Influence of ZrO2 Nanoparticle Addition on the Optical Properties of Denture Base Materials Fabricated Using Additive Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nanocomposite Mixture
2.1.1. Preparation of Heat-Polymerized Acrylic Resin Specimens
2.1.2. Preparation of 3D-Printed Specimens
2.2. NP Distribution and Bonding Analysis
2.3. Thermal Cycling Procedures
2.4. Translucency Test
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slade, G.D.; Akinkugbe, A.A.; Sanders, A.E. Projections of US edentulism prevalence following 5 decades of decline. J. Dent. Res. 2014, 93, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F. The current and future treatment of edentulism. J. Prosthodont. 2009, 18, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Al-Rafee, M.A. The epidemiology of edentulism and the associated factors: A literature review. J. Fam. Med. Prim. Care 2020, 9, 1841–1843. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Saponaro, P.C. Management of edentulous patients. Dent. Clin. N. Am. 2019, 63, 249–261. [Google Scholar] [CrossRef]
- Anadioti, E.; Musharbash, L.; Blatz, M.B.; Papavasiliou, G.; Kamposiora, P. 3D-printed complete removable dental prostheses: A narrative review. BMC Oral Health 2020, 20, 343. [Google Scholar] [CrossRef]
- Alzayyat, S.T.; Almutiri, G.A.; Aljandan, J.K.; Algarzai, R.M.; Khan, S.Q.; Akhtar, S.; Matin, A.; Gad, M.M. Antifungal efficacy and physical properties of poly (methylmethacrylate) denture base material reinforced with SiO2 nanoparticles. J. Prosthodont. 2021, 30, 500–508. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic applications of polymethyl methacrylate (PMMA): An update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Shah, R.; Aras, M. Esthetics in removable partial denture—A review. Kathmandu Univ. Med. J. 2013, 11, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Alshehri, S.Z.; Alhamid, S.A.; Albarrak, A.; Khan, S.Q.; Alshahrani, F.A.; Alqarawi, F.K. Water sorption, solubility, and translucency of 3D-printed denture base resins. Dent. J. 2022, 10, 42. [Google Scholar] [CrossRef]
- Patras, M.; Kourtis, S.; Sykaras, N. Creating natural-looking removable prostheses: Combining art and science to imitate nature. J. Esthet. Restor. Dent. 2012, 24, 160–168. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Alqarawi, F.K.; Emam, A.M.; Khan, S.Q.; Akhtar, S.; Mahrous, A.A.; Al-harbi, F.A. Translucency of nanoparticle-reinforced PMMA denture base material: An in-vitro comparative study. Dent. Mater. J. 2021, 40, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Lee, Y.K. Translucency of varied brand and shade of resin composites. Am. J. Dent. 2008, 21, 229–232. [Google Scholar] [PubMed]
- Johnston, W.M.; Ma, T.; Kienle, B.H. Translucency parameter of colorants for maxillofacial prostheses. Int. J. Prosthodont. 1995, 8, 79–86. [Google Scholar] [PubMed]
- Yu, B.; Ahn, J.S.; Lee, Y.K. Measurement of translucency of tooth enamel and dentin. Acta Odontol. Scand. 2009, 67, 57–64. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Al-Abidi, K.S.; Akhtar, S. Effect of zirconium oxide nanoparticles addition on the optical and tensile properties of polymethyl methacrylate denture base material. Int. J. Nanomed. 2018, 13, 283–292. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ebrahim, M.I. Effect of zirconium oxide nano-fillers addition on the flexural strength, fracture toughness, and hardness of heat-polymerized acrylic resin. World J. Nano Sci. Eng. 2014, 4, 50–57. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, X.J.; Huang, Z.L.; Zhu, B.S.; Chen, R.R. Hybrid effects of zirconia nanoparticles with aluminum borate whiskers on mechanical properties of denture base resin PMMA. Dent. Mater. J. 2014, 33, 141–146. [Google Scholar] [CrossRef]
- Abushowmi, T.H.; AlZaher, Z.A.; Almaskin, D.F.; Qaw, M.S.; Abualsaud, R.; Akhtar, S.; Al-Thobity, A.M.; Al-Harbi, F.A.; Gad, M.M.; Baba, N.Z. Comparative effect of glass fiber and nano-filler addition on denture repair strength. J. Prosthodont. 2020, 29, 261–268. [Google Scholar] [CrossRef]
- Gad, M.M.; Rahoma, A.; Al-Thobity, A.M.; ArRejaie, A.S. Influence of incorporation of ZrO2 nanoparticles on the repair strength of polymethyl methacrylate denture bases. Int. J. Nanomed. 2016, 11, 5633–5643. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef]
- Jordan, J.; Jacob, K.I.; Tannenbaum, R.; Sharaf, M.A.; Jasiuk, I. Experimental trends in polymer nanocomposites—A review. Mater. Sci. Eng. A 2005, 393, 1–11. [Google Scholar] [CrossRef]
- Kim, J.J.; Moon, H.J.; Lim, B.S.; Lee, Y.K.; Rhee, S.H.; Yang, H.C. The effect of nanofiller on the opacity of experimental composites. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 332–338. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Y.; Xie, C.; Powers, J.M.; Kiat-amnuay, S. Color stability of pigmented maxillofacial silicone elastomer: Effects of nano-oxides as opacifiers. J. Dent. 2010, 38 (Suppl. 2), e100–e105. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Aszrin, F.N.; Takarini, V.; Hasratiningsih, Z.; Purwasasmita, B.S. Translucency evaluation of polymethyl methacrylate (PMMA) reinforced with ZrO2-Al2O3-SiO2 filler system in fabricating indirect restoration. UI Proc. Health Med. 2016, 1, 48–52. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.K. Influence of scattering/absorption characteristics on the color of resin composites. Dent. Mater. 2007, 23, 124–131. [Google Scholar] [CrossRef]
- Ayad, N.M.; Badawi, M.F.; Fatah, A.A. Effect of reinforcement of high impact acrylic resin with micro-zirconia on some physical and mechanical properties. Arch. Oral Res. 2008, 4, 145–151. [Google Scholar]
- Dawood, A.; Marti Marti, B.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef]
- Kessler, A.; Hickel, R.; Reymus, M. 3D Printing in dentistry–State of the art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef]
- Bassoli, E.; Gatto, A.; Iuliano, L.; Grazia Violante, M.G. 3D printing technique applied to rapid casting. Rapid Prototyp. J. 2007, 13, 148–155. [Google Scholar] [CrossRef]
- Bae, E.J.; Jeong, I.D.; Kim, W.C.; Kim, J.H. A comparative study of additive and subtractive manufacturing for dental restorations. J. Prosthet. Dent. 2017, 118, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Goodacre, B.J.; Goodacre, C.J.; Baba, N.Z.; Kattadiyil, M.T. Comparison of denture base adaptation between CAD-CAM and conventional fabrication techniques. J. Prosthet. Dent. 2016, 116, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, M. Review on polymethyl methacrylate as denture base materials. Malays. J. Microsc. 2018, 31, 14. [Google Scholar]
- Vojdani, M.; Bagheri, R.; Khaledi, A.A.R. Effects of aluminum oxide addition on the flexural strength, surface hardness, and roughness of heat-polymerized acrylic resin. J. Dent. Sci. 2012, 7, 238–244. [Google Scholar] [CrossRef]
- Meng, T.R.; Latta, M.A. Physical properties of four acrylic denture base resins. J. Contemp. Dent. Pract. 2005, 6, 93–100. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Jia, Y.G.; Lu, B.; Ren, L. A study of 3D-printable reinforced composite resin: PMMA modified with silver nanoparticles loaded cellulose nanocrystal. Materials 2018, 11, 2444. [Google Scholar] [CrossRef]
- Mubarak, S.; Dhamodharan, D.; Kale, M.B.; Divakaran, N.; Senthil, T.; Wu, L.; Wang, J. A novel approach to enhance mechanical and thermal properties of SLA 3D-printed structure by incorporation of metal-metal oxide nanoparticles. Nanomaterials 2020, 10, 217. [Google Scholar] [CrossRef]
- Aati, S.; Akram, Z.; Ngo, H.; Fawzy, A.S. Development of 3D-printed resin reinforced with modified ZrO2 nanoparticles for long-term provisional dental restorations. Dent. Mater. 2021, 37, e360–e374. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Harbi, F.A.; Akhtar, S.; Fouda, S.M. 3D-printable denture base resin containing SiO2 nanoparticles: An in vitro analysis of mechanical and surface properties. J. Prosthodont. 2022. [Google Scholar] [CrossRef]
- Alshaikh, A.A.; Khattar, A.; Almindil, I.A.; Alsaif, M.H.; Akhtar, S.; Khan, S.Q.; Gad, M.M. 3D-printed nanocomposite denture-base resins: Effect of ZrO2 nanoparticles on the mechanical and surface properties in vitro. Nanomaterials 2022, 12, 2451. [Google Scholar] [CrossRef]
- Zidan, S.; Silikas, N.; Alhotan, A.; Haider, J.; Yates, J. Investigating the mechanical properties of ZrO2-impregnated PMMA nanocomposite for denture-based applications. Materials 2019, 12, 1344. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Akhtar, S.; Fouda, S.M. Double-layered acrylic resin denture base with nanoparticle additions: An in vitro study. J. Prosthet. Dent. 2022, 127, 174–183. [Google Scholar] [CrossRef]
- Alshahrani, F.A.; Gad, M.M.; Al-Thobity, A.M.; Akhtar, S.; Kashkari, A.; Alzoubi, F.; Yilmaz, B. Effect of treated zirconium dioxide nanoparticles on the flexural properties of autopolymerized resin for interim fixed restorations: An in vitro study. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Rahman, H.A. The effect of addition nano particle ZrO2 on some properties of autoclave processed heat cure acrylic denture base material. J. Bagh. Coll. Dent. 2015, 27, 32–39. [Google Scholar]
- Unkovskiy, A.; Bui, P.H.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects build orientation, positioning, and curing influence dimensional accuracy and flexural properties of stereolithographically printed resin. Dent. Mater. 2018, 34, e324–e333. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, J.Y.; Mangal, U.; Seo, J.Y.; Lee, M.J.; Jin, J.; Yu, J.H.; Choi, S.H. Durable oral biofilm resistance of 3D-Printed dental base polymers containing zwitterionic materials. Int. J. Mol. Sci. 2021, 22, 417. [Google Scholar] [CrossRef]
- Gale, M.S.; Darvell, B.W. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Silva, C.d.S.; Machado, A.L.; Chaves, C.d.A.L.; Pavarina, A.C.; Vergani, C.E. Effect of thermal cycling on denture base and autopolymerizing reline resins. J. Appl. Oral Sci. 2013, 21, 219–224. [Google Scholar] [CrossRef]
- Jurišić, S.; Jurišić, G.; Zlatarić, D.K. In vitro evaluation and comparison of the translucency of two different all-ceramic systems. Acta Stomatol. Croat. 2015, 49, 195–203. [Google Scholar] [CrossRef]
- Kawai, N.; Lin, J.; Youmaru, H.; Shinya, A.; Shinya, A. Effects of three luting agents and cyclic impact loading on shear bond strengths to zirconia with tribochemical treatment. J. Dent. Sci. 2012, 7, 118–124. [Google Scholar] [CrossRef]
- Mallineni, S.K.; Nuvvula, S.; Matinlinna, J.P.; Yiu, C.K.; King, N.M. Bio compatibility of various dental materials in contemporary dentistry: A narrative insight. J. Investig. Clin. Dent. 2013, 4, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hada, T.; Kanazawa, M.; Miyamoto, N.; Liu, H.; Iwaki, M.; Komagamine, Y.; Minakuchi, S. Effect of different filler contents and printing directions on the mechanical properties for photopolymer resins. Int. J. Mol. Sci. 2022, 23, 2296. [Google Scholar] [CrossRef] [PubMed]
- El-Tamimi, K.M.; Bayoumi, D.A.; Ahmed, M.M.Z.; Albaijan, I.; El-Sayed, M.E. The effect of salinized nano ZrO2 Particles on the microstructure, hardness, and wear behavior of acrylic denture tooth nanocomposite. Polymers 2022, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Gowri, S.; Rajiv Gandhi, R.R.; Sundrarajan, M. Structural, optical, antibacterial and antifungal properties of zirconia nanoparticles by biobased protocol. J. Mater. Sci. Technol. 2014, 30, 782–790. [Google Scholar] [CrossRef]
- Kelly, J.R.; Nishimura, I.; Campbell, S.D. Ceramics in dentistry: Historical roots and current perspectives. J. Prosthet. Dent. 1996, 75, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Altonbary, G.Y.; Emera, R.M.K. Patient satisfaction and masticatory performance of zirconia bar compared to cobalt chromium bar retaining mandibular implant overdenture: A crossover study. J. Oral Rehabil. 2021, 48, 827–835. [Google Scholar] [CrossRef]
- Reymus, M.; Lümkemann, N.; Stawarczyk, B. 3D-printed material for temporary restorations: Impact of print layer thickness and post-curing method on degree of conversion. Int. J. Comput. Dent. 2019, 22, 231–237. [Google Scholar]
- Zeidan, A.A.E.; Sherif, A.F.; Baraka, Y.; Abualsaud, R.; Abdelrahim, R.A.; Gad, M.M.; Helal, M.A. Evaluation of the effect of different construction techniques of CAD-CAM milled, 3D-printed, and polyamide denture base resins on flexural strength: An in vitro comparative study. J. Prosthodont. 2022. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Abualsaud, R.; Alshahrani, F.A.; Al-Thobity, A.M.; Khan, S.Q.; Akhtar, S.; Ateeq, I.S.; Helal, M.A.; Al-Harbi, F.A. Strength and surface properties of a 3D-printed denture base polymer. J. Prosthodont. 2022, 31, 412–418. [Google Scholar] [CrossRef]
- Asopa, V.; Suresh, S.; Khandelwal, M.; Sharma, V.; Asopa, S.S.; Kaira, L.S. A comparative evaluation of properties of zirconia reinforced high impact acrylic resin with that of high impact acrylic resin. Saudi J. Dent. Res. 2015, 6, 146–151. [Google Scholar] [CrossRef]
- Shirkavand, S.; Moslehifard, E. Effect of TiO2 nanoparticles on tensile strength of dental acrylic resins. J. Dent. Res. Dent. Clin. Dent. Prospects 2014, 8, 197–203. [Google Scholar] [CrossRef]
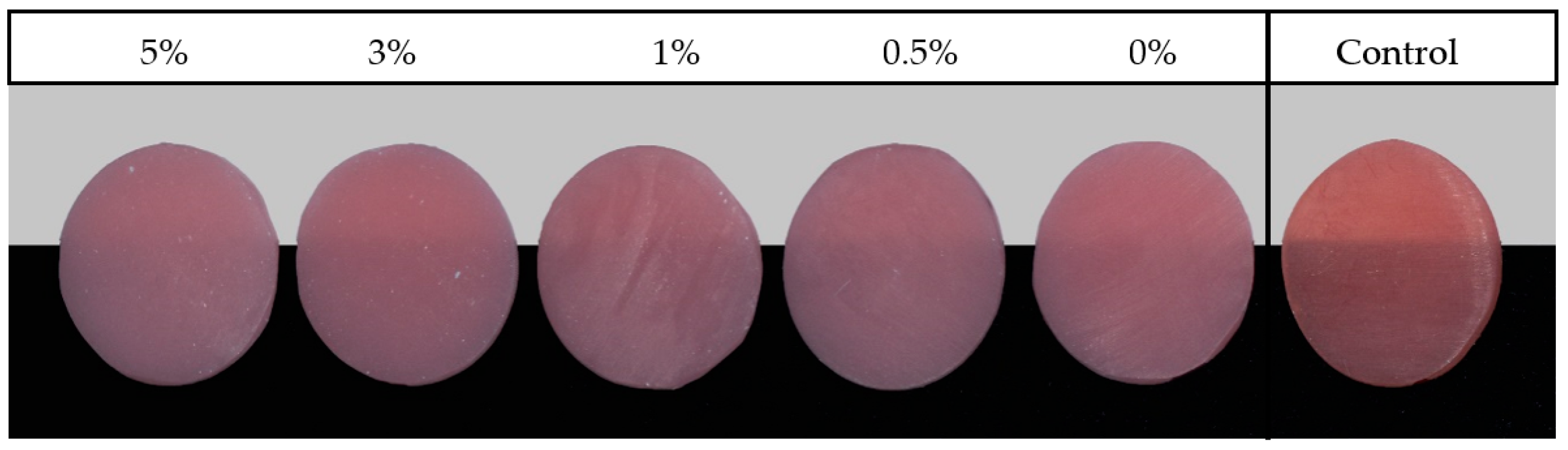
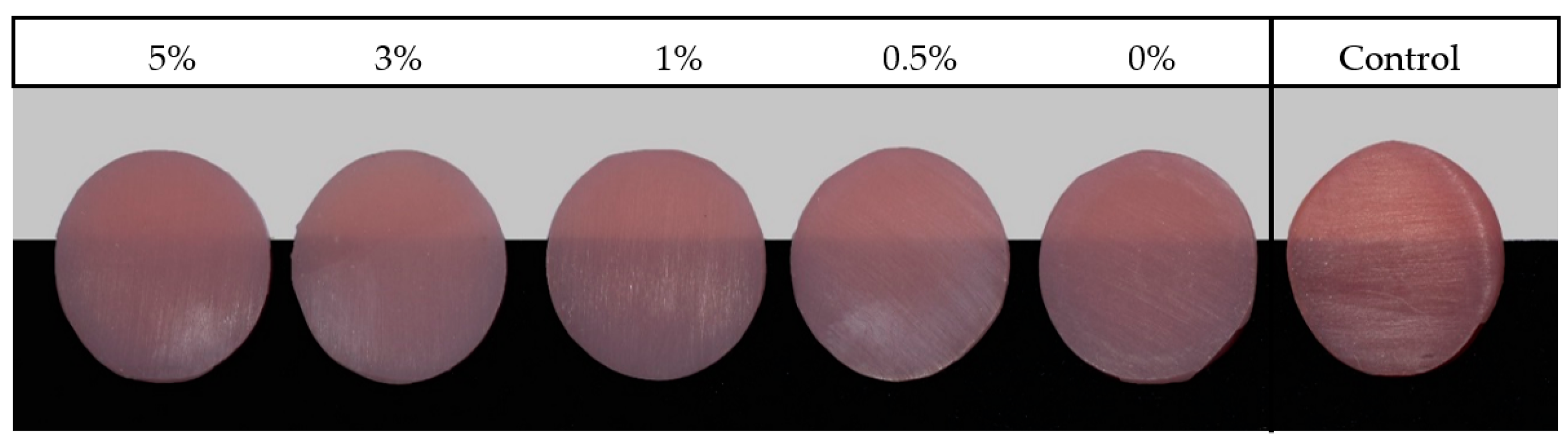
| Material Brand Name/Printers/Manufacture/Printing Technology | Composition | Printing Parameters | Post Printing Conditions | ||||
|---|---|---|---|---|---|---|---|
| Layer Thickness | Orientations | Light Source /Wavelength | Rinsing/ Cleaning | Post Curing Machine | Post Curing Time | ||
| NextDent Denture 3D+/ NextDent 5100 3D NextDent B.V Soesterberg, The Netherlands/ Stereolithography | Methacrylic oligomers, methacrylate monomer, inorganic filler, phosphine oxides, pigments | 50 m | 90° | UV light/405 nm | Isopropyl alcohol 99.9%/ glycerol | LC-3DPrint Box, NextDent, Soesterberg, The Netherlands | 10 min |
| ASIGA DentaBASE ASIGA MAX UV/ASIGA, Erfurt, Germany/Digital light processing (DLP) | 7,7,9(or 7,9,9)-trimethyl-4,13-dioxo-3,14-dioxa-5,12-diazahexadecane- 1,16-diyl bismethacrylate; Diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide; Tetrahydrofurfuryl methacrylate | 50 m | 90° | UV light/405 nm | Isopropyl alcohol 99.9%/ glycerol | ASIGA Flash, ASIGA, Sydney, Australia | 20 min |
| Finishing and Polishing | Thermocycling | |||||
|---|---|---|---|---|---|---|
| Finishing Paper | Polishing Suspension | Polishing Cloth | Polishing Machine | Machine | Cycles | Temperature/Time |
| Silicon carbide grinding paper 800, 1500, and 2000 grit | 0.050 m - Master Prep polishing suspension; Buehler GmbH | TexMet C10in, 42-3210; Buehler GmbH, Düsseldorf, Germany | Metaserv 250 grinder-polisher; Buehler GmbH, Lake Bluff, IL, USA | Thermocycler THE-1100/THE-1200, SD Mechatronik GMBH Miesbacher Str. 34 83,620 Feldkirchen-Westerham Germany | 5000 cycles | 5–55 °C /30 s of dwell time and 5 s for dripping |
| Material | ZrO2NP Concentration | Mean (SD) | p-Value |
|---|---|---|---|
| HP | Control | 11.04 (1.3) | 0.000 * |
| NextDent | 0% | 6.32 ± 0.48 a,b,c | |
| 0.5% | 6.40 ± 0.55 a,d,e | ||
| 1% | 6.12 ± 0.33 b,d,f | ||
| 3% | 5.66 ± 0.27 c,e,f,g | ||
| 5% | 4.91 ± 0.35 g |
| Material | ZrO2NP Concentration | Mean (SD) | p-Value |
|---|---|---|---|
| HP | Control | 11.04 ± 1.3 | 0.000 * |
| ASIGA | 0% | 9.26 ± 0.48 a,b | |
| 0.5% | 8.86 ± 0.75 a,c | ||
| 1% | 8.40 ± 0.47 b,c | ||
| 3% | 7.13 ± 0.47 d | ||
| 5% | 7.46 ± 0.23 d |
| ASIGA | ||||||
|---|---|---|---|---|---|---|
| 0% | 0.5% | 1% | 3% | 5% | ||
| NextDent | 0% | 0.000 * | 0.000 * | 0.000 * | 0.005 * | 0.000 * |
| 0.5% | 0.000 * | 0.000 * | 0.000 * | 0.02 * | 0.000 * | |
| 1% | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| 3% | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| 5% | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| Source | Type III Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Corrected Model | 329.752a | 10 | 32.975 | 94.621 | 0.000 * |
| Intercept | 5839.598 | 1 | 5839.598 | 16,756.418 | 0.000 * |
| Material | 137.476 | 1 | 137.476 | 394.479 | 0.000 * |
| Concentration | 42.095 | 4 | 10.524 | 30.198 | 0.000 * |
| Material * concentration | 5.880 | 4 | 1.470 | 4.218 | 0.003 * |
| Error | 34.501 | 99 | 0.348 | ||
| Total | 6412.446 | 110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattar, A.; Alsaif, M.H.; Alghafli, J.A.; Alshaikh, A.A.; Alsalem, A.M.; Almindil, I.A.; Alsalman, A.M.; Alboori, A.J.; Al-Ajwad, A.M.; Almuhanna, H.M.; et al. Influence of ZrO2 Nanoparticle Addition on the Optical Properties of Denture Base Materials Fabricated Using Additive Technologies. Nanomaterials 2022, 12, 4190. https://doi.org/10.3390/nano12234190
Khattar A, Alsaif MH, Alghafli JA, Alshaikh AA, Alsalem AM, Almindil IA, Alsalman AM, Alboori AJ, Al-Ajwad AM, Almuhanna HM, et al. Influence of ZrO2 Nanoparticle Addition on the Optical Properties of Denture Base Materials Fabricated Using Additive Technologies. Nanomaterials. 2022; 12(23):4190. https://doi.org/10.3390/nano12234190
Chicago/Turabian StyleKhattar, Abdulrahman, Majed H. Alsaif, Jawad A. Alghafli, Ali A. Alshaikh, Ali M. Alsalem, Ibrahim A. Almindil, Abdulsalam M. Alsalman, Ali J. Alboori, Abdullah M. Al-Ajwad, Hussain M Almuhanna, and et al. 2022. "Influence of ZrO2 Nanoparticle Addition on the Optical Properties of Denture Base Materials Fabricated Using Additive Technologies" Nanomaterials 12, no. 23: 4190. https://doi.org/10.3390/nano12234190
APA StyleKhattar, A., Alsaif, M. H., Alghafli, J. A., Alshaikh, A. A., Alsalem, A. M., Almindil, I. A., Alsalman, A. M., Alboori, A. J., Al-Ajwad, A. M., Almuhanna, H. M., Khan, S. Q., AlRumaih, H. S., & Gad, M. M. (2022). Influence of ZrO2 Nanoparticle Addition on the Optical Properties of Denture Base Materials Fabricated Using Additive Technologies. Nanomaterials, 12(23), 4190. https://doi.org/10.3390/nano12234190







