Visible-Light-Active N-Doped TiO2 Photocatalysts: Synthesis from TiOSO4, Characterization, and Enhancement of Stability Via Surface Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Photocatalysts
2.3. Characterization Techniques
2.4. Photocatalytic Experiments
3. Results and Discussion
3.1. Effect of Preparation Conditions on the Characteristics and Photocatalytic Activity of N-Doped TiO2
3.2. Enhancement of Stability Via Surface Modification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Cao, S.; Piao, L.; Chen, X. Emerging Photocatalysts for Hydrogen Evolution. Trends Chem. 2020, 2, 57–70. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 Photocatalysis: Design and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous Photocatalytic Degradation of Organic Contaminants over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Selishchev, D.; Svintsitskiy, D.; Kovtunova, L.; Gerasimov, E.; Gladky, A.; Kozlov, D. Surface Modification of TiO2 with Pd Nanoparticles for Enhanced Photocatalytic Oxidation of Benzene Micropollutants. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125959. [Google Scholar] [CrossRef]
- Paz, Y. Application of TiO2 Photocatalysis for Air Treatment: Patents’ Overview. Appl. Catal. B 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Selishchev, D.S.; Kolinko, P.A.; Kozlov, D.V. Adsorbent as an Essential Participant in Photocatalytic Processes of Water and Air Purification: Computer Simulation Study. Appl. Catal. A Gen. 2010, 377, 140–149. [Google Scholar] [CrossRef]
- Friedmann, D.; Mendive, C.; Bahnemann, D. TiO2 for Water Treatment: Parameters Affecting the Kinetics and Mechanisms of Photocatalysis. Appl. Catal. B 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Shi, Y.; Sun, H.; Cheng, X.; Shi, W.; Chen, L. Formation of Unique Hollow ZnSnO3@ZnIn2S4 Core-Shell Heterojunction to Boost Visible-Light-Driven Photocatalytic Water Splitting for Hydrogen Production. J. Colloid Interface Sci. 2021, 602, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Weon, S.; He, F.; Choi, W. Status and Challenges in Photocatalytic Nanotechnology for Cleaning Air Polluted with Volatile Organic Compounds: Visible Light Utilization and Catalyst Deactivation. Environ. Sci. Nano 2019, 6, 3185–3214. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Verbruggen, S.W. TiO2 Photocatalysis for the Degradation of Pollutants in Gas Phase: From Morphological Design to Plasmonic Enhancement. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Ong, W.L.; Wang, J.; Ho, G.W. Structural Design of TiO2-Based Photocatalyst for H2 Production and Degradation Applications. Catal. Sci. Technol. 2015, 5, 4703–4726. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification Strategies of TiO2 for Potential Applications in Photocatalysis: A Critical Review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Etacheri, V.; di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, W.; Mi, Y.; Yan, J.; Li, X.; Shangguan, W. Enhanced Photocatalytic Overall Water Splitting by Tuning the Relative Concentration Ratio of Bulk Defects to Surface Defects in SrTiO3. Int. J. Hydrog. Energy 2022, in press. [Google Scholar] [CrossRef]
- Sato, S. Photocatalytic Activity of NOx-Doped TiO2 in the Visible Light Region. Chem. Phys. Lett. 1986, 123, 126–128. [Google Scholar] [CrossRef]
- Asahi, R. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 269, 269–271. [Google Scholar] [CrossRef] [PubMed]
- di Valentin, C.; Pacchioni, G.; Selloni, A. Origin of the Different Photoactivity of N-Doped Anatase and Rutile TiO2. Phys. Rev. B 2004, 70, 085116. [Google Scholar] [CrossRef]
- di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of Paramagnetic Species in N-Doped TiO2 Powders by EPR Spectroscopy and DFT Calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef]
- di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-Doped TiO2: Theory and Experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Sato, S.; Nakamura, R.; Abe, S. Visible-Light Sensitization of TiO2 Photocatalysts by Wet-Method N Doping. Appl. Catal. A Gen. 2005, 284, 131–137. [Google Scholar] [CrossRef]
- Zhengpeng, W.; Wenqi, G.; Xiaoting, H.; Weimin, C.; Juhui, J.; Baoxue, Z. Preparation, Characterization and Visible Light Photocatalytic Activity of Nitrogen-Doped TiO2. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2006, 21, 71–77. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Di Valentin, C.; Pacchioni, G. Origin of Photoactivity of Nitrogen-Doped Titanium Dioxide under Visible Light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef]
- Huan, Y.; Xuxu, Z.; Zhongyi, Y.; Feng, T.; Fang, B.; Keshan, H. Preparation of Nitrogen-Doped TiO2 Nanoparticle Catalyst and Its Catalytic Activity under Visible Light. Chin. J. Chern. Eng. 2007, 15, 802–807. [Google Scholar]
- Cheng, X.; Yu, X.; Xing, Z.; Wan, J. Enhanced Photocatalytic Activity of Nitrogen Doped TiO2 Anatase Nano-Particle under Simulated Sunlight Irradiation. Energy Procedia 2012, 16, 598–605. [Google Scholar] [CrossRef]
- Gole, J.L.; Stout, J.D.; Burda, C.; Lou, Y.; Chen, X. Highly Efficient Formation of Visible Light Tunable TiO2-xNx Photocatalysts and Their Transformation at the Nanoscale. J. Phys. Chem. B 2004, 108, 1230–1240. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Kajitvichyanukul, P.; Seraphin, S. Visible Light Absorption Ability and Photocatalytic Oxidation Activity of Various Interstitial N-Doped TiO2 Prepared from Different Nitrogen Dopants. J. Hazard. Mater. 2009, 168, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, M.; Shi, J.; Shangguan, W. Preparations and Photocatalytic Hydrogen Evolution of N-Doped TiO2 from Urea and Titanium Tetrachloride. Int. J. Hydrog. Energy 2006, 31, 1326–1331. [Google Scholar] [CrossRef]
- Mrowetz, M.; Balcerski, W.; Colussi, A.J.; Hoffmann, M.R. Oxidative Power of Nitrogen-Doped TiO2 Photocatalysts under Visible Illumination. J. Phys. Chem. B 2004, 108, 17269–17273. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and Characterization of Nitrogen-Doped TiO2 Nanophotocatalyst with High Visible Light Activity. J. Phys. Chem. C 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Quesada-Cabrera, R.; Sotelo-Vázquez, C.; Quesada-González, M.; Melián, E.P.; Chadwick, N.; Parkin, I.P. On the Apparent Visible-Light and Enhanced UV-Light Photocatalytic Activity of Nitrogen-Doped TiO2 Thin Films. J. Photochem. Photobiol. A Chem. 2017, 333, 49–55. [Google Scholar] [CrossRef][Green Version]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P.; Gopinath, C.S. Synthesis, Characterization, Electronic Structure, and Photocatalytic Activity of Nitrogen-Doped TiO2 Nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P. Influence of Heteroatom Doping of TiO2 Nanoparticle on the Red Shift and the Related Catalytic Activity. Int. J. Nanosci. 2007, 6, 137–141. [Google Scholar] [CrossRef]
- Chainarong, S.; Sikong, L.; Pavasupree, S.; Niyomwas, S. Synthesis and Characterization of Nitrogen-Doped TiO2 Nanomaterials for Photocatalytic Activities under Visible Light. Energy Procedia 2011, 9, 418–427. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Nanostructured N-Doped TiO2 Coated on Glass Spheres for the Photocatalytic Removal of Organic Dyes under UV or Visible Light Irradiation. Appl. Catal. B 2015, 170, 153–161. [Google Scholar] [CrossRef]
- Sirisaksoontorn, W.; Thachepan, S.; Songsasen, A. Photodegradation of Phenanthrene by N-Doped TiO2 Photocatalyst. J. Environ. Sci. Health Part A 2009, 44, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Synthesis and Characterization of N-Doped TiO2 Nanoparticles and Their Application in Photocatalytic Oxidation of Dibenzothiophene under Visible Light. Ceram. Int. 2016, 42, 14834–14842. [Google Scholar] [CrossRef]
- Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Kinetics of Ethyl Paraben Degradation by Simulated Solar Radiation in the Presence of N-Doped TiO2 Catalysts. Water Res. 2015, 81, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Senthilnathan, J.; Philip, L. Photocatalytic Degradation of Lindane under UV and Visible Light Using N-Doped TiO2. Chem. Eng. J. 2010, 161, 83–92. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Han, C.; Sannino, D.; Dionysiou, D.D. Photocatalytic Removal of Atrazine Using N-Doped TiO2 Supported on Phosphors. Appl. Catal. B 2015, 164, 462–474. [Google Scholar] [CrossRef]
- Šojić, D.; Despotović, V.; Abramović, B.; Todorova, N.; Giannakopoulou, T.; Trapalis, C. Photocatalytic Degradation of Mecoprop and Clopyralid in Aqueous Suspensions of Nanostructured N-Doped TiO2. Molecules 2010, 15, 2994–3009. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Osorio-Vargas, P.; Pulgarin, C. A Critical Review on N-Modified TiO2 Limits to Treat Chemical and Biological Contaminants in Water. Evidence That Enhanced Visible Light Absorption Does Not Lead to Higher Degradation Rates under Whole Solar Light. J. Hazard. Mater. 2022, 425, 127979. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a Comprehensive Software Package for Spectral Simulation and Analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Lyulyukin, M.; Filippov, T.; Cherepanova, S.; Solovyeva, M.; Prosvirin, I.; Bukhtiyarov, A.; Kozlov, D.; Selishchev, D. Synthesis, Characterization and Visible-Light Photocatalytic Activity of Solid and TiO2-Supported Uranium Oxycompounds. Nanomaterials 2021, 11, 1036. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Braun, A.M.; Cassano, A.E.; Emeline, A.V.; Litter, M.I.; Palmisano, L.; Parmon, V.N.; Serpone, N. Glossary of Terms Used in Photocatalysis and Radiation Catalysis (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 931–1014. [Google Scholar] [CrossRef]
- Lyulyukin, M.; Kovalevskiy, N.; Timofeeva, S.; Gusachenko, E.; Solovyeva, M.; Selishchev, D.; Kozlov, D. Method for Correction of Experimental Action Spectrum Using Actual Overlapping Spectra of Radiation Sources. MethodsX 2021, 8, 101221. [Google Scholar] [CrossRef] [PubMed]
- Lyulyukin, M.; Kovalevskiy, N.; Selishchev, D.; Kozlov, D. Correction of Experimental Action Spectra for TiO2 Photocatalysts Measured Using Single-Peak LEDs. J. Photochem. Photobiol. A Chem. 2021, 405, 112981. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Bahnemann, D. Highly Photoactive Supported TiO2 Prepared by Thermal Hydrolysis of TiOSO4: Optimisation of the Method and Comparison with Other Synthetic Routes. Appl. Catal. B 2005, 61, 259–266. [Google Scholar] [CrossRef]
- Li, J.G.; Ishigaki, T.; Sun, X. Anatase, Brookite, and Rutile Nanocrystals via Redox Reactions under Mild Hydrothermal Conditions: Phase-Selective Synthesis and Physicochemical Properties. J. Phys. Chem. C 2007, 111, 4969–4976. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Seabra, M.P.; Otero-Irurueta, G.; de Miguel, Y.R.; Ball, R.J.; Singh, M.K.; Pullar, R.C.; Labrincha, J.A. Quantitative XRD Characterisation and Gas-Phase Photocatalytic Activity Testing for Visible-Light (Indoor Applications) of KRONOClean 7000®. RSC Adv. 2015, 5, 102911–102918. [Google Scholar] [CrossRef]
- Batalović, K.; Bundaleski, N.; Radaković, J.; Abazović, N.; Mitrić, M.; Silva, R.A.; Savić, M.; Belošević-Čavor, J.; Rakočević, Z.; Rangel, C.M. Modification of N-Doped TiO2 Photocatalysts Using Noble Metals (Pt, Pd)—A Combined XPS and DFT Study. Phys. Chem. Chem. Phys. 2017, 19, 7062–7071. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhang, J.; Zhang, Q.; Liu, Z. An N-Doped Anatase/Rutile TiO2 Hybrid from Low-Temperature Direct Nitridization: Enhanced Photoactivity under UV-/Visible-Light. RSC Adv. 2013, 4, 420–427. [Google Scholar] [CrossRef]
- Cetinorgu-Goldenberg, E.; Burstein, L.; Chayun-Zucker, I.; Avni, R.; Boxman, R.L. Structural and Optical Characteristics of Filtered Vacuum Arc Deposited N:TiOx Thin Films. Thin Solid Film 2013, 537, 28–35. [Google Scholar] [CrossRef]
- Lee, S.; Cho, I.S.; Lee, D.K.; Kim, D.W.; Noh, T.H.; Kwak, C.H.; Park, S.; Hong, K.S.; Lee, J.K.; Jung, H.S. Influence of Nitrogen Chemical States on Photocatalytic Activities of Nitrogen-Doped TiO2 Nanoparticles under Visible Light. J. Photochem. Photobiol. A Chem. 2010, 213, 129–135. [Google Scholar] [CrossRef]
- Jaeger, D.; Patscheider, J. A Complete and Self-Consistent Evaluation of XPS Spectra of TiN. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 523–534. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T. Nitrogen Complex Species and Its Chemical Nature in TiO2 for Visible-Light Sensitized Photocatalysis. Chem. Phys. 2007, 339, 57–63. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, Z.; Geng, J.; Wang, Q.; Yang, D. Carbon and Nitrogen Co-Doped TiO2 with Enhanced Visible-Light Photocatalytic Activity. Ind. Eng. Chem. Res. 2007, 46, 2741–2746. [Google Scholar] [CrossRef]
- Viswanathan, B.; Krishanmurthy, K.R. Nitrogen Incorporation in TiO2: Does It Make a Visible Light Photo-Active Material? Int. J. Photoenergy 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Liu, S.J.; Ma, Q.; Gao, F.; Song, S.H.; Gao, S. Relationship between N-Doping Induced Point Defects by Annealing in Ammonia and Enhanced Thermal Stability for Anodized Titania Nanotube Arrays. J. Alloys Compd. 2012, 543, 71–78. [Google Scholar] [CrossRef]
- Peng, F.; Cai, L.; Yu, H.; Wang, H.; Yang, J. Synthesis and Characterization of Substitutional and Interstitial Nitrogen-Doped Titanium Dioxides with Visible Light Photocatalytic Activity. J. Solid State Chem. 2008, 181, 130–136. [Google Scholar] [CrossRef]
- Graciani, J.; Álvarez, L.J.; Rodriguez, J.A.; Sanz, J.F. N Doping of Rutile TiO2 (110) Surface. A Theoretical DFT Study. J. Phys. Chem. C 2008, 112, 2624–2631. [Google Scholar] [CrossRef]
- Gobaut, B.; Orgiani, P.; Sambri, A.; di Gennaro, E.; Aruta, C.; Borgatti, F.; Lollobrigida, V.; Céolin, D.; Rueff, J.-P.; Ciancio, R.; et al. Role of Oxygen Deposition Pressure in the Formation of Ti Defect States in TiO2 (001) Anatase Thin Films. ACS Appl. Mater. Interfaces 2017, 9, 23099–23106. [Google Scholar] [CrossRef]
- Hashimoto, S.; Tanaka, A. Alteration of Ti 2p XPS Spectrum for Titanium Oxide by Low-Energy Ar Ion Bombardment. Surf. Interface Anal. 2002, 34, 262–265. [Google Scholar] [CrossRef]
- Kumar, C.P.; Gopal, N.O.; Wang, T.C.; Wong, M.S.; Ke, S.C. EPR Investigation of TiO2 Nanoparticles with Temperature-Dependent Properties. J. Phys. Chem. B 2006, 110, 5223–5229. [Google Scholar] [CrossRef]
- Ahmed, H.; Pepper, M.; Broers, A. Photoinduced Defects in Semiconductors; Cambridge Studies in Semiconductor Physics and Microelectronic Engineering: 4; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Funabiki, H.; Ozawa, K.; Sekiya, T. Electronic State of Nitrogen in Doped Titanium Dioxide. In Proceedings of the 12th International Conference on Excitonic and Photonic Processes in Condensed Matter and Nano Materials (EXCON 2018), Nara City, Japan, 8–13 July 2018; Institute of Physics Publishing: Bristol, UK, 2019; Volume 1220. [Google Scholar]
- Yuan, H.; He, J.; Li, R.; Ma, X. Characterization of SO42−/TiO2 and Its Catalytic Activity in the Epoxidation Reaction. Res. Chem. Intermed. 2017, 43, 4353–4368. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Altynnikov, A.A.; Savinov, E.N.; Kurkin, E.N. Correlation of TiO2 Photocatalytic Activity and Diffuse Reflectance Spectra. J. Photochem. Photobiol. A Chem. 2001, 144, 193–196. [Google Scholar] [CrossRef]
- di Valentin, C.; Pacchioni, G. Trends in Non-Metal Doping of Anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Xiu, Z.; Guo, M.; Zhao, T.; Pan, K.; Xing, Z.; Li, Z.; Zhou, W. Recent Advances in Ti3+ Self-Doped Nanostructured TiO2 Visible Light Photocatalysts for Environmental and Energy Applications. Chem. Eng. J. 2020, 382, 123011. [Google Scholar] [CrossRef]
- Na, S.; Seo, S.; Lee, H. Recent Developments of Advanced Ti3+-Self-Doped TiO2 for Efficient Visible-Light-Driven Photocatalysis. Catalysts 2020, 10, 679. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Hou, Y.; Huang, J.; Wu, L.; Fu, X. The Effect of Postnitridation Annealing on the Surface Property and Photocatalytic Performance of N-Doped TiO2 under Visible Light Irradiation. J. Catal. 2008, 255, 59–67. [Google Scholar] [CrossRef]
- Kovalevskiy, N.; Cherepanova, S.; Gerasimov, E.; Lyulyukin, M.; Solovyeva, M.; Prosvirin, I.; Kozlov, D.; Selishchev, D. Enhanced Photocatalytic Activity and Stability of Bi2WO6—TiO2-N Nanocomposites in the Oxidation of Volatile Pollutants. Nanomaterials 2022, 12, 359. [Google Scholar] [CrossRef]
- Kovalevskiy, N.S.; Lyulyukin, M.N.; Kozlov, D.V.; Selishchev, D.S. Cu-Grafted TiO2 Photocatalysts: Effect of Cu on the Action Spectrum of Composite Materials. Mendeleev Commun. 2021, 31, 644–646. [Google Scholar] [CrossRef]
- Schon, G. ESCA Studies of Cu, Cu2O and CuO. Surf. Sci. 1973, 35, 96–108. [Google Scholar] [CrossRef]
- Svintsitskiy, D.A.; Stadnichenko, A.I.; Demidov, D.V.; Koscheev, S.V.; Boronin, A.I. Investigation of Oxygen States and Reactivities on a Nanostructured Cupric Oxide Surface. Appl. Surf. Sci. 2011, 257, 8542–8549. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Imagawa, H. ESR Studies of Cupric Ion in Various Oxide Glasses. Phys. Status Solidi 1968, 30, 469–478. [Google Scholar] [CrossRef]
- Bagratashvili, V.N.; Bogomolova, L.D.; Jachkin, V.A.; Krasi’nikova, N.A.; Rybaltovskii, A.O.; Tsypina, S.I.; Chutko, E.A. Electron Paramagnetic Resonance of Color Centers in Nanoporous Glasses Impregnated with Copper Beta-Diketonate with the Use of Supercritical Carbon Dioxide. Glass Phys. Chem. 2004, 30, 500–505. [Google Scholar] [CrossRef]
- Chen, C.S.; Chen, T.C.; Chen, C.C.; Lai, Y.T.; You, J.H.; Chou, T.M.; Chen, C.H.; Lee, J.F. Effect of Ti3+ on TiO2-Supported Cu Catalysts Used for CO Oxidation. Langmuir 2012, 28, 9996–10006. [Google Scholar] [CrossRef] [PubMed]
- Endo-Kimura, M.; Karabiyik, B.; Wang, K.; Wei, Z.; Ohtani, B.; Markowska-Szczupak, A.; Kowalska, E. Vis-Responsive Copper-Modified Titania for Decomposition of Organic Compounds and Microorganisms. Catalysts 2020, 10, 1194. [Google Scholar] [CrossRef]
- Nishikawa, M.; Mitani, Y.; Nosaka, Y. Photocatalytic Reaction Mechanism of Fe(III)-Grafted TiO2 Studied by Means of ESR Spectroscopy and Chemiluminescence Photometry. J. Phys. Chem. C 2012, 116, 14900–14907. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Energy-Level Matching of Fe(III) Ions Grafted at Surface and Doped in Bulk for Efficient Visible-Light Photocatalysts. J. Am. Chem. Soc. 2013, 135, 10064–10072. [Google Scholar] [CrossRef]
- Yu, H.; Irie, H.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; Miyauchi, M.; Hashimoto, K. An Efficient Visible-Light-Sensitive Fe(III)-Grafted TiO2 Photocatalyst. J. Phys. Chem. C 2010, 114, 16481–16487. [Google Scholar] [CrossRef]
- Jung, S.M.; Grange, P. The investigation of mechanism of SCR reaction on a TiO2-SO42− catalyst by DRIFTS. Appl. Catal. B 2000, 27, L11–L16. [Google Scholar] [CrossRef]
- Kolinko, P.A.; Kozlov, D.V. Products distribution during the gas phase photocatalytic oxidation of ammonia over the various titania based photocatalysts. Appl. Catal. B. 2009, 90, 126–131. [Google Scholar] [CrossRef]
- Tan, X.; Cheng, G.; Song, X.; Chen, X.; Dai, W.; Fu, X. The promoting effect of visible light on the CO + NO reaction over the Pd/N–TiO2 catalyst. Catal. Sci. Technol. 2019, 9, 3637–3646. [Google Scholar] [CrossRef]
- Ramis, G.; Busca, G.; Lorenzelli, V.; Forzatti, P. Fourier transform infrared study of the adsorption and coadsorption of nitric oxide, nitrogen dioxide and ammonia on TiO2 anatase. Appl. Catal. 1990, 64, 243–257. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wang, Y.; Cen, W.; Wu, Z.; Wang, H.; Weng, X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B. 2014, 148–149, 582–588. [Google Scholar] [CrossRef]
- Kamaei, M.; Rashedi, H.; Dastgheib, S.M.M.; Tasharrofi, S. Comparing Photocatalytic Degradation of Gaseous Ethylbenzene Using N-doped and Pure TiO2 Nano-Catalysts Coated on Glass Beads under Both UV and Visible Light Irradiation. Catalysts 2018, 8, 466. [Google Scholar] [CrossRef]
- Belver, C.; Bellod, R.; Stewart, S.J.; Requejo, F.G.; Fernández-García, M. Nitrogen-containing TiO2 photocatalysts: Part 2. Photocatalytic behavior under sunlight excitation. Appl. Catal. B: Environ. 2006, 65, 309–314. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Wang, X.; Liu, W.; Yu, K.; Liang, C. Facile synthesis of nitrogen-doped titanium dioxide with enhanced photocatalytic properties. Mater. Res. Express 2019, 6, 115019. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, W.; Hong, X.; Zhao, X.; Xu, F.; Cai, C. Photocatalytic degradation of phenol in aqueous nitrogen-doped TiO2 suspensions with various light sources. Appl. Catal. B: Environ. 2005, 57, 223–231. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Jin, Z.; Wu, Z.; Zhang, S. Visible light photocatalytic decoloration of methylene blue on novel N-doped TiO2. Chin. Sci. Bull. 2007, 52, 2157–2160. [Google Scholar] [CrossRef]
- Khan, T.T.; Bari, G.A.K.M.R.; Kang, H.J.; Lee, T.G.; Park, J.W.; Hwang, H.J.; Hossain, S.M.; Mun, J.S.; Suzuki, N.; Fujishima, A.; et al. Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx. Catalysts 2021, 11, 109. [Google Scholar] [CrossRef]
- Ai, H.Y.; Shi, J.W.; Duan, R.X.; Chen, J.W.; Cui, H.J.; Fu, M.L. Sol-gel to prepare nitrogen doped TiO2 nanocrystals with exposed {001} facets and high visible-light photocatalytic performance. Int. J. Photoenergy 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Chen, C.; Bai, H.; Chang, S.M.; Chang, C.; Den, W. Preparation of N-doped TiO2 photocatalyst by atmospheric pressure plasma process for VOCs decomposition under UV and visible light sources. J. Nanoparticle Res. 2006, 9, 365–375. [Google Scholar] [CrossRef]
- Liu, S.H.; Tang, W.T.; Lin, W.X. Self-assembled ionic liquid synthesis of nitrogen-doped mesoporous TiO2 for visible-light-responsive hydrogen production. Int. J. Hydrogen Energy 2017, 42, 24006–24013. [Google Scholar] [CrossRef]
- Japa, M.; Tantraviwat, D.; Phasayavan, W.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Simple preparation of nitrogen-doped TiO2 and its performance in selective oxidation of benzyl alcohol and benzylamine under visible light. Colloids Surfaces A: Physicochem. Eng. Asp. 2020, 610, 125743. [Google Scholar] [CrossRef]
- Bellardita, M.; Addamo, M.; di Paola, A.; Palmisano, L.; Venezia, A.M. Preparation of N-doped TiO2: Characterization and photocatalytic performance under UV and visible light. Phys. Chem. Chem. Phys. 2009, 11, 4084–4093. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.M.; Naik, B. Synthesis of mesoporous TiO2−xNx spheres by template free homogeneous co-precipitation method and their photo-catalytic activity under visible light illumination. J. Colloid Interface Sci. 2009, 333, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]

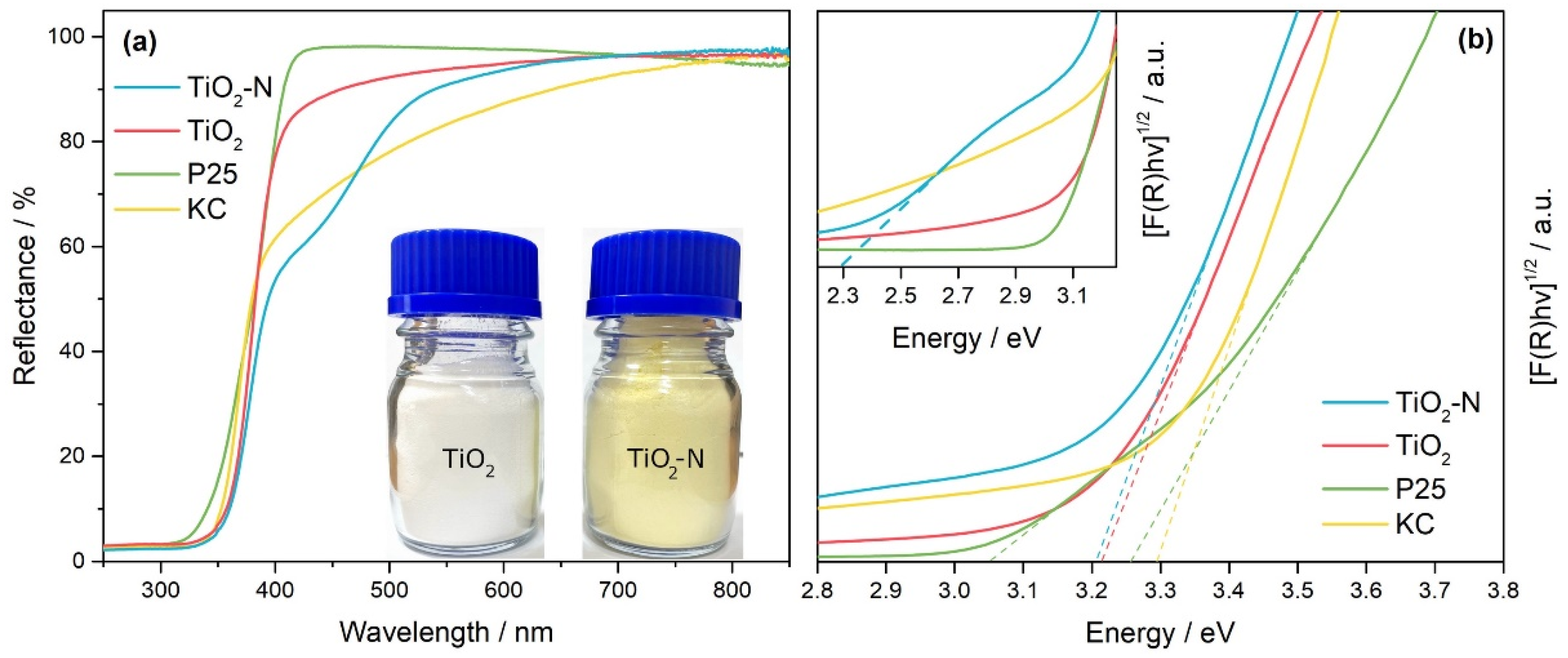
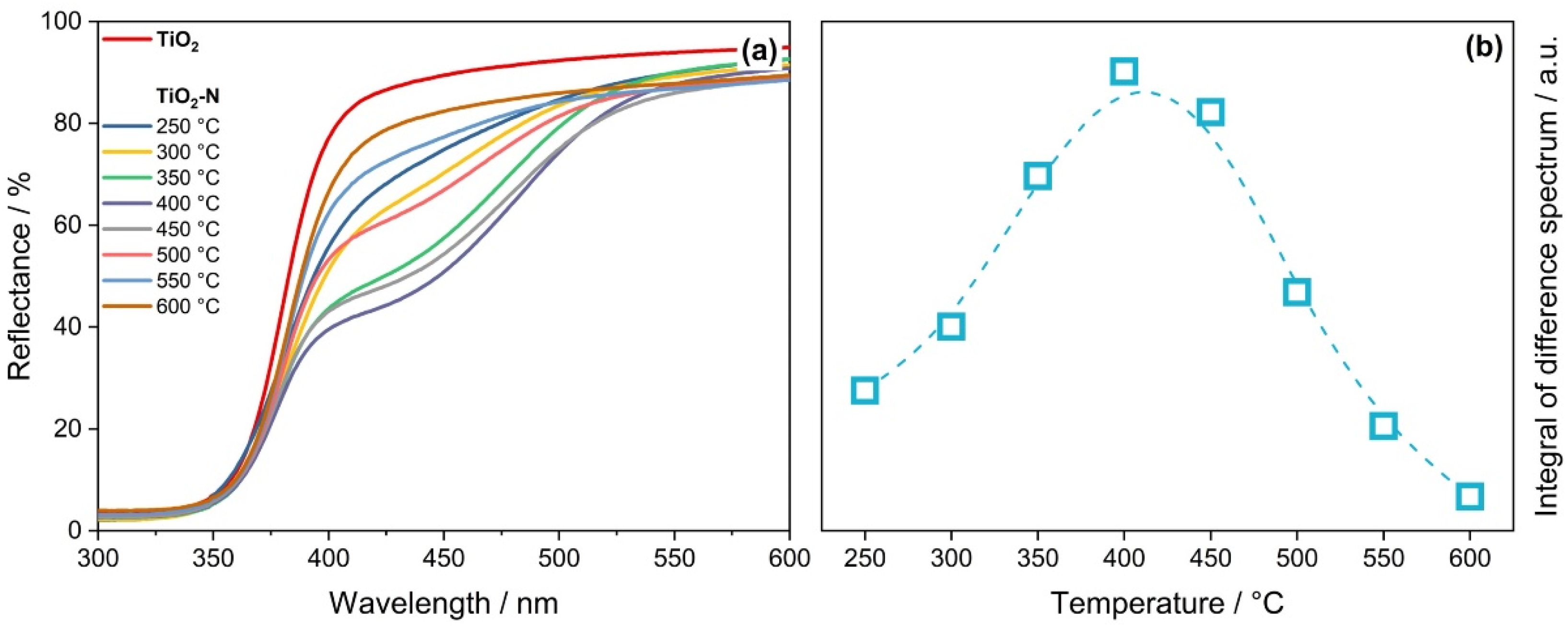
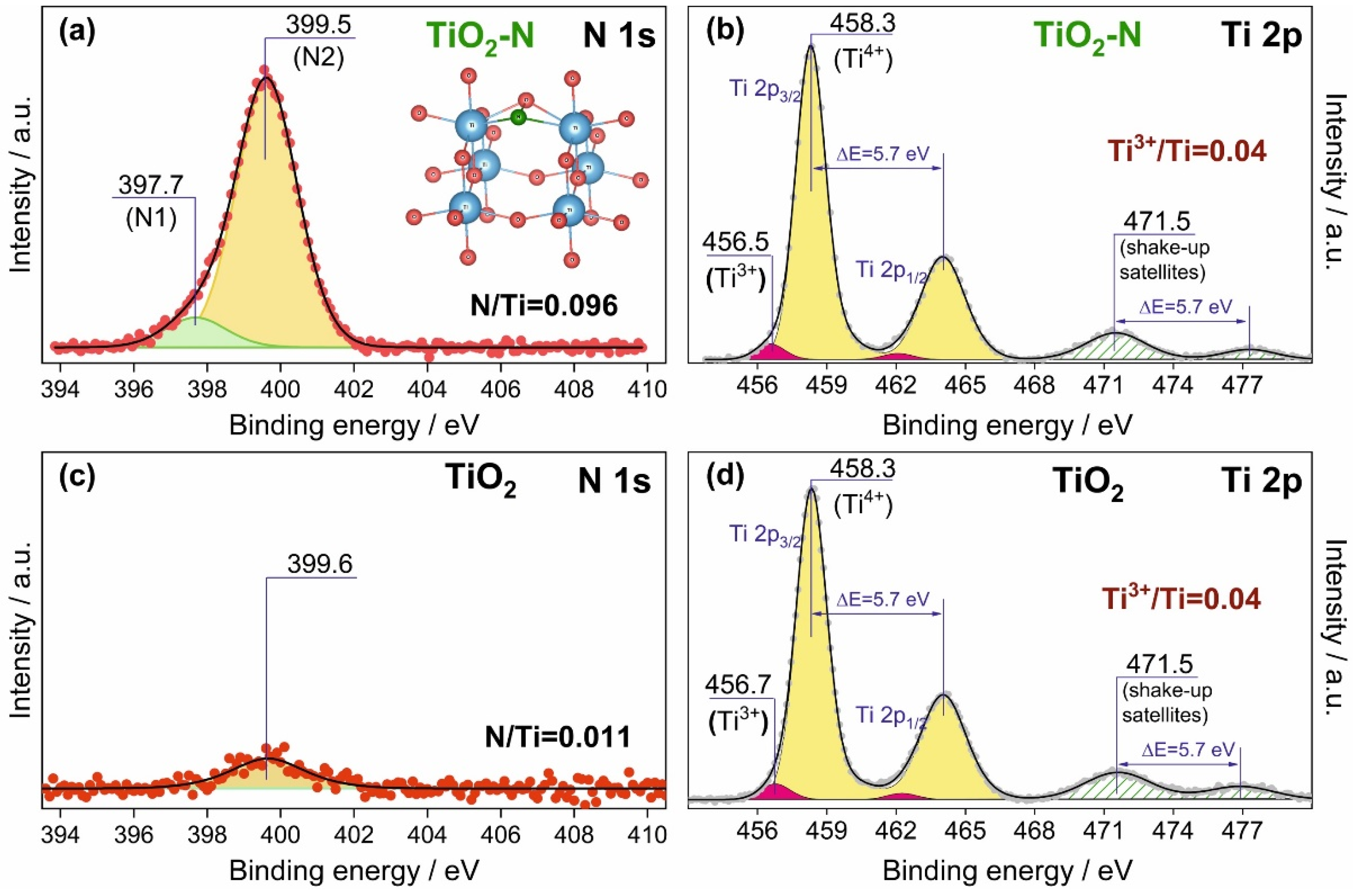
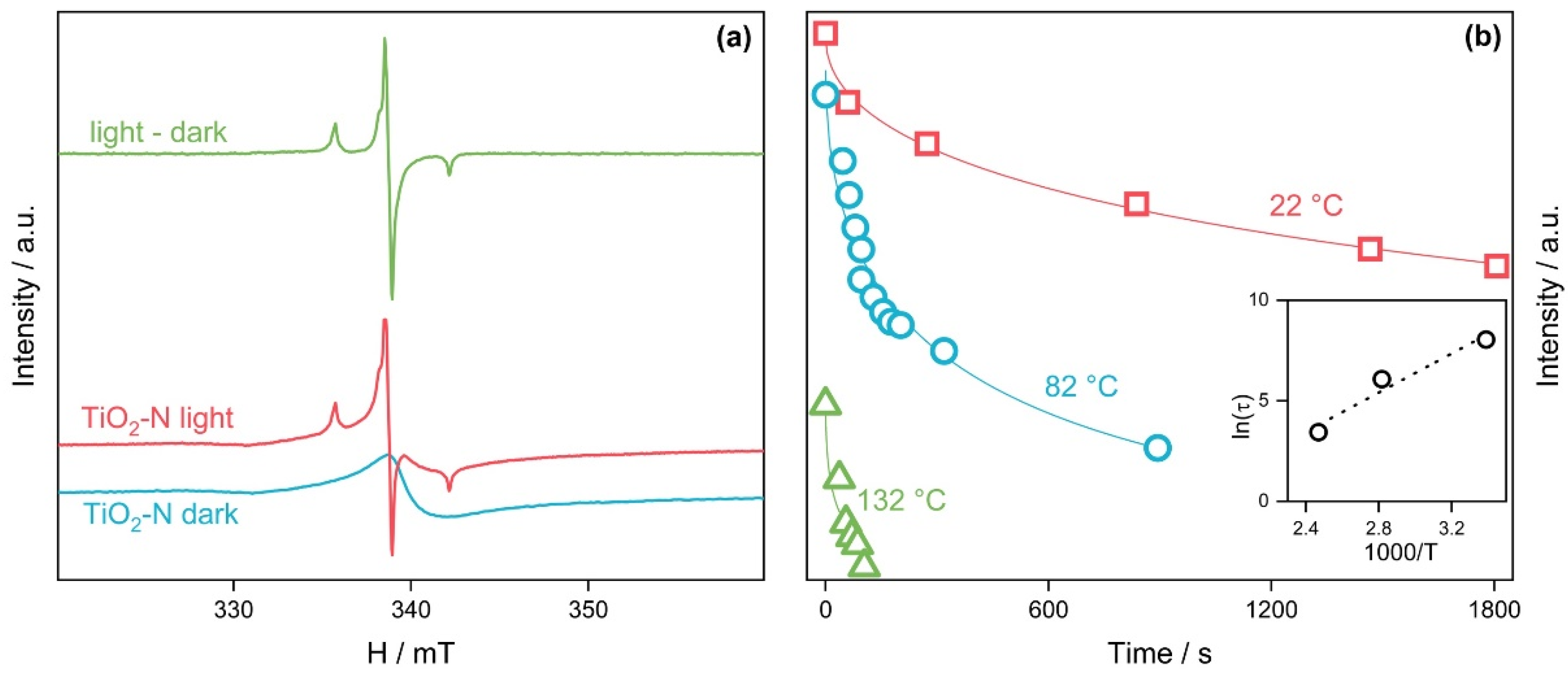

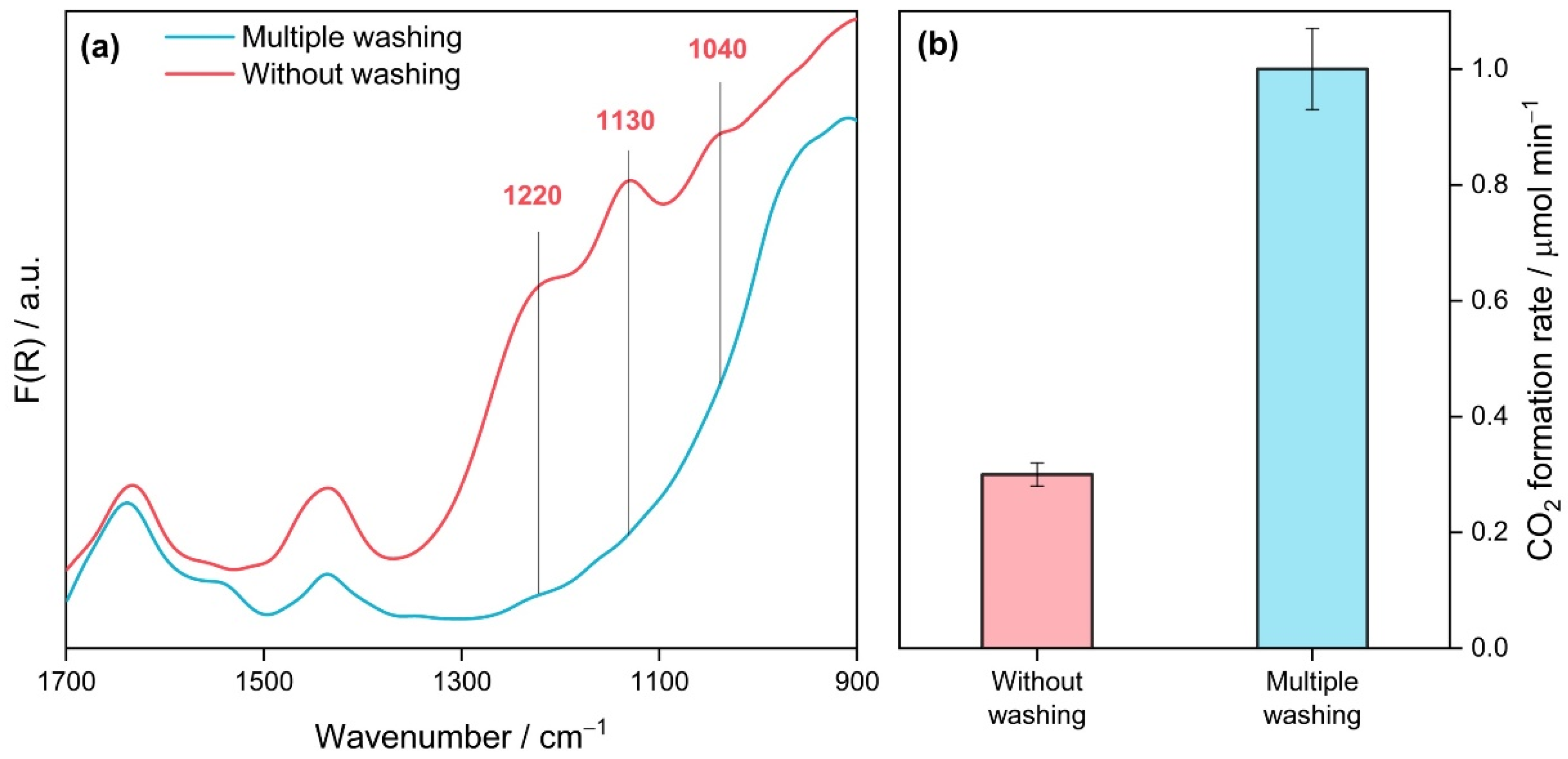

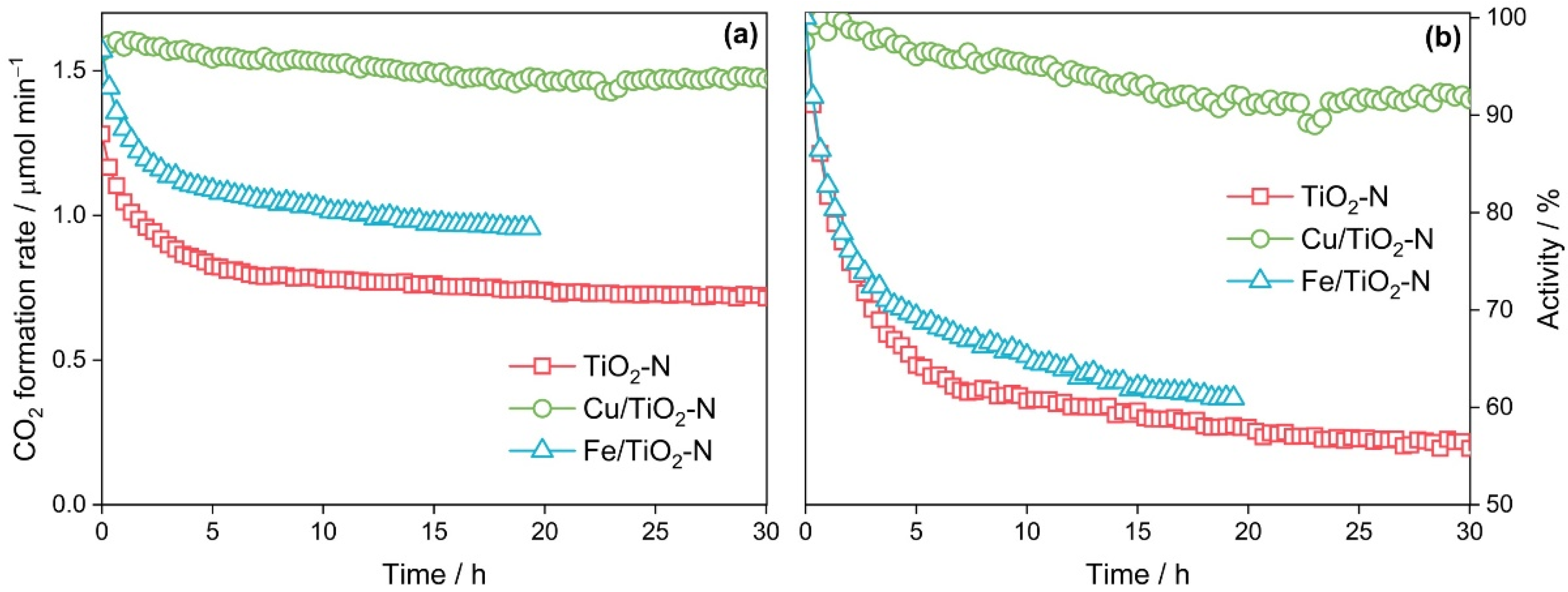

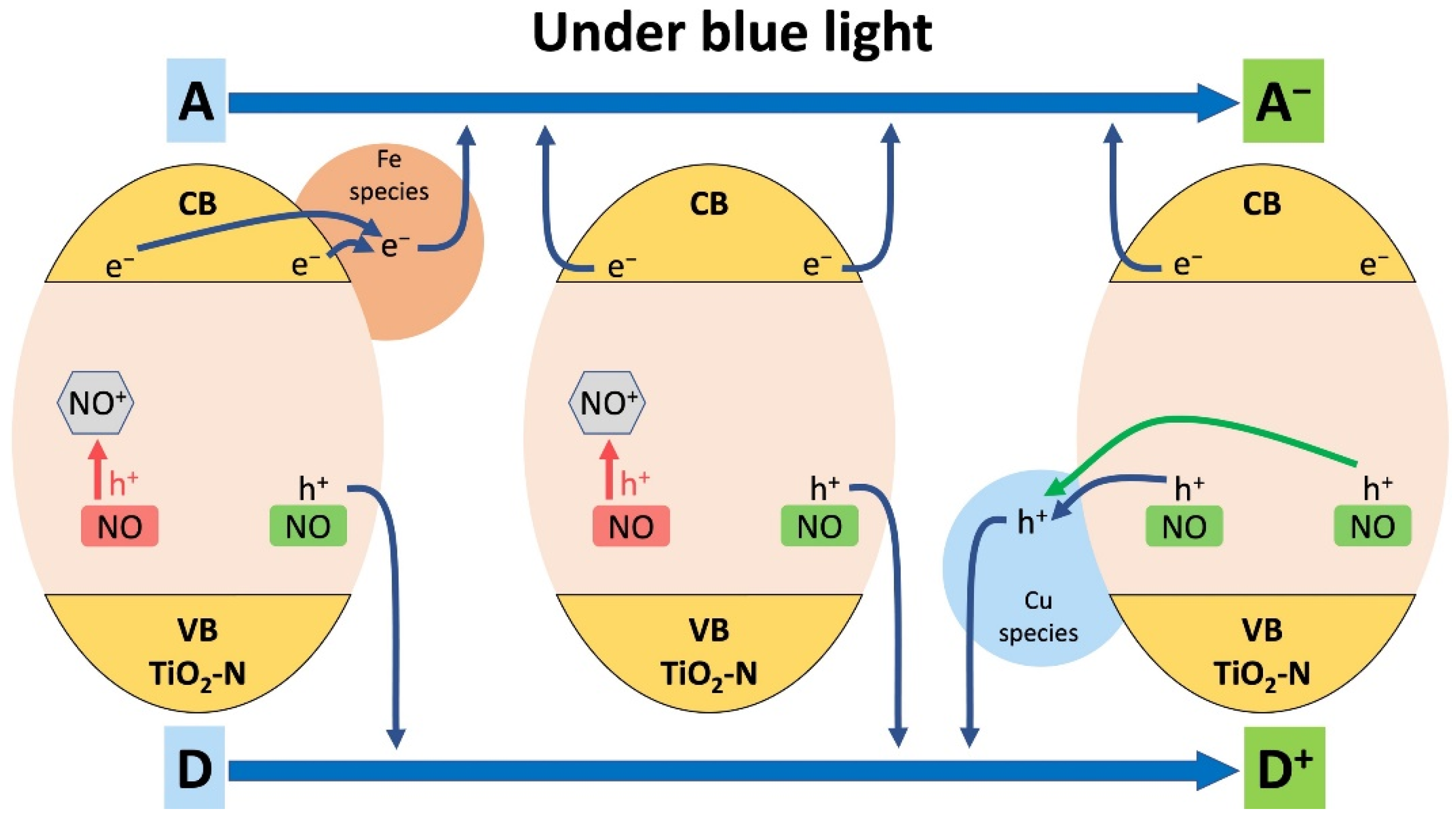
| Calcination Temperature, °C | Crystal Size, nm | Stain Values (ε) | Surface Area, m2 g−1 | Pore Volume, cm3 g−1 | Pore Diameter, Å | Nitrogen Content, wt.% | Sulfur Content, wt.% | Band Gap, eV | MEE **, eV |
|---|---|---|---|---|---|---|---|---|---|
| 250 | n.d. * | n.d. | 344 | 0.59 | 69 | 0.8 ± 0.1 | 0.14 ± 0.04 | 3.22 | 2.22 |
| 300 | 0.6 ± 0.2 | 0.16 ± 0.06 | 3.20 | 2.27 | |||||
| 350 | 16 | 0.0031 | 151 | 0.43 | 115 | 0.5 ± 0.1 | 0.16 ± 0.04 | 3.19 | 2.32 |
| 400 | 0.4 ± 0.1 | 0.16 ± 0.06 | 3.17 | 2.30 | |||||
| 450 | 20 | 0.0020 | 113 | 0.38 | 136 | 0.36 ± 0.03 | 0.11 ± 0.03 | 3.18 | 2.27 |
| 500 | 23 | 0.0017 | 0.27 ± 0.03 | 0.08 ± 0.01 | 3.19 | 2.24 | |||
| 550 | 73 | 0.31 | 172 | 0.14 ± 0.01 | 0.14 ± 0.04 | 3.19 | n.d. | ||
| 600 | 25 | 0.0010 | n.d. | 0.11 ± 0.03 | 3.18 | n.d. |
| Paramagnetic Center | g-Factor ± g-Strain | Hyperfine Splitting, MHz |
|---|---|---|
| Ti3+ | 1.99 | |
| N | {2.003 ± 0.001; 2.004 ± 0.008; 2.002 ± 0.006} | {2.3; 12.3; 88.9} |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalevskiy, N.; Svintsitskiy, D.; Cherepanova, S.; Yakushkin, S.; Martyanov, O.; Selishcheva, S.; Gribov, E.; Kozlov, D.; Selishchev, D. Visible-Light-Active N-Doped TiO2 Photocatalysts: Synthesis from TiOSO4, Characterization, and Enhancement of Stability Via Surface Modification. Nanomaterials 2022, 12, 4146. https://doi.org/10.3390/nano12234146
Kovalevskiy N, Svintsitskiy D, Cherepanova S, Yakushkin S, Martyanov O, Selishcheva S, Gribov E, Kozlov D, Selishchev D. Visible-Light-Active N-Doped TiO2 Photocatalysts: Synthesis from TiOSO4, Characterization, and Enhancement of Stability Via Surface Modification. Nanomaterials. 2022; 12(23):4146. https://doi.org/10.3390/nano12234146
Chicago/Turabian StyleKovalevskiy, Nikita, Dmitry Svintsitskiy, Svetlana Cherepanova, Stanislav Yakushkin, Oleg Martyanov, Svetlana Selishcheva, Evgeny Gribov, Denis Kozlov, and Dmitry Selishchev. 2022. "Visible-Light-Active N-Doped TiO2 Photocatalysts: Synthesis from TiOSO4, Characterization, and Enhancement of Stability Via Surface Modification" Nanomaterials 12, no. 23: 4146. https://doi.org/10.3390/nano12234146
APA StyleKovalevskiy, N., Svintsitskiy, D., Cherepanova, S., Yakushkin, S., Martyanov, O., Selishcheva, S., Gribov, E., Kozlov, D., & Selishchev, D. (2022). Visible-Light-Active N-Doped TiO2 Photocatalysts: Synthesis from TiOSO4, Characterization, and Enhancement of Stability Via Surface Modification. Nanomaterials, 12(23), 4146. https://doi.org/10.3390/nano12234146










