Iron and Copper Doped Zinc Oxide Nanopowders as a Sensitizer of Industrial Energetic Materials to Visible Laser Radiation
Abstract
1. Introduction
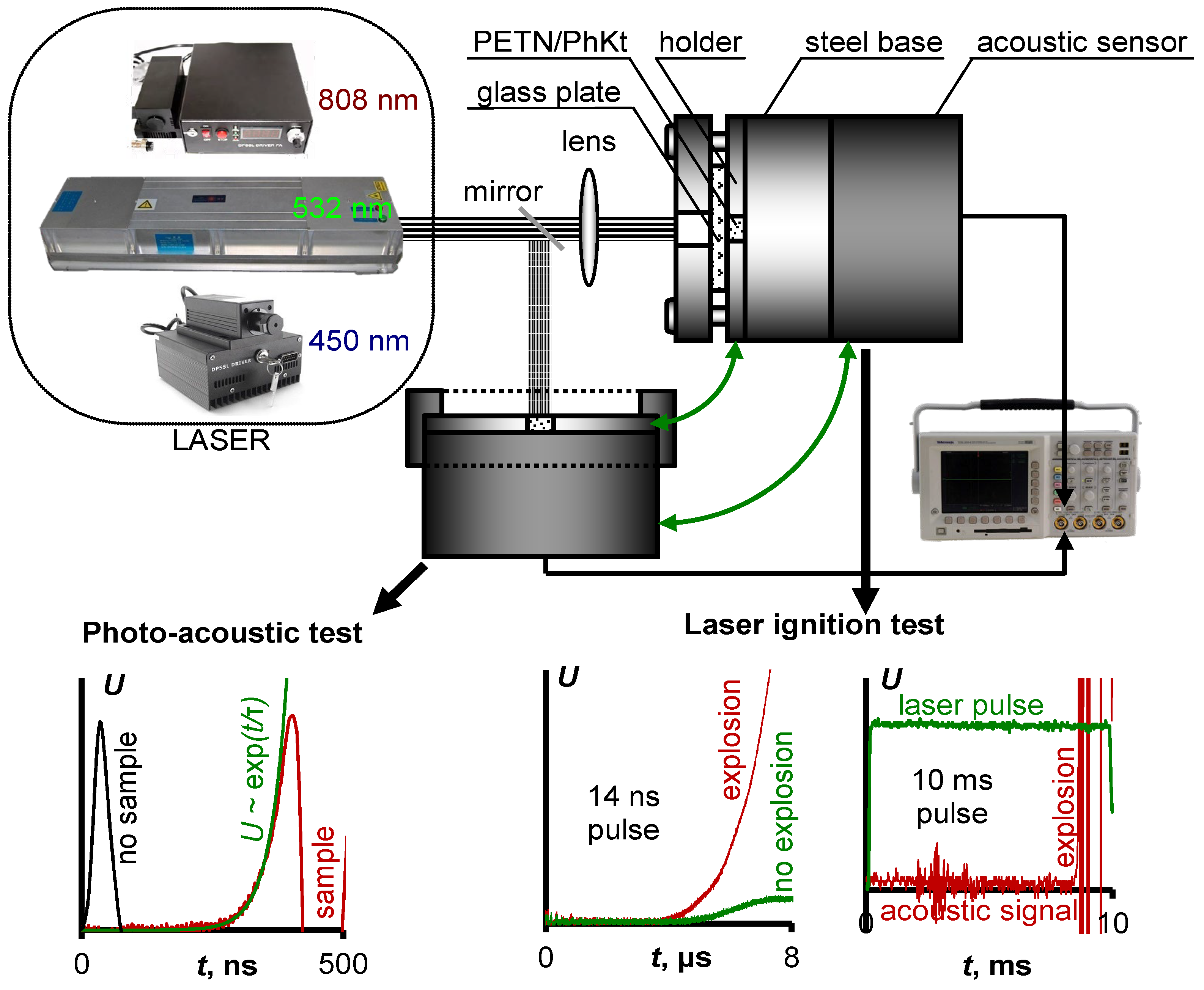
2. Materials and Methods
2.1. Materials Synthesis and Characterisation
2.2. Diffuse Reflection Spectroscopy
2.3. Photoacoustic Measurements
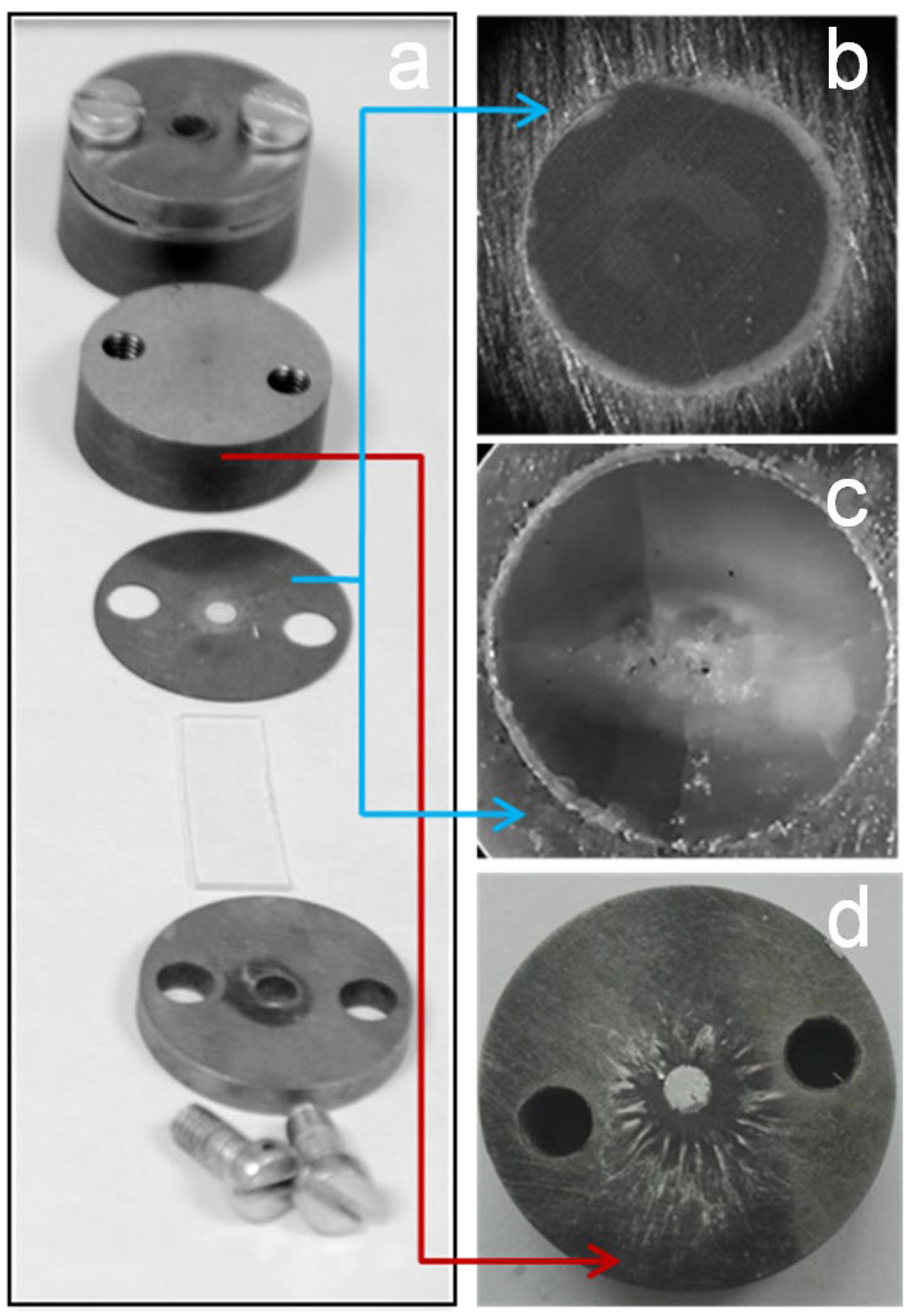
2.4. Laser Initiation
3. Results
3.1. Phase Composition, Morphology and Optical Properties of ZnO Based Nanopowders
3.2. Laser Initiation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, S.R.; Cartwright, M. Laser Ignition of Energetic Materials; John Wiley & Sons: Hoboken, NJ, USA, 2014; 304p. [Google Scholar]
- O’Briant, S.A.; Gupta, S.B.; Vasu, S.S. Laser ignition for aerospace propulsion. Propuls. Power Res. 2016, 5, 1–21. [Google Scholar] [CrossRef]
- Sivan, J.; Yehuda, H. Laser ignition of various pyrotechnic mixtures–an experimental study. Propellants Explos. Pyrotech. 2015, 40, 755–758. [Google Scholar] [CrossRef]
- Collard, D.N.; Uhlenhake, K.E.; Örnek, M.; Rhoads, J.F.; Son, S.F. Photoflash and laser ignition of Al/PVDF films and additively manufactured igniters for solid propellant. Combust. Flame 2022, 244, 112252. [Google Scholar] [CrossRef]
- Ulas, A.; Kuo, K.K. Laser-induced ignition of solid propellants for gas generators. Fuel 2008, 87, 639–646. [Google Scholar] [CrossRef]
- Aduev, B.P.; Nurmukhametov, D.R.; Liskov, I.Y.; Tupitsyn, A.V.; Belokurov, G.M. Laser pulse initiation of RDX-Al and PETN-Al composites explosion. Combust. Flame 2020, 216, 468–471. [Google Scholar] [CrossRef]
- Wilkins, P.R. Laser deflagration-to-detonation in keto-RDX doped with resonant hollow gold nanoshells. In Proceedings of the 15th International Detonation Symposium, San Francisco, CA, USA, 13–18 July 2014. [Google Scholar]
- Aluker, E.D.; Zverev, A.S.; Krechetov, A.G.; Mitrofanov, A.Y.; Terent’eva, A.O.; Tupitsyn, A.V.; Sakharchuk, Y.P. Initiation of tetranitropentaerythrit by millisecond laser pulses. Russ. Phys. J. 2014, 56, 1357–1362. [Google Scholar] [CrossRef]
- Cooper, J.K.; Grant, C.D.; Zhang, J.Z. Experimental and TD-DFT study of optical absorption of six explosive molecules: RDX, HMX, PETN, TNT, TATP, and HMTD. J. Phys. Chem. A 2013, 117, 6043–6051. [Google Scholar] [CrossRef] [PubMed]
- Tarzhanov, V.I.; Sdobnov, V.I.; Zinchenko, A.D.; Pogrebov, A.I. Laser initiation of low-density mixtures of PETN with metal additives. Combust. Explos. Shock Waves 2017, 53, 229–235. [Google Scholar] [CrossRef]
- Churchyard, S.; Fang, X.; Vrcelj, R. Laser ignitibility of energetic crystals doped with gold nanoparticles. Opt. Laser. Technol. 2019, 113, 281–288. [Google Scholar] [CrossRef]
- Fang, X.; McLuckie, W.G. Laser ignitibility of insensitive secondary explosive 1, 1-diamino-2, 2-dinitroethene (FOX-7). J. Hazard. Mater. 2015, 285, 375–382. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Yang, Y.; Zhao, F.; Li, H.; Xu, K. Enhanced thermal decomposition, laser ignition and combustion properties of NC/Al/RDX composite fibers fabricated by electrospinning. Cellulose 2021, 28, 6089–6105. [Google Scholar] [CrossRef]
- Aluker, E.D.; Zverev, A.S.; Krechetov, A.G.; Mitrofanov, A.Y.; Terentyeva, A.O.; Tupitsyn, A.V. Photo-and thermochemical initiation of PETN under laser excitation. Russ. J. Phys. Chem. B 2014, 8, 687–691. [Google Scholar] [CrossRef]
- Kang, Z.; Si, H.; Zhang, S.; Wu, J.; Sun, Y.; Liao, Q.; Zheng, Z.; Zhang, Y. Interface engineering for modulation of charge carrier behavior in ZnO photoelectrochemical water splitting. Adv. Funct. Mater. 2019, 29, 1808032. [Google Scholar] [CrossRef]
- Saleem, Z.; Pervaiz, E.; Yousaf, M.U.; Niazi, M.B.K. Two-dimensional materials and composites as potential water splitting photocatalysts: A review. Catalysts 2020, 10, 464. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Sun, L. Recent advances in photocatalytic decomposition of water and pollutants for sustainable application. Chemosphere 2021, 276, 130201. [Google Scholar] [CrossRef]
- Sun, J.; Chen, X.; Mo, S.; Zhang, Z.; Guo, D.; Li, Y.; Liu, L. Recent Advances of Bismuth Halide Based Nanomaterials for Photocatalytic Antibacterial and Photodynamic Therapy. Adv. Mater. Interfaces 2022, 9, 2200704. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Latest innovations and nanotechnologies with curcumin as a nature-inspired photosensitizer applied in the photodynamic therapy of cancer. Pharmaceutics 2021, 13, 1562. [Google Scholar] [CrossRef]
- Brish, A.A.; Galeev, I.A.; Zaitsev, B.N.; Sbitnev, E.A.; Tatarintsev, L.V. Mechanism of initiation of condensed explosives by laser radiation. Combust. Explos. Shock Waves 1969, 5, 326–328. [Google Scholar] [CrossRef]
- McGrane, S.D.; Bolme, C.A.; Greenfield, M.T.; Chavez, D.E.; Hanson, S.K.; Scharff, R.J. Photoactive high explosives: Substituents effects on tetrazine photochemistry and photophysics. J. Phys. Chem. A 2016, 120, 895–902. [Google Scholar] [CrossRef]
- Evers, J.; Gospodinov, I.; Joas, M.; Klapötke, T.M.; Stierstorfer, J. Cocrystallization of photosensitive energetic copper (II) perchlorate complexes with the nitrogen-rich ligand 1, 2-di (1 H-tetrazol-5-yl) ethane. Inorg. Chem. 2014, 53, 11749–11756. [Google Scholar] [CrossRef]
- Tsyshevsky, R.; Zverev, A.S.; Mitrofanov, A.Y.; Ilyakova, N.N.; Kostyanko, M.V.; Luzgarev, S.V.; Garifzianova, G.G.; Kuklja, M.M. Role of Hydrogen Abstraction Reaction in Photocatalytic Decomposition of High Energy Density Materials. J. Phys. Chem. C 2016, 120, 24835–24846. [Google Scholar] [CrossRef]
- Kuklja, M.M.; Tsyshevsky, R.; Zverev, A.S.; Mitrofanov, A.; Ilyakova, N.; Nurmukhametov, D.R.; Rashkeev, S.N. Achieving tunable chemical reactivity through photo-initiation of energetic materials at metal oxide surfaces. Phys. Chem. Chem. Phys. 2020, 22, 25284–25296. [Google Scholar] [CrossRef] [PubMed]
- Tsyshevsky, R.V.; Rashkeev, S.N.; Kuklja, M.M. Defect-induced decomposition of energetic nitro compounds at MgO Surface. Surf. Sci. 2022, 722, 122085. [Google Scholar] [CrossRef]
- Tsyshevsky, R.V.; Zverev, A.; Mitrofanov, A.; Rashkeev, S.N.; Kuklja, M.M. Photochemistry of the α-Al2O3-PETN Interface. Molecules 2016, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Tsyshevsky, R.V.; Rashkeev, S.N.; Kuklja, M.M. Defect states at organic–inorganic interfaces: Insight from first principles calculations for pentaerythritol tetranitrate on MgO surface. Surf. Sci. 2015, 637, 19–28. [Google Scholar] [CrossRef]
- Chai, H.Y.; Lam, S.M.; Sin, J.C. Green synthesis of magnetic Fe-doped ZnO nanoparticles via Hibiscus rosa-sinensis leaf extracts for boosted photocatalytic, antibacterial and antifungal activities. Mater. Lett. 2019, 242, 103–106. [Google Scholar] [CrossRef]
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef]
- Sin, J.C.; Tan, S.Q.; Quek, J.A.; Lam, S.M.; Mohamed, A.R. Facile fabrication of hierarchical porous ZnO/Fe3O4 composites with enhanced magnetic, photocatalytic and antibacterial properties. Mater. Lett. 2018, 228, 207–211. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Vo, D.V.N.; Nguyen, L.T.; Duong, A.T.; Nguyen, H.Q.; Chu, N.M.; Nguyen, D.T.C.; Van Tran, T. Synthesis, characterization, and application of ZnFe2O4@ ZnO nanoparticles for photocatalytic degradation of Rhodamine B under visible-light illumination. Environ. Technol. Innov. 2022, 25, 102130. [Google Scholar] [CrossRef]
- Prabhu, Y.T.; Rao, V.N.; Shankar, M.; Sreedhar, B.; Pal, U. The facile hydrothermal synthesis of CuO@ ZnO heterojunction nanostructures for enhanced photocatalytic hydrogen evolution. N. J. Chem. 2019, 43, 6794–6805. [Google Scholar] [CrossRef]
- Taraka, T.P.Y.; Gautam, A.; Jain, S.L.; Bojja, S.; Pal, U. Controlled addition of Cu/Zn in hierarchical CuO/ZnO pn heterojunction photocatalyst for high photoreduction of CO2 to MeOH. J. CO2 Util. 2019, 31, 207–214. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Epstein, P.; Dinka, P. Solution combustion synthesis of nanomaterials. Proc. Combust. Inst. 2007, 31, 1789–1795. [Google Scholar] [CrossRef]
- Kang, X.; Li, C.; Zheng, Z.; Cui, X. Synthesis of ZnO and Cu-doped ZnO nanocrystalline by solution combustion method and their catalytic effects on thermal behavior of KClO4. Combust. Sci. Technol. 2021, 193, 75–89. [Google Scholar] [CrossRef]
- Aduev, B.P.; Nurmukhametov, D.R.; Belokurov, G.M.; Nelyubina, N.V.; Tupitsyn, A.V. Optoacoustic Effects in Pentaerythritol Tetranitrate with Ultrafine Aluminum-Particle Inclusions under Pulsed-Laser Action. Opt. Spectrosc. 2018, 124, 412–417. [Google Scholar] [CrossRef]
- Aluker, E.D.; Krechetov, A.G.; Mitrofanov, A.Y.; Zverev, A.S.; Kuklja, M.M. Topography of photochemical initiation in molecular materials. Molecules 2013, 18, 14148–14160. [Google Scholar] [CrossRef] [PubMed]
- Åsbrink, S.; Norrby, L.J. A refinement of the crystal structure of copper (II) oxide with a discussion of some exceptional esd’s. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 1970, 26, 8–15. [Google Scholar] [CrossRef]
- Kihara, K.; Donnay, G. Anharmonic thermal vibrations in ZnO. Can. Mineral. 1985, 23, 647–654. [Google Scholar]
- Hill, R.J.; Craig, J.R.; Gibbs, G.V. Systematics of the spinel structure type. Phys. Chem. Miner 1979, 4, 317–339. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: Material, physics and applications. ChemPhysChem 2007, 8, 782–803. [Google Scholar] [CrossRef]
- Sheikov, Y.V.; Bat’yanov, S.M.; Kalashnikova, O.N.; Lukovkin, O.M.; Mil’chenko, D.V.; Vakhmistrov, S.A.; Mikhailov, A.L. Initiating Aluminized High Explosives by Laser Radiation. Combust. Explos. Shock Waves 2018, 54, 563–569. [Google Scholar] [CrossRef]
- Aduev, B.P.; Nurmukhametov, D.R.; Zvekov, A.A.; Liskov, I.Y. Influence of the size of inclusions of ultrafine nickel particles on the laser initiation threshold of PETN. Combust. Explos. Shock Waves 2015, 51, 472–475. [Google Scholar] [CrossRef]
- Aduev, B.P.; Nurmukhametov, D.R.; Zvekov, A.A.; Nikitin, A.P.; Kovalev, R.Y. Laser initiation of PETN–iron nanoparticle composites. Russ. J. Phys. Chem. B 2016, 10, 615–620. [Google Scholar] [CrossRef]
- Conner, R.W.; Dlott, D.D. Time-resolved spectroscopy of initiation and ignition of flash-heated nanoparticle energetic materials. J. Phys. Chem. C 2012, 116, 14737–14747. [Google Scholar] [CrossRef]
- Gerasimov, S.I.; Ilyushin, M.A.; Kuz’min, V.A. A laser diode beam initiates a high-energy mercury perchlorate-polymer complex. Tech. Phys. Lett. 2015, 41, 338–340. [Google Scholar] [CrossRef]
- Das, J.; Shem-Tov, D.; Zhang, S.; Gao, C.Z.; Zhang, L.; Yao, C.; Flaxer, E.; Stierstorfer, J.; Wurzenberger, M.; Rahinov, I.; et al. Power of sulfur–Chemistry, properties, laser ignition and theoretical studies of energetic perchlorate-free 1, 3, 4-thiadiazole nitramines. Chem. Eng. J. 2022, 443, 136246. [Google Scholar] [CrossRef]
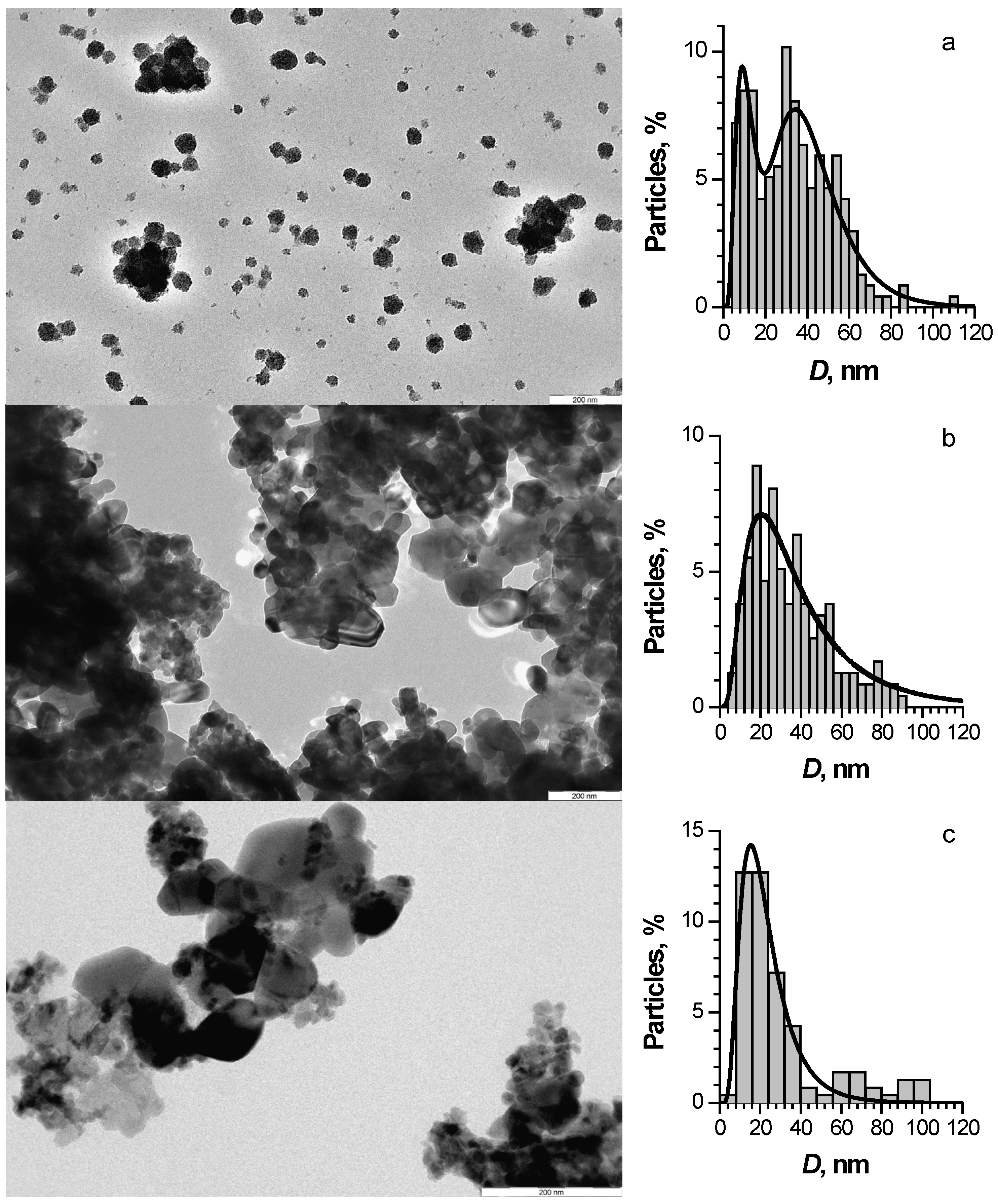
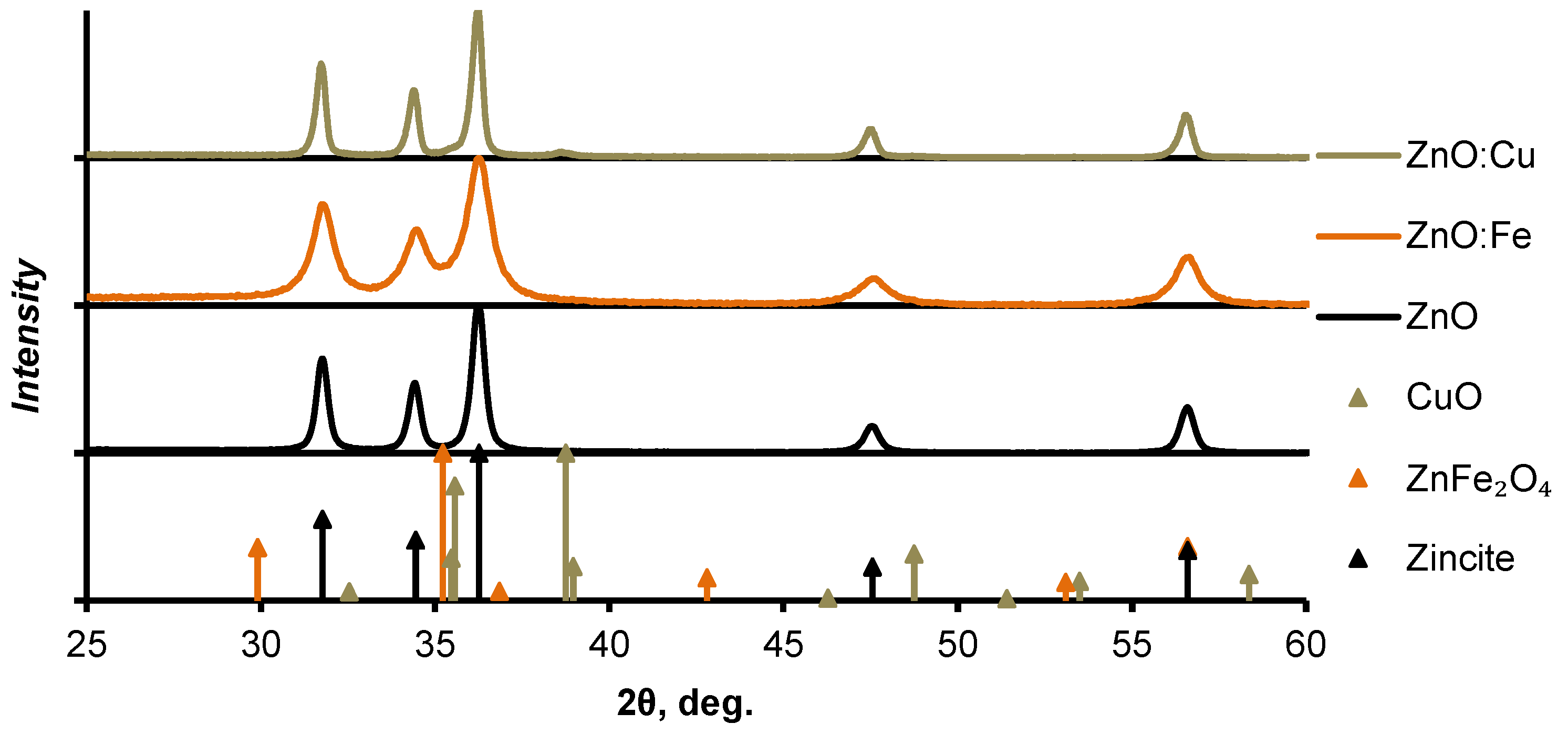
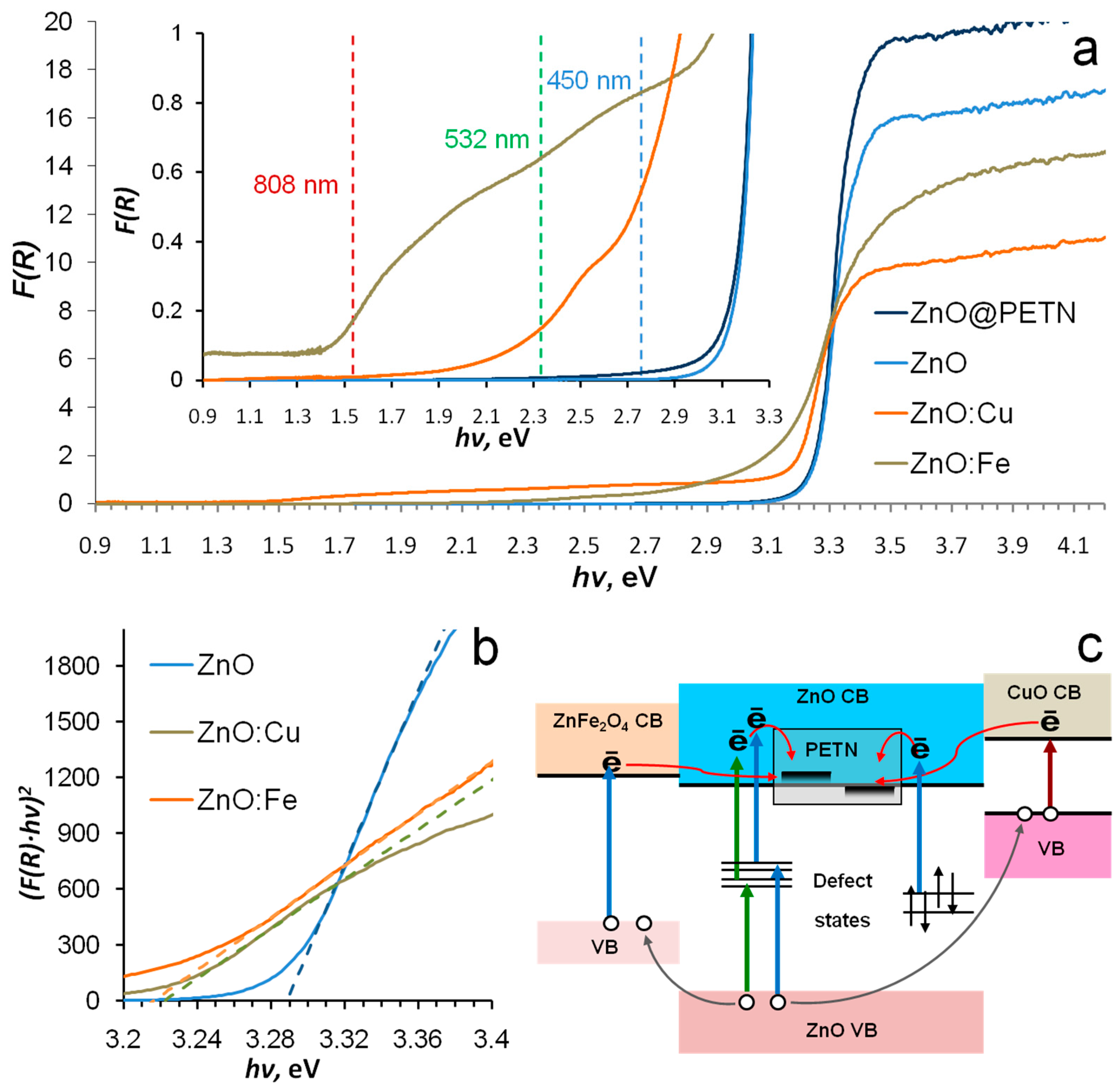

| Sample | SBET, m2/g | VΣ, cm3/g | Vmicro, cm3/g | Vmeso, cm3/g | Dpores, Å |
|---|---|---|---|---|---|
| ZnO | 17.0 | 0.0876 | 0.0005 | 0.0860 | 202 |
| ZnO:Fe | 30.0 | 0.1455 | 0.0015 | 0.1430 | 191 |
| ZnO:Cu | 9.5 | 0.0319 | 0.0003 | 0.0299 | 126 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zverev, A.S.; Ilyakova, N.N.; Nurmukhametov, D.R.; Dudnikova, Y.N.; Russakov, D.M.; Pugachev, V.M.; Mitrofanov, A.Y. Iron and Copper Doped Zinc Oxide Nanopowders as a Sensitizer of Industrial Energetic Materials to Visible Laser Radiation. Nanomaterials 2022, 12, 4176. https://doi.org/10.3390/nano12234176
Zverev AS, Ilyakova NN, Nurmukhametov DR, Dudnikova YN, Russakov DM, Pugachev VM, Mitrofanov AY. Iron and Copper Doped Zinc Oxide Nanopowders as a Sensitizer of Industrial Energetic Materials to Visible Laser Radiation. Nanomaterials. 2022; 12(23):4176. https://doi.org/10.3390/nano12234176
Chicago/Turabian StyleZverev, Anton S., Natalya N. Ilyakova, Denis R. Nurmukhametov, Yulia N. Dudnikova, Dmitry M. Russakov, Valery M. Pugachev, and Anatoly Y. Mitrofanov. 2022. "Iron and Copper Doped Zinc Oxide Nanopowders as a Sensitizer of Industrial Energetic Materials to Visible Laser Radiation" Nanomaterials 12, no. 23: 4176. https://doi.org/10.3390/nano12234176
APA StyleZverev, A. S., Ilyakova, N. N., Nurmukhametov, D. R., Dudnikova, Y. N., Russakov, D. M., Pugachev, V. M., & Mitrofanov, A. Y. (2022). Iron and Copper Doped Zinc Oxide Nanopowders as a Sensitizer of Industrial Energetic Materials to Visible Laser Radiation. Nanomaterials, 12(23), 4176. https://doi.org/10.3390/nano12234176







