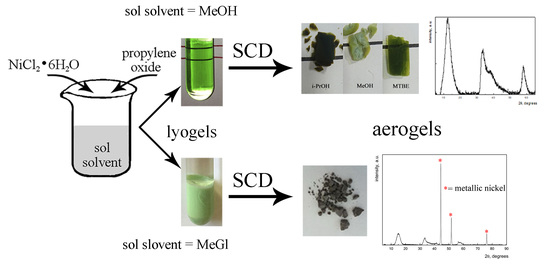
NiO-Based Aerogels—Unexpected Formation of Metallic Nickel Nanoparticles during Supercritical Drying Process
Abstract
1. Introduction
2. Experimental Section
2.1. NiO-Based Aerogels Preparation
2.2. Investigation Methods
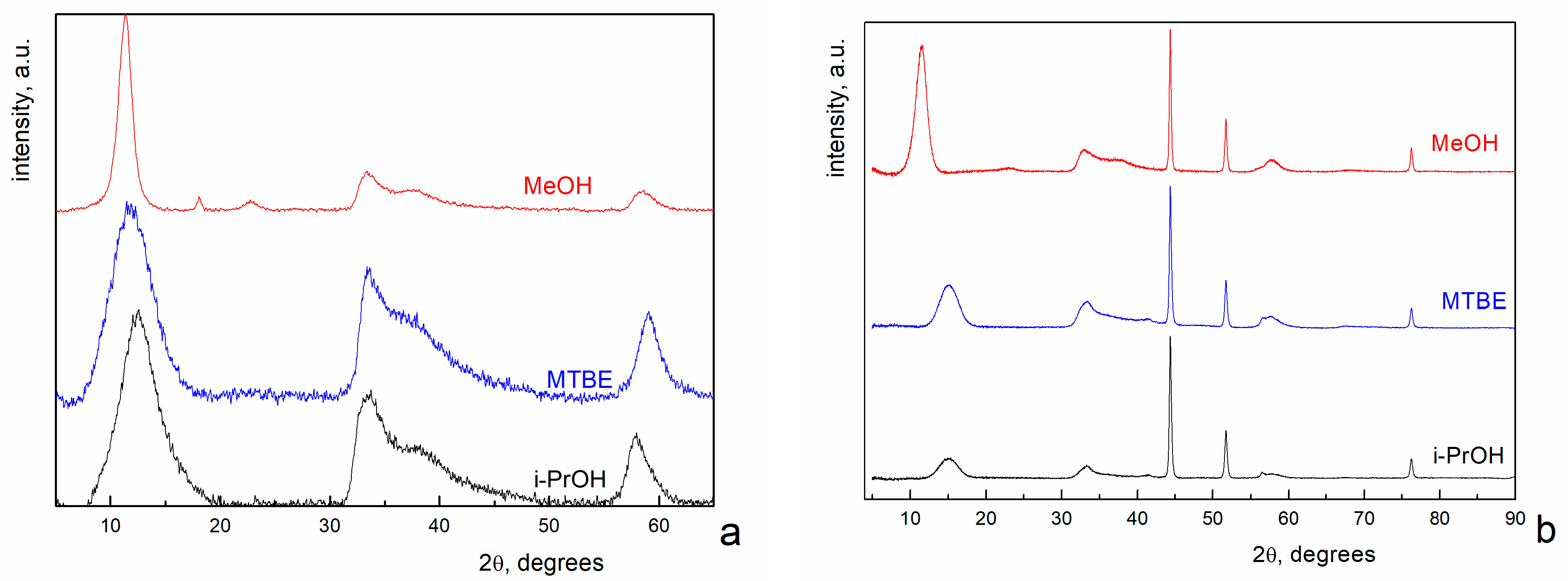
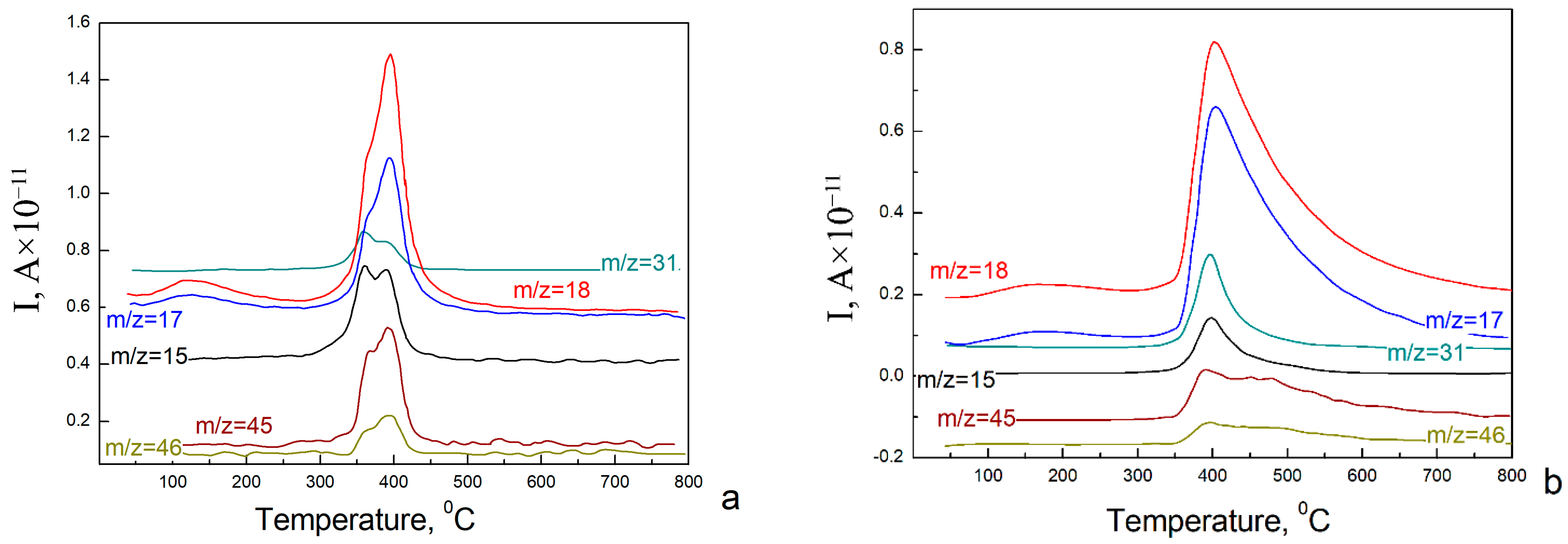
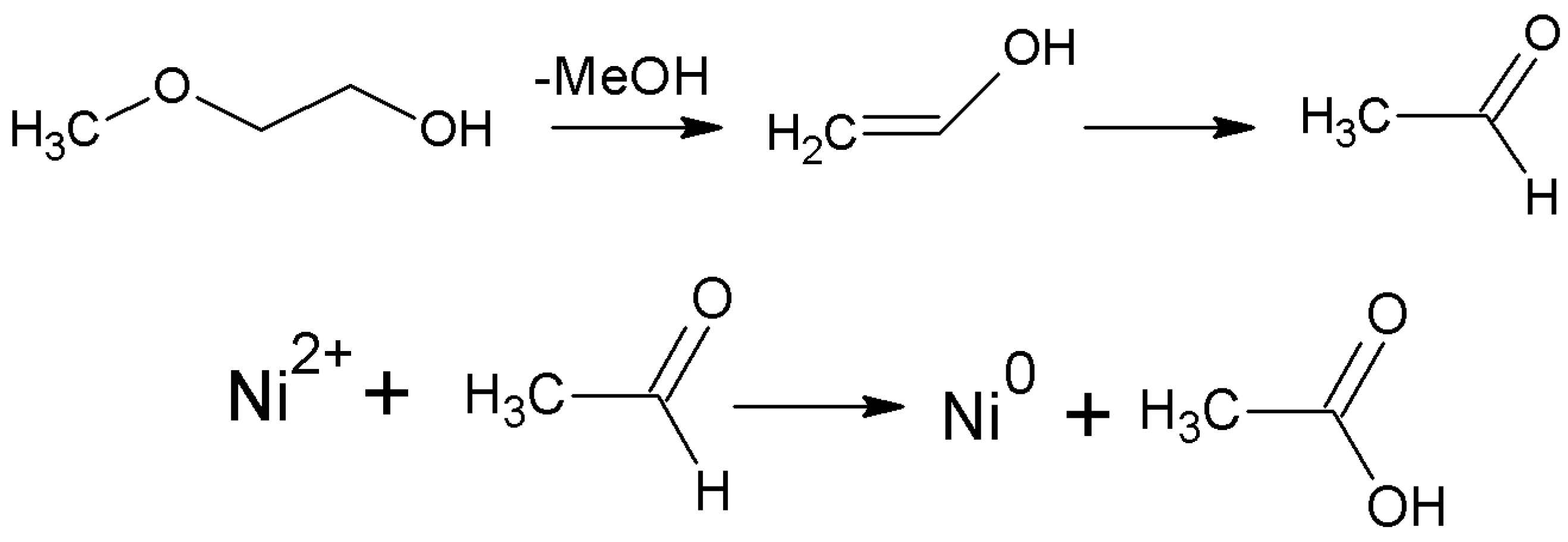
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osaki, T.; Horiuchi, T.; Sugiyama, T.; Suzuki, K.; Mori, T. Catalysis of NiO–Al2O3 aerogels for the CO2-reforming of CHF4. Catal. Lett. 1998, 52, 171–180. [Google Scholar] [CrossRef]
- Danilova, M.; Fedorova, Z.; Zaikovskii, V.; Porsin, A.; Kirillov, V.; Krieger, T. Porous nickel-based catalysts for combined steam and carbon dioxide reforming of methane. Appl. Catal. B Environ. 2014, 147, 858–863. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Lebedev, M.Y.; Rudina, N.A.; Kuvshinov, G.G. Filamentous carbon templated SiO2–NiO aerogel: Structure and catalytic properties for direct oxidation of hydrogen sulfide into sulfur. Catal. Lett. 2000, 70, 83–91. [Google Scholar] [CrossRef]
- Estelle, J. Comparative study of the morphology and surface properties of nickel oxide prepared from different precursors. Solid State Ion. 2003, 156, 233–243. [Google Scholar] [CrossRef]
- Das, D.; Pál, M.; Di Bartolomeo, E.; Traversa, E.; Chakravorty, D. Synthesis of nanocrystalline nickel oxide by controlled oxidation of nickel nanoparticles and their humidity sensing properties. J. Appl. Phys. 2000, 88, 6856–6860. [Google Scholar] [CrossRef]
- Gash, A.E.; Satcher, J.H.; Simpson, R.L. Monolithic nickel(II)-based aerogels using an organic epoxide: The importance of the counterion. J. Non-Cryst. Solids 2004, 350, 145–151. [Google Scholar] [CrossRef]
- David Levy, M.Z. The Sol-Gel Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Yoldas, B.E.; Annen, M.J.; Bostaph, J. Chemical engineering of aerogel morphology formed under nonsupercritical conditions for thermal insulation. Chem. Mater. 2000, 12, 2475–2484. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels—Airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Conroy, J.F.; Hosticka, B.; Davis, S.C.; Smith, A.N.; Norris, P.M. Microscale thermal relaxation during acoustic propagation in aerogel and other porous media. Microscale Thermophys. Eng. 1999, 3, 199–215. [Google Scholar] [CrossRef]
- Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Restuccia, G.; Tokarev, M.M.; Cacciola, G.J.R.K. Selective water sorbents for multiple applications, 10. Energy storage ability. React. Kinet. Catal. Lett. 2000, 69, 345–353. [Google Scholar] [CrossRef]
- Antczak, T.; Mrowiec-Białoń, J.; Bielecki, S.; Jarzebski, A.B.; Malinowski, J.J.; Lachowski, A.I.; Galas, E. Thermostability and esterification activity ofMucor javanicus lipase entrapped in silica aerogel matrix and in organic solvents. Biotechnol. Tech. 1997, 11, 9–11. [Google Scholar] [CrossRef]
- Pierre, M.; Buisson, P.; Fache, F.; Pierre, A. Influence of the drying technique of silica gels on the enzymatic activity of encapsulated lipase. Biocatal. Biotransform. 2000, 18, 237–251. [Google Scholar] [CrossRef]
- Lermontov, S.; Malkova, A.; Yurkova, L.; Straumal, E.; Gubanova, N.; Baranchikov, A.; Smirnov, M.; Tarasov, V.; Buznik, V.; Ivanov, V. Hexafluoroisopropyl alcohol as a new solvent for aerogels preparation. J. Supercrit. Fluids 2014, 89, 28–32. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Straumal, E.A.; Mazilkin, A.A.; Zverkova, I.I.; Baranchikov, A.E.; Straumal, B.B.; Ivanov, V.K. How to tune the alumina aerogels structure by the variation of a supercritical solvent. Evolution of the structure during heat treatment. J. Phys. Chem. C 2016, 120, 3319–3325. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Malkova, A.N.; Yurkova, L.L.; Straumal, E.A.; Gubanova, N.N.; Baranchikov, A.Y.; Ivanov, V.K. Diethyl and methyl-tert-buthyl ethers as new solvents for aerogels preparation. Mater. Lett. 2014, 116, 116–119. [Google Scholar] [CrossRef]
- Cutrufello, M.G.; Rombi, E.; Ferino, I.; Loche, D.; Corrias, A.; Casula, M.F. Ni-based xero- and aerogels as catalysts for nitroxidation processes. J. Sol-Gel Sci. Technol. 2011, 60, 324–332. [Google Scholar] [CrossRef]
- Gill, S.K.; Brown, P.; Ogundiya, M.T.; Hope-Weeks, L.J. High surface area alumina-supported nickel (II) oxide aerogels using epoxide addition method. J. Sol-Gel Sci. Technol. 2010, 53, 635–640. [Google Scholar] [CrossRef]
- Osaki, T.; Mori, T. Characterization of nickel–alumina aerogels with high thermal stability. J. Non-Cryst. Solids 2009, 355, 1590–1596. [Google Scholar] [CrossRef]
- Abouarnadasse, S.; Pajonk, G.; Teichner, S. Support effects in the catalytic nitroxidation of toluene into benzonitrile on nickel oxide based catalysts. Appl. Catal. 1985, 16, 237–247. [Google Scholar] [CrossRef]
- Osaki, T.; Horiuchi, T.; Sugiyama, T.; Suzuki, K.; Mori, T. NiO–Al2O3 aerogel from (CH2O)2Ni and AlOOH sol. J. Non-Cryst. Solids 1998, 225, 111–114. [Google Scholar] [CrossRef]
- Chaaban, M.; El-Rassy, H. Nickel–Aluminum Oxide Aerogels: Super-adsorbents for Azo Dyes for Water Remediation. ACS Omega 2020, 5, 27401–27412. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, S. Humidity sensing properties of NiO/Al2O3 nanocomposite materials. Solid State Ionics 2003, 164, 97–106. [Google Scholar] [CrossRef]
- Krompiec, S.; Mrowiec-Białoń, J.; Skutil, K.; Dukowicz, A.; Pająk, L.; Jarzębski, A. Nickel–alumina composite aerogel catalysts with a high nickel load: A novel fast sol–gel synthesis procedure and screening of catalytic properties. J. Non-Cryst. Solids 2003, 315, 297–303. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Q.; Gao, H.; Shi, Z.; Wu, J.; Zhi, M.; Hong, Z. Nickel oxide aerogel for high performance supercapacitor electrodes. RSC Adv. 2016, 6, 112620–112624. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. (Eds.) Aerogels Handbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; p. 23. [Google Scholar] [CrossRef]
- Lermontov, S.; Straumal, E.; Mazilkin, A.; Baranchikov, A.; Straumal, B.; Ivanov, V. An approach for highly transparent titania aerogels preparation. Mater. Lett. 2018, 215, 19–22. [Google Scholar] [CrossRef]
- Asano, T.; Kitahara, S. The dissolution of heattreated silica gel powders and change of their surface induced by treatment with methanol at 150–250 °C. Nippon. Kagaku Zassi 1970, 91, 109–117. [Google Scholar] [CrossRef]
- Carter, C.B.; Williams, D.B. (Eds.) Transmission Electron Microscopy: Diffraction, Imaging, and Spectrometry; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Alymov, M.I.; Rubtsov, N.M.; Seplyarskii, B.S.; Zelensky, V.A.; Ankudinov, A.B.; Kovalev, I.D.; Kochetkov, R.A.; Shchukin, A.S.; Petrov, E.V.; Kochetov, N.A. Synthesis of Ni nanoparticles with controlled pyrophoricity and mean size. Dokl. Chem. 2019, 484, 19–23. [Google Scholar] [CrossRef]
- Mohamed, M.; Halawy, S.; Ebrahim, M. Non-isothermal decomposition of nickel acetate tetrahydrate. J. Anal. Appl. Pyrolysis 1993, 27, 109–110. [Google Scholar] [CrossRef]
- Pronin, A.S.; Semenov, S.A.; Drobot, D.V.; Volchkova, E.V.; Dzhardimalieva, G.I. Synthesis and Thermal Conversions of Unsaturated Nickel(II) Monocarboxylates—Precursors of Metal-Containing Nanocomposites. Russ. J. Inorg. Chem. 2020, 65, 1173–1185. [Google Scholar] [CrossRef]
- Baraldi, P. Thermal behavior of metal carboxylates: III-metal acetates. Spectrochim. Acta Part A Mol. Spectrosc. 1982, 38, 51–55. [Google Scholar] [CrossRef]
- Alymov, M.I.; Zelensky, V.A.; Tregubova, I.V.; Ankudinov, A.B. Effect of Reduction Regimes on Dispersion and Degree of Reduction of Ni Nanopowders. Phys. Chem. Mater. Process. (RUS) 2009, 5, 55–58. [Google Scholar]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; Chichester & Wiley: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Robson, R.; Vince, D. Complexes of binucleating ligands. IX. Some tetranuclear nickel(II), cobalt(H) and mixed cobalt(II)-cobalt(III) complexes. Inorg. Chim. Acta 1977, 25, 191–201. [Google Scholar] [CrossRef]




| SCD Solvent | |||
|---|---|---|---|
| i-PrOH | MTBE | MeOH | |
| MeOH sol | 336 | 405 | 139 |
| MeGl sol | 650 | 430 | 178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straumal, E.A.; Mazilkin, A.A.; Gozhikova, I.O.; Yurkova, L.L.; Kottsov, S.Y.; Lermontov, S.A. NiO-Based Aerogels—Unexpected Formation of Metallic Nickel Nanoparticles during Supercritical Drying Process. Nanomaterials 2022, 12, 4033. https://doi.org/10.3390/nano12224033
Straumal EA, Mazilkin AA, Gozhikova IO, Yurkova LL, Kottsov SY, Lermontov SA. NiO-Based Aerogels—Unexpected Formation of Metallic Nickel Nanoparticles during Supercritical Drying Process. Nanomaterials. 2022; 12(22):4033. https://doi.org/10.3390/nano12224033
Chicago/Turabian StyleStraumal, Elena A., Andrey A. Mazilkin, Inna O. Gozhikova, Lyudmila L. Yurkova, Sergey Yu. Kottsov, and Sergey A. Lermontov. 2022. "NiO-Based Aerogels—Unexpected Formation of Metallic Nickel Nanoparticles during Supercritical Drying Process" Nanomaterials 12, no. 22: 4033. https://doi.org/10.3390/nano12224033
APA StyleStraumal, E. A., Mazilkin, A. A., Gozhikova, I. O., Yurkova, L. L., Kottsov, S. Y., & Lermontov, S. A. (2022). NiO-Based Aerogels—Unexpected Formation of Metallic Nickel Nanoparticles during Supercritical Drying Process. Nanomaterials, 12(22), 4033. https://doi.org/10.3390/nano12224033