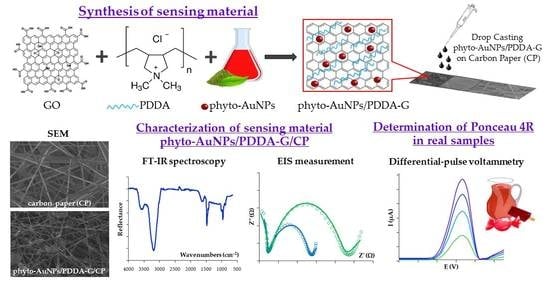
Carbon Paper Modified with Functionalized Poly(diallyldimethylammonium chloride) Graphene and Gold Phytonanoparticles as a Promising Sensing Material: Characterization and Electroanalysis of Ponceau 4R in Food Samples
Abstract
1. Introduction
2. Methods and Materials
2.1. Chemicals and Reagents
2.2. Instruments
2.3. Synthetic Procedures
2.3.1. PDDA Synthesis
2.3.2. PDDA-G Synthesis
2.3.3. Phyto-AuNPs Synthesis
2.3.4. Preparation of Phyto-AuNPs/PDDA-G
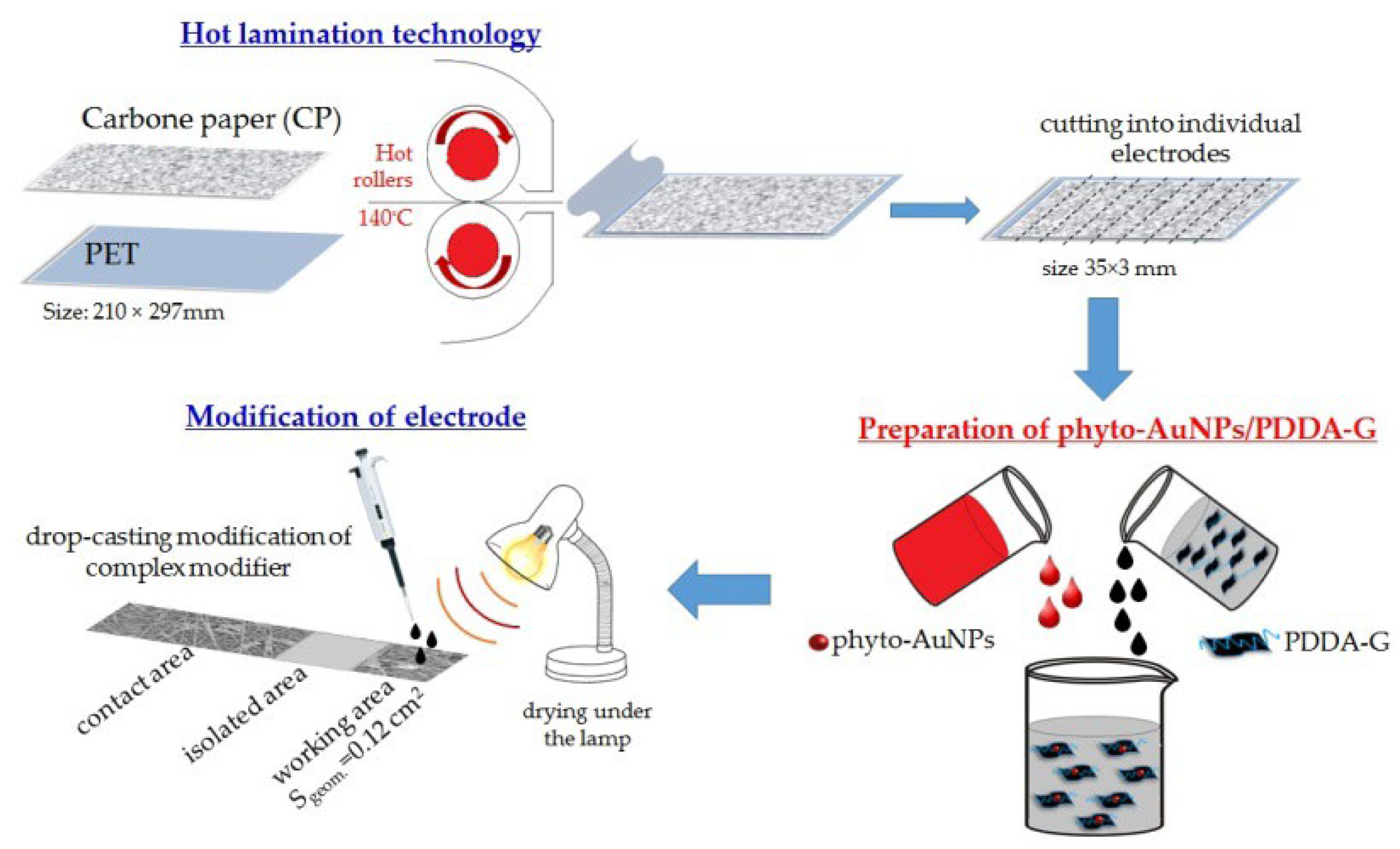
2.4. Fabrication of Electrodes
2.5. Electrochemical Procedures
2.5.1. Cyclic Voltammetry
2.5.2. Linear Sweep and Voltammetry
2.5.3. Differential-Pulse Voltammetry
2.5.4. Electrochemical Impedance Spectroscopy (EIS)
2.6. Real Samples
2.7. Results Processing
3. Results
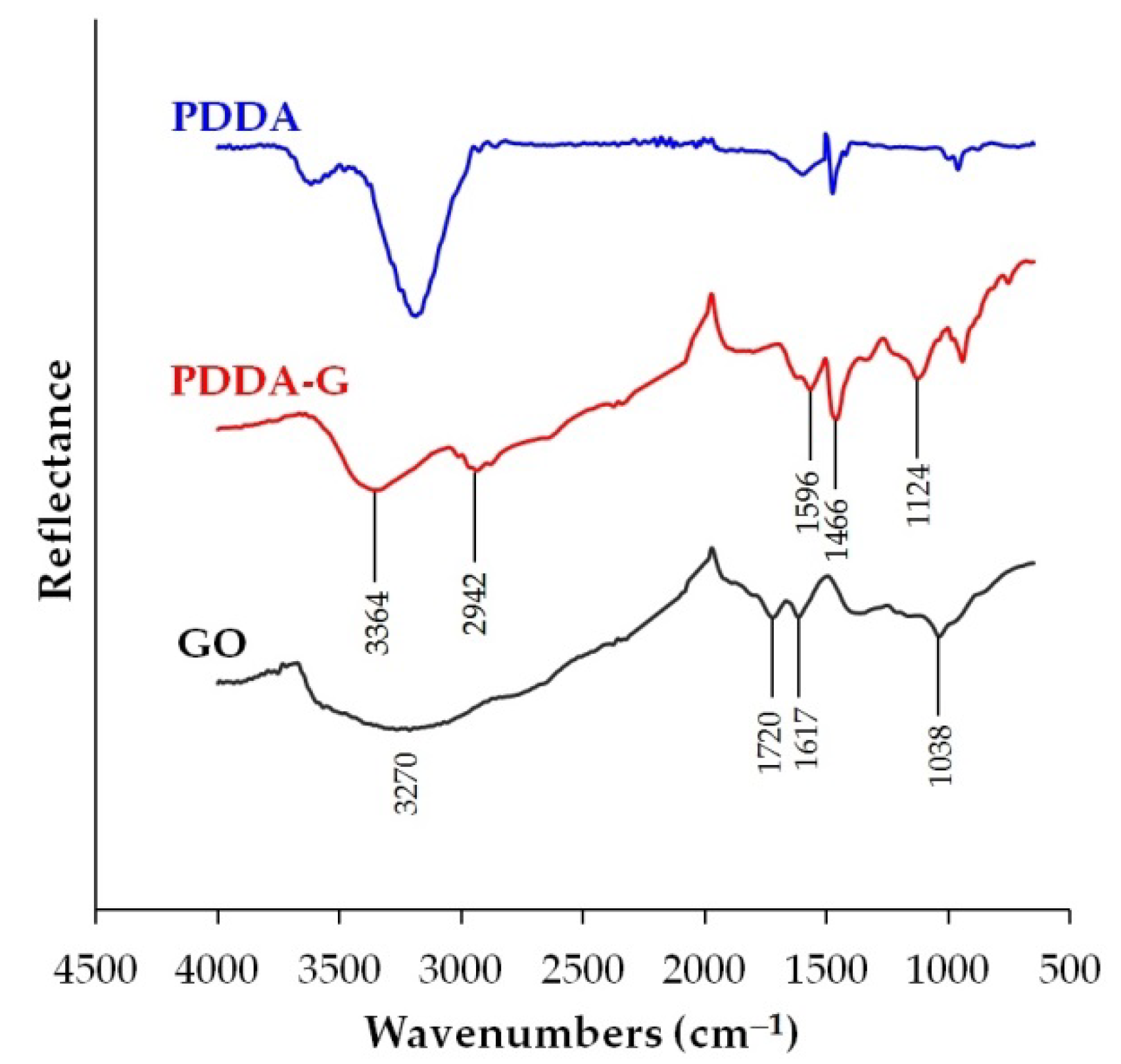
3.1. PDDA-G Characteristics
FT-IR Spectroscopy
3.2. Features of Modified Carbon Paper
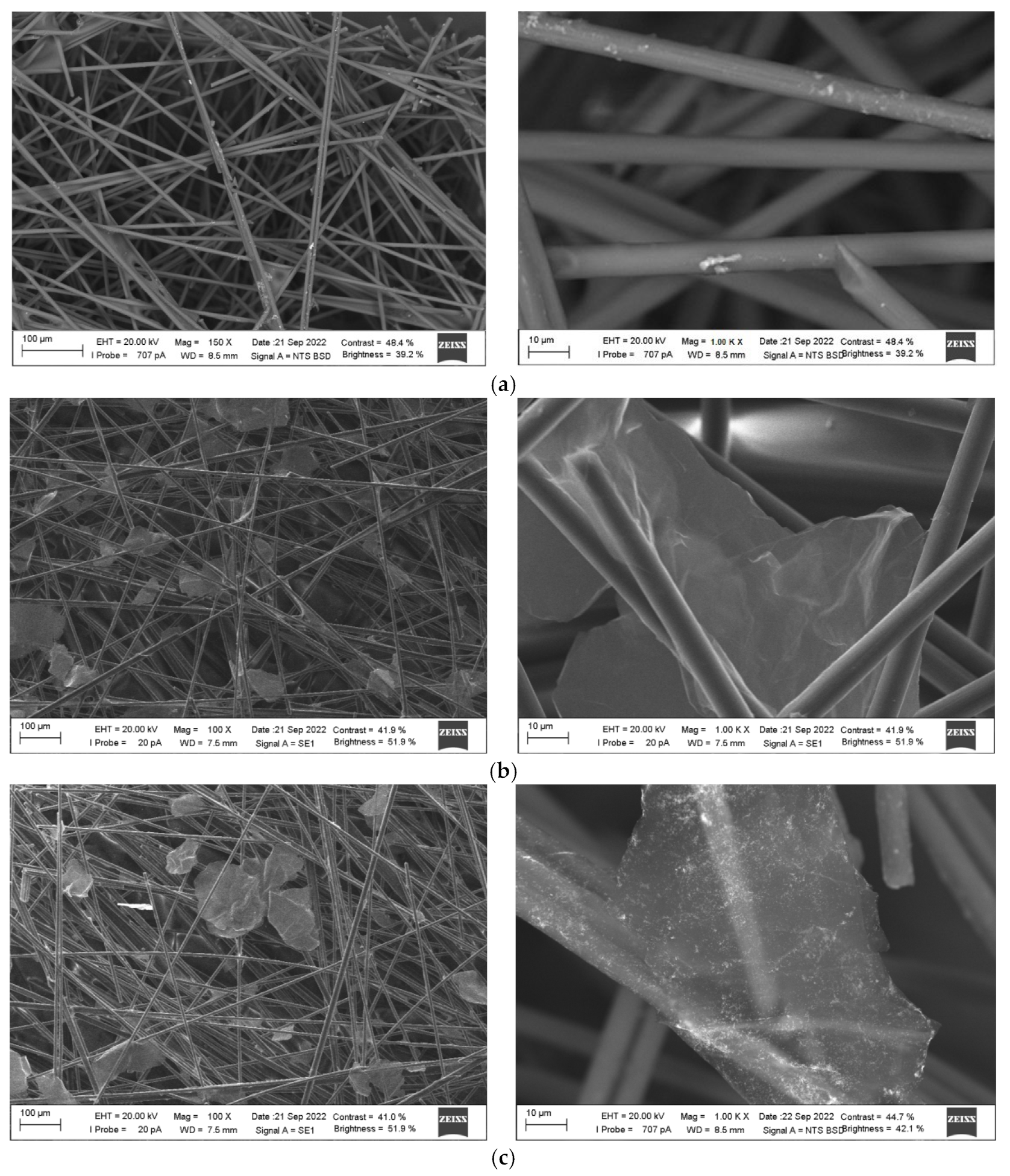
3.2.1. Scanning Electron Microscopy (SEM)
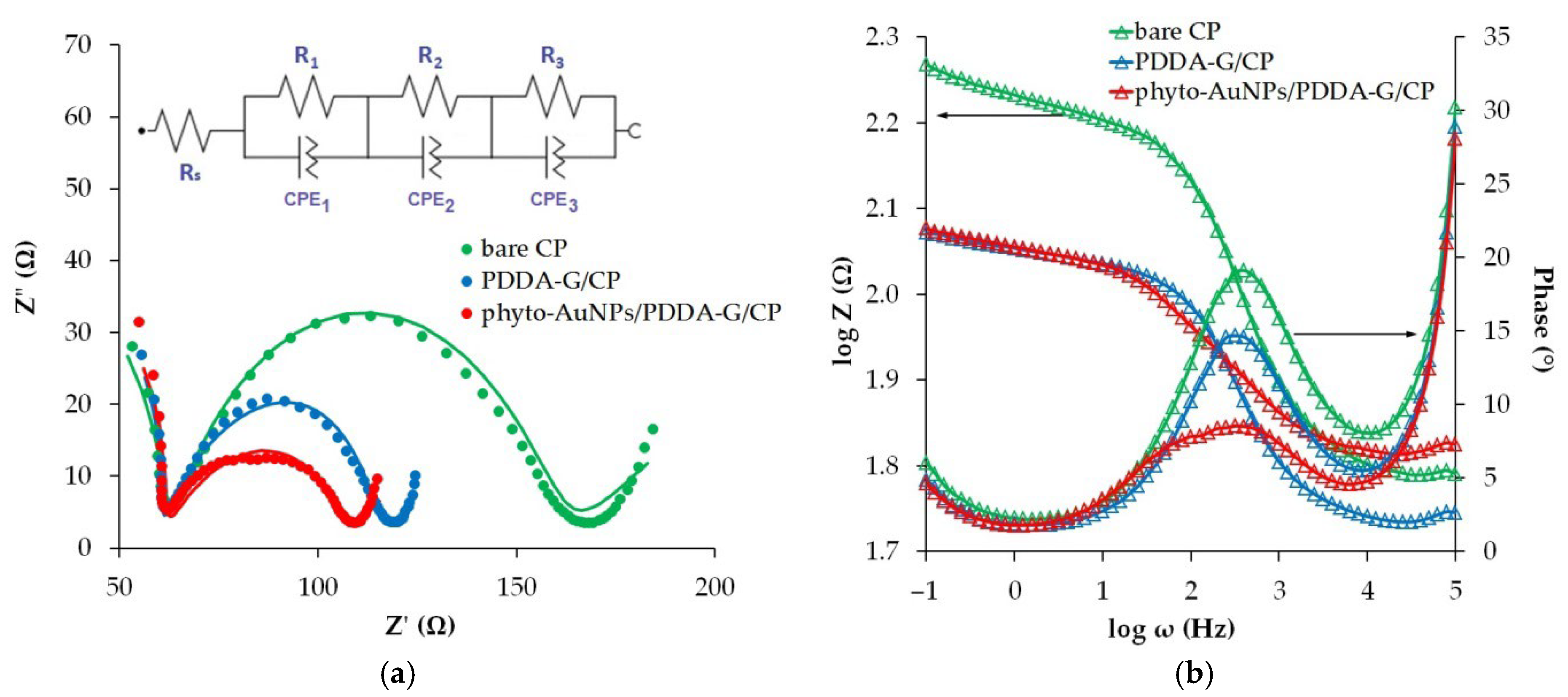
3.2.2. EIS Measurement
3.2.3. Evaluation of Electroactive Area
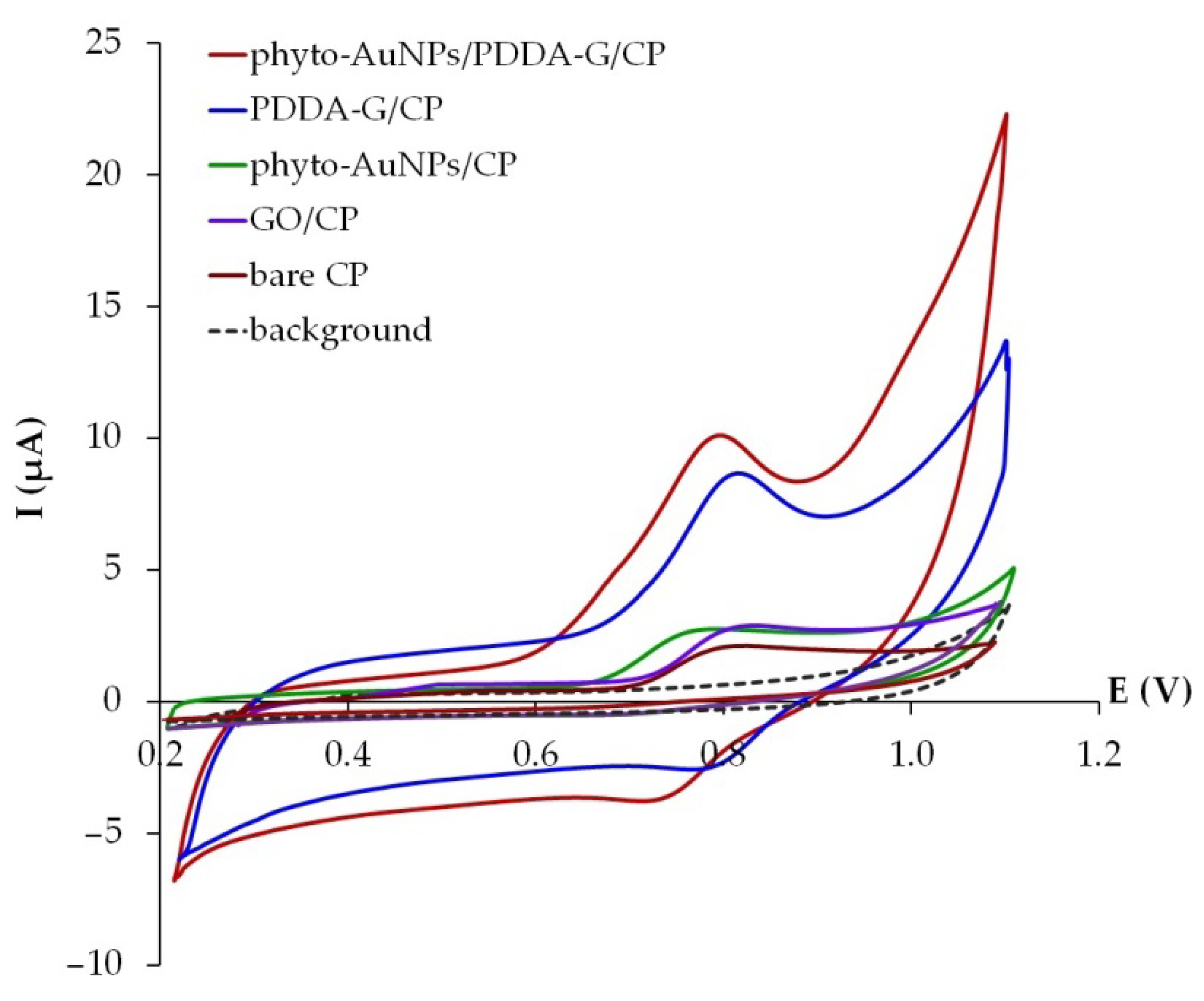
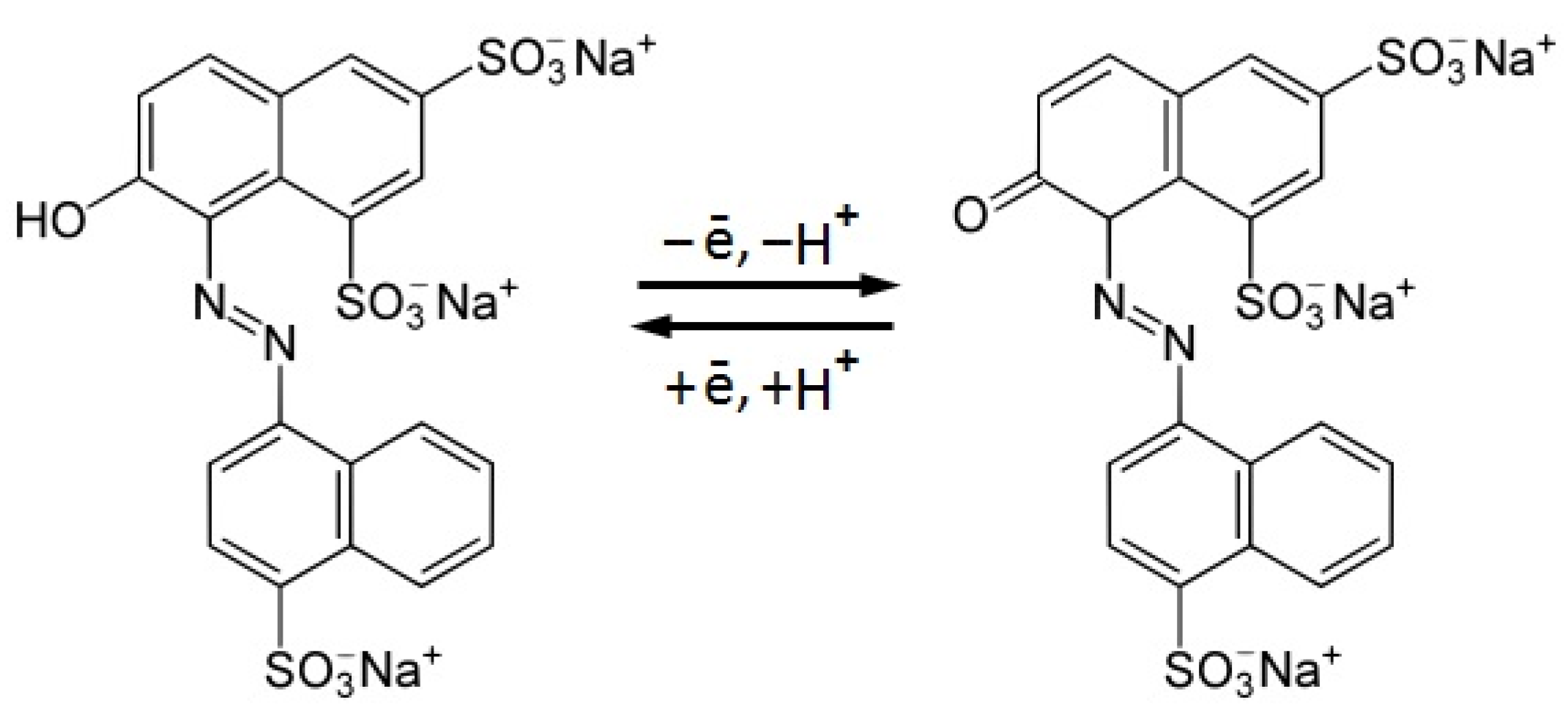
3.3. Ponceau 4R Electrochemical Behavior
3.4. Effect of Modifier Quantity
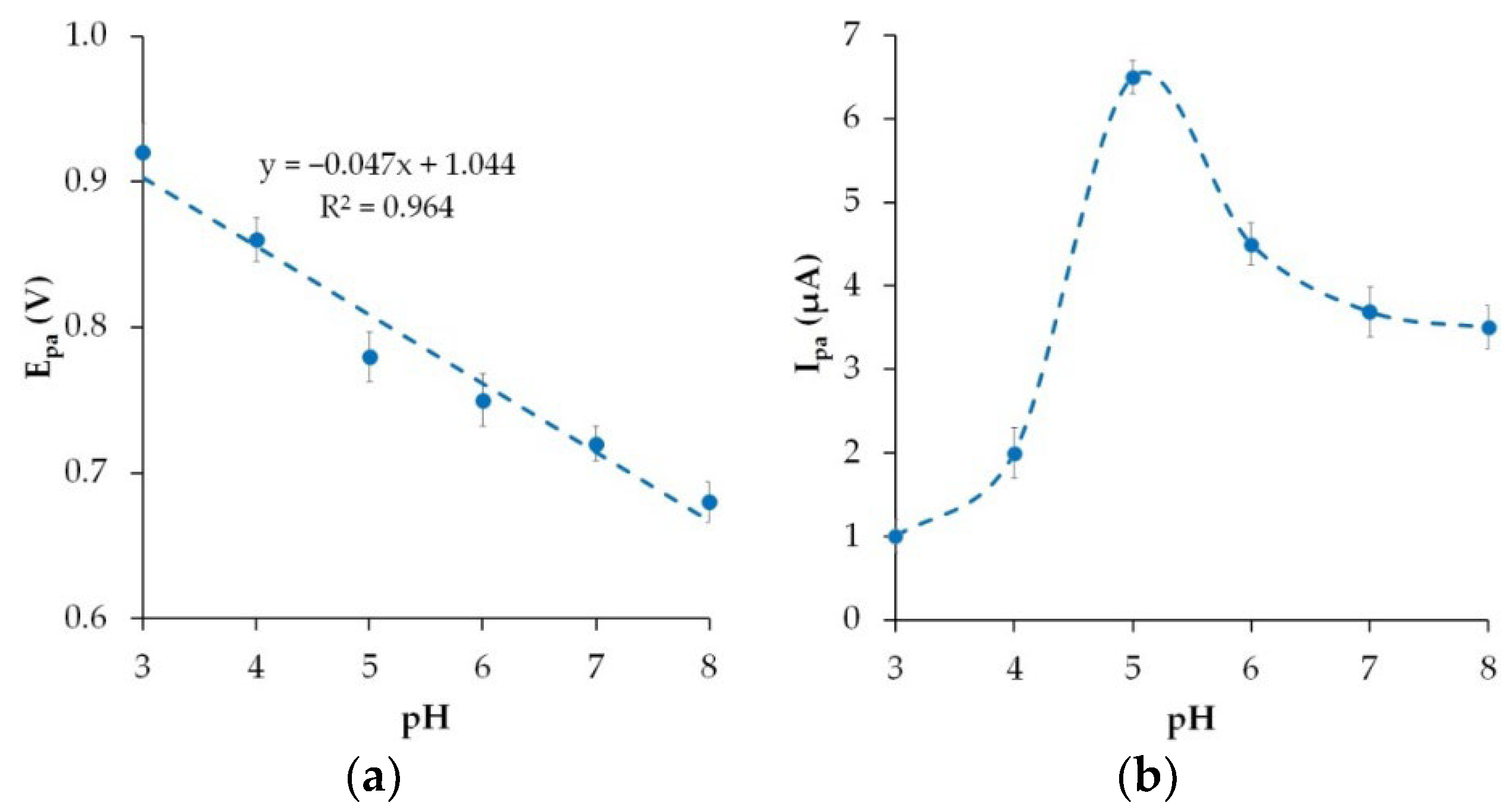
3.5. Effect of pH Value
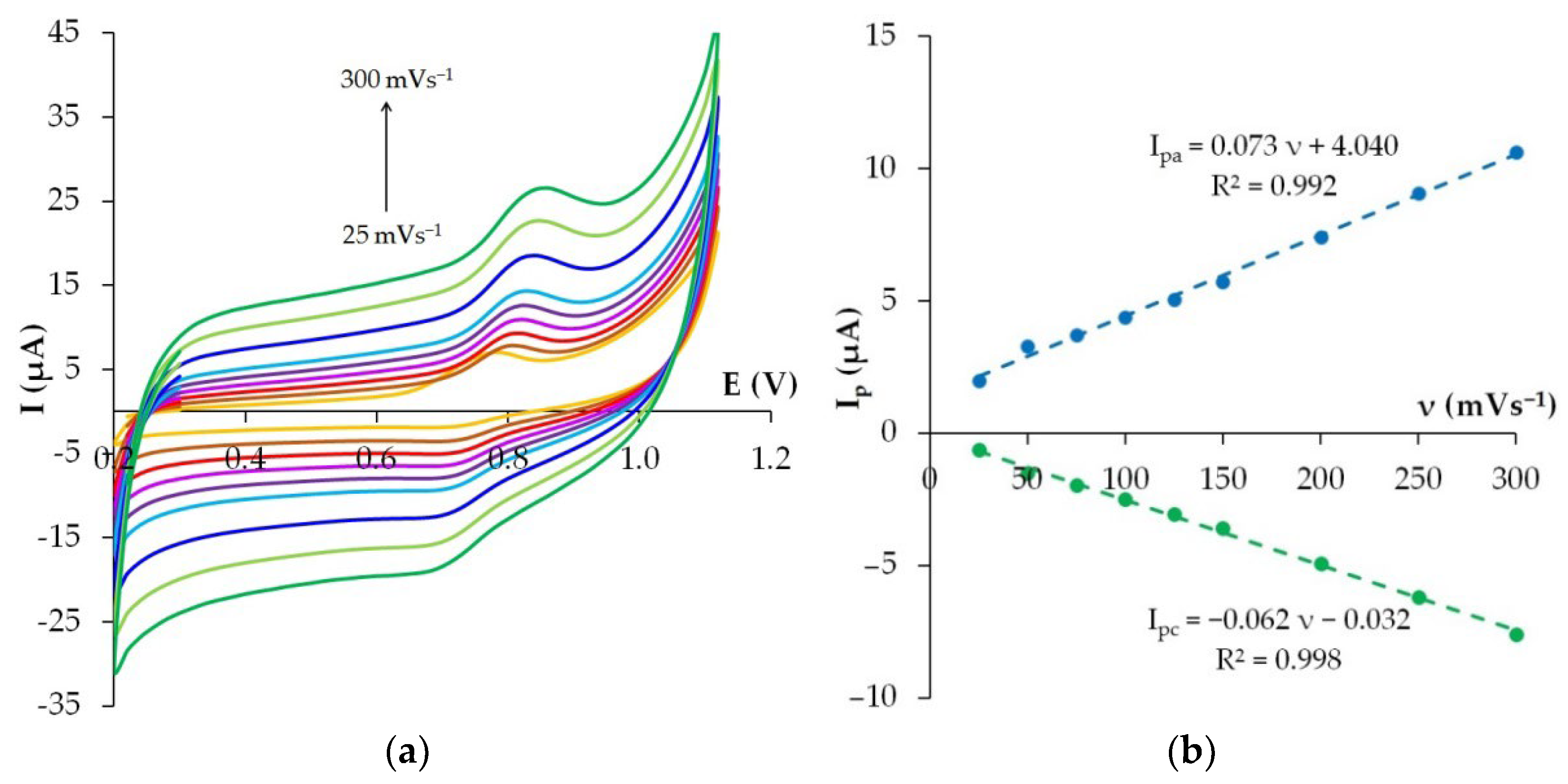
3.6. Effect of Potential Scan Rate
3.7. Effect of Accumulation Parameters
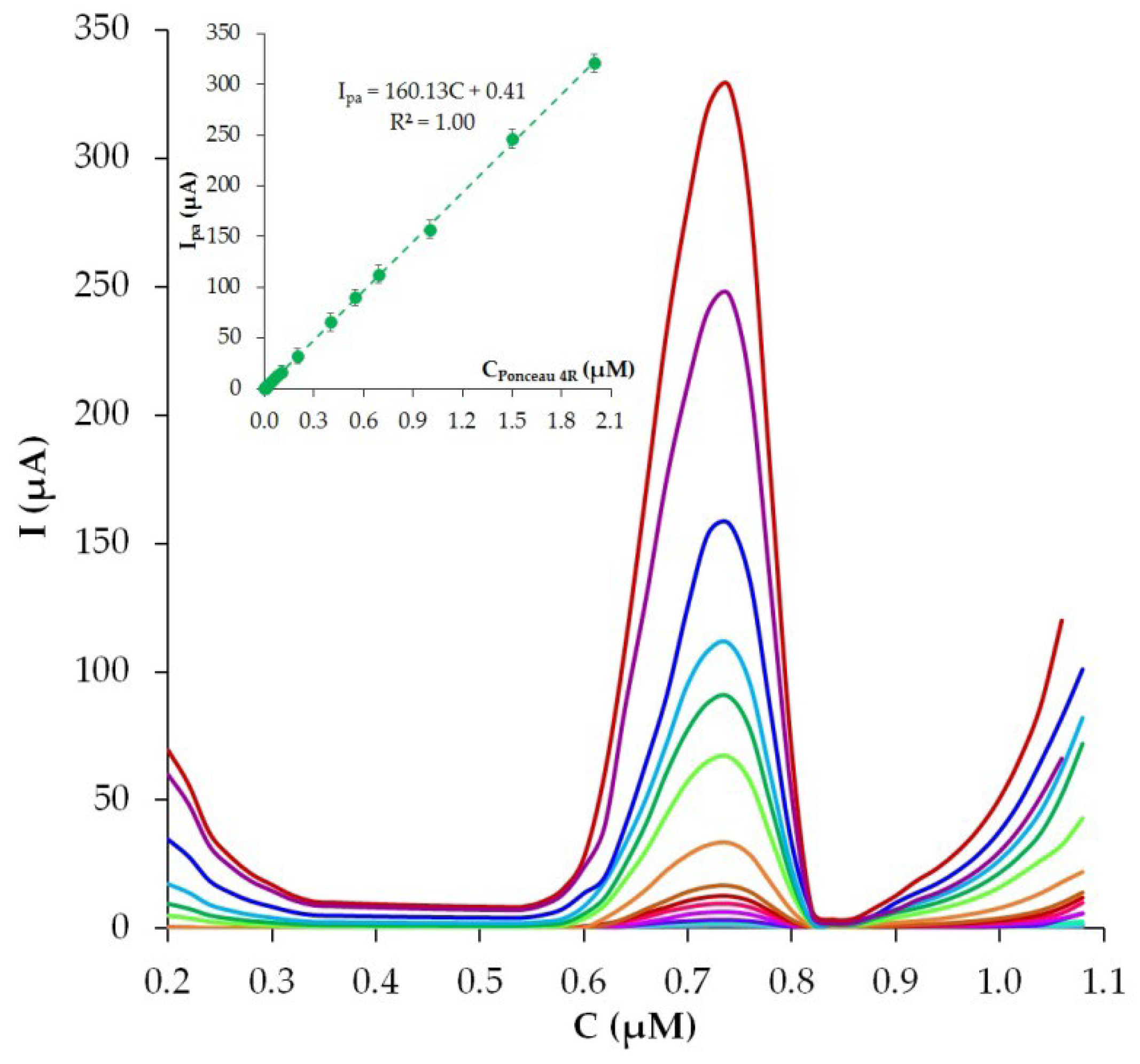
3.8. Analytical Characteristics of the Phyto-AuNPs/PDDA-G/CP Electrode
3.8.1. Optimization of Experimental Conditions
- -
- A modulation amplitude of 60 mV
- -
- A step potential of 15 mV
- -
- A modulation time of 100 ms
3.8.2. Interference
3.8.3. Reproducibility, Repeatability, and Stability
3.8.4. Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhang, S.; Wang, X.; Wang, W.; Chen, Z. Simultaneous determination of Ponceau-4R and Allura Red in soft drinks based on the ionic liquid modified expanded graphite paste electrode. Int. J. Environ. Anal. Chem. 2015, 95, 581–591. [Google Scholar] [CrossRef]
- Sharifpour, E.; Arabkhani, P.; Sadegh, F.; Mousavizadeh, A.; Asfaram, A. In-situ hydrothermal synthesis of CNT decorated by nano ZnS/CuO for simultaneous removal of acid food dyes from binary water samples. Sci. Rep. 2022, 12, 12381. [Google Scholar] [CrossRef]
- John, A.; Yang, H.-H.; Muhammad, S.; Khan, Z.I.; Yu, H.; Luqman, M.; Tofail, M.; Hussain, M.I.; Awan, M.U.F. Cross Talk between Synthetic Food Colors (Azo Dyes), Oral Flora, and Cardiovascular Disorders. Appl. Sci. 2022, 12, 7084. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Refined exposure assessment for Ponceau 4R (E 124). EFSA J. 2015, 13, 4073. [Google Scholar] [CrossRef]
- Yamjala, K.; Nainar, M.S.; Ramisetti, N.R. Methods for the analysis of azo dyes employed in food industry—A review. Food Chem. 2016, 192, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Neto, G.F.D.S.; Rodrigues, M.L.D.A.; Fonseca, A. A new quantitative gel electrophoresis method with image-based detection for the determination of food dyes and metallic ions. Talanta 2021, 221, 121602. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Mao, X.; Zhang, H.; Zeng, H.; Lin, Z.; Zhang, X.; Qi, P. Magnetic Solid-Phase Extraction Based on Magnetic Sulfonated Reduced Graphene Oxide for HPLC–MS/MS Analysis of Illegal Basic Dyes in Foods. Molecules 2021, 26, 7427. [Google Scholar] [CrossRef]
- De Moraes, P.; Hudari, F.; Silva, J.; Zanoni, M. Enhanced Detection of Ponceau 4R Food Dye by Glassy Carbon Electrode Modified with Reduced Graphene Oxide. J. Braz. Chem. Soc. 2018, 29, 1237–1244. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.; Liu, Y.; Wu, J.; Li, G.; Liu, J.; He, Q. A Simple but Efficient Voltammetric Sensor for Simultaneous Detection of Tartrazine and Ponceau 4R Based on TiO2/Electro-Reduced Graphene Oxide Nanocomposite. Chemosensors 2020, 8, 70. [Google Scholar] [CrossRef]
- Ma, X.; Xie, Y.; Yu, Y.; Lu, L.; Liu, G.; Yu, Y.; Hu, C. Three-dimensional β-cyclodextrin functionalized graphene aerogels: An enhanced electrochemical sensing platform for quantification of Ponceau 4R. J. Electroanal. Chem. 2018, 823, 437–444. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Lu, X.; Yang, J.; Wu, K. Multi-wall carbon nanotube film-based electrochemical sensor for rapid detection of Ponceau 4R and Allura Red. Food Chem. 2020, 122, 909–913. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J. A Facile Method Used for Simultaneous Determination of Ponceau 4R, Allura Red and Tartrazine in Alcoholic Beverages. J. Electrochem. Soc. 2015, 162, H321–H327. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Wang, Z.; Zhang, J.; Duan, X.; Xu, J.; Wen, Y. Trace analysis of Ponceau 4R in soft drinks using differential pulse stripping voltammetry at SWCNTs composite electrodes based on PEDOT:PSS derivatives. Food Chem. 2015, 180, 186–193. [Google Scholar] [CrossRef]
- Cheng, Q.; Xia, S.; Tong, J.; Wu, K. Highly-sensitive electrochemical sensing platforms for food colourants based on the property-tuning of porous carbon. Anal. Chim. Acta 2015, 887, 75–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, L.; Liu, X.; Liu, B.; Wu, K. Highly-sensitive and rapid detection of ponceau 4R and tartrazine in drinks using alumina microfibers-based electrochemical sensor. Food Chem. 2015, 166, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zeng, Q.; Wang, L. Ultrasensitive electrochemical determination of Ponceau 4R with a novel ε-MnO2 microspheres/chitosan modified glassy carbon electrode. Electrochim. Acta 2016, 206, 176–183. [Google Scholar] [CrossRef]
- Blanco, E.; Arias, L.; Vazquez, L.; del Pozo, M.; Sánchez, L.; Petit-Domínguez, M.D.; Quintana, C.; Casero, E. Sensor based on diamond nanoparticles and WS2 for ponceau 4R and tartrazine determination: Influence of green solvents employed for WS2 exfoliation. FlatChem 2020, 23, 100185. [Google Scholar] [CrossRef]
- Cyriac, S.T.; Sivasankaran, U.; Kumar, K.G. Biopolymer Based Electrochemical Sensor for Ponceau 4R: An Insight into Electrochemical Kinetics. J. Electrochem. Soc. 2018, 165, B746–B752. [Google Scholar] [CrossRef]
- Darabi, R.; Shabani-Nooshabadi, M.; Karimi-Maleh, H.; Gholami, A. The potential of electrochemistry for one-pot and sensitive analysis of patent blue V, tartrazine, acid violet 7 and ponceau 4R in foodstuffs using IL/Cu-BTC MOF modified sensor. Food Chem. 2022, 368, 130811. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Jin, Y.; Wu, K.; Wan, C.; Yang, N.; Tang, Y. Morphology-dependent electrochemical sensing performance of metal (Ni, Co, Zn)-organic frameworks. Anal. Chim. Acta 2018, 1031, 60–66. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Zare, N.; Karimi, F.; Karaman, C.; Alizadeh, M.; Vasseghian, Y. Recent advances in Ponceau dyes monitoring as food colorant substances by electrochemical sensors and developed procedures for their removal from real samples. Food Chem. Toxicol. 2022, 161, 112830. [Google Scholar] [CrossRef]
- Torrinha, Á.; Morais, S. Electrochemical (bio)sensors based on carbon cloth and carbon paper: An overview. TrAC Trends Anal. Chem. 2021, 142, 116324. [Google Scholar] [CrossRef]
- Stozhko, N.Y.; Bukharinova, M.A.; Khamzina, E.I.; Tarasov, A.V.; Sokolkov, S.V. Film Carbon Veil-Based Electrode Modified with Triton X-100 for Nitrite Determination. Chemosensors 2020, 8, 78. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Bukharinova, M.A.; Stozhko, N.Y.; Sokolkov, S.V.; Tarasov, A.V.; Vidrevich, M.B. Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid. Sensors 2020, 20, 1800. [Google Scholar] [CrossRef]
- Tarasov, A.; Khamzina, E.; Bukharinova, M.; Stozhko, N. Flexible Potentiometric Sensor System for Non-Invasive Determination of Antioxidant Activity of Human Skin: Application for Evaluating the Effectiveness of Phytocosmetic Products. Chemosensors 2021, 9, 76. [Google Scholar] [CrossRef]
- Stozhko, N.Y.; Khamzina, E.I.; Bukharinova, M.A.; Tarasov, A.V. An Electrochemical Sensor Based on Carbon Paper Modified with Graphite Powder for Sensitive Determination of Sunset Yellow and Tartrazine in Drinks. Sensors 2022, 22, 4092. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Y.; Liao, H.; Engelhard, M.H.; Yin, G.; Lin, Y. Polyelectrolyte-Induced Reduction of Exfoliated Graphite Oxide: A Facile Route to Synthesis of Soluble Graphene Nanosheets. ACS Nano 2011, 5, 1785–1791. [Google Scholar] [CrossRef]
- Valade, D.; Boschet, F.; Roualdès, S.; Ameduri, B. Preparation of solid alkaline fuel cell binders based on fluorinated poly(diallyldimethylammonium chloride)s [poly(DADMAC)] or poly(chlorotrifluoroethylene-co-DADMAC) copolymers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2043–2058. [Google Scholar] [CrossRef]
- Liu, L.-J.; Gao, X.; Zhang, P.; Feng, S.-L.; Hu, F.-D.; Li, Y.-D.; Wang, C.-M. Ultrasensitive Detection of Ferulic Acid Using Poly(diallyldimethylammonium chloride) Functionalized Graphene-Based Electrochemical Sensor. J. Anal. Methods Chem. 2014, 2014, 424790. [Google Scholar] [CrossRef]
- Peng, X.; Zou, J.; Liu, Z.; Guo, Y. Electrochemical sensor for facile detection of trace N-nitrosodiphenylamine based on poly(diallyldimethylammonium chloride)-stabilized graphene/platinum nanoparticles. New J. Chem. 2019, 43, 820–826. [Google Scholar] [CrossRef]
- Liu, X.-P.; Tong, J.; Yuan, Z.; Yang, Y.; Mao, C.-J.; Niu, H.-L.; Jin, B.-K.; Zhang, S.-Y. Highly Sensitive Electrochemical Dopamine Sensor from Poly(diallyldimethylammonium chloride)-Functionalized Graphene Nanoribbon/Gold Nanoparticle Nanocomposite. J. Nanosci. Nanotechnol. 2016, 16, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shi, M.; Yue, X.; Qu, L. Detection of allura red based on the composite of poly (diallyldimethylammonium chloride) functionalized graphene and nickel nanoparticles modified electrode. Sens. Actuators B Chem. 2016, 225, 398–404. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Wang, A.; Cai, W.; Lin, H. Sensitive determination of quinoline yellow using poly (diallyldimethylammonium chloride) functionalized reduced graphene oxide modified grassy carbon electrode. Food Chem. 2015, 181, 127–132. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Vikulova, E. Nanomaterials: Electrochemical Properties and Application in Sensors. Phys. Sci. Rev. 2018, 3, 1–39. [Google Scholar] [CrossRef]
- Ghosh, T.; Raj, G.B.; Dash, K.K. A comprehensive review on nanotechnology based sensors for monitoring quality and shelf life of food products. Meas. Food 2022, 7, 100049. [Google Scholar] [CrossRef]
- Stozhko, N.Y.; Bukharinova, M.A.; Khamzina, E.I.; Tarasov, A.V. Electrochemical Properties of Phytosynthesized Gold Nanoparticles for Electrosensing. Sensors 2022, 22, 311. [Google Scholar] [CrossRef]
- Wandrey, C.; Hernández-Barajas, J.; Hunkeler, D. Diallyldimethylammonium Chloride and its Polymers. In Radical Polymerisation Polyelectrolytes. Advances in Polymer Science; Capek, I., Hernfández-Barajas, J., Hunkeler, D., Reddinger, J.L., Reynolds, J.R., Wandrey, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 145, pp. 123–183. [Google Scholar] [CrossRef]
- Wandrey, C.; Jaeger, W.; Reinisch, G. Zur Bestimmung der relativen Molmasse von Poly(dimethyl-diallyl-ammoniumchlorid) durch Lösungsviskosimetrie. Acta Polym. 1982, 33, 156–158. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Khamzina, E.; Vidrevich, M. Potentiometric method of plant microsuspensions antioxidant activity determination. Food Chem. 2019, 278, 653–658. [Google Scholar] [CrossRef]
- Burns, D.T.; Danzer, K.; Townshend, A. Use of the term “recovery” and “apparent recovery” in analytical procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 2002, 74, 2201–2205. [Google Scholar] [CrossRef]
- Yang, R.; Miao, D.; Liang, Y.; Qu, L.; Li, J.; Harrington, P.D.B. Ultrasensitive electrochemical sensor based on CdTe quantum dots-decorated poly(diallyldimethylammonium chloride)-functionalized graphene nanocomposite modified glassy carbon electrode for the determination of puerarin in biological samples. Electrochim. Acta 2015, 173, 839–846. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, L.; Zhu, Y.; Ai, F.; Li, H.; Li, Y.; Jiang, Z. The Effect of Immersion Corrosion Time on Electrochemical Corrosion Behavior and the Corrosion Mechanism of EH47 Ship Steel in Seawater. Metals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Tolouei, N.E.; Ghamari, S.; Shavezipur, M. Development of circuit models for electrochemical impedance spectroscopy (EIS) responses of interdigitated MEMS biochemical sensors. J. Electroanal. Chem. 2020, 878, 114598. [Google Scholar] [CrossRef]
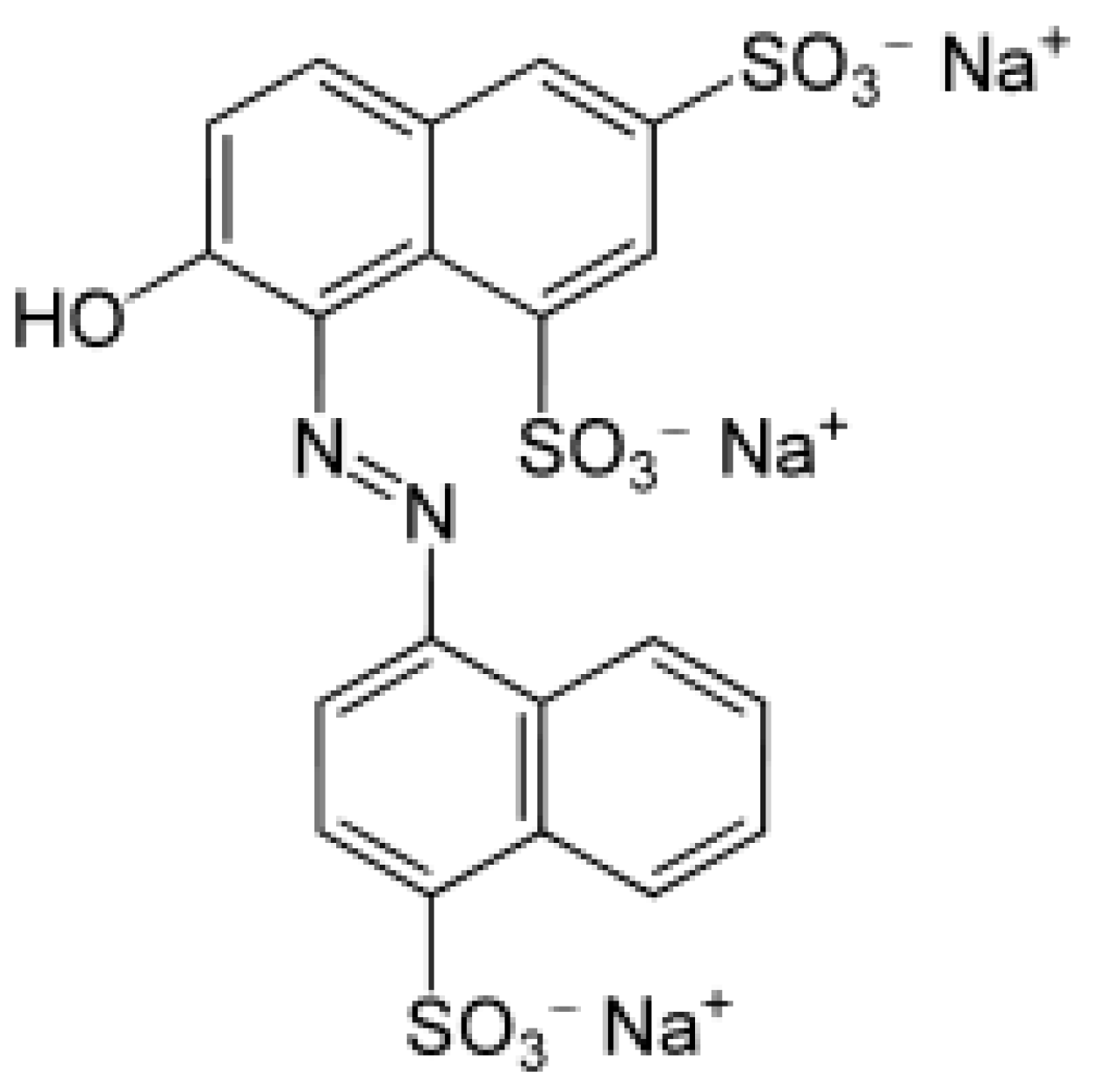
- Bevziuk, K.; Chebotarev, A.; Snigur, D.; Bazel, Y.; Fizer, M.; Sidey, V. Spectrophotometric and theoretical studies of the protonation of Allura Red AC and Ponceau 4R. J. Mol. Struct. 2017, 1144, 216–224. [Google Scholar] [CrossRef]
- Hata, H.; Kobayashi, Y.; Mallouk, T.E. Encapsulation of Anionic Dye Molecules by a Swelling Fluoromica through Intercalation of Cationic Polyelectrolytes. Chem. Mater. 2007, 19, 79–87. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Sun, Q.; Zhao, J. Ultrasensitive and simultaneous determination of the isomers of Amaranth and Ponceau 4R in foods based on new carbon nanotube/polypyrrole composites. Food Chem. 2015, 172, 873–879. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Shentu, C.; Wang, W.; Chen, Z. Simultaneous determination of the isomers of Ponceau 4R and Amaranth using an expanded graphite paste electrode. Food Chem. 2014, 160, 11–15. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Y.; Feng, J.; Liu, J.; Tian, Y.; Xiao, J.; He, Q. An ultrasensitive ponceau 4R detection sensor based on molecularly imprinted electrode using pod-like cerium molybdate and multi-walled carbon nanotubes hybrids. J. Food Compos. Anal. 2022, 114, 104849. [Google Scholar] [CrossRef]

| Sensor | LOD (nM) | LR (µM) | Method | Accumulation Time/Potential | Reference |
|---|---|---|---|---|---|
| Alumina microfibers/CPE | 0.8 | 0.001–0.1 | DPV | 180 s/0.4 V | [15] |
| CNT-ppy/GCE | 1 | 0.008–0.1, 0.1–1.0 | SWV | 300 s | [46] |
| Poly(EDOT-AA-co-EDOT):PSS– SWCNTs–PVA/GCE | 1.8 | 0.0055–110.6 | DPV | 120 s | [13] |
| EGPE | 2 | 0.06–4 | SWV | 400 s/0.4 V | [47] |
| GAs/GCE β-CD/GAs/GCE | 3 0.3 | 0.01–1.0 0.001–1.0 | DPV | 180 s/0.2 V | [10] |
| PC/GCE | 3.5 | 0.004–1.654 | DPV | 240 s/0.1 V | [14] |
| TiO2/ERGO/GCE | 4 | 0.01–5 | AdSDPV | 120 s/−0.1 V | [9] |
| MIPs/Ce2Mo3O13/MWCNTs/GCE | 7 | 0.01–1 | DPV | 65 s | [48] |
| r-GO/GCE | 28.4 | 0.2–20 | SWV | 30 s | [8] |
| poly(L-Cysteine)/GCE | 37.3 | 1–10 | Chronocoulometry | 240 s | [18] |
| WS2/DNP/GCE | 7800 | 5–50 | DPV | 15 s | [17] |
| phyto-AuNPs/PDDA-G/CP | 0.6 | 0.001–2 | DPV | 180 s/0 V | This work |
| Sample | Found in Sample (µM) | RSD (%) | Added (µM) | Found in Sample with Additives (µM) | RSD (%) | Found Additive (µM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|---|---|---|---|
| Carbonated soft drink “Barbaris” | 83.1 ± 3.1 | 3.1 | 85.0 | 169.8 ± 5.8 | 1.4 | 84.8 ± 5.4 | 2.8 | 99 |
| Fruit drink | 102.1 ± 4.9 | 4.9 | 100.0 | 198.2 ± 2.6 | 1.1 | 98.2 ± 2.6 | 2.1 | 98 |
| Popsicle | 40.8 ± 1.2 | 1.2 | 40.0 | 79.9 ± 3.1 | 1.5 | 39.2 ± 3.0 | 3.1 | 98 |
| Chewing candy 1 | 24.4 ± 1.6 | 5.2 | 25.0 | 48.9 ± 2.5 | 4.1 | 25.2 ± 0.9 | 2.9 | 102 |
| Chewing candy 2 | 20.4 ± 1.1 | 4.1 | 20.0 | 40.1 ± 1.8 | 3.6 | 19.9 ± 1.5 | 6.2 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stozhko, N.Y.; Khamzina, E.I.; Bukharinova, M.A.; Tarasov, A.V.; Kolotygina, V.Y.; Lakiza, N.V.; Kuznetcova, E.D. Carbon Paper Modified with Functionalized Poly(diallyldimethylammonium chloride) Graphene and Gold Phytonanoparticles as a Promising Sensing Material: Characterization and Electroanalysis of Ponceau 4R in Food Samples. Nanomaterials 2022, 12, 4197. https://doi.org/10.3390/nano12234197
Stozhko NY, Khamzina EI, Bukharinova MA, Tarasov AV, Kolotygina VY, Lakiza NV, Kuznetcova ED. Carbon Paper Modified with Functionalized Poly(diallyldimethylammonium chloride) Graphene and Gold Phytonanoparticles as a Promising Sensing Material: Characterization and Electroanalysis of Ponceau 4R in Food Samples. Nanomaterials. 2022; 12(23):4197. https://doi.org/10.3390/nano12234197
Chicago/Turabian StyleStozhko, Natalia Yu., Ekaterina I. Khamzina, Maria A. Bukharinova, Aleksey V. Tarasov, Veronika Yu. Kolotygina, Natalia V. Lakiza, and Ekaterina D. Kuznetcova. 2022. "Carbon Paper Modified with Functionalized Poly(diallyldimethylammonium chloride) Graphene and Gold Phytonanoparticles as a Promising Sensing Material: Characterization and Electroanalysis of Ponceau 4R in Food Samples" Nanomaterials 12, no. 23: 4197. https://doi.org/10.3390/nano12234197
APA StyleStozhko, N. Y., Khamzina, E. I., Bukharinova, M. A., Tarasov, A. V., Kolotygina, V. Y., Lakiza, N. V., & Kuznetcova, E. D. (2022). Carbon Paper Modified with Functionalized Poly(diallyldimethylammonium chloride) Graphene and Gold Phytonanoparticles as a Promising Sensing Material: Characterization and Electroanalysis of Ponceau 4R in Food Samples. Nanomaterials, 12(23), 4197. https://doi.org/10.3390/nano12234197