Plasmon-Enhanced Ultraviolet Luminescence in Colloid Solutions and Nanostructures Based on Aluminum and ZnO Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement Procedure
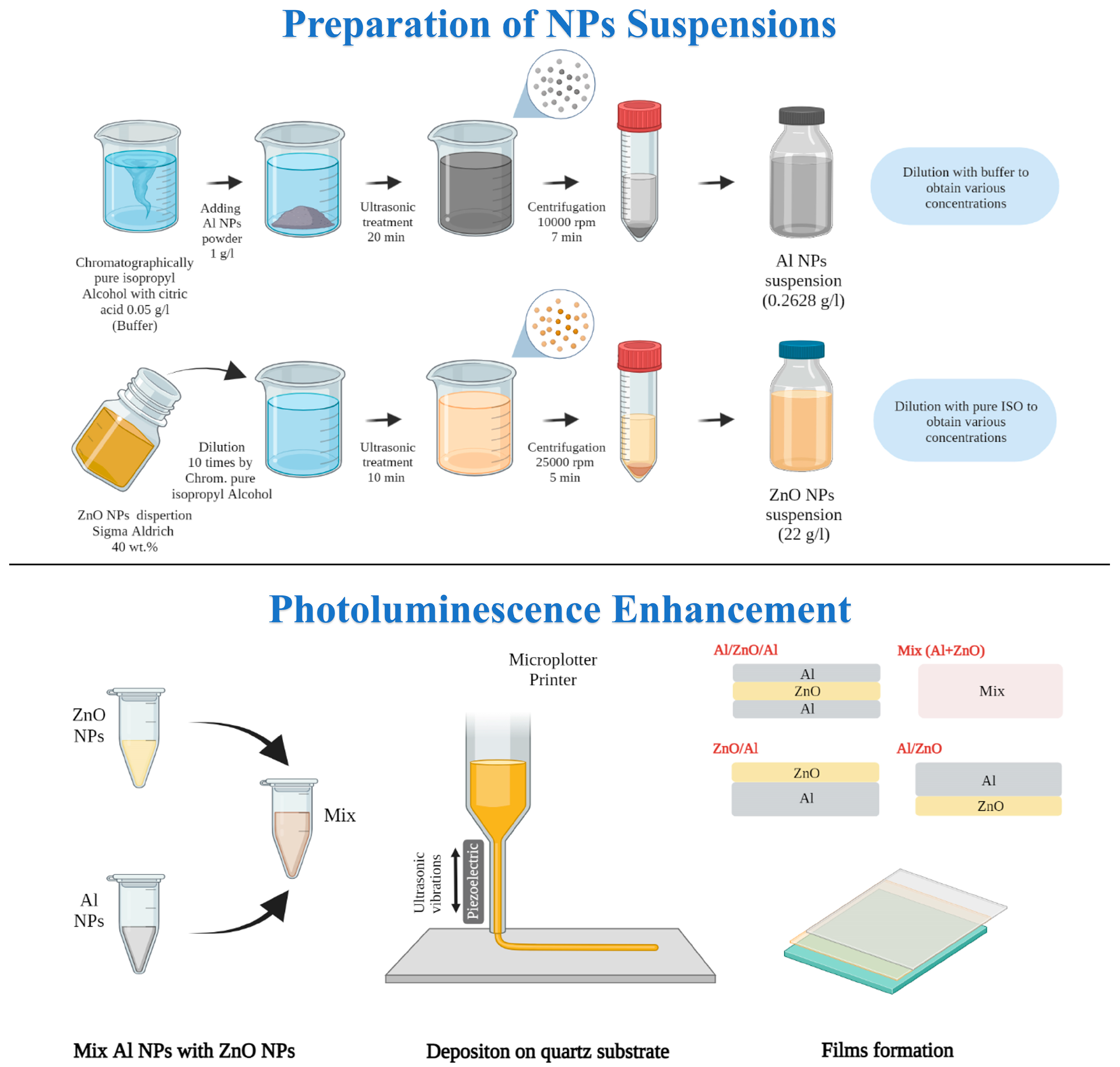
2.2. Preparation of Colloids and Films
3. Results and Discussion
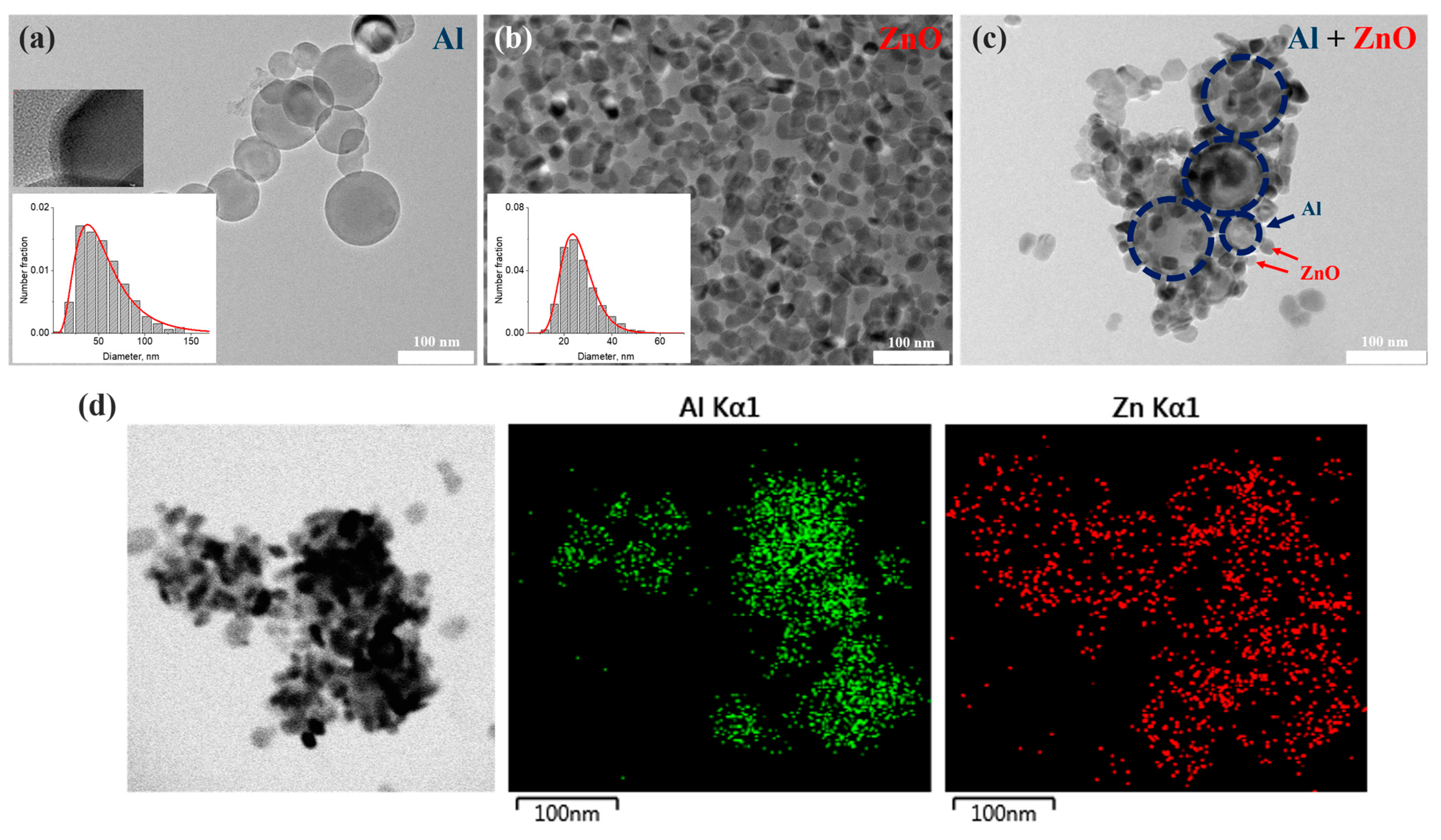
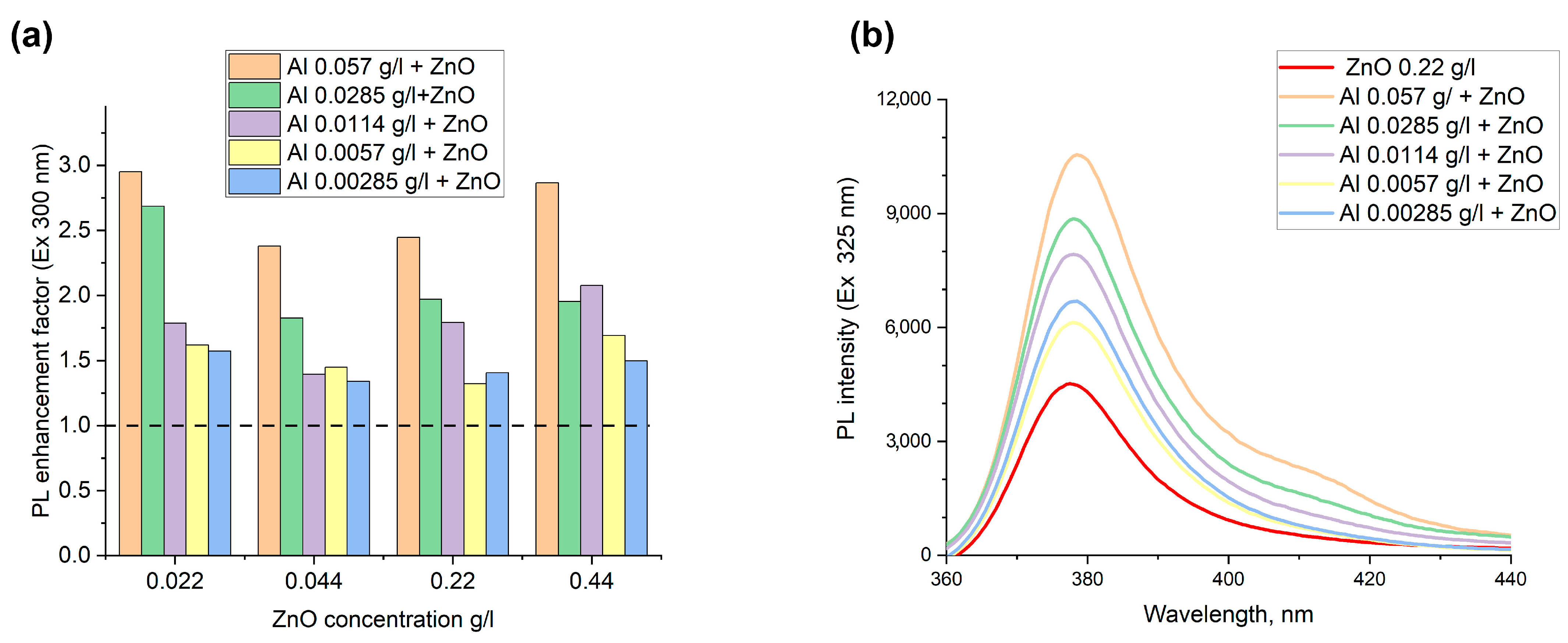
3.1. Luminescence in Colloids
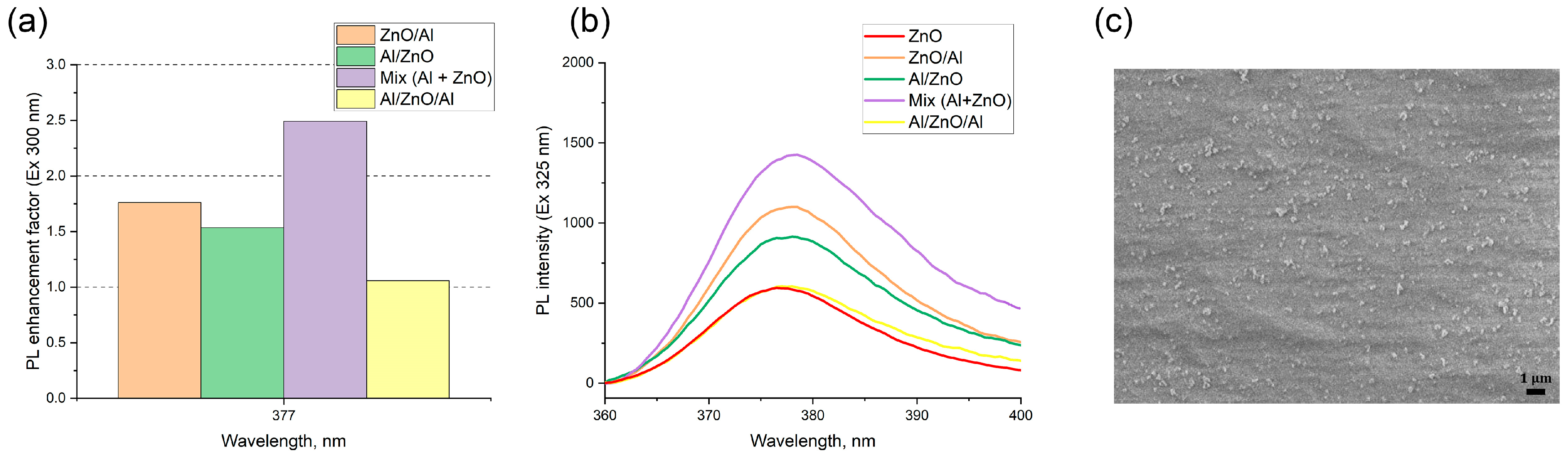
3.2. Luminescence in Film Nanostructures
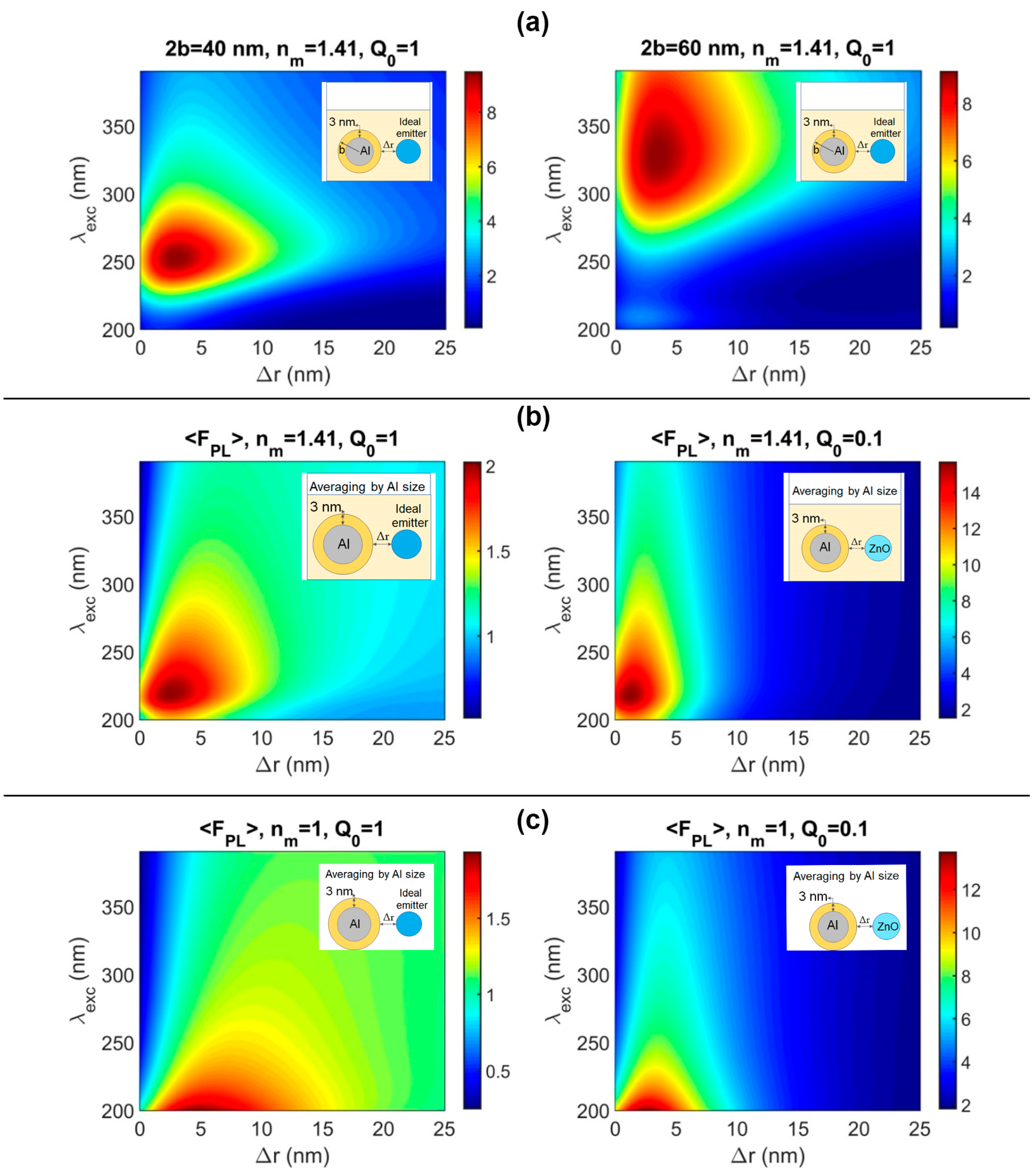
3.3. Numerical Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klimov, V. Surface plasmons. In Nanoplasmonics; Jenny Stanford Publishing: Singapore, 2014; pp. 79–106. [Google Scholar]
- Li, S.; Shan, S.; Chen, S.; Li, H.; Li, Z.; Liang, Y.; Fei, J.; Xie, L.; Li, J. Photocatalytic degradation of hazardous organic pollutants in water by Fe-MOFs and their composites: A review. J. Environ. Chem. Eng. 2021, 9, 105967. [Google Scholar] [CrossRef]
- Yasmeen, H.; Zada, A.; Ali, S.; Khan, I.; Ali, W.; Khan, W.; Khan, M.; Anwar, N.; Ali, A.; Huerta-Flores, A.M.; et al. Visible light-excited surface plasmon resonance charge transfer significantly improves the photocatalytic activities of ZnO semiconductor for pollutants degradation. J. Chin. Chem. Soc. 2020, 67, 1611–1617. [Google Scholar] [CrossRef]
- Gangadharan, D.T.; Xu, Z.; Liu, Y.; Izquierdo, R.; Ma, D. Recent advancements in plasmon-enhanced promising third-generation solar cells. Nanophotonics 2017, 6, 153–175. [Google Scholar] [CrossRef]
- Li, Y.-F.; Kou, Z.-L.; Feng, J.; Sun, H.-B. Plasmon-enhanced organic and perovskite solar cells with metal nanoparticles. Nanophotonics 2020, 9, 3111–3133. [Google Scholar] [CrossRef]
- Gaponenko, S.V. Introduction to Nanophotonics; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Yesudasu, V.; Pradhan, H.S.; Pandya, R.J. Recent progress in surface plasmon resonance based sensors: A comprehensive review. Heliyon 2021, 7, e06321. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.-Y. Plasmon-enhanced light–matter interactions and applications. NPJ Comput. Mater. 2019, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Badshah, M.A.; Koh, N.Y.; Zia, A.W.; Abbas, N.; Zahra, Z.; Saleem, M.W. Recent Developments in Plasmonic Nanostructures for Metal Enhanced Fluorescence-Based Biosensing. Nanomaterials 2020, 10, 1749. [Google Scholar] [CrossRef]
- Gaponenko, S.V.; Demir, H.V. Applied Nanophotonics; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Knight, M.W.; King, N.S.; Liu, L.; Everitt, H.O.; Nordlander, P.; Halas, N.J. Aluminum for plasmonics. ACS Nano 2014, 8, 834–840. [Google Scholar] [CrossRef]
- Gérard, D.; Gray, S.K. Aluminium plasmonics. J. Phys. D Appl. Phys. 2014, 48, 184001. [Google Scholar] [CrossRef]
- Afanasyev, D.A.; Ibrayev, N.K.; Serik, A.F. Optical properties of Al nanoparticles prepared by laser ablation method. In IOP Conference Series: Materials Science and Engineering, Proceedings of the Open School-Conference of NIS Countries Ultrafine Grained and Nanostructured Materials, Ufa, Russia, 1–5 October 2018; IOP Publishing: Bristol, UK, 2018; Volume 447, p. 012032. [Google Scholar] [CrossRef]
- Ramanenka, A.A.; Lizunova, A.A.; Mazharenko, A.K.; Kerechanina, M.F.; Ivanov, V.V.; Gaponenko, S.V. Preparation and Optical Properties of Isopropanol Suspensions of Aluminum Nanoparticles. J. Appl. Spectrosc. 2020, 87, 662–667. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Tan, Y.N. Metal nanoparticles-enhanced biosensors: Synthesis, design and applications in fluorescence enhancement and surface-enhanced Raman scattering. Chem.–Asian J. 2020, 15, 3180–3208. [Google Scholar] [CrossRef] [PubMed]
- Guzatov, D.V.; Gaponenko, S.V.; Demir, H.V. Plasmonic enhancement of electroluminescence. AIP Adv. 2018, 8, 015324. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cai, B.; Jia, B. Ultraviolet Plasmonic Aluminium Nanoparticles for Highly Efficient Light Incoupling on Silicon Solar Cells. Nanomaterials 2016, 6, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yang, G.; Zhang, Q.; Gao, S.; Zhang, R.; Zheng, Y. Localized Surface Plasmon-Enhanced Deep-UV Light-Emitting Diodes with Al/Al2O3 Asymmetrical Nanoparticles. Plasmonics 2017, 12, 843–848. [Google Scholar] [CrossRef]
- Mondal, R.K.; Adhikari, S.; Chatterjee, V.; Pal, S. Recent advances and challenges in AlGaN-based ultra-violet light emitting diode technologies. Mater. Res. Bull. 2021, 140, 111258. [Google Scholar] [CrossRef]
- Cho, C.Y.; Zhang, Y.; Cicek, E.; Rahnema, B.; Bai, Y.; McClintock, R.; Razeghi, M. Surface plasmon enhanced light emission from AlGaN-based ultraviolet light-emitting diodes grown on Si (111). Appl. Phys. Lett. 2013, 102, 211110. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Chen, S.; Li, J.; Kang, J.; Li, W.; Jin, P.; Chen, Y.; Wu, Z.; Dai, J.; et al. Surface Plasmon Enhanced Hot Exciton Emission in Deep UV-Emitting AlGaN Multiple Quantum Wells. Adv. Opt. Mater. 2014, 2, 451–458. [Google Scholar] [CrossRef]
- Chen, Y.; Xin, X.; Zhang, N.; Xu, Y.J. Aluminum-Based Plasmonic Photocatalysis. Part. Part. Syst. Charact. 2017, 34, 1600357. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Yu, J.; Li, Z.; Zhao, X.; Liu, A.; Jiang, S.; Yang, C.; Zhang, C.; Man, B. Aluminum nanoparticle films with an enhanced hot-spot intensity for high-efficiency SERS. Opt. Express 2020, 28, 9174–9185. [Google Scholar] [CrossRef]
- Wang, X.; Ye, Q.; Bai, L.-H.; Su, X.; Wang, T.-T.; Peng, T.-W.; Zhai, X.-Q.; Huo, Y.; Wu, H.; Liu, C.; et al. Enhanced UV Emission from ZnO on Silver Nanoparticle Arrays by the Surface Plasmon Resonance Effect. Nanoscale Res. Lett. 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Lu, J.; Li, J.; Xu, C.; Li, Y.; Dai, J.; Wang, Y.; Lin, Y.; Wang, S. Direct Resonant Coupling of Al Surface Plasmon for Ultraviolet Photoluminescence Enhancement of ZnO Microrods. ACS Appl. Mater. Interfaces 2014, 6, 18301–18305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cui, S.; Zhang, X.; Li, W. Significantly enhanced UV luminescence by plasmonic metal on ZnO nanorods patterned by screen-printing. Nanotechnology 2018, 29, 355703. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Khlopin, D.; Zhang, F.; Schuermans, S.; Proust, J.; Maurer, T.; Gérard, D.; Plain, J. Aluminum nanostructures for ultraviolet plasmonics. In UV and Higher Energy Photonics: From Materials to Applications 2017, Proceedings of the SPIE NanoScience + Engineering Conference, San Diego, CA, USA, 6–10 August 2017; International Society for Optics and Photonics: St. Bellingham, WA, USA, 2017; Volume 2017, p. 10351. [Google Scholar]
- Muravitskaya, A.; Gokarna, A.; Movsesyan, A.; Kostcheev, S.; Rumyantseva, A.; Couteau, C.; Lerondel, G.; Baudrion, A.-L.; Gaponenko, S.; Adam, P.-M. Refractive index mediated plasmon hybridization in an array of aluminium nanoparticles. Nanoscale 2020, 12, 6394–6402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Cao, R.-Y.; Wang, X.; Zhai, X.-Q.; Wang, T.-T.; Xu, Y.; He, Y.; Jia, M.; Su, X.; Bai, L.-H.; et al. Localized surface plasmon and transferred electron enhanced UV emission of ZnO by periodical aluminum nanoparticle arrays. J. Lumin 2022, 244. [Google Scholar] [CrossRef]
- Bhattarai, J.K.; Maruf MH, U.; Stine, K.J. Plasmonic-active nanostructured thin films. Processes 2020, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Piot, A.; Earl, S.K.; Ng, C.; Dligatch, S.; Roberts, A.; Davis, T.J.; Gómez, D.E. Collective excitation of plasmonic hot-spots for enhanced hot charge carrier transfer in metal/semiconductor contacts. Nanoscale 2015, 7, 8294–8298. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-W.; Raja, S.S.; Chang, C.-W.; Zhang, X.-Q.; Liu, P.-Y.; Lee, Y.-H.; Shih, C.-K.; Gwo, S. Epitaxial aluminum plasmonics covering full visible spectrum. Nanophotonics 2021, 10, 627–637. [Google Scholar] [CrossRef]
- Maidecchi, G.; Gonella, G.; Zaccaria, R.P.; Moroni, R.; Anghinolfi, L.; Giglia, A.; Nannarone, S.; Mattera, L.; Dai, H.-L.; Canepa, M.; et al. Deep Ultraviolet Plasmon Resonance in Aluminum Nanoparticle Arrays. ACS Nano 2013, 7, 5834–5841. [Google Scholar] [CrossRef]
- Mayer, M.; Schnepf, M.J.; König, T.A.F.; Fery, A. Colloidal Self-Assembly Concepts for Plasmonic Metasurfaces. Adv. Opt. Mater. 2019, 7, 1800564. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Wang, L.; Fang, F.; Fu, Y.; Yin, Z. A Review on Colloidal Self-Assembly and their Applications. Curr. Nanosci. 2016, 12, 725–746. [Google Scholar] [CrossRef]
- Vaschenko, S.V.; Ramanenka, A.; Guzatov, D.; Stankevich, V.V.; Lunevich, A.Y.; Glukhov, Y.F.; Sveklo, I.; Gaponenko, S.V. Plasmon-enhanced fluorescence of labeled biomolecules on top of a silver sol-gel film. J. Nanophotonics 2012, 6, 061710. [Google Scholar] [CrossRef]
- Lee, J.; You, E.-A.; Hwang, D.W.; Kang, S.; Wi, J.-S. Active Accumulation of Spherical Analytes on Plasmonic Hot Spots of Double-Bent Au Strip Arrays by Multiple Dip-Coating. Nanomaterials 2019, 9, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanenka, A.A.; Vaschenko, S.V.; Stankevich, V.V.; Lunevich, A.Y.; Glukhov, Y.F.; Gaponenko, S.V. Plasmonic Enhancement of Luminescence of Fluorscein Isothiocyanate and Human Immunoglobulin Conjugates. J. Appl. Spectrosc. 2014, 81, 222–225. [Google Scholar] [CrossRef]
- Markelonis, A.R.; Wang, J.S.; Ullrich, B.; Wai, C.M.; Brown, G.J. Nanoparticle film deposition using a simple and fast centrifuge sedimentation method. Appl. Nanosci. 2015, 5, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.; Byun, H.; Kim, J.-H. Rapid preparation of paper-based plasmonic platforms for SERS applications. Mater. Chem. Phys. 2020, 240, 122124. [Google Scholar] [CrossRef]
- Saito, K.; McGehee, K.; Manabe, K.; Norikane, Y. Facile fabrication of self-assembled nanostructures of vertically aligned gold nanorods by using inkjet printing. RSC Adv. 2021, 11, 22376–22380. [Google Scholar] [CrossRef]
- Ivanov, V.; Lizunova, A.; Rodionova, O.; Kostrov, A.; Kornyushin, D.; Aybush, A.; Golodyayeva, A.; Efimov, A.; Nadtochenko, V. Aerosol Dry Printing for SERS and Photoluminescence-Active Gold Nanostructures Preparation for Detection of Traces in Dye Mixtures. Nanomaterials 2022, 12, 448. [Google Scholar] [CrossRef]
- Khabarov, K.M.; Nouraldeen, M.; Lizunova, A.A.; Urazov, M.N.; Ivanov, V.V. Formation of planar plasmon microstructures by dry aerosol printing. In Journal of Physics: Conference Series, Proceedings of the 8th International School and Conference "Saint Petersburg OPEN 2021": Optoelectronics, Photonics, Engineering and Nanostructures (SPbOPEN 2021), Saint Petersburg, Russia, 25–28 May 2021; IOP Publishing: Bristol, UK, 2021; Volume 2086, p. 2086. [Google Scholar] [CrossRef]
- Грoмoв, А.А.; Хабас, Т.А.; Ильин, А.П. Combustion of Metal Nanopowders; Tomsk Polytechnic University: Tomsk, Russia, 2008. [Google Scholar]
- Altuwirqi, R.M.; Baatiyah, B.; Nugali, E.; Hashim, Z.; Al-Jawhari, H. Synthesis and Characterization of Aluminum Nanoparticles Prepared in Vinegar Using a Pulsed Laser Ablation Technique. J. Nanomater. 2020, 2020, 1327868. [Google Scholar] [CrossRef] [Green Version]
- Mozaffari, H.; Mahdieh, M.H. Synthesis of colloidal aluminum nanoparticles by nanosecond pulsed laser and the effect of external electric field and laser fluence on ablation rate. Opt. Laser Technol. 2020, 126, 106083. [Google Scholar] [CrossRef]
- Kotov, Y.A. The Electrical Explosion of Wire: A method for the synthesis of weakly aggregated nanopowders. Nanotechnologies in Russia. 2009, 4, 415–424. [Google Scholar]
- Borisov, V.I.; Lizunova, A.A.; Mazharenko, A.K.; Malo, D.; Ramanenka, A.A.; Shuklov, I.A.; Ivanov, V.V. Aluminum nanoparticles synthesis in spark discharge for ultraviolet plasmonics. J. Physics Conf. Ser. 2020, 1695, 012021. [Google Scholar] [CrossRef]
- Kotov, Y.A. Electric Explosion of Wires as a Method for Preparation of Nanopowders. J. Nanoparticle Res. 2003, 5, 539–550. [Google Scholar] [CrossRef]
- Lizunova, A.A.; Efimov, A.A.; Urazov, M.N.; Sivodedov, D.A.; Lisovskii, S.V.; Scidin, D.O.; Loshkarev, A.A.; Volkov, I.A.; Ivanov, V.V. Development and application of nanoparticle diameter certified reference materials of alumina, titania, silica, and zinc oxide colloidal solutions. Certified Reference Materials. 2013, 3, 15–20. [Google Scholar]
- Lizunova, A.A.; Loshkarev, A.A.; Tokunov, Y.M.; Ivanov, V.V. Comparison of the Results of Measurements of the Sizes of Nanoparticles in Stable Colloidal Solutions by the Methods of Acoustic Spectroscopy, Dynamic Light Scattering, and Transmission Electron Microscopy. Meas. Tech. 2017, 59, 1151–1155. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; University of Maryland School of Medicine: Baltimore, MD, USA, 2006. [Google Scholar]
- Joyce, C.; Fothergill, S.M.; Xie, F. Recent advances in gold-based metal enhanced fluorescence platforms for diagnosis and imaging in the near-infrared. Mater. Today Adv. 2020, 7, 100073. [Google Scholar] [CrossRef]
- Sultangaziyev, A.; Bukasov, R. Review: Applications of surface-enhanced fluorescence (SEF) spectroscopy in bio-detection and biosensing. Sens. Bio-Sens. Res. 2020, 30, 100382. [Google Scholar] [CrossRef]
- Park, K.H.; Oh, S.; Kim, J.; Yang, S.Y.; An, B.-S.; Hwang, D.Y.; Lee, J.H.; Kim, H.S.; Lee, J.; Seo, S. Effect of surface charge of gold nanoparticles on fluorescence amplification of polydiacetylene-based liposomes. J. Exp. Nanosci. 2020, 15, 174–181. [Google Scholar] [CrossRef]
- Starukhin, A.; Apyari, V.; Gorski, A.; Ramanenka, A.; Furletov, A. Plasmon enhancement of fluorescence of phthalocyanines metallocomplexes in solutions of silver nanoparticles. In EPJ Web of Confernces, Proceedings of the XIII International Workshop on Quantum Optics (IWQO-2019), Vladimir, Russia, 9–14 September 2019; EDP Sciences: Les Ulis, France, 2019; Volume 220, p. 03003. [Google Scholar] [CrossRef] [Green Version]
- Chu, V.H.; Fort, E.; Nghiem TH, L.; Tran, H.N. Photoluminescence enhancement of dye-doped nanoparticles by surface plasmon resonance effects of gold colloidal nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 045010. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Yun, J.-H.; Ahn, H.-K.; Usikov, A.S.; Yakimov, E.B.; Tarelkin, S.A.; Smirnov, N.B.; Shcherbachev, K.D.; Helava, H.; Makarov, Y.N.; et al. Photoluminescence enhancement by localized surface plasmons in AlGaN/GaN/AlGaN double heterostructures. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2015, 9, 575–579. [Google Scholar] [CrossRef]
- Gaponenko, S.V.; Guzatov, D.V. Colloidal Plasmonics for Active Nanophotonics. Proc. IEEE 2020, 108, 704–720. [Google Scholar] [CrossRef]
- Guzatov, D.V.; Vaschenko, S.V.; Stankevich, V.V.; Lunevich, A.Y.; Glukhov, Y.F. Gaponenko, Plasmonic enhancement of molecular fluorescence near silver nanoparticles: Theory, modeling, and experiment. J. Phys. Chem. C 2012, 116, 10723–10733. [Google Scholar] [CrossRef]
- Guzatov, D.; Gaponenko, S.V.; Demir, H.V. Colloidal Photoluminescent Refractive Index Nanosensor Using Plasmonic Effects. Z. Phys. Chem. 2018, 232, 1431–1441. [Google Scholar] [CrossRef] [Green Version]
- Palik, E.D. (Ed.) Handbook of Optical Constants of Solids; Academic Press: New York, NY, USA, 1998; 805p. [Google Scholar]
- Gaponenko, S.V.; Adam, P.-M.; Guzatov, D.; Muravitskaya, A.O. Possible nanoantenna control of chlorophyll dynamics for bioinspired photovoltaics. Sci. Rep. 2019, 9, 7138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley-Interscience: New York, NY, USA, 1983; 530p. [Google Scholar]
- Song, T.; Qu, Y.; Ren, Z.; Yu, S.; Sun, M.; Yu, X.; Yu, X. Synthesis and Characterization of Polyvinylpyrrolidone-Modified ZnO Quantum Dots and Their In Vitro Photodynamic Tumor Suppressive Action. Int. J. Mol. Sci. 2021, 22, 8106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizunova, A.A.; Malo, D.; Guzatov, D.V.; Vlasov, I.S.; Kameneva, E.I.; Shuklov, I.A.; Urazov, M.N.; Ramanenka, A.A.; Ivanov, V.V. Plasmon-Enhanced Ultraviolet Luminescence in Colloid Solutions and Nanostructures Based on Aluminum and ZnO Nanoparticles. Nanomaterials 2022, 12, 4051. https://doi.org/10.3390/nano12224051
Lizunova AA, Malo D, Guzatov DV, Vlasov IS, Kameneva EI, Shuklov IA, Urazov MN, Ramanenka AA, Ivanov VV. Plasmon-Enhanced Ultraviolet Luminescence in Colloid Solutions and Nanostructures Based on Aluminum and ZnO Nanoparticles. Nanomaterials. 2022; 12(22):4051. https://doi.org/10.3390/nano12224051
Chicago/Turabian StyleLizunova, Anna A., Dana Malo, Dmitry V. Guzatov, Ivan S. Vlasov, Ekaterina I. Kameneva, Ivan A. Shuklov, Maxim N. Urazov, Andrei A. Ramanenka, and Victor V. Ivanov. 2022. "Plasmon-Enhanced Ultraviolet Luminescence in Colloid Solutions and Nanostructures Based on Aluminum and ZnO Nanoparticles" Nanomaterials 12, no. 22: 4051. https://doi.org/10.3390/nano12224051
APA StyleLizunova, A. A., Malo, D., Guzatov, D. V., Vlasov, I. S., Kameneva, E. I., Shuklov, I. A., Urazov, M. N., Ramanenka, A. A., & Ivanov, V. V. (2022). Plasmon-Enhanced Ultraviolet Luminescence in Colloid Solutions and Nanostructures Based on Aluminum and ZnO Nanoparticles. Nanomaterials, 12(22), 4051. https://doi.org/10.3390/nano12224051





