, T.; Holm, R.; Kiekens, F. Application of solid lipid nanoparticles as a long-term drug delivery platform for intramuscular and subcutaneous administration: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2021, 163, 158–170. [Google Scholar] [CrossRef]
- Mohamed, H.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.; Sharma, K.; Dhasmana, D.; Garg, N.; Siril, P.F. Preparation, characterization and in vitro cytotoxicity of Fenofibrate and Nabumetone loaded solid lipid nanoparticles. Mater. Sci. Eng. C 2020, 106, 110184. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Burilov, V.A.; Artemenko, A.A.; Garipova, R.I.; Amirova, R.R.; Fatykhova, A.M.; Borisova, J.A.; Mironova, D.A.; Sultanova, E.D.; Evtugyn, V.G.; Solovieva, S.E.; et al. New calix[4]arene-fluoresceine conjugate by click approach-synthesis and preparation of photocatalytically active solid lipid nanoparticles. Molecules 2022, 27, 2436. [Google Scholar] [CrossRef]
- Pojarova, M.; Ananchenko, G.S.; Udachin, K.A.; Daroszewska, M.; Perret, F.; Coleman, A.W.; Ripmeester, J.A. Solid lipid nanoparticles of p-hexanoyl calix[4]arene as a controlling agent in the photochemistry of a sunscreen blocker. Chem. Mater. 2006, 18, 5817–5819. [Google Scholar] [CrossRef]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Mannelli, L.D.C. Development and in vivo evaluation of an innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef]
- Helttunen, K.; Galán, A.; Ballester, P.; Bergenholtz, J.; Nissinen, M. Solid lipid nanoparticles from amphiphilic calixpyrroles. J. Colloid Interface Sci. 2016, 464, 59–65. [Google Scholar] [CrossRef]
- Montasser, I.; Shahgaldian, P.; Perret, F.; Coleman, A.W. Solid lipid nanoparticle-based calix[n]arenes and calix-resorcinarenes as building blocks: Synthesis, formulation and characterization. Int. J. Mol. Sci. 2013, 14, 21899–21942. [Google Scholar] [CrossRef]
- Pandey, A. Cyclodextrin-based nanoparticles for pharmaceutical applications: A review. Environ. Chem. Lett. 2021, 19, 4297–4310. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Shurpik, D.N.; Guralnik, E.G.; Evtugyn, V.G.; Osin, Y.N.; Stoikov, I.I. Fluorescein-loaded solid lipid nanoparticles based on monoamine pillar[5]arene: Synthesis and interaction with DNA. ChemNanoMat 2018, 4, 919–923. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Guralnik, E.G.; Shurpik, D.N.; Evtugyn, V.G.; Osin, Y.N.; Subakaeva, E.V.; Sokolova, E.A.; Zelenikhin, P.V.; Stoikov, I.I. Morphology, structure and cytotoxicity of dye-loaded lipid nanoparticles based on monoamine pillar[5]arenes. Mater. Chem. Front. 2020, 4, 2962–2970. [Google Scholar] [CrossRef]
- Nazarova, A.; Yakimova, L.; Filimonova, D.; Stoikov, I. Surfactant effect on the physicochemical characteristics of solid lipid nanoparticles based on pillar[5]arenes. Int. J. Mol. Sci. 2022, 23, 779. [Google Scholar] [CrossRef]
- Demay-Drouhard, P.; Du, K.; Samanta, K.; Wan, X.; Yang, W.; Srinivasan, R.; Sue, A.C.-H.; Zuihof, H. Functionalization at will of rim-differentiated pillar[5]arenes. Org. Lett. 2019, 21, 3976–3980. [Google Scholar] [CrossRef] [PubMed]
- Nazarova, A.A.; Makhmutova, L.I.; Stoikov, I.I. Synthesis of pillar[5]arenes with a PH-containing fragment. Russ. J. Gen. Chem. 2017, 87, 1941–1945. [Google Scholar] [CrossRef]
- Strutt, N.L.; Zhang, H.; Schneebeli, S.T.; Stoddart, J.F. Functionalizing pillar[n]arenes. Acc. Chem. Res. 2014, 47, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Nazarova, A.A.; Shibaeva, K.S.; Stoikov, I.I. Synthesis of the monosubstituted pillar[5]arenes with 1-aminophosphonate fragment. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1583–1584. [Google Scholar] [CrossRef]
- Wei, T.-B.; Chen, J.-F.; Cheng, X.-B.; Li, H.; Han, B.-B.; Zhang, Y.-M.; Yao, H.; Lin, Q. A novel functionalized pillar[5]arene-based selective amino acid sensor for l-tryptophan. Org. Chem. Front. 2017, 4, 210–213. [Google Scholar] [CrossRef]
- Lia, C. Pillararene-based supramolecular polymers: From molecular recognition to polymeric aggregates. Chem. Commun. 2014, 50, 12420–12433. [Google Scholar] [CrossRef]
- Chen, J.-F.; Lin, Q.; Zhang, Y.-M.; Yao, H.; Wei, T.-B. Pillararene-based fluorescent chemosensors: Recent advances and perspectives. Chem. Commun. 2017, 53, 13296–13311. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Gao, L.; Mosel, S.; Ehlers, M.; Zellermann, E.; Jiang, H.; Knauer, S.K.; Wang, L.; Scmuck, C. From supramolecular vesicles to micelles: Controllable construction of tumor-targeting nanocarriers based on host–guest interaction between a pillar[5]arene-based prodrug and a RGD-sulfonate guest. Small 2018, 14, 1803952. [Google Scholar] [CrossRef]
- Xia, D.; Wang, L.; Lv, X.; Chao, J.; Wei, X.; Wang, P. Dual-responsive [2]pseudorotaxane on the basis of a pH-sensitive pillar[5]arene and its application in the fabrication of metallosupramolecular polypseudorotaxane. Macromolecules 2018, 51, 2716–2722. [Google Scholar] [CrossRef]
- Pearce, N.; Reynolds, K.E.A.; Kayal, S.; Sun, X.Z.; Davies, E.S.; Malagreca, F.; Schürmann, C.J.; Ito, S.; Yamano, A.; Argent, S.P.; et al. Selective photoinduced charge separation in perylenediimide-pillar[5]arene rotaxanes. Nat. Commun. 2022, 13, 415. [Google Scholar] [CrossRef]
- Nazarova, A.A.; Yakimova, L.S.; Padnya, P.L.; Evtugyn, V.G.; Osin, Y.N.; Cragg, P.J.; Stoikov, I.I. Monosubstituted pillar[5]arene functionalized with (amino)phosphonate fragments are “smart” building blocks for constructing nanosized structures with some s-and p-metal cations in the organic phase. New J. Chem. 2019, 43, 14450–14458. [Google Scholar] [CrossRef]
- Nazarova, A.; Khannanov, A.; Boldyrev, A.; Yakimova, L.; Stoikov, I. Self-assembling systems based on pillar[5]arenes and surfactants for encapsulation of diagnostic dye dapi. Int. J. Mol. Sci. 2021, 22, 6038. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Zhao, Y. Pillararene-based self-assembled amphiphiles. Chem. Soc. Rev. 2018, 47, 5491–5528. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Wang, R.; Wang, L.; He, X.; Jiang, H.; Tang, H.; Cao, D.; Tang, Z. A conjugated polymeric supramolecular network with aggregation-induced emission enhancement: An efficient light-harvesting system with an ultrahigh antenna effect. Angew. Chem. 2020, 59, 9908–9913. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Liang, T.; Wen, H.; Tian, W. Pillararene-based supramolecular polymers. Chem. Commun. 2019, 55, 271–285. [Google Scholar] [CrossRef]
- Wang, P.; Liang, B.; Xia, D. A linear AIE supramolecular polymer based on a salicylaldehyde azine-containing pillararene and its reversible cross-linking by CuII and cyanide. Inorg. Chem. 2019, 58, 2252–2256. [Google Scholar] [CrossRef]
- Ho, F.-C.; Huang, Y.-J.; Weng, C.-C.; Wu, C.-H.; Li, Y.-K.; Wu, J.I.; Lin, H.-C. Efficient FRET approaches toward copper(II) and cyanide detections via host-guest interactions of photo-switchable [2]pseudo-rotaxane polymers containing naphthalimide and merocyanine moieties. ACS Appl. Mater. Interfaces 2020, 12, 53257–53273. [Google Scholar] [CrossRef]
- Lu, F.; Chen, Y.; Fu, B.; Chen, S.; Wang, L. Multistimuli responsive supramolecular polymer networks via host-guest complexation of pillararene-containing polymers and sulfonium salts. Chin. Chem. Lett. 2022, 33, 5111–5115. [Google Scholar] [CrossRef]
- Strutt, N.L.; Zhang, H.; Giesener, M.A.; Lei, J.; Stoddart, J.F. A self-complexing and self-assembling pillar[5]arene. Chem. Commun. 2012, 48, 1647–1649. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Khalil-Cruz, L.E.; Khashab, N.M.; Yu, G.; Huang, F. Pillararene-based supramolecular systems for theranostics and bioapplications. Sci. China Chem. 2021, 64, 688–700. [Google Scholar] [CrossRef]
- Liu, P.; Li, Z.; Shi, B.; Liu, J.; Zhu, H.; Huang, F. Formation of linear side-chain polypseudorotaxane with supramolecular polymer backbone through neutral halogen bonds and pillar[5]arene-based host–guest interactions. Chem. Eur. J. 2018, 24, 4264–4267. [Google Scholar] [CrossRef] [PubMed]
- Dal Pizzol, C.; Filippin-Monteiro, F.B.; Restrepo, J.A.S.; Pittella, F.; Silva, A.H.; de Souza, P.A.; de Campos, A.M.; Creczynski-Pasa, T.B. Influence of surfactant and lipid type on the physicochemical properties and biocompatibility of solid lipid nanoparticles. Int. J. Environ. Res. Public Health 2014, 11, 8581–8596. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Ledinski, M.; Marić, I.; Štefanić, P.P.; Ladan, I.; Mihalić, K.C.; Jurkin, T.; Gotić, M.; Urlić, I. Synthesis and in vitro characterization of ascorbyl palmitate-loaded solid lipid nanoparticles. Polymers 2022, 14, 1751. [Google Scholar] [CrossRef]
- Cao, S.; Liu, X.; Li, X.; Lin, C.; Zhang, W.; Tan, C.H.; Liang, S.; Luo, B.; Xu, X.; Saw, P.E. Shape matters: Comprehensive analysis of star-shaped lipid nanoparticles. Front. Pharmacol. 2020, 11, 539. [Google Scholar] [CrossRef]
- Galukhin, A.; Erokhin, A.; Imatdinov, I.; Osin, Y. Investigation of DNA binding abilities of solid lipid nanoparticles based on p-tert-butylthiacalix[4]arene platform. RSC Adv. 2015, 5, 33351–33355. [Google Scholar] [CrossRef]
- Trotta, M.; Debernardi, F.; Caputo, O. Preparation of solid lipid nanoparticles by a solvent emulsification–diffusion technique. Int. J. Pharm. 2003, 257, 153–160. [Google Scholar] [CrossRef]

| Macrocycles | Solvent | C, M | d, nm (S, %) | PDI |
|---|---|---|---|---|
| 2 | CHCl3 | 1 × 10−5 | 363 ± 50 (60%) 3636 ± 589 (40%) | 0.63 ± 0.06 |
| 1 × 10−4 | 308 ± 34 (73%) 3519 ± 149 (27%) | 0.46 ± 0.09 | ||
| 1 × 10−3 | 192 ± 20 (100%) | 0.26 ± 0.01 | ||
| DMSO | 1 × 10−5 | 851 ± 99 (100%) | 0.36 ± 0.04 | |
| 1 × 10−4 | 439 ± 59 (100%) | 0.26 ± 0.03 | ||
| 1 × 10−3 | 289 ± 9 (93%) 5050 ± 254 (7%) | 0.44 ± 0.03 | ||
| 3 | CHCl3 | 1 × 10−5 | 4226 ± 253 (66%) 346 ± 47 (34%) | 0.78 ± 0.11 |
| 1 × 10−4 | 152 ± 46 (100%) | 0.92 ± 0.09 | ||
| 1 × 10−3 | 155 ± 32 (100%) | 0.85 ± 0.11 | ||
| DMSO | 1 × 10−5 | 367 ± 38 (77%) 4722 ± 115 (23%) | 0.63 ± 0.12 | |
| 1 × 10−4 | 344 ± 26 (84%) 4924 ± 84 (16%) | 0.52 ± 0.05 | ||
| 1 × 10−3 | 332 ± 7 (94%) 5150 ± 128 (6%) | 0.39 ± 0.03 |
| C, M | d, nm | PDI | ζ, mV | |
|---|---|---|---|---|
| SLN-2 | 3 × 10−6 | 364 ± 39 | 0.24 ± 0.02 | −25 ± 2 |
| 3 × 10−5 | 382 ± 8 | 0.21 ± 0.01 | −33 ± 1 | |
| 3 × 10−4 | 454 ± 19 | 0.18 ± 0.03 | −33 ± 1 | |
| SLN-3 | 3 × 10−6 | 582 ± 23 | 0.27 ± 0.01 | −3 ± 0 |
| 3 × 10−5 | 444 ± 10 | 0.21 ± 0.01 | −21 ± 1 | |
| 3 × 10−4 | 209 ± 2 | 0.14 ± 0.01 | −20 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filimonova, D.; Nazarova, A.; Yakimova, L.; Stoikov, I. Solid Lipid Nanoparticles Based on Monosubstituted Pillar[5]arenes: Chemoselective Synthesis of Macrocycles and Their Supramolecular Self-Assembly. Nanomaterials 2022, 12, 4266. https://doi.org/10.3390/nano12234266
Filimonova D, Nazarova A, Yakimova L, Stoikov I. Solid Lipid Nanoparticles Based on Monosubstituted Pillar[5]arenes: Chemoselective Synthesis of Macrocycles and Their Supramolecular Self-Assembly. Nanomaterials. 2022; 12(23):4266. https://doi.org/10.3390/nano12234266
Chicago/Turabian StyleFilimonova, Darya, Anastasia Nazarova, Luidmila Yakimova, and Ivan Stoikov. 2022. "Solid Lipid Nanoparticles Based on Monosubstituted Pillar[5]arenes: Chemoselective Synthesis of Macrocycles and Their Supramolecular Self-Assembly" Nanomaterials 12, no. 23: 4266. https://doi.org/10.3390/nano12234266
APA StyleFilimonova, D., Nazarova, A., Yakimova, L., & Stoikov, I. (2022). Solid Lipid Nanoparticles Based on Monosubstituted Pillar[5]arenes: Chemoselective Synthesis of Macrocycles and Their Supramolecular Self-Assembly. Nanomaterials, 12(23), 4266. https://doi.org/10.3390/nano12234266