Pseudocapacitance-Enhanced Storage Kinetics of 3D Anhydrous Iron (III) Fluoride as a Cathode for Li/Na-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
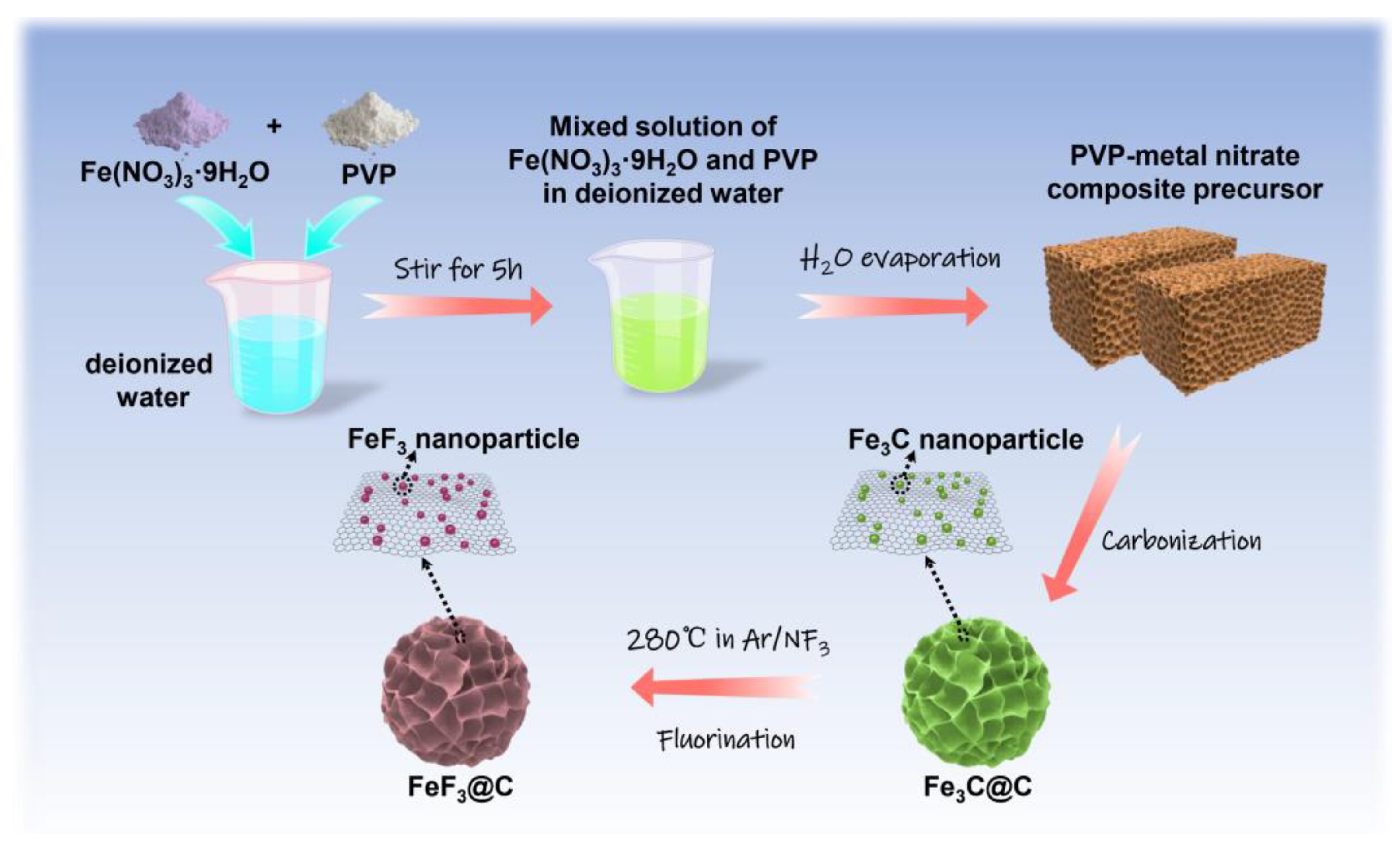
2.1. Synthesis of Fe3C@C Composite
2.2. Synthesis of FeF3@C Composite
2.3. Material Characterization
2.4. Electrochemical Characterization
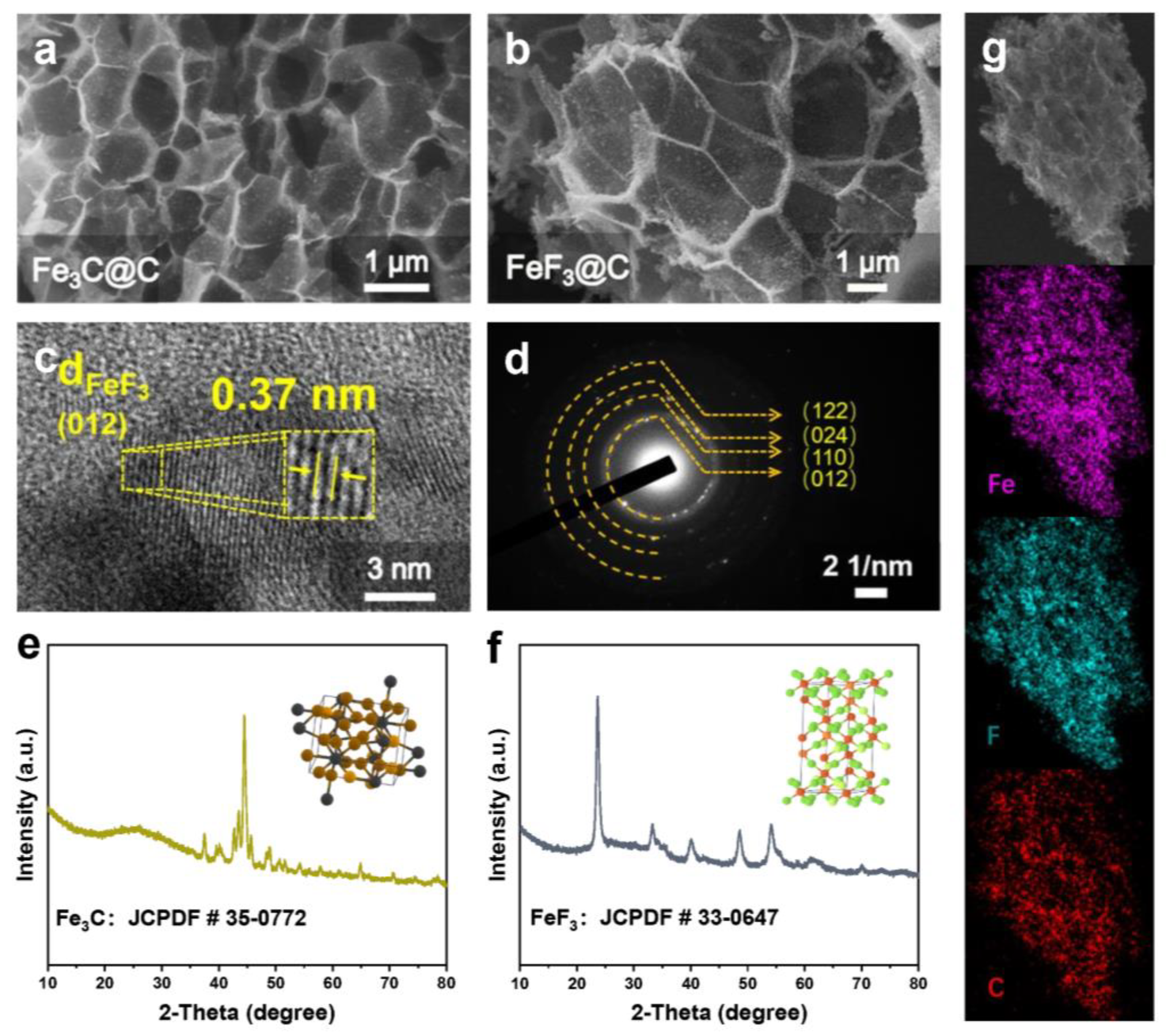
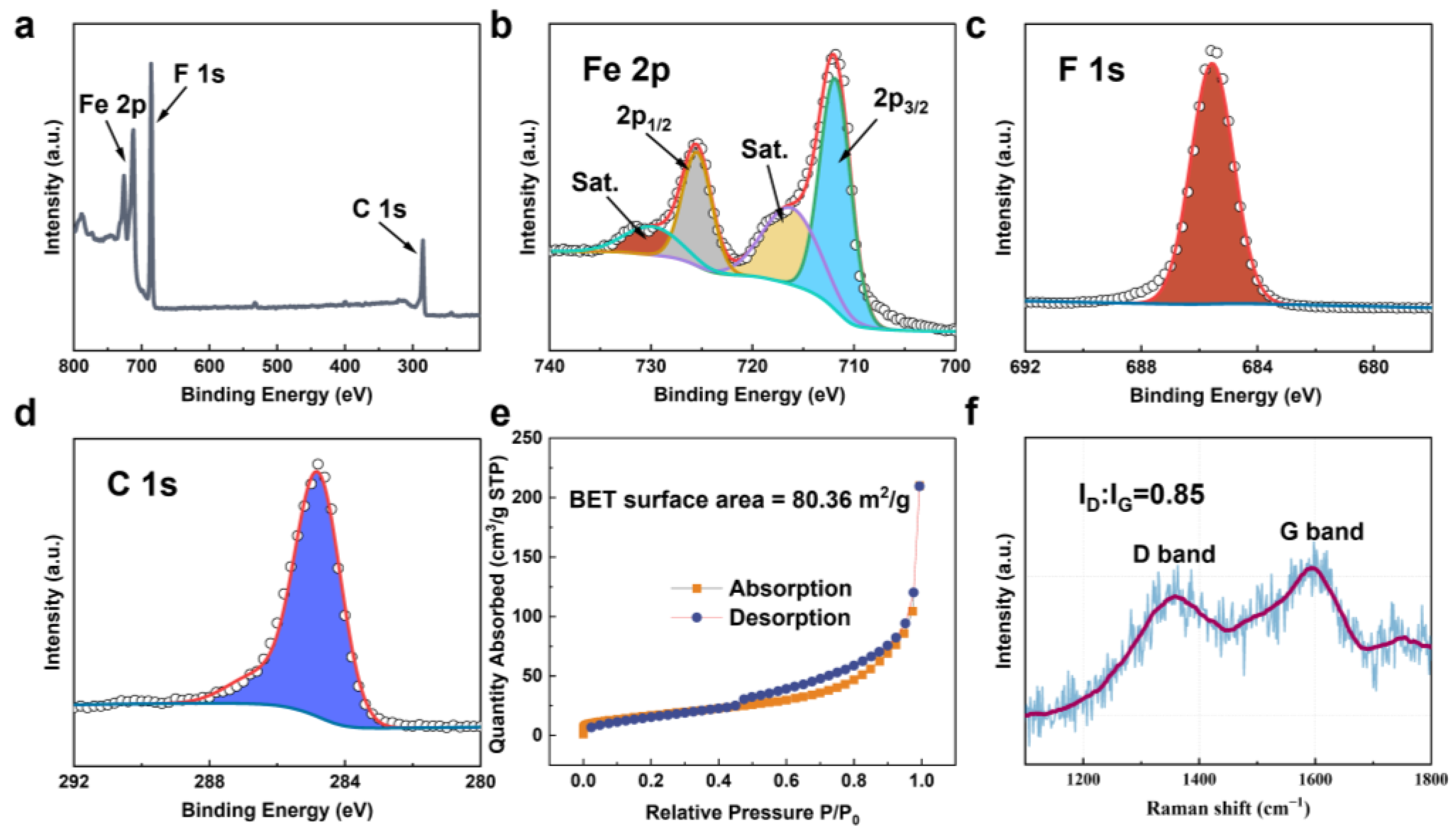
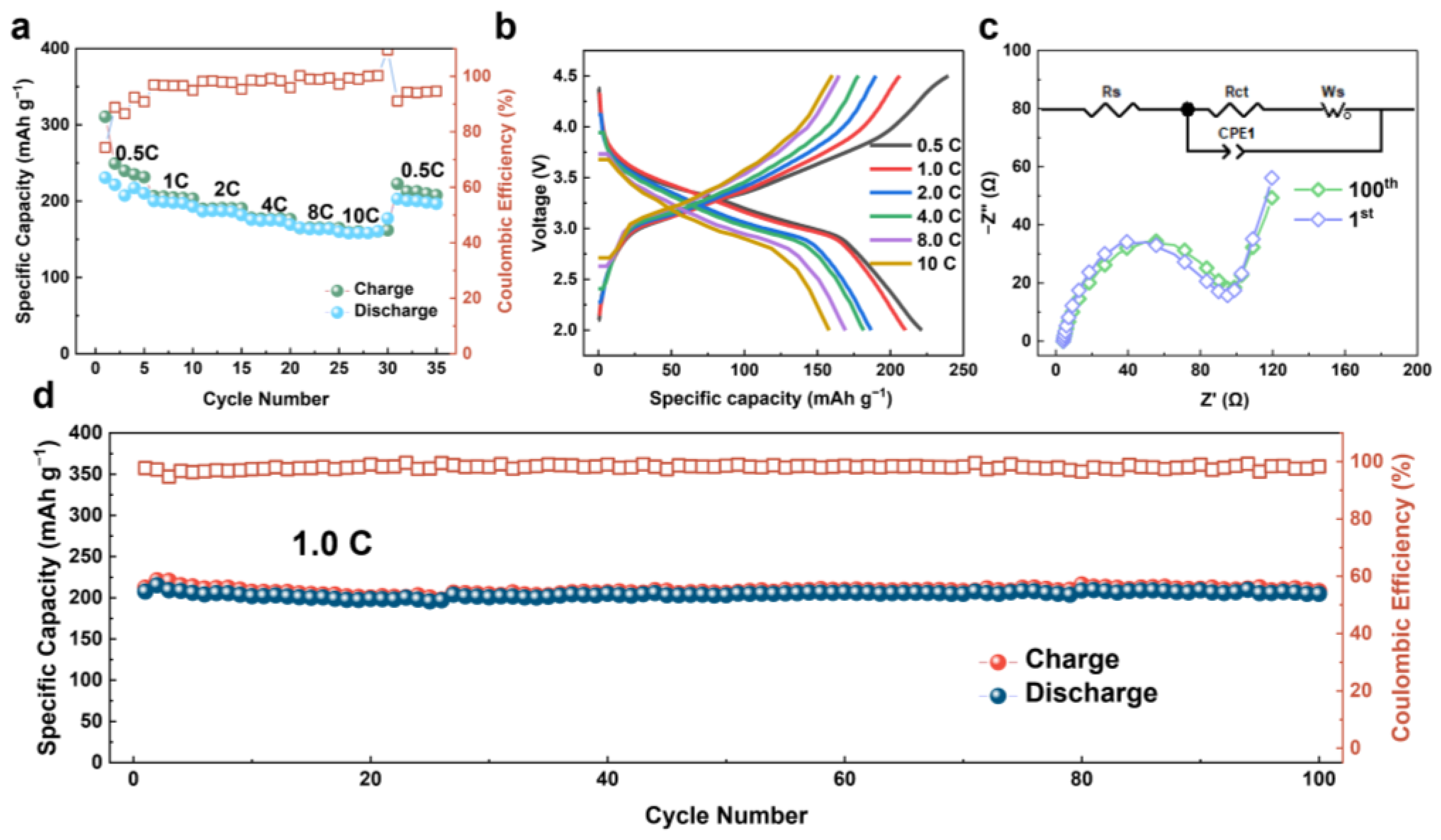
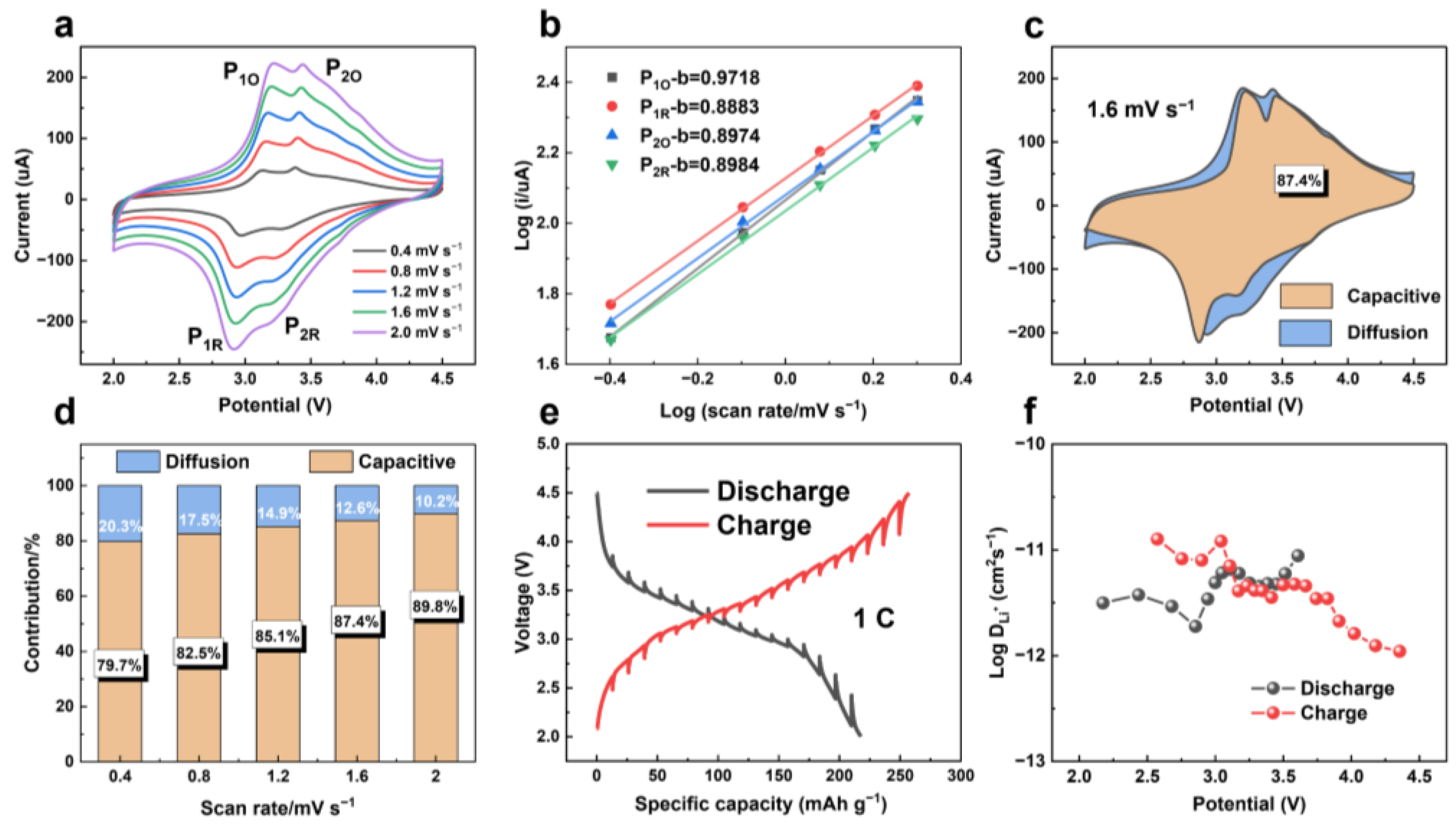
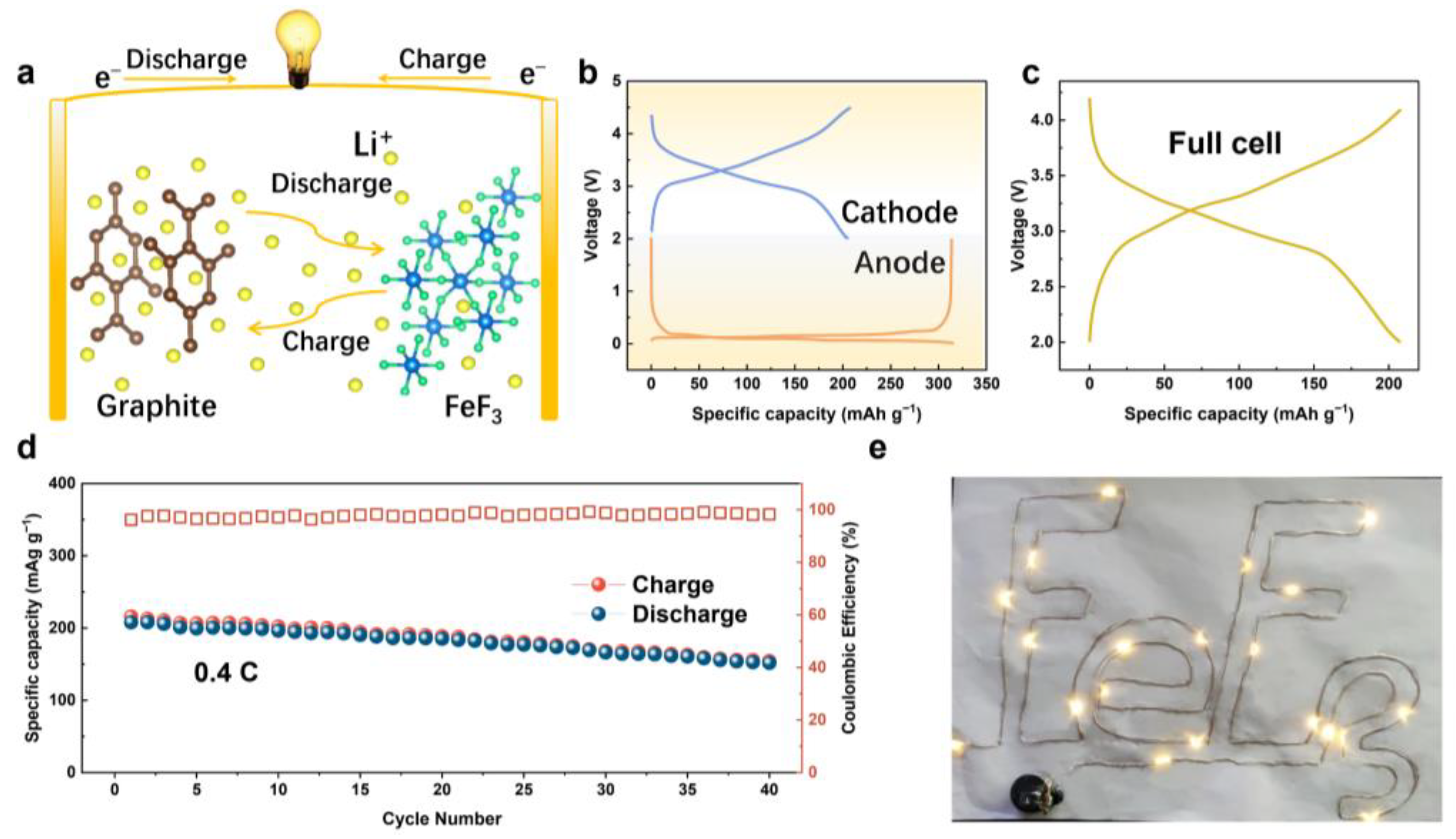
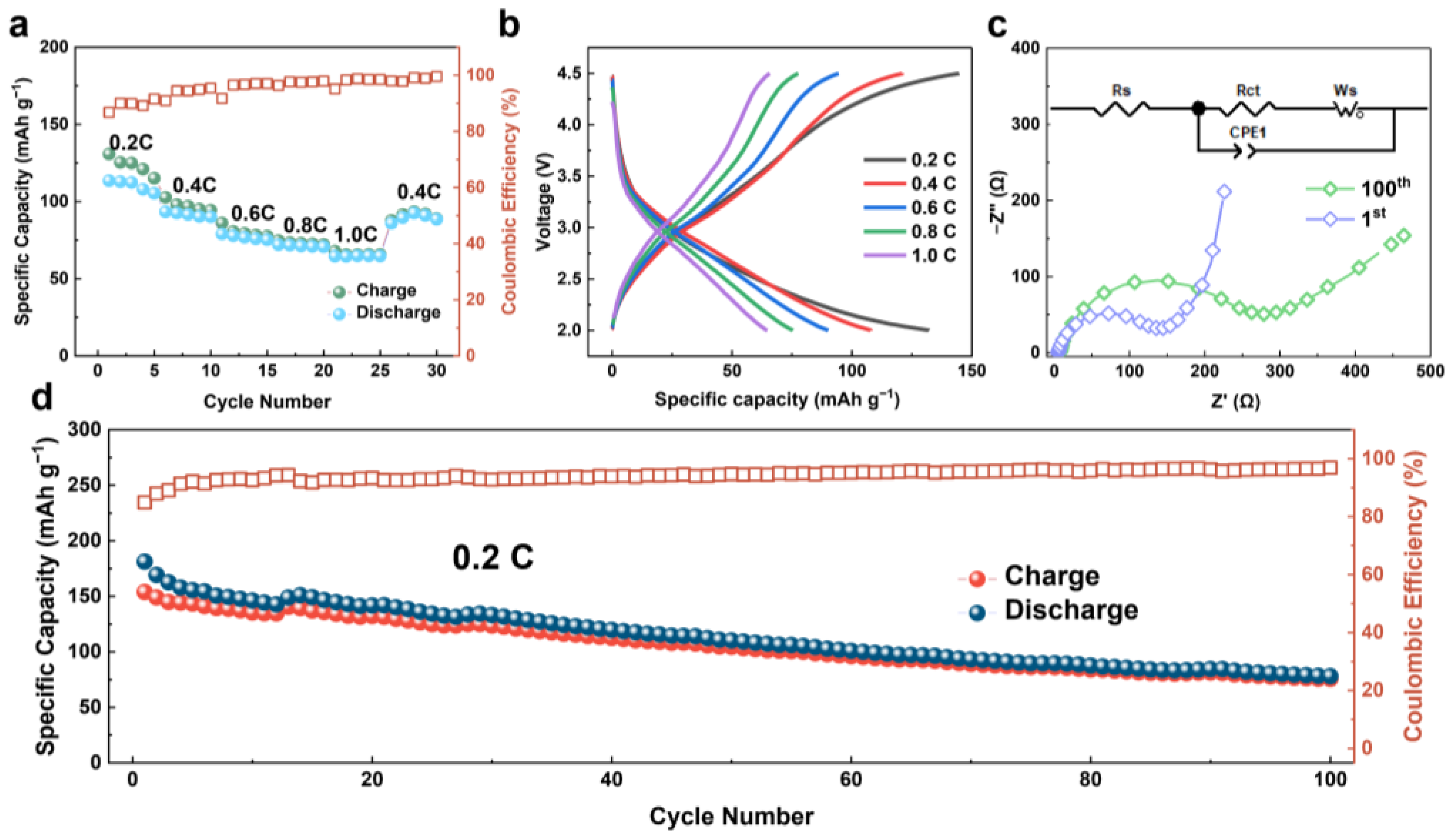
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Zhang, Y.; Li, X.; Gu, F.; Zhang, L.; Liu, H.; Xia, Q.; Li, Q.; Ye, W.; Ge, C.; et al. Reacquainting the Electrochemical Conversion Mechanism of FeS2 Sodium-Ion Batteries by Operando Magnetometry. J. Am. Chem. Soc. 2021, 143, 12800–12808. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-P.; Xie, K.-X.; He, J.-P.; Wang, Q.-P.; Ma, J.-M.; Yang, S.; Wang, Q.-H. SnO2 quantum dots modified N-doped carbon as high-performance anode for lithium ion batteries by enhanced pseudocapacitance. Rare Metals 2020, 40, 48–56. [Google Scholar] [CrossRef]
- Li, X.; Su, J.; Li, Z.; Zhao, Z.; Zhang, F.; Zhang, L.; Ye, W.; Li, Q.; Wang, K.; Wang, X.; et al. Revealing interfacial space charge storage of Li+/Na+/K+ by operando magnetometry. Sci. Bull. 2022, 67, 1145–1153. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Zhao, Y.; Meng, J.; Wen, B.; Muttaqi, K.M.; Islam, M.R.; Cai, Q.; Zhang, S. High-Performance Rechargeable Aluminum-Ion Batteries Enabled by Composite FeF3 @ Expanded Graphite Cathode and Carbon Nanotube-Modified Separator. Adv. Energy Mater. 2022, 12, 2200959. [Google Scholar] [CrossRef]
- Xu, X.; Li, F.; Zhang, D.; Liu, Z.; Zuo, S.; Zeng, Z.; Liu, J. Self-Sacrifice Template Construction of Uniform Yolk-Shell ZnS@C for Superior Alkali-Ion Storage. Adv. Sci. 2022, 9, 2200247. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Kang, K. Conversion-Based Cathode Materials for Rechargeable Sodium Batteries. Adv. Energy Mater. 2018, 8, 1702646. [Google Scholar] [CrossRef]
- Odoom-Wubah, T.; Rubio, S.; Tirado, J.L.; Ortiz, G.F.; Akoi, B.J.; Huang, J.; Li, Q. Waste Pd/Fish-Collagen as anode for energy storage. Renew. Sustain. Energ. Rev. 2020, 131, 109968. [Google Scholar] [CrossRef]
- Yang, H.; Xu, G.; Wei, X.; Cao, J.; Yang, L.; Chu, P.K. Ultrafast hetero-assembly of monolithic interwoven V2O5 nanobelts/carbon nanotubes architectures for high-energy alkali-ion batteries. J. Power Sources 2018, 395, 295–304. [Google Scholar] [CrossRef]
- Liang, L.; Li, X.; Zhao, F.; Zhang, J.; Liu, Y.; Hou, L.; Yuan, C. Construction and Operating Mechanism of High-Rate Mo-Doped Na3V2(PO4)3@C Nanowires toward Practicable Wide-Temperature-Tolerance Na-Ion and Hybrid Li/Na-Ion Batteries. Adv. Energy Mater. 2021, 11, 2100287. [Google Scholar] [CrossRef]
- Martin, A.; Doublet, M.-L.; Kemnitz, E.; Pinna, N. Reversible Sodium and Lithium Insertion in Iron Fluoride Perovskites. Adv. Funct. Mater. 2018, 28, 1802057. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Z.; Sun, X.; Lin, Y.; Tan, K.; Sun, J.; Liang, L.; Hou, L.; Yuan, C. Construction of Hierarchical Nanotubes Assembled from Ultrathin V3 S4 @C Nanosheets towards Alkali-Ion Batteries with Ion-Dependent Electrochemical Mechanisms. Angew. Chem. Int. Ed. 2020, 59, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, S.; Pan, Y.; Wang, X.; Liu, H.; Wang, Q.; Zhang, Z.; Deng, B.; Guo, C.; Shi, X. Atomistic Insights into FeF3 Nanosheet: An Ultrahigh-Rate and Long-Life Cathode Material for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 3142–3151. [Google Scholar] [CrossRef]
- Wu, F.; Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Li, T.; Chen, Z.X.; Cao, Y.L.; Ai, X.P.; Yang, H.X. Transition-metal chlorides as conversion cathode materials for Li-ion batteries. Electrochim. Acta 2012, 68, 202–205. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 4949, 1569–1614. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, E.; Sun, Z.; Ren, X.; Magasinski, A.; Yushin, G. Iron Fluoride-Carbon Nanocomposite Nanofibers as Free-Standing Cathodes for High-Energy Lithium Batteries. Adv. Funct. Mater. 2018, 28, 1801711. [Google Scholar] [CrossRef]
- Fan, X.; Hu, E.; Ji, X.; Zhu, Y.; Han, F.; Hwang, S.; Liu, J.; Bak, S.; Ma, Z.; Gao, T.; et al. High energy-density and reversibility of iron fluoride cathode enabled via an intercalation-extrusion reaction. Nat. Commun. 2018, 9, 2324. [Google Scholar] [CrossRef] [Green Version]
- Hua, X.; Eggeman, A.S.; Castillo-Martinez, E.; Robert, R.; Geddes, H.S.; Lu, Z.; Pickard, C.J.; Meng, W.; Wiaderek, K.M.; Pereira, N.; et al. Revisiting metal fluorides as lithium-ion battery cathodes. Nat. Mater. 2021, 20, 841–850. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Zou, J.; Gao, P.; Niu, X.; Li, H.; Chen, L. Li-free Cathode Materials for High Energy Density Lithium Batteries. Joule 2019, 3, 2086–2102. [Google Scholar] [CrossRef]
- Zhang, N.; Xiao, X.; Pang, H. Transition metal (Fe, Co, Ni) fluoride-based materials for electrochemical energy storage. Nanoscale Horiz. 2019, 4, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, J.; Xu, M.; Jiang, J.; Li, C.M. In Situ Engineering Toward Core Regions: A Smart Way to Make Applicable FeF3@Carbon Nanoreactor Cathodes for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 17992–18000. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mu, X.; van Aken, P.A.; Maier, J. A High-Capacity Cathode for Lithium Batteries Consisting of Porous Microspheres of Highly Amorphized Iron Fluoride Densified from Its Open Parent Phase. Adv. Energy Mater. 2013, 3, 113–119. [Google Scholar] [CrossRef]
- Shimoda, K.; Shikano, M.; Murakami, M.; Sakaebe, H. Capacity fading mechanism of conversion-type FeF3 electrode: Investigation by electrochemical operando nuclear magnetic resonance spectroscopy. J. Power Sources 2020, 477, 228772. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Gwon, H.; Kim, J.; Kang, K. Fabrication of FeF3 Nanoflowers on CNT branches and their application to high power lithium rechargeable batteries. Adv. Mater. 2010, 22, 5260–5264. [Google Scholar] [CrossRef]
- Wu, F.; Srot, V.; Chen, S.; Lorger, S.; van Aken, P.A.; Maier, J.; Yu, Y. 3D Honeycomb Architecture Enables a High-Rate and Long-Life Iron (III) Fluoride-Lithium Battery. Adv. Mater. 2019, 31, 1905146. [Google Scholar] [CrossRef]
- Li, J.; Fu, L.; Xu, Z.; Zhu, J.; Yang, W.; Li, D.; Zhou, L. Electrochemical properties of carbon-wrapped FeF3 nanocomposite as cathode material for lithium ion battery. Electrochim. Acta 2018, 281, 88–98. [Google Scholar] [CrossRef]
- Sun, Z.; Fu, W.; Liu, M.Z.; Lu, P.; Zhao, E.; Magasinski, A.; Liu, M.; Luo, S.; McDaniel, J.; Yushin, G. A nanoconfined iron(iii) fluoride cathode in a NaDFOB electrolyte: Towards high-performance sodium-ion batteries. J. Mater. Chem. A 2020, 8, 4091–4098. [Google Scholar] [CrossRef]
- Rubio, S.; Odoom-Wubah, T.; Li, Q.; Tirado, J.L.; Lavela, P.; Huang, J.; Ortiz, G.F. Marine shrimp/tin waste as a negative electrode for rechargeable sodium-ion batteries. J. Clean. Prod. 2022, 359, 131994. [Google Scholar] [CrossRef]
- Ortiz, G.F.; Lavela, P.; Knauth, P.; Djenizian, T.; Alcántara, R.; Tirado, J.L. Tin-Based composite Materials Fabricated by Anodic Oxidation for the Negative Electrode of Li-Ion Batteries. J. Electrochem. Soc. 2011, 158, A1094. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Su, Y.; Zheng, C.; Zhu, D.; Zhang, X.; Zhou, X.; Ouyang, R.; Huang, Q.; He, Y.; et al. Exploiting the Iron Difluoride Electrochemistry by Constructing Hierarchical Electron Pathways and Cathode Electrolyte Interface. Small 2022, 18, 2202006. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.n.; Guo, H.; Wang, X.; Zhu, Y.; Hu, J.; Yang, M.; Zhao, L.; Wang, J. Iron Trifluoride as a High Voltage Cathode Material for Thermal Batteries. J. Electrochem. Soc. 2019, 166, A3599–A3605. [Google Scholar] [CrossRef]
- Li, B.; Cheng, Z.; Zhang, N.; Sun, K. Self-supported, binder-free 3D hierarchical iron fluoride flower-like array as high power cathode material for lithium batteries. Nano Energy 2014, 4, 7–13. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, L.; Li, O.L.; Choi, S.-Y.; Saeed, G.; Kim, K.H. FeF3·0.33H2O@carbon nanosheets with honeycomb architectures for high-capacity lithium-ion cathode storage by enhanced pseudocapacitance. J. Mater. Chem. A 2021, 9, 16370–16383. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Zhang, Y.; Wang, Y.; Liu, W.; Yang, C.; Kondratiev, V.; Wu, F. Enhancing Electrochemical Performance of CoF2–Li Batteries via Honeycombed Nanocomposite Cathode. Energy Fuels 2022, 36, 8439–8448. [Google Scholar] [CrossRef]
- Wei, H.; Yang, H.-Y.; Zhang, X.-Q.; Zhu, J.-F.; Qiu, P.-P.; Luo, W. Hydrogen peroxide enabled two-dimensional molybdenum trioxide nanosheet clusters for enhanced surface Li-ion storage. Tungsten 2021, 3, 338–347. [Google Scholar] [CrossRef]
- Thomas, S.; Nam, E.B.; Lee, S.U. Atomistic Dynamics Investigation of the Thermomechanical Properties and Li Diffusion Kinetics in psi-Graphene for LIB Anode Material. ACS Appl. Mater. Interfaces 2018, 10, 36240–36248. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Sun, C.; Zhao, W.; Zhao, T.; Zhang, M.; Wang, H.; Ma, Y. Honeycomb Inspired Lithiophilic Scaffold for Ultra-Stable, High-Areal-Capacity Metallic Deposition. Energy Stor. Mater. 2021, 35, 378–387. [Google Scholar] [CrossRef]
- Qiu, D.; Fu, L.; Zhan, C.; Lu, J.; Wu, D. Seeding Iron Trifluoride Nanoparticles on Reduced Graphite Oxide for Lithium-Ion Batteries with Enhanced Loading and Stability. ACS Appl. Mater. Interfaces 2018, 10, 29505–29510. [Google Scholar] [CrossRef]
- Li, L.; Meng, F.; Jin, S. High-capacity lithium-ion battery conversion cathodes based on iron fluoride nanowires and insights into the conversion mechanism. Nano Lett. 2012, 12, 6030–6037. [Google Scholar] [CrossRef]
- Knehr, K.W.; Hodson, T.; Bommier, C.; Davies, G.; Kim, A.; Steingart, D.A. Understanding Full-Cell Evolution and Non-chemical Electrode Crosstalk of Li-Ion Batteries. Joule 2018, 2, 1146–1159. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Lu, Z.; Wang, C.; Wang, H.E.; Yang, S.; Xi, L.; Chung, J.C. Large-scale fabrication of graphene-wrapped FeF3 nanocrystals as cathode materials for lithium ion batteries. Nanoscale 2013, 5, 6338–6343. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Jae, W.J.; Kim, H.; Park, M.; Han, J.M.; Kim, J. A cathode material for lithium-ion batteries based on graphitized carbon-wrapped FeF3 nanoparticles prepared by facile polymerization. J. Mater. Chem. A 2016, 4, 14857–14864. [Google Scholar] [CrossRef]
- Li, J.; Meng, Y.; Wang, Y.; Li, X.; Lai, Y.; Guo, Y.; Wen, X.; Xiao, D. The fluorination-assisted dealloying synthesis of porous reduced graphene oxide-FeF2@carbon for high-performance lithium-ion battery and the exploration of its electrochemical mechanism. Inorg. Chem. Front. 2021, 8, 3273–3283. [Google Scholar] [CrossRef]
- Tawa, S.; Matsumoto, K.; Hagiwara, R. Reaction Pathways of Iron Trifluoride Investigated by Operation at 363 K Using an Ionic Liquid Electrolyte. J. Electrochem. Soc. 2019, 166, A2105–A2110. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Zhu, Y.F.; Yao, H.R.; Wang, P.F.; Zhang, X.D.; Li, H.; Yang, X.; Gu, L.; Li, Y.C.; Wang, T.; et al. A Stable Layered Oxide Cathode Material for High-Performance Sodium-Ion Battery. Adv. Energy Mater. 2019, 9, 1803978. [Google Scholar] [CrossRef]
- Du, K.; Tao, R.; Guo, C.; Li, H.; Liu, X.; Guo, P.; Wang, D.; Liang, J.; Li, J.; Dai, S.; et al. In-situ synthesis of porous metal fluoride@carbon composite via simultaneous etching/fluorination enabled superior Li storage performance. Nano Energy 2022, 103, 107862. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, Y.; Lin, X.; Liu, J.; Yuan, Z.; Cai, Y. Hybrid Cobalt(II) Fluoride Derived from a Bimetallic Zeolitic Imidazolate Framework as a High-Performance Cathode for Lithium–Ion Batteries. J. Phys. Chem. C 2020, 124, 8624–8632. [Google Scholar] [CrossRef]
- Wang, X.; Gu, W.; Lee, J.T.; Nitta, N.; Benson, J.; Magasinski, A.; Schauer, M.W.; Yushin, G. Carbon Nanotube-CoF2 Multifunctional Cathode for Lithium Ion Batteries: Effect of Electrolyte on Cycle Stability. Small 2015, 11, 5164–5173. [Google Scholar] [CrossRef]
- Li, J.; Xu, S.; Huang, S.; Lu, L.; Lan, L.; Li, S. In situ synthesis of Fe(1−x)CoxF3/MWCNT nanocomposites with excellent electrochemical performance for lithium-ion batteries. J. Mater. Sci. 2017, 53, 2697–2708. [Google Scholar] [CrossRef]
- Tan, J.; Liu, L.; Guo, S.; Hu, H.; Yan, Z.; Zhou, Q.; Huang, Z.; Shu, H.; Yang, X.; Wang, X. The electrochemical performance and mechanism of cobalt (II) fluoride as anode material for lithium and sodium ion batteries. Electrochim. Acta 2015, 168, 225–233. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Adelhelm, P.; Titirici, M.M.; Hu, Y.S. Intercalation chemistry of graphite: Alkali metal ions and beyond. Chem. Soc. Rev. 2019, 48, 4655–4687. [Google Scholar] [CrossRef] [PubMed]
- Rubio, S.; Ruiz, R.; Zuo, W.; Li, Y.; Liang, Z.; Cosano, D.; Gao, J.; Yang, Y.; Ortiz, G.F. Insights into the Reaction Mechanisms of Nongraphitic High-Surface Porous Carbons for Application in Na- and Mg-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 43127–43140. [Google Scholar] [CrossRef] [PubMed]
- Guntlin, C.P.; Zünd, T.; Kravchyk, K.V.; Wörle, M.; Bodnarchuk, M.I.; Kovalenko, M.V. Nanocrystalline FeF3 and MF2 (M = Fe, Co, and Mn) from metal trifluoroacetates and their Li(Na)-ion storage properties. J. Mater. Chem. A 2017, 5, 7383–7393. [Google Scholar] [CrossRef]
- Ma, D.-l.; Wang, H.-g.; Li, Y.; Xu, D.; Yuan, S.; Huang, X.-l.; Zhang, X.-b.; Zhang, Y. In situ generated FeF3 in homogeneous iron matrix toward high-performance cathode material for sodium-ion batteries. Nano Energy 2014, 10, 295–304. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, S.; Yu, L.; Xu, X.; Liu, J. Amorphous FeF3/C nanocomposite cathode derived from metal–organic frameworks for sodium ion batteries. RSC Adv. 2017, 7, 24004–24010. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Chen, S.; Zhu, L.; Yuan, Z.; Liu, J. Soft-template fabrication of hierarchical nanoparticle iron fluoride as high-capacity cathode materials for Li-ion batteries. Electrochim. Acta 2020, 364, 137293. [Google Scholar] [CrossRef]
- Cheng, Q.; Pan, Y.; Chen, Y.; Zeb, A.; Lin, X.; Yuan, Z.; Liu, J. Nanostructured Iron Fluoride Derived from Fe-Based Metal-Organic Framework for Lithium Ion Battery Cathodes. Inorg. Chem. 2020, 59, 12700–12710. [Google Scholar] [CrossRef]
- Zhang, C.; An, S.; Li, W.; Xu, H.; Hao, W.; Liu, W.; Li, Z.; Qiu, X. Hierarchical Mesoporous Iron Fluoride and Reduced Graphene Oxide Nanocomposite as Cathode Materials for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 17538–17546. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, X.; Hu, H.; Jiang, M.; Bai, Y.; Yang, X.; Shu, H. Sheet-like structure FeF3/graphene composite as novel cathode material for Na ion batteries. RSC Adv. 2015, 5, 38277–38282. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Wang, X.; Liu, M.; Wei, S.; Wang, Y.; Hu, H. Iron Fluoride Packaged into 3D Order Mesoporous Carbons as High-Performance Sodium-Ion Battery Cathode Material. J. Electrochem. Soc. 2018, 165, A89–A96. [Google Scholar] [CrossRef] [Green Version]
- Yanuar Maulana, A.; Song, J.; Futalan, C.M.; Kim, J. Improved reversibility of phase transformations using electron-rich graphitic carbon matrix in FeF2 cathode for sodium-ion batteries. Chem. Eng. J. 2022, 434, 134727. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Liu, Y.; Chen, G.; Liu, H.; Han, Y.; Zhai, S.; Zhang, L.; Pan, Y.; Li, Q.; Li, Q. Pseudocapacitance-Enhanced Storage Kinetics of 3D Anhydrous Iron (III) Fluoride as a Cathode for Li/Na-Ion Batteries. Nanomaterials 2022, 12, 4041. https://doi.org/10.3390/nano12224041
Zhang T, Liu Y, Chen G, Liu H, Han Y, Zhai S, Zhang L, Pan Y, Li Q, Li Q. Pseudocapacitance-Enhanced Storage Kinetics of 3D Anhydrous Iron (III) Fluoride as a Cathode for Li/Na-Ion Batteries. Nanomaterials. 2022; 12(22):4041. https://doi.org/10.3390/nano12224041
Chicago/Turabian StyleZhang, Tao, Yan Liu, Guihuan Chen, Hengjun Liu, Yuanyuan Han, Shuhao Zhai, Leqing Zhang, Yuanyuan Pan, Qinghao Li, and Qiang Li. 2022. "Pseudocapacitance-Enhanced Storage Kinetics of 3D Anhydrous Iron (III) Fluoride as a Cathode for Li/Na-Ion Batteries" Nanomaterials 12, no. 22: 4041. https://doi.org/10.3390/nano12224041
APA StyleZhang, T., Liu, Y., Chen, G., Liu, H., Han, Y., Zhai, S., Zhang, L., Pan, Y., Li, Q., & Li, Q. (2022). Pseudocapacitance-Enhanced Storage Kinetics of 3D Anhydrous Iron (III) Fluoride as a Cathode for Li/Na-Ion Batteries. Nanomaterials, 12(22), 4041. https://doi.org/10.3390/nano12224041








