Design of Nanostructured Hybrid Electrodes Based on a Liquid Crystalline Zn(II) Coordination Complex-Carbon Nanotubes Composition for the Specific Electrochemical Sensing of Uric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hybrids
2.3. Electrochemical Tests
2.4. Characterisation Techniques
3. Results
3.1. Mesomorphic Properties
3.1.1. DSC Studies
3.1.2. S/WAXS Studies
3.2. Electrochemical Studies
3.2.1. Electrochemical Characterization of Paste Electrodes (PE_0i)
3.2.2. Electrochemical Behavior of Uric Acid (UA) on Paste Electrodes (PE_0i)-Cyclic Voltammetry Study
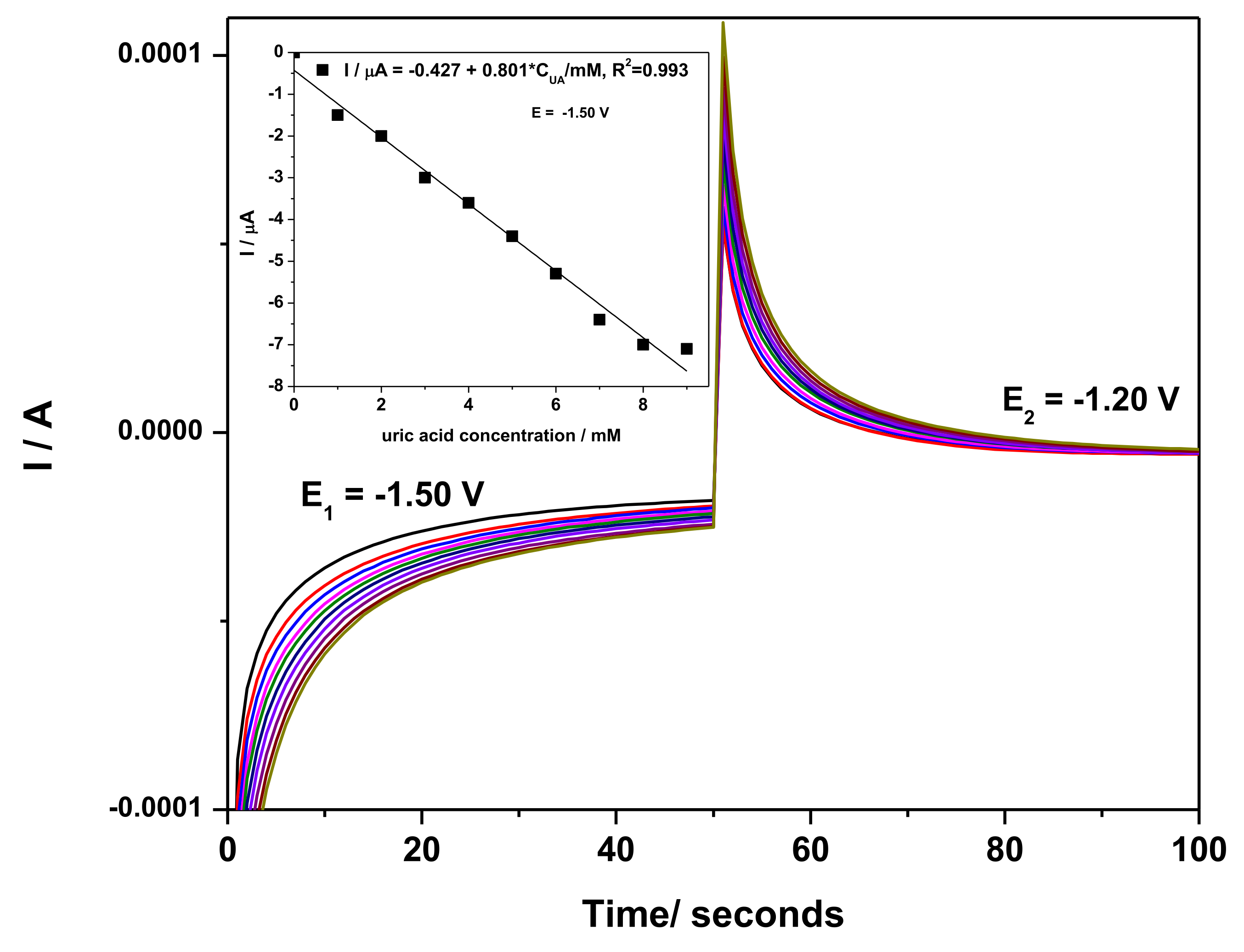
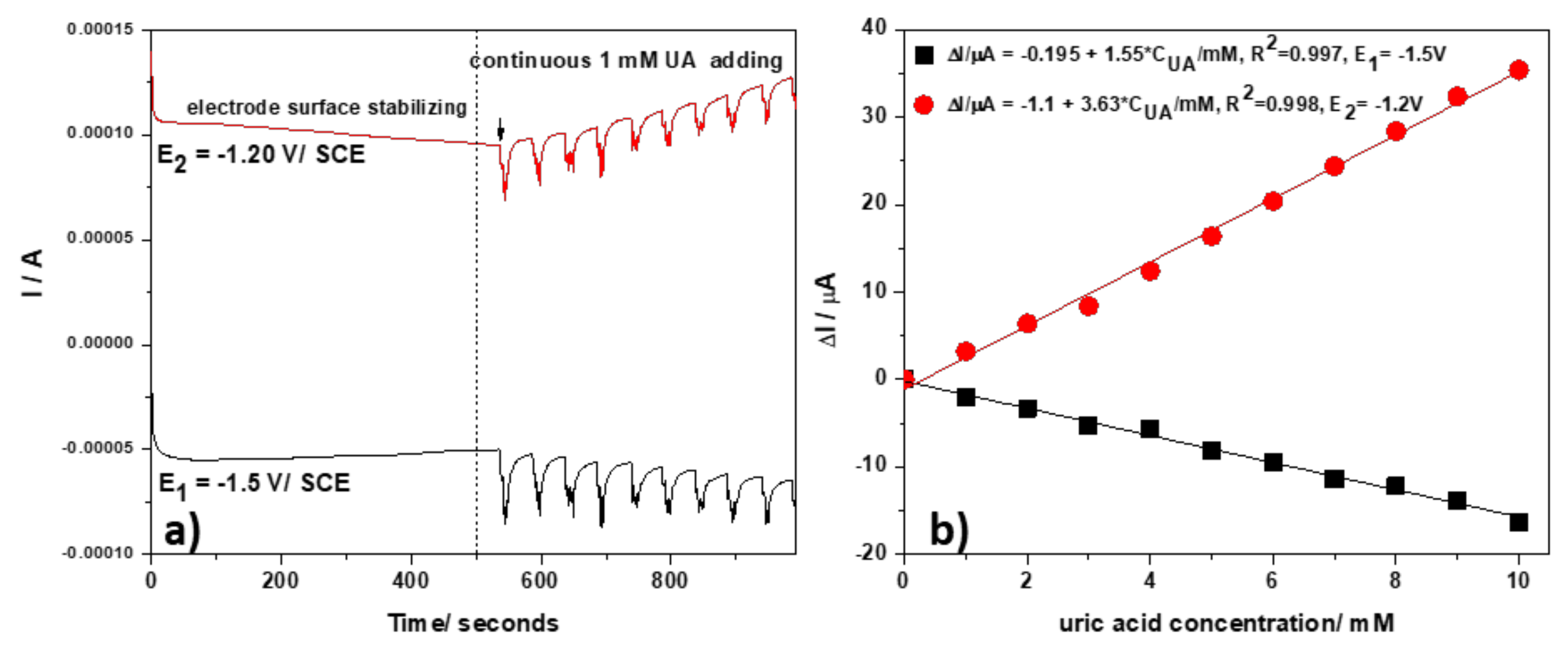
3.2.3. Development of Selective Amperometric Detection of UA
- −1.50 V/SCE (pulse time of 0.1 s) for electrode surface renewing by Zn and ZnO formation
- −1.20 V/SCE (pulse time of 0.03 s) where Zn2+ is stripping and Zn:urate complex is forming
- +0.10 V/SCE (pulse time of 0.05 s) where UA oxidation is starting
- +0.70 V/SCE (pulse time of 0.05 s) corresponding to the zinc oxide dissolution with the zincate formation, which is controlled by the HO- anions diffusion at the electrode surface [29] and UA oxidation.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramya, B.; Gomathi Priya, P. Rapid phytochemical microwave-assisted synthesis of zinc oxide nano flakes with excellent electrocatalytic activity for non-enzymatic electrochemical sensing of uric acid. Mater. Sci. Mater. Electron. 2021, 32, 21406–21424. [Google Scholar] [CrossRef]
- Pan, Y.Z.; Zuo, J.L.; Hou, Z.Y.; Huang, Y.Z.; Huang, C.C. Preparation of Electrochemical Sensor Based on Zinc Oxide Nanoparticles for Simultaneous Determination of AA, DA, and UA. Front. Chem. 2020, 8, 592538. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, R.M.A.; Ab Ghani, S. Voltammetric analysis of uric acid by zinc-nickel nanoalloy coated composite graphite. Sens. Actuators B Chem. 2010, 145, 20–24. [Google Scholar] [CrossRef]
- Babitha, K.B.; Soorya, P.S.; Peer Mohamed, A.; Rakhi, R.B.; Ananthakumar, S. Development of ZnO@rGO nanocomposites for the enzyme free electrochemical detection of urea and glucose. Mater. Adv. 2020, 1, 1939–1951. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Rocha, D.P.; Silva, M.N.T.; Martins, P.R.; Nossol, E.; Angnes, L.; Rout, C.S.; Munoz, R.A.A. Feasible strategies to promote the sensing performances of spinel MCo2O4 (M = Ni, Fe, Mn, Cu and Zn) based electrochemical sensors: A review. J. Mater. Chem. C 2021, 9, 7852–7887. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, D.; Suo, G.; Wei, W.; Huang, T. Nanocomposite Ag@Zn-TSA as highly efficient immobilization matrixes for sensitive electrochemical biosensing. Anal. Chim. Acta 2016, 934, 203–211. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Osman, D.I.; Ali, O.I.; Shousha, W.G.; Shoeib, M.A.; Shawky, S.M.; Sheta, S.M. A novel Ag/Zn bimetallic MOF as a superior sensitive biosensing platform for HCV-RNA electrochemical detection. Appl. Surf. Sci. 2021, 562, 150202. [Google Scholar] [CrossRef]
- Kumar, P.; Maikap, S.; Singh, K.; Chatterjee, S.; Chen, Y.-Y.; Cheng, H.-M.; Mahapatra, R.; Qiu, J.-T.; Yang, J.-R. Highly Reliable Label-Free Detection of Urea/Glucose and Sensing Mechanism Using SiO2 and CdSe-ZnS Nanoparticles in Electrolyte-Insulator-Semiconductor Structure. J. Electrochem. Soc. 2016, 163, B580–B587. [Google Scholar] [CrossRef]
- Rezaeia, R.; Foroughia, M.M.; Beitollahib, H.; Alizadeh, R. Electrochemical Sensing of Uric Acid Using a ZnO/Graphene Nanocomposite Modified Graphite Screen Printed Electrode. Russ. J. Electrochem. 2018, 54, 860–866. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajheidari, N. ZnO-CuxO/polypyrrole nanocomposite modified electrode for simultaneous determination of ascorbic acid, dopamine, and uric acid. Analyt. Biochem. 2015, 473, 53–62. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajian, A. Electrochemical characterization of Au/ZnO/PPy/RGO nanocomposite and its application for simultaneous determination of ascorbic acid, epinephrine, and uric acid. J. Electroanalyt. Chem. 2017, 801, 466–479. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.C.; Ma, L.X. One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivity for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2016, 227, 488–496. [Google Scholar] [CrossRef]
- Rais, N.S.M.; Isa, I.M.; Hashim, N.; Saidin, M.I.; Yazid, S.N.A.M.; Ahmad, M.S.; Zainul, R.; Suyanta; Mukdasai, S. Simultaneously determination of bisphenol A and uric acid by zinc/aluminum-layered double hydroxide-2-(2,4-dichlorophenoxy) propionate paste electrode. Int. J. Electrochem. Sci. 2019, 14, 7911–7924. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.; Wang, Y.; Li, P.; Zhang, W.; Lian, K. Controllable synthesis of branched hierarchical ZnO nanorod arrays for highly sensitive hydrazine detection. Appl. Surf. Sci. 2016, 364, 434–441. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Li, P.; Sang, S.; Zhang, W.; Hu, J.; Lian, K. A highly sensitive electrochemical sensor based on Cu/Cu2O@carbon nanocomposite structures for hydrazine detection. Anal. Methods 2015, 7, 9040–9046. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Wang, W.; Sun, Y.; Li, P.; Hu, J.; Chen, L.; Gong, W. Synthesis and electrochemical properties of Co3O4-rGO/CNTs composites towards highly sensitive nitrite detection. Appl. Surf. Sci. 2019, 485, 274–282. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Li, P.; Zhang, W.; Lian, K.; Hu, J.; Chen, Y. Preparation and characterization of AuNPs/CNTs-ErGO electrochemical sensors for highly sensitive detection of hydrazine. Talanta 2016, 158, 283–291. [Google Scholar] [CrossRef]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef]
- Pop, A.; Motoc, S.; Manea, F. Carbon nanomaterials-based sensors for water treatment. In Carbon Nanomaterials-Based Sensors: Emerging Research Trends in Devices and Applications; Manjunatha, J.G., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 125–148. [Google Scholar]
- Cho, G.; Azzouzi, S.; Zucchi, G.; Lebental, B. Electrical and Electrochemical Sensors Based on Carbon Nanotubes for the Monitoring of Chemicals in Water—A Review. Sensors 2022, 22, 218. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Safaei, M.; Zhang, K.; Le, Q.V.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Developments and applications of nanomaterial-based carbon paste electrodes. RSC Adv. 2020, 10, 21561–21581. [Google Scholar] [CrossRef]
- Hu, C.; Hu, S. Carbon Nanotube-Based Electrochemical Sensors: Principles and Applications in Biomedical Systems. J. Sens. 2009, 2009, 187615. [Google Scholar] [CrossRef]
- Amatatongchai, M.; Sroysee, W.; Chairam, S.; Nacapricha, D. Amperometric flow injection analysis of glucose using immobilized glucose oxidase on nano-composite carbon nanotubes-platinum nanoparticles carbon paste electrode. Talanta 2017, 166, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Ganjali, M.R.; Rfiei, F. Trace level and highly selective determination of urea in various real samples based upon voltammetric analysis of diacetylmonoxime-urea reaction product on the carbon nanotube/carbon paste electrode. Anal. Chim. Acta 2017, 974, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour-Mobarakeh, L.; Nezamzadeh-Ejhieh, A. A zeolite modified carbon paste electrode as useful sensor for voltammetric determination of acetaminophen. Mater. Sci. Eng. C 2015, 49, 493–499. [Google Scholar] [CrossRef]
- Saadat, M.; Nezamzadeh-Ejhieh, A. Clinoptilolite nanoparticles containing HDTMA and Arsenazo III as a sensitive carbon paste electrode modifier for indirect voltammetric measurement of Cesium ions. Electrochim. Acta 2016, 217, 163–170. [Google Scholar] [CrossRef]
- Motoc, S.; Cretu, C.; Costisor, O.; Baciu, A.; Manea, F.; Szerb, E.I. Cu(I) Coordination Complex Precursor for Randomized CuOx Microarray Loaded on Carbon Nanofiber with Excellent Electrocatalytic Performance for Electrochemical Glucose Detection. Sensors 2019, 19, 5353. [Google Scholar] [CrossRef]
- Motoc Ilies, S.; Schinteie, B.; Pop, A.; Negrea, S.; Cretu, C.; Szerb, E.I.; Manea, F. Graphene Quantum Dots and Cu(I) Liquid Crystal for Advanced Electrochemical Detection of Doxorubicine in Aqueous Solutions. Nanomaterials 2021, 11, 2788. [Google Scholar] [CrossRef]
- Andelescu, A.A.; Ilies, S.; Cretu, C.; Popa, P.; Marinescu, S.; Heinrich, B.; Manea, F.; Negrea, S.; Donnio, B.; Szerb, E.I. Pentacoordinated Liquid Crystalline Zn(II) Complex Organized in Smectic Mesophase: Synthesis, Structural and Electrochemical Properties. Appl. Sci. 2022, 12, 8306. [Google Scholar] [CrossRef]
- Cuerva, C.; Cano, M.; Lodeiro, C. Advanced Functional Luminescent Metallomesogens: The Key Role of the Metal Center. Chem. Rev. 2021, 121, 12966–13010. [Google Scholar] [CrossRef]
- Szerb, E.I.; Crispini, A.; Aiello, I.; La Deda, M. Part XII: Inorganic Materials for Optoelectronics, 62: Liquid Crystals. In Springer Handbook of Inorganic Photochemistry; Bahnemann, D.W., Patrocinio, A.O.T., Zysman-Colman, E., Eds.; Springer: Cham, Switzerland, 2022; ISSN1 2522-8692. ISSN2 2522-8706. ISBN1 978-3-030-63712-5. ISBN2 978-3-030-63713-2. [Google Scholar] [CrossRef]
- Pucci, D.; Donnio, B. 4. Metal-Containing Liquid Crystals. In Handbook of Liquid Crystals; Goodby, J.W., Tschierske, C., Raynes, P., Gleeson, H., Kato, T., Collings, P.J., Eds.; Wiley-VCH Verlag Gmbh: Weinheim, Germany, 2014; pp. 1–67. [Google Scholar]
- Lakshmi, D.; Whitcombe, M.J.; Davis, F.; Sharma, P.S.; Prasa, B.B. Electrochemical Detection of Uric Acid in Mixed and Clinical Samples: A Review. Electroanalysis 2011, 23, 305–320. [Google Scholar] [CrossRef]
- Abrori, S.A.; Septiani, N.L.W.; Hakim, F.N.; Maulana, A.; Suyatman, S.; Nugraha, S.; Anshori, I.; Yuliarto, B. Non-Enzymatic Electrochemical Detection for Uric Acid Based on a Glassy Carbon Electrode Modified With MOF-71. IEEE Sens. J. 2021, 21, 170–177. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Orha, C.; Pop, A. Enhanced electrochemical response of diclofenac at a fullerene–carbon nanofiber paste electrode. Sensors 2019, 19, 1332. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Baciu, A.; Orha, C.; Pop, A. Electrochemical method for ease determination of sodium diclofenac trace levels in water using graphene—Multi-walled carbon nanotubes paste electrode. Int. J. Environ. Res. Public Health 2022, 19, 29. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Baciu, A.; Vasilie, S.; Pop, A. Highly sensitive and simultaneous electrochemical determinations of non-steroidal anti-inflammatory drugs in water using nanostructured carbon-based paste electrodes. Sci. Total Environ. 2022, 846, 157412. [Google Scholar] [CrossRef]
- Moawad, M.M. Complexation and Thermal Studies of Uric Acid with Some Divalent and Trivalent Metal Ions of Biological Interest in the Solid State. J. Coord. Chem. 2002, 55, 61–78. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, X.; Kang, Z.; Lin, P.; Fang, X.; Lei, Y.; Ma, S.; Zhang, Y. Highly sensitive uric acid biosensor based on individual zinc oxide micro/nanowires. Microchim. Acta 2013, 180, 759–766. [Google Scholar] [CrossRef]
- Jindal, K.; Tomar, M.; Gupta, V. Nitrogen-doped zinc oxide thin films biosensor for determination of uric acid. Analyst 2013, 138, 4353. [Google Scholar] [CrossRef]
- Ali, M.; Shah, I.; Kim, S.W.; Sajid, M.; Lim, J.H.; Choi, K.H. Quantitative detection of uric acid through ZnO quantum dots based highly sensitive electrochemical biosensor. Sens. Actuators A Phys. 2018, 283, 282–290. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Zhu, Z.; Zhang, J.; Zhu, J. Novel Uric Acid Sensor Based on Enzyme Electrode Modified by ZnO Nanoparticles and Multiwall Carbon Nanotubes. Anal. Lett. 2009, 42, 775–789. [Google Scholar] [CrossRef]
- Mazzara, F.; Patella, B.; Aiello, G.; O’Riordan, A.; Torino, C.; Vilasi, A.; Inguanta, R. Electrochemical detection of uric acid and ascorbic acid using r-GO/NPs based sensors. Electrochim. Acta 2021, 388, 138652. [Google Scholar] [CrossRef]
- La Deda, M.; Di Maio, G.; Candreva, A.; Heinrich, B.; Andelescu, A.-A.; Popa, E.; Voirin, E.; Badea, V.; Amati, M.; Costişor, O.; et al. Very intense polarized emission in self-assembled room temperature metallomesogens based on Zn(II) coordination complexes: An experimental and computational study. J. Mater. Chem. C 2022, 10, 115–125. [Google Scholar] [CrossRef]

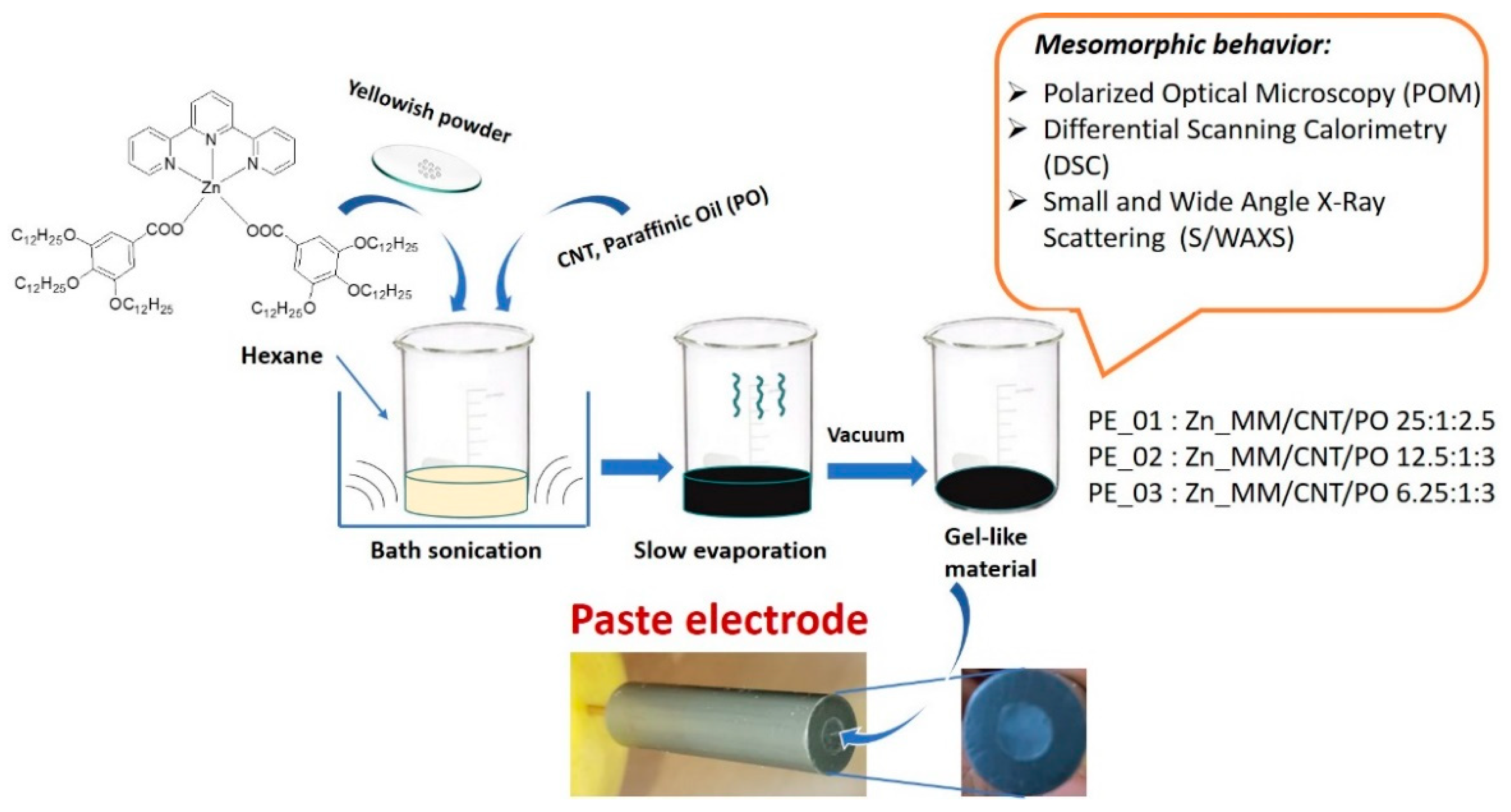

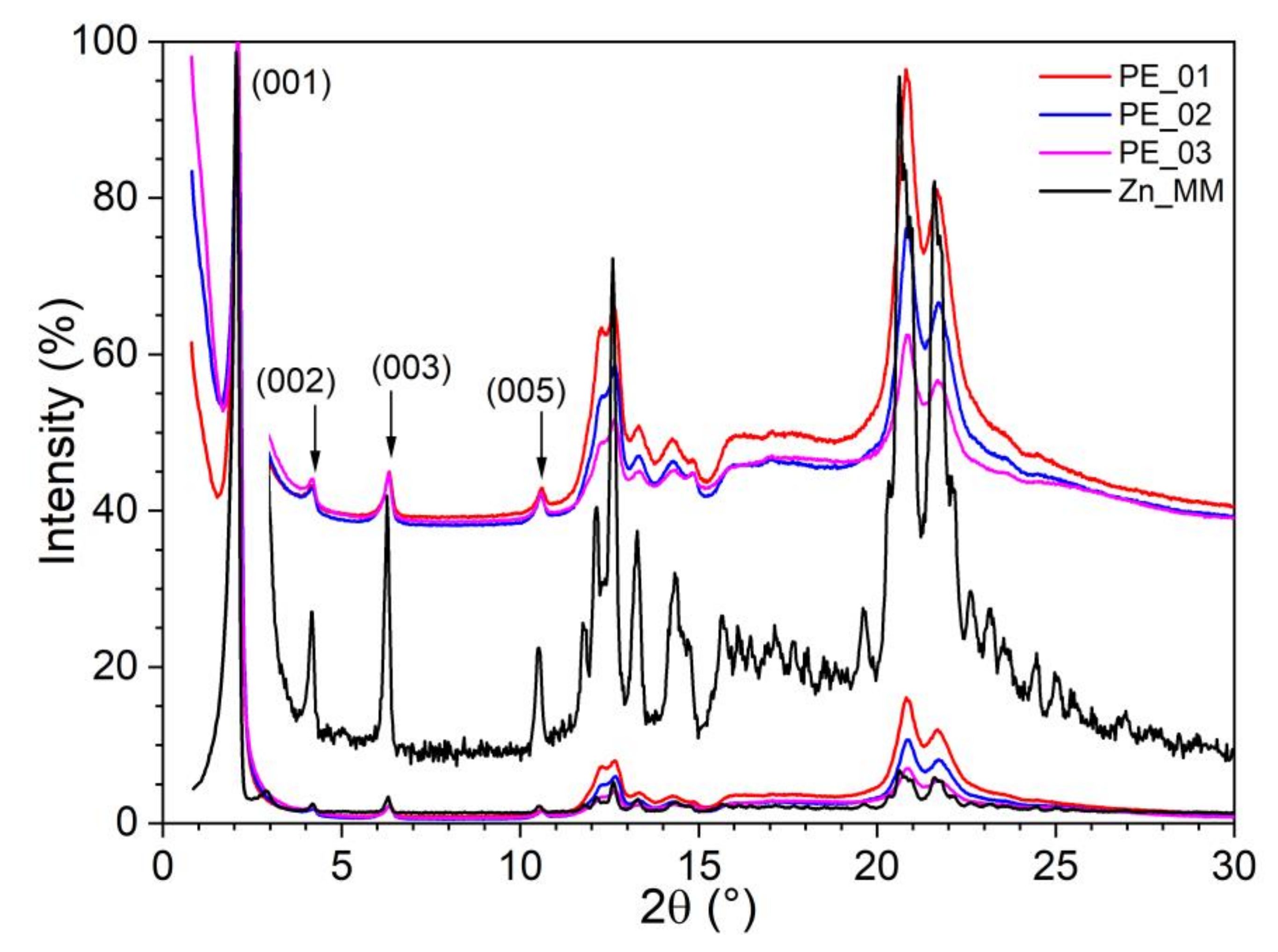
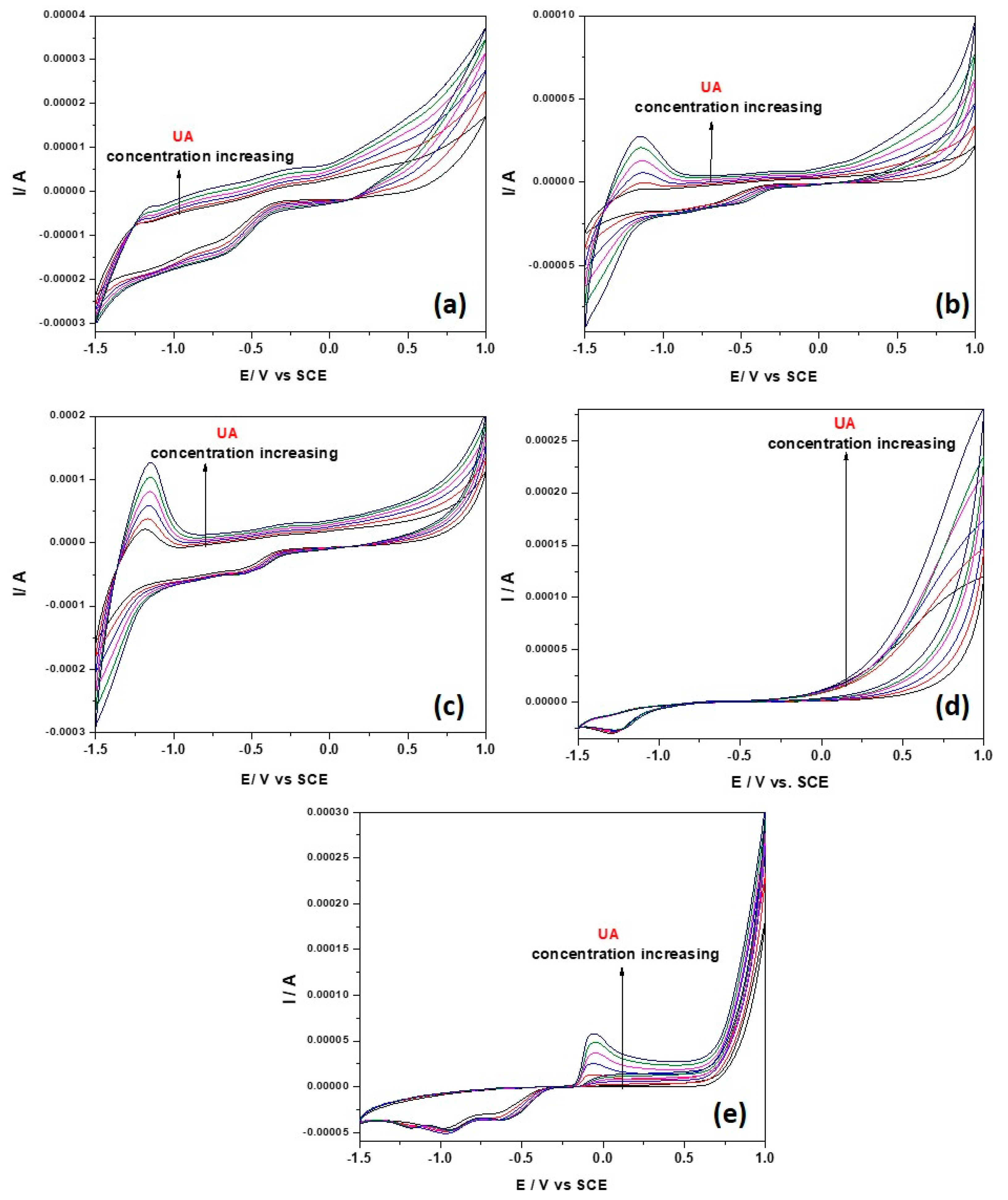

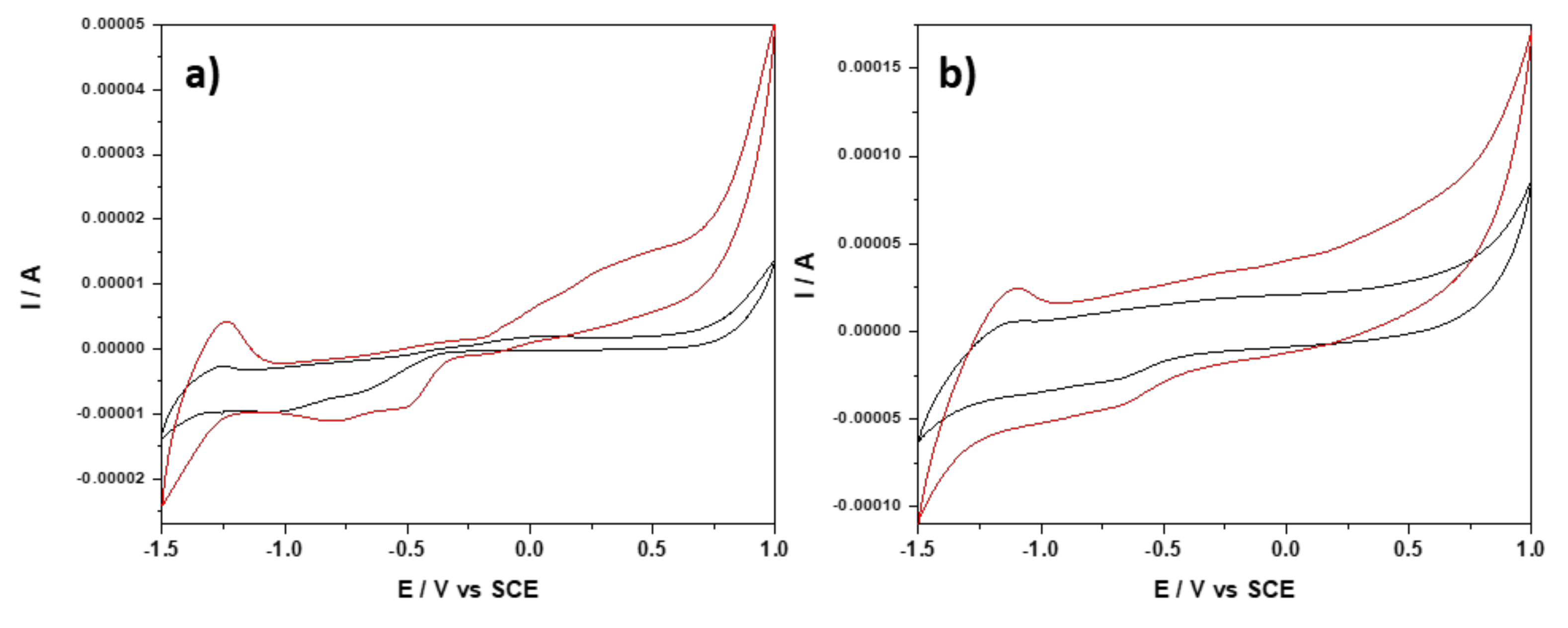

| Paste Electrode | Zn_MM | CNT | Paraffinic Oil | |||
|---|---|---|---|---|---|---|
| Weight Ratio [%] | Mass [mg] | Weight Ratio [%] | Mass [mg] | Weight Ratio [%] | Mass [mg] | |
| PE_01 | 25 | 500 | 1 | 20 | 2.5 | 50 |
| PE_02 | 12.5 | 500 | 1 | 40 | 3 | 120 |
| PE_03 | 6.25 | 500 | 1 | 80 | 3 | 240 |
| Cpd. | Heating/ Cooling Cycle | Mesophases,1 Transition Temperatures (°C) and Enthalpies [ΔH (kJ·mol−1)] |
|---|---|---|
| Zn_MM | I II | Cr 49.0 [3.8] Cr’ 62.5 [53.0] CrSm 94.3 [13.5] SmA 116.5 [1.6] Iso Iso 115.7 [−2.4] SmA 73.7 [−7.4] CrSm CrSm 89 [12.0] SmA 109 [2.5] Iso Iso 114 [−2.6] SmA 73 [−10.2] CrSm |
| PE_01 | I II | Cr 36.2 [5.7] CrSm 80.4 (43.2) SmA [-] 2 Iso Iso [-] 2 SmA 82.6 [−0.9] CrSm 45.0 [−3.5] Cr Cr 64.3 [−4.4] CrSm 80.4 [2.4] SmA [-] 2 Iso Iso [-] 2 82.8 [−0.7] SmA 45.1 [−3.7] CrSm |
| PE_02 | I II | Cr 35.9 [7.2] CrSm 78.5 [45.5] SmA [-] 2 Iso Iso [-]2 74.3 [−0.5] SmA [-]2 CrSm CrSm 37.2 [−3.9] Cr 61.9 [4.2] CrSm 87.2 [1.0] SmA [-] 2 Iso Iso [-]2 SmA 74.0 [0.5] CrSm |
| PE_03 | I II | Cr 34.2 [5.9] CrSm 66.2 [43.2] SmA [-] 2 Iso Iso [-] 2 SmA [-] 2 CrSm CrSm 79.3 [3.1] SmA [-] 2 Iso Iso [-] 2 SmA [-] 2 CrSm |
| Electrode Type | Zn_MM:CNT:Parrafin Oil/Weight Ratio | Electroactive Surface Area/cm2 | Electroactive/Geometrical Surface Areas Ratio |
|---|---|---|---|
| PE_01 | 25:1:3 | 0.016 | 0.225 |
| PE_02 | 12.5:1:3 | 0.036 | 0.507 |
| PE_03 | 6.25:1:3 | 0.051 | 0.720 |
| CNT | 1:3 | 0.117 | 1.53 |
| Electrode Composition | Applied Technique | Detection Potential/V vs. SCE | Sensitivity/µA∙mM−1∙cm−2 | LOD/µM | Ref. |
|---|---|---|---|---|---|
| Nafion/Uricase/Zn O micro/NWs/Au | CA | +0.80 | 89.74 | 25.6 | [39] |
| Nitrogen-doped zinc oxide thin films | CV | +0.50 | 1.1 | 40 | [40] |
| Nafion/ZnO QD/Uricase | CA | +0.60 | 4 | 22.97 | [41] |
| Cationic polydiallyldimethylammonium chloride/Uricase/ZnO NPs/MWNTs/Pyrolytic graphite wafers | DPV | +0.33 | 393 | 2 | [42] |
| ITO-r-GO-AuNPs | LSV | +0.26 | 0.31∙10−3 | 10.9 | [43] |
| PE_03: 6.25 Zn_MM:1 CNT:3 Parrafinic Oil w/w% | MPA | −1.50 | 479 | 33 | This work |
| −1.20 | 229 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negrea, S.; Andelescu, A.A.; Ilies, S.; Cretu, C.; Cseh, L.; Rastei, M.; Donnio, B.; Szerb, E.I.; Manea, F. Design of Nanostructured Hybrid Electrodes Based on a Liquid Crystalline Zn(II) Coordination Complex-Carbon Nanotubes Composition for the Specific Electrochemical Sensing of Uric Acid. Nanomaterials 2022, 12, 4215. https://doi.org/10.3390/nano12234215
Negrea S, Andelescu AA, Ilies S, Cretu C, Cseh L, Rastei M, Donnio B, Szerb EI, Manea F. Design of Nanostructured Hybrid Electrodes Based on a Liquid Crystalline Zn(II) Coordination Complex-Carbon Nanotubes Composition for the Specific Electrochemical Sensing of Uric Acid. Nanomaterials. 2022; 12(23):4215. https://doi.org/10.3390/nano12234215
Chicago/Turabian StyleNegrea, Sorina, Adelina A. Andelescu, Sorina Ilies (b. Motoc), Carmen Cretu, Liliana Cseh, Mircea Rastei, Bertrand Donnio, Elisabeta I. Szerb, and Florica Manea. 2022. "Design of Nanostructured Hybrid Electrodes Based on a Liquid Crystalline Zn(II) Coordination Complex-Carbon Nanotubes Composition for the Specific Electrochemical Sensing of Uric Acid" Nanomaterials 12, no. 23: 4215. https://doi.org/10.3390/nano12234215
APA StyleNegrea, S., Andelescu, A. A., Ilies, S., Cretu, C., Cseh, L., Rastei, M., Donnio, B., Szerb, E. I., & Manea, F. (2022). Design of Nanostructured Hybrid Electrodes Based on a Liquid Crystalline Zn(II) Coordination Complex-Carbon Nanotubes Composition for the Specific Electrochemical Sensing of Uric Acid. Nanomaterials, 12(23), 4215. https://doi.org/10.3390/nano12234215




.png)





