Nanosecond Laser-Textured Copper Surfaces Hydrophobized with Self-Assembled Monolayers for Enhanced Pool Boiling Heat Transfer
Abstract
1. Introduction
1.1. Boiling Heat Transfer
1.2. Boiling Heat Transfer Enhancement via Surface Functionalization
1.3. Laser-Textured Surfaces for Boiling Enhancement
1.4. Motivation and Goals of the Present Study
2. Materials and Methods
2.1. Samples
2.2. Surface Laser Texturing
2.3. Surface Hydrophobization
2.4. Surface Analysis
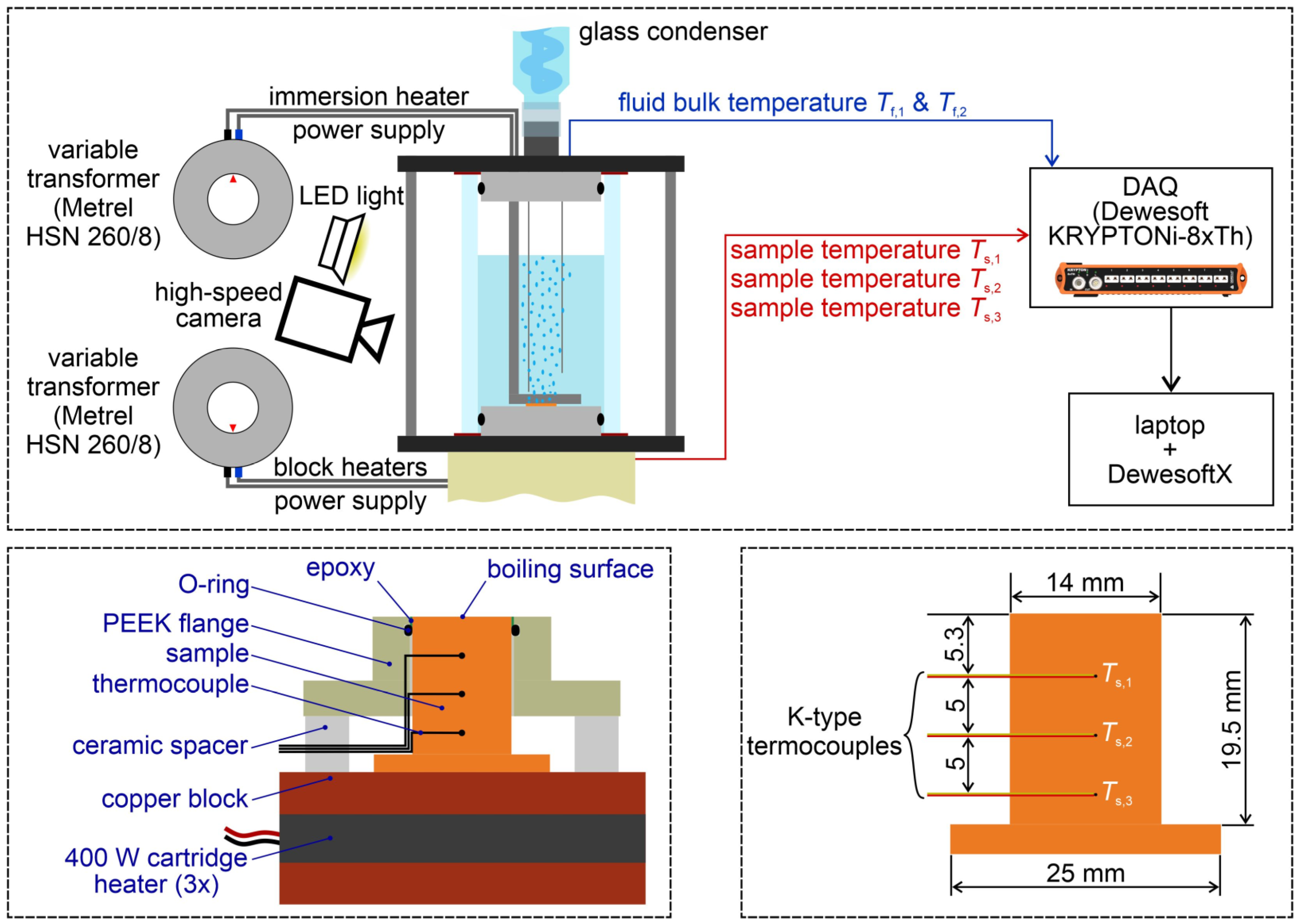
2.5. Pool Boiling Experimental Setup
2.6. Data Reduction and Measurement Uncertainty
2.7. Measurement Protocol
3. Results
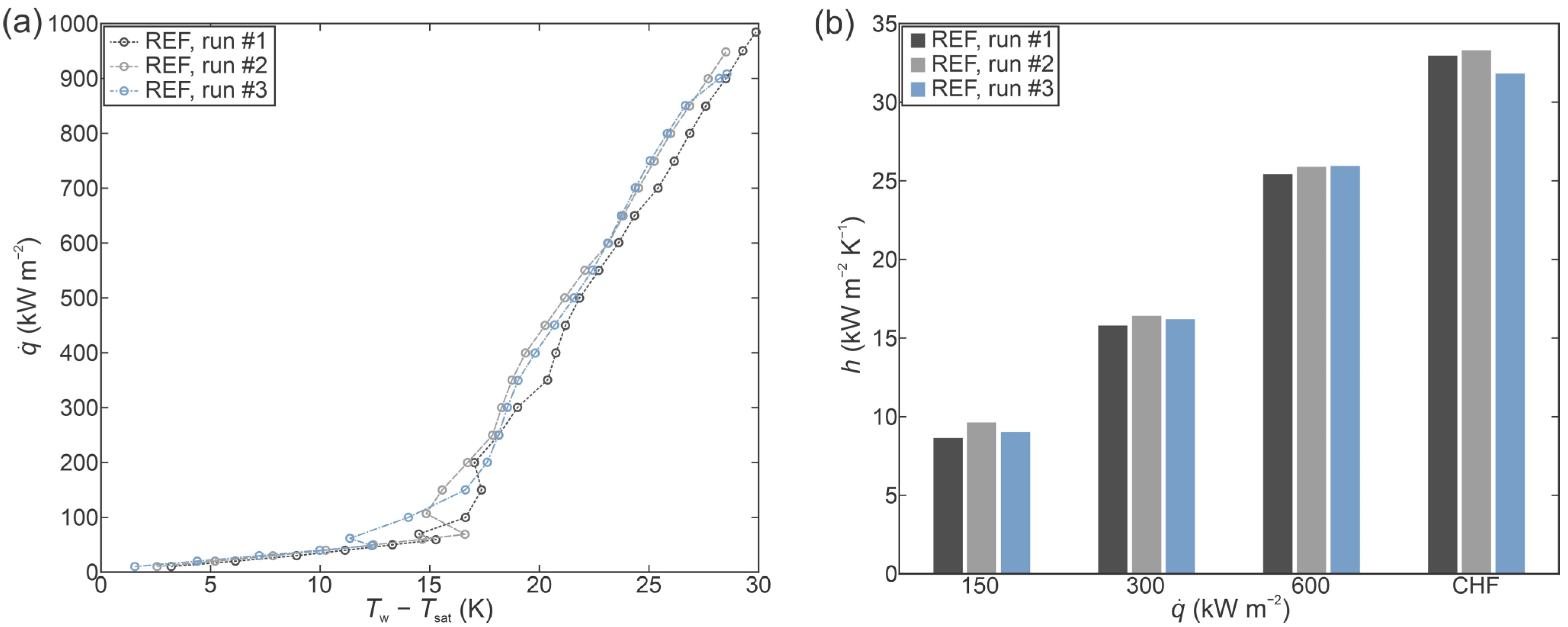
3.1. Reference Experiments
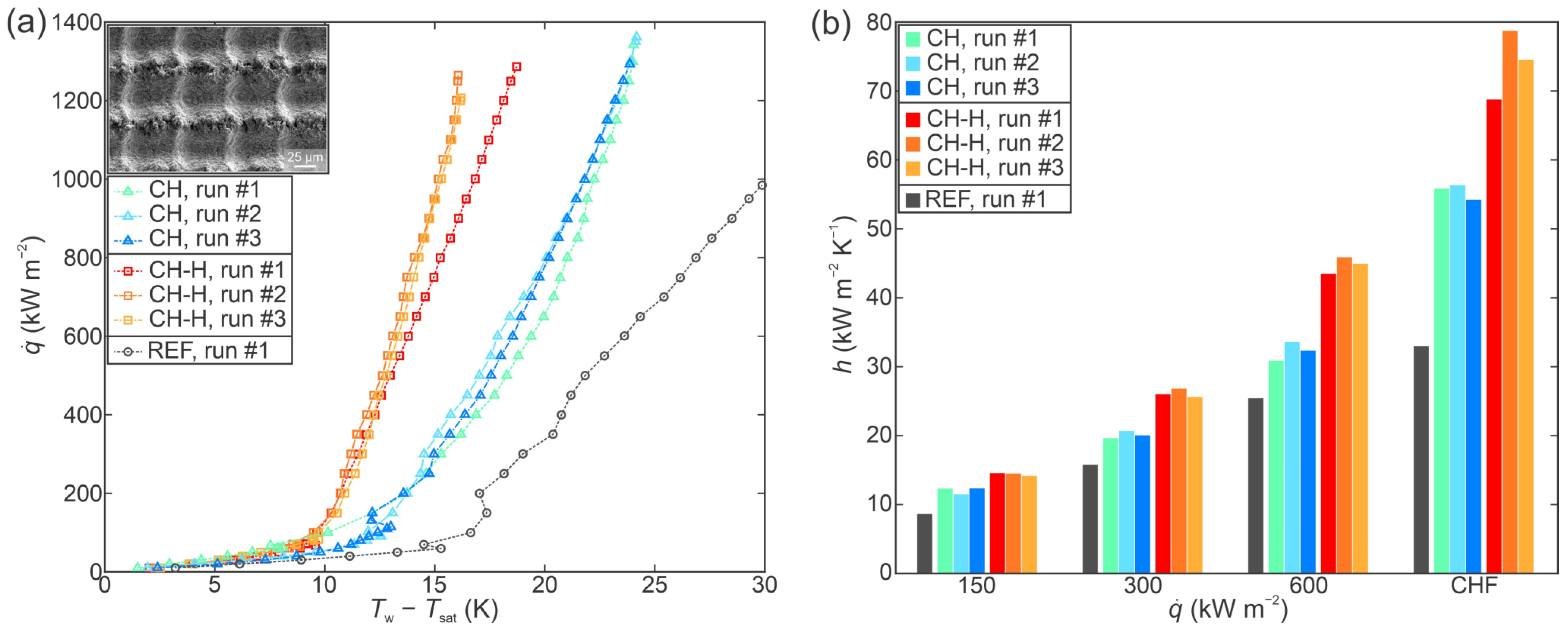
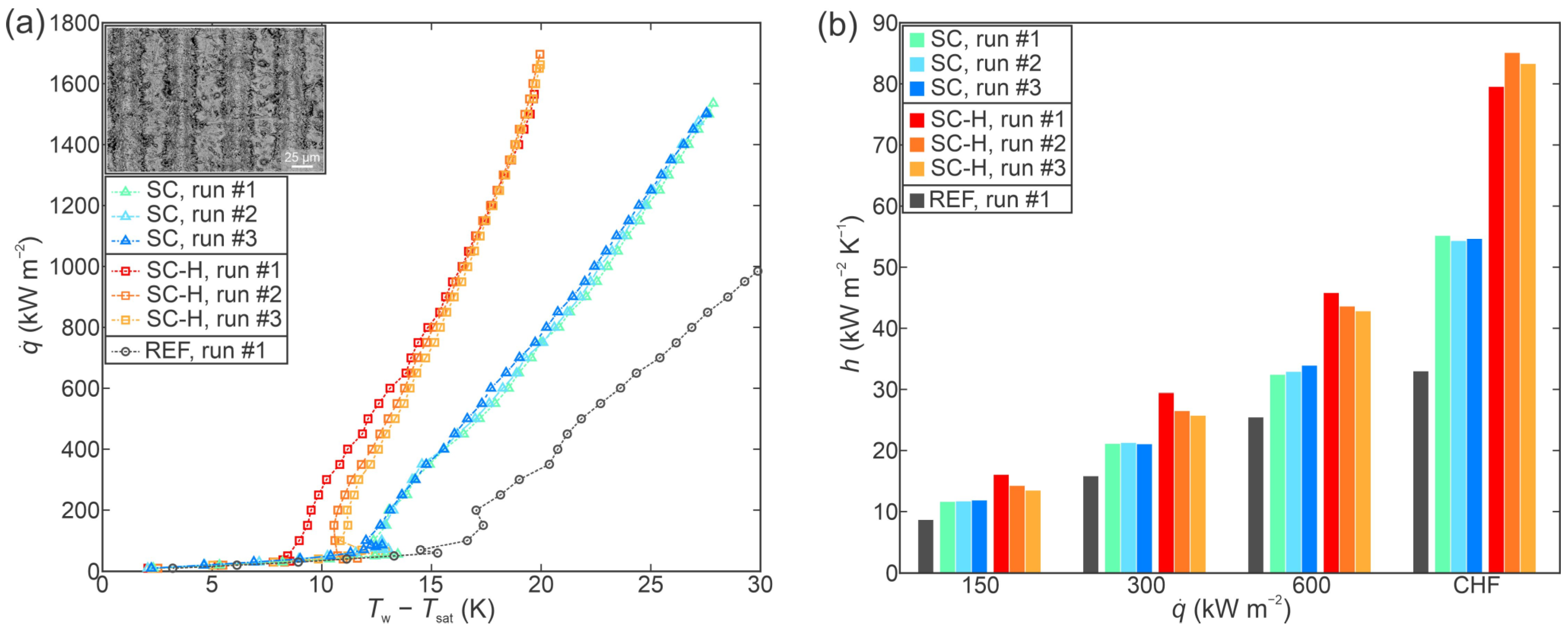
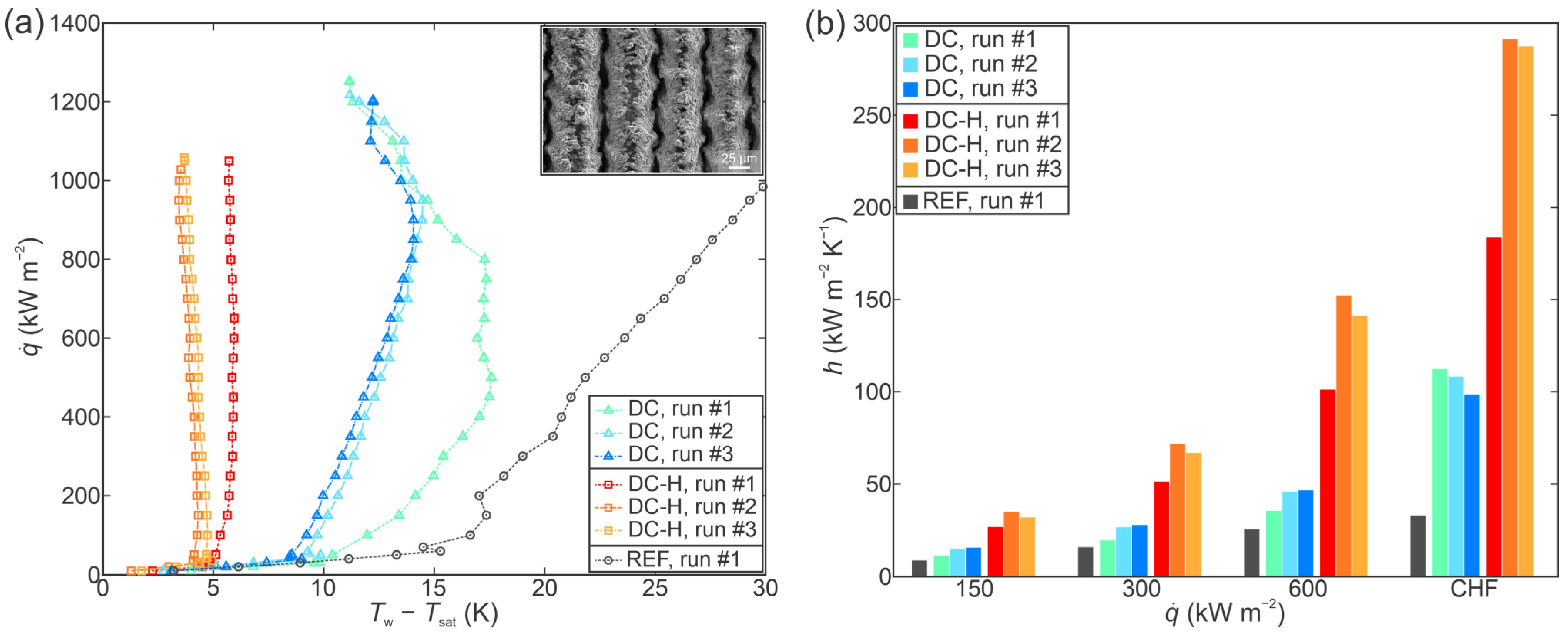
3.2. Performance of Individual Functionalized Surfaces
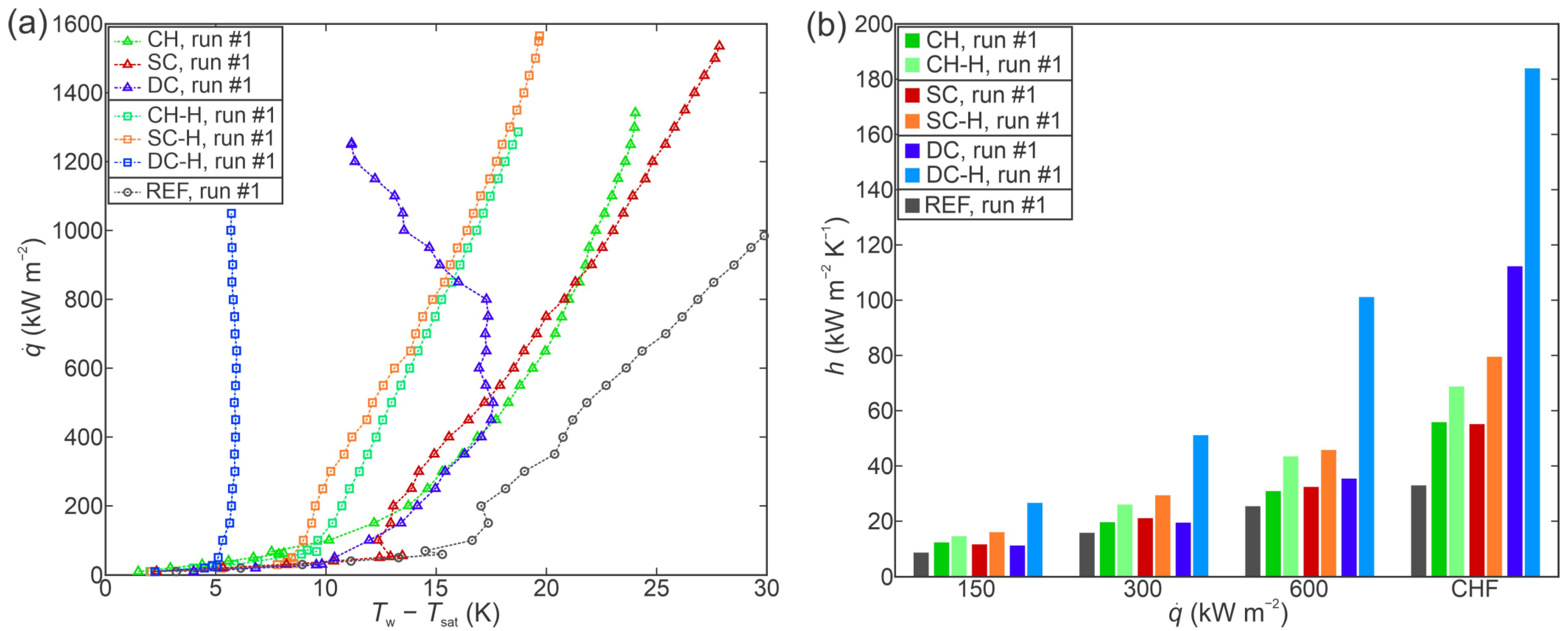
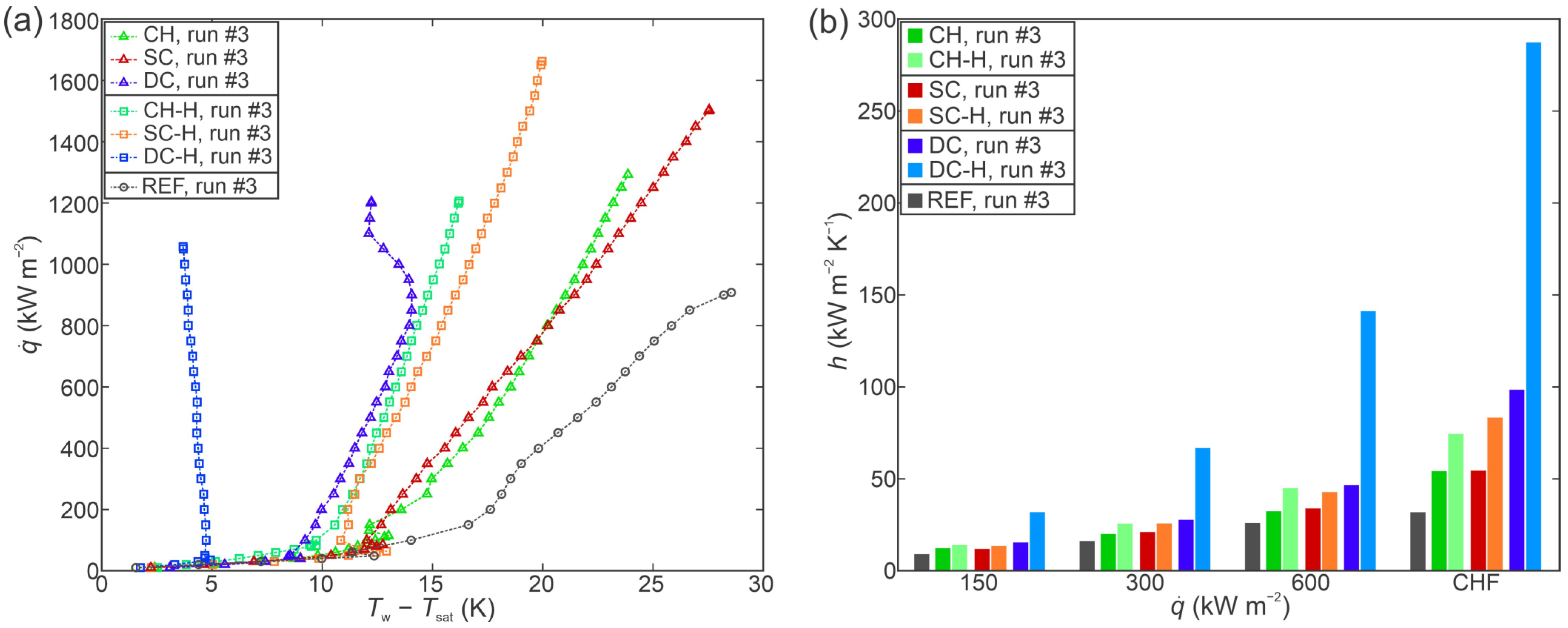
3.3. Comparison of Boiling Performance
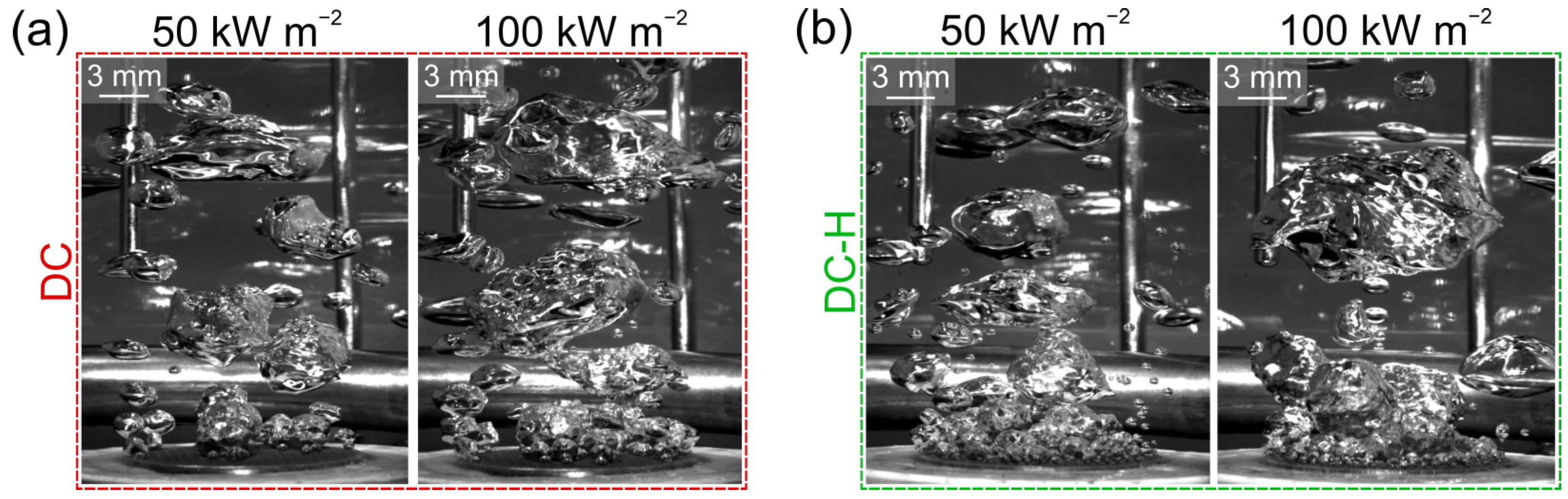
3.4. High-Speed Videography Analysis
4. Discussions
5. Conclusions
- All laser-functionalized surfaces exhibited enhanced boiling heat transfer performance in comparison with an untreated reference surface. The recorded highest critical heat flux enhancement was achieved on a hydrophobized shallow channel surface (SC-H), where a CHF of 1697 kW m−2 was recorded during the third experimental run.
- In addition to increased critical heat flux, significant enhancements of the heat transfer coefficient were observed. Specifically, the hydrophobized surface with deep channels (DC-H) provided an HTC of 291.4 kW m−2 K−1 at CHF incipience, which represents a 775% enhancement over the highest values recorded on the untreated reference.
- Functionalized surfaces exhibited positive stability and repeatable boiling curves. A shift towards lower superheat values was observed on the deep channel surfaces after the first experimental run, which may be attributed to additional degassing of the surface during the post-CHF transition towards film boiling.
- Surface microstructure was identified as the key reason for enhanced heat transfer parameters. Despite large differences in surface wettability, hydrophobized surfaces exhibited comparable (and in one case even higher) CHF values in comparison with their hydrophilic counterparts, which are traditionally considered as more favorable for achieving high CHF values.
- A significant reduction in bubble departure diameter was observed on the best-performing surface DC-H, which exhibited the highest HTC value. The small bubble departure diameter is attributed to effective vapor entrapment in the deep surface structures and is pointed out as a major contributing reason for the observed extreme boiling heat transfer performance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tong, L.S. Heat transfer in water-cooled nuclear reactors. Nucl. Eng. Des. 1967, 6, 301–324. [Google Scholar] [CrossRef]
- Trojer, M.; Azizian, R.; Paras, J.; McKrell, T.; Atkhen, K.; Bucci, M.; Buongiorno, J. A margin missed: The effect of surface oxidation on CHF enhancement in IVR accident scenarios. Nucl. Eng. Des. 2018, 335, 140–150. [Google Scholar] [CrossRef]
- Anderson, T.M.; Mudawar, I. Microelectronic cooling by enhanced pool boiling of a dielectric fluorocarbon liquid. J. Heat Transf. 1989, 111, 752–759. [Google Scholar] [CrossRef]
- A Bar-Cohen, K.J.L.G. Cooling the electronic brain. Mech. Eng. 2011, 133, 38–41. [Google Scholar] [CrossRef]
- Allred, T.P.; Weibel, J.A.; Garimella, S.V. Enabling Highly Effective Boiling from Superhydrophobic Surfaces. Phys. Rev. Lett. 2018, 120, 174501. [Google Scholar] [CrossRef]
- Može, M.; Senegačnik, M.; Gregorčič, P.; Hočevar, M.; Zupančič, M.; Golobič, I. Laser-Engineered Microcavity Surfaces with a Nanoscale Superhydrophobic Coating for Extreme Boiling Performance. ACS Appl. Mater. Interfaces 2020, 12, 24419–24431. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.; Chakraborty, A.; Lee, S.E.; Kim, G.; Yu, C. Recent Advances in Two-Phase Immersion Cooling with Surface Modifications for Thermal Management. Energies 2022, 15, 1214. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ebrahim, S.; Mishra, P.C.; Ali, N.; Chaudhuri, P. A Review on Pool and Flow Boiling Enhancement Using Nanofluids: Nuclear Reactor Application. Processes 2022, 10, 177. [Google Scholar] [CrossRef]
- Moghadasi, H.; Malekian, N.; Saffari, H.; Gheitaghy, A.M.; Zhang, G.Q. Recent advances in the critical heat flux amelioration of pool boiling surfaces using metal oxide nanoparticle deposition. Energies 2020, 13, 4026. [Google Scholar] [CrossRef]
- Khan, S.A.; Atieh, M.A.; Koç, M. Micro-nano scale surface coating for nucleate boiling heat transfer: A critical review. Energies 2018, 11, 3189. [Google Scholar] [CrossRef]
- Meena, C.S.; Kumar, A.; Roy, S.; Cannavale, A.; Ghosh, A. Review on Boiling Heat Transfer Enhancement Techniques. Energies 2022, 15, 5759. [Google Scholar] [CrossRef]
- Ahmadi, V.E.; Aboubakri, A.; Sadaghiani, A.K.; Sefiane, K.; Koşar, A. Effect of functional surfaces with gradient mixed wettability on flow boiling in a high aspect ratio microchannel. Fluids 2020, 5, 5–9. [Google Scholar] [CrossRef]
- Le Ba, T.; Baqer, A.; Kamel, M.S.; Gróf, G.; Odhiambo, V.O.; Wongwises, S.; Ferenc, L.; Szilágyi, I.M. Experimental Study of Halloysite Nanofluids in Pool Boiling Heat Transfer. Molecules 2022, 27, 729. [Google Scholar] [CrossRef]
- Cho, H.; Godinez, J.; Han, J.S.; Fadda, D.; You, S.M.; Lee, J.; Park, S.J. Fabrication of micro-patterned surface for pool-boiling enhancement by using powder injection molding process. Materials 2019, 12, 507. [Google Scholar] [CrossRef]
- Može, M.; Hadžić, A.; Zupančič, M.; Golobič, I. Boiling heat transfer enhancement on titanium through nucleation-promoting morphology and tailored wettability. Int. J. Heat Mass Transf. 2022, 195, 123161. [Google Scholar] [CrossRef]
- Freitas, E.; Pontes, P.; Cautela, R.; Bahadur, V.; Miranda, J.; Ribeiro, A.; Souza, R.; Oliveira, J.; Copetti, J.; Lima, R.; et al. Pool Boiling of Nanofluids on Biphilic Surfaces: An Experimental and Numerical Study. Nanomaterials 2021, 11, 125. [Google Scholar] [CrossRef]
- Galicia, E.S.; Otomo, Y.; Saiwai, T.; Takita, K.; Orito, K.; Enoki, K. Subcooled Flow Boiling Heat Flux Enhancement Using High Porosity Sintered Fiber. Appl. Sci. 2021, 11, 5883. [Google Scholar] [CrossRef]
- Otomo, Y.; Galicia, E.S.; Enoki, K. Enhancement of subcooled flow boiling heat transfer with high porosity sintered fiber metal. Appl. Sci. 2021, 11, 1237. [Google Scholar] [CrossRef]
- Može, M.; Vajc, V.; Zupančič, M.; Šulc, R.; Golobič, I. Pool boiling performance of water and self-rewetting fluids on hybrid functionalized aluminum surfaces. Processes 2021, 9, 1058. [Google Scholar] [CrossRef]
- Kaniowski, R.; Pastuszko, R. Pool Boiling of Water on Surfaces with Open Microchannels. Energies 2021, 14, 3062. [Google Scholar] [CrossRef]
- Kamel, M.S.; Lezsovits, F. Experimental Investigation on Pool Boiling Heat Transfer Performance Using Tungsten Oxide WO3 Nanomaterial-Based Water Nanofluids. Materials 2020, 13, 1922. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hong, D.; Badshah, M.A.; Lu, X.; Kim, Y.K.; Kim, S.M. Direct metal forming of a microdome structure with a glassy carbon mold for enhanced boiling heat transfer. Micromachines 2018, 9, 376. [Google Scholar] [CrossRef]
- Može, M.; Vajc, V.; Zupančič, M.; Golobič, I. Hydrophilic and hydrophobic nanostructured copper surfaces for efficient pool boiling heat transfer with water, water/butanol mixtures and novec 649. Nanomaterials 2021, 11, 3216. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ali, N.; Aljuwayhel, N.F.; Mishra, P.C.; Sen, S.; Chaudhuri, P. Pool boiling amelioration by aqueous dispersion of silica nanoparticles. Nanomaterials 2021, 11, 2138. [Google Scholar] [CrossRef]
- Pranoto, I.; Rahman, M.A.; Waluyo, J. The Role of Pin Fin Array Configurations and Bubble Characteristics on the Pool Boiling Heat Transfer Enhancement. Fluids 2022, 7, 232. [Google Scholar] [CrossRef]
- Hadžić, A.; Može, M.; Arhar, K.; Zupančič, M.; Golobič, I. Effect of Nanoparticle Size and Concentration on Pool Boiling Heat Transfer with TiO2 Nanofluids on Laser-Textured Copper Surfaces. Nanomaterials 2022, 12, 2611. [Google Scholar] [CrossRef] [PubMed]
- Orman, Ł.J.; Radek, N.; Pietraszek, J.; Szczepaniak, M. Analysis of enhanced pool boiling heat transfer on laser-textured surfaces. Energies 2020, 13, 2700. [Google Scholar] [CrossRef]
- Sajjad, U.; Hussain, I.; Hamid, K.; Ali, H.M.; Wang, C.-C.; Yan, W.-M. Liquid-to-vapor phase change heat transfer evaluation and parameter sensitivity analysis of nanoporous surface coatings. Int. J. Heat Mass Transf. 2022, 194, 123088. [Google Scholar] [CrossRef]
- Duarte, M.; Lasagni, A.; Giovanelli, R.; Narciso, J.; Louis, E.; Mücklich, F. Increasing Lubricant Film Lifetime by Grooving Periodical Patterns Using Laser Interference Metallurgy. Adv. Eng. Mater. 2008, 10, 554–558. [Google Scholar] [CrossRef]
- Jereb, S.; Zupančič, M.; Može, M.; Golobič, I. Predicting the drop size passing through a superhydrophobic orifice. Phys. Fluids 2022, 34, 117117. [Google Scholar] [CrossRef]
- Kumar, G.U.; Suresh, S.; Kumar, C.S.S.; Back, S.; Kang, B.; Lee, H.J. A review on the role of laser textured surfaces on boiling heat transfer. Appl. Therm. Eng. 2020, 174, 115274. [Google Scholar] [CrossRef]
- Costa-Greger, J.; Tsubaki, A.; Gerdes, J.; Anderson, M.; Zuhlke, C.; Alexander, D.; Shield, J.; Gogos, G. Pool Boiling Inversion and Hysteresis with Femtosecond Laser Processed 304 Stainless Steel Surfaces for Heat Transfer Enhancement. In Proceedings of the 2020 19th IEEE InterSociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems, Orlando, FL, USA, 21–23 July 2020; pp. 298–305. [Google Scholar]
- Kruse, C.; Peng, E.; Zuhlke, C.; Shield, J.; Alexander, D.; Ndao, S.; Gogos, G. Role of copper oxide layer on pool boiling performance with femtosecond laser processed surfaces. In Proceedings of the 15th International Conference on Nanochannels Microchannels–Minichannels, ICNMM 2017, ASME, Cambridge, MA, USA, 27–30 August 2017. [Google Scholar]
- Gogos, G.; Zuhlke, C.; Kruse, C.; Ndao, S.; Shield, J.E.; Lucis, M.; Anderson, T.; Alexander, D. Effects of Femtosecond Laser Surface Processed Nanoparticle Layers on Pool Boiling Heat Transfer Performance. J. Therm. Sci. Eng. Appl. 2017, 10, 031009. [Google Scholar]
- Kruse, C.; Tsubaki, A.; Zuhlke, C.; Anderson, T.; Alexander, D.; Gogos, G.; Ndao, S. Secondary pool boiling effects. Appl. Phys. Lett. 2016, 108, 051602. [Google Scholar] [CrossRef]
- Kruse, C.; Tsubaki, A.; Zuhlke, C.; Alexander, D.; Anderson, M.; Peng, E.; Shield, J.; Ndao, S.; Gogos, G. Influence of Copper Oxide on Femtosecond Laser Surface Processed Copper Pool Boiling Heat Transfer Surfaces. J. Heat Transf. 2019, 141, 1–9. [Google Scholar] [CrossRef]
- Kong, D.; Kang, M.; Kim, K.Y.; Jang, J.; Cho, J.; In, J.B.; Lee, H. Hierarchically Structured Laser-Induced Graphene for Enhanced Boiling on Flexible Substrates. ACS Appl. Mater. Interfaces 2020, 12, 37784–37792. [Google Scholar] [CrossRef]
- Eid, E.I.; Al-Nagdy, A.A.; Khalaf-Allah, R.A. Nucleate pool boiling heat transfer above laser machining heating surfaces with different micro-cavity geometric shape for water-aluminum oxide nanofluid. Exp. Heat Transf. 2022, 35, 688–707. [Google Scholar] [CrossRef]
- Zupančič, M.; Može, M.; Gregorčič, P.; Golobič, I. Nanosecond laser texturing of uniformly and non-uniformly wettable micro structured metal surfaces for enhanced boiling heat transfer. Appl. Surf. Sci. 2017, 399, 480–490. [Google Scholar] [CrossRef]
- Gregorčič, P.; Zupančič, M.; Golobič, I. Scalable Surface Microstructuring by a Fiber Laser for Controlled Nucleate Boiling Performance of High- and Low-Surface-Tension Fluids. Sci. Rep. 2018, 8, 7461. [Google Scholar] [CrossRef]
- Može, M. Effect of boiling-induced aging on pool boiling heat transfer performance of untreated and laser-textured copper surfaces. Appl. Therm. Eng. 2020, 181, 116025. [Google Scholar] [CrossRef]
- Može, M.; Zupančič, M.; Hočevar, M.; Golobič, I.; Gregorčič, P. Surface chemistry and morphology transition induced by critical heat flux incipience on laser-textured copper surfaces. Appl. Surf. Sci. 2019, 490, 220–230. [Google Scholar] [CrossRef]
- Voglar, J.; Gregorčič, P.; Zupančič, M.; Golobič, I. Boiling performance on surfaces with capillary-length-spaced one- and two-dimensional laser-textured patterns. Int. J. Heat Mass Transf. 2018, 127, 1188–1196. [Google Scholar] [CrossRef]
- Može, M.; Zupančič, M.; Golobič, I. Pattern geometry optimization on superbiphilic aluminum surfaces for enhanced pool boiling heat transfer. Int. J. Heat Mass Transfer 2020, 161, 120265. [Google Scholar] [CrossRef]
- Zakšek, P.; Zupančič, M.; Gregorčič, P.; Golobič, I. Investigation of Nucleate Pool Boiling of Saturated Pure Liquids and Ethanol-Water Mixtures on Smooth and Laser-Textured Surfaces. Nanoscale Microscale Thermophys. Eng. 2020, 24, 29–42. [Google Scholar] [CrossRef]
- Zupančič, M.; Gregorčič, P.; Bucci, M.; Wang, C.; Aguiar, G.M.; Bucci, M. The wall heat flux partitioning during the pool boiling of water on thin metallic foils. Appl. Therm. Eng. 2022, 200, 117638. [Google Scholar] [CrossRef]
- Sitar, A.; Može, M.; Crivellari, M.; Schille, J.; Golobič, I. Nucleate pool boiling heat transfer on etched and laser structured silicon surfaces. Int. J. Heat Mass Transfer 2020, 147, 118956. [Google Scholar] [CrossRef]
- Serdyukov, V.; Starinskiy, S.; Malakhov, I.; Safonov, A.; Surtaev, A. Laser texturing of silicon surface to enhance nucleate pool boiling heat transfer. Appl. Thermal Eng. 2021, 194, 117102. [Google Scholar] [CrossRef]
- Senegačnik, M.; Gregorčič, P. Diffraction-driven laser surface nanostructuring: Towards patterning with curved periodic surface structures. Appl. Surface Sci. 2023, 610, 155486. [Google Scholar] [CrossRef]
- Boyina, K.S.; Mahvi, A.J.; Chavan, S.; Park, D.; Kumar, K.; Lira, M.; Yu, Y.; Gunay, A.A.; Wang, X.; Miljkovic, N. Condensation frosting on meter-scale superhydrophobic and superhydrophilic heat exchangers. Int. J. Heat Mass Transfer 2019, 145, 118694. [Google Scholar] [CrossRef]
- Gregorčič, P.; Šetina-Batič, B.; Hočevar, M. Controlling the stainless steel surface wettability by nanosecond direct laser texturing at high fluences. Appl. Phys. A Mater. Sci. Process. 2017, 123, 766. [Google Scholar] [CrossRef]
- Hsu, Y.Y. On the Size Range of Active Nucleation Cavities on a Heating Surface. J. Heat Transf. 1962, 84, 207–213. [Google Scholar] [CrossRef]
- Jones, S.F.; Evans, G.M.; Galvin, K.P. Bubble nucleation from gas cavities—A review. Adv. Colloid Interface Sci. 1999, 80, 27–50. [Google Scholar] [CrossRef]
- Attinger, D.; Frankiewicz, C.; Betz, A.R.; Schutzius, T.M.; Ganguly, R.; Das, A.; Kim, C.-J.; Megaridis, C.M. Surface engineering for phase change heat transfer: A review. MRS Energy Sustain. 2014, 1, 1–40. [Google Scholar] [CrossRef]
- Može, M.; Zupančič, M.; Golobič, I. Investigation of the scatter in reported pool boiling CHF measurements including analysis of heat flux and measurement uncertainty evaluation methodology. Appl. Thermal Eng. 2020, 169, 114938. [Google Scholar] [CrossRef]
- Gregorčič, P.; Conradi, M.; Hribar, L.; Hočevar, M. Long-term influence of laser-processing parameters on (Super)hydrophobicity development and stability of stainless-steel surfaces. Materials 2018, 11, 2240. [Google Scholar] [CrossRef]
- Gregorčič, P. Comment on “Bioinspired Reversible Switch between Underwater Superoleophobicity/Superaerophobicity and Oleophilicity/Aerophilicity and Improved Antireflective Property on the Nanosecond Laser-Ablated Superhydrophobic Titanium Surfaces. ” ACS Appl. Mater. Interfaces 2020, 13, 2117–2127. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Tian, Y. Insights into the wettability transition of nanosecond laser ablated surface under ambient air exposure. J. Colloid Interface Sci. 2019, 533, 268–277. [Google Scholar] [CrossRef]
- Long, J.; Zhong, M.; Fan, P.; Gong, D.; Zhang, H. Wettability conversion of ultrafast laser structured copper surface. J. Laser Appl. 2015, 27, S29107. [Google Scholar] [CrossRef]
- Chen, R.; Lu, M.-C.; Srinivasan, V.; Wang, Z.; Cho, H.H.; Majumdar, A. Nanowires for Enhanced Boiling Heat Transfer. Nano Lett. 2009, 9, 548–553. [Google Scholar] [CrossRef]
- Betz, A.R.; Jenkins, J.; Kim, C.J.; Attinger, D. Boiling heat transfer on superhydrophilic, superhydrophobic, and superbiphilic surfaces. Int. J. Heat Mass Transf. 2013, 57, 733–741. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ölçeroğlu, E.; McCarthy, M. Role of wickability on the critical heat flux of structured superhydrophilic surfaces. Langmuir 2014, 30, 11225–11234. [Google Scholar] [CrossRef]
- Carvalho, L.; Pacquentin, W.; Tabarant, M.; Maskrot, H.; Semerok, A. Growth of micrometric oxide layers for the study of metallic surfaces decontamination by laser. EPJ Web Conf. 2017, 153, 07038. [Google Scholar] [CrossRef]
- Florian, C.; Wonneberger, R.; Undisz, A.; Kirner, S.V.; Wasmuth, K.; Spaltmann, D.; Krüger, J.; Bonse, J. Chemical effects during the formation of various types of femtosecond laser-generated surface structures on titanium alloy. Appl. Phys. A Mater. Sci. Process. 2020, 126, 1–11. [Google Scholar] [CrossRef]
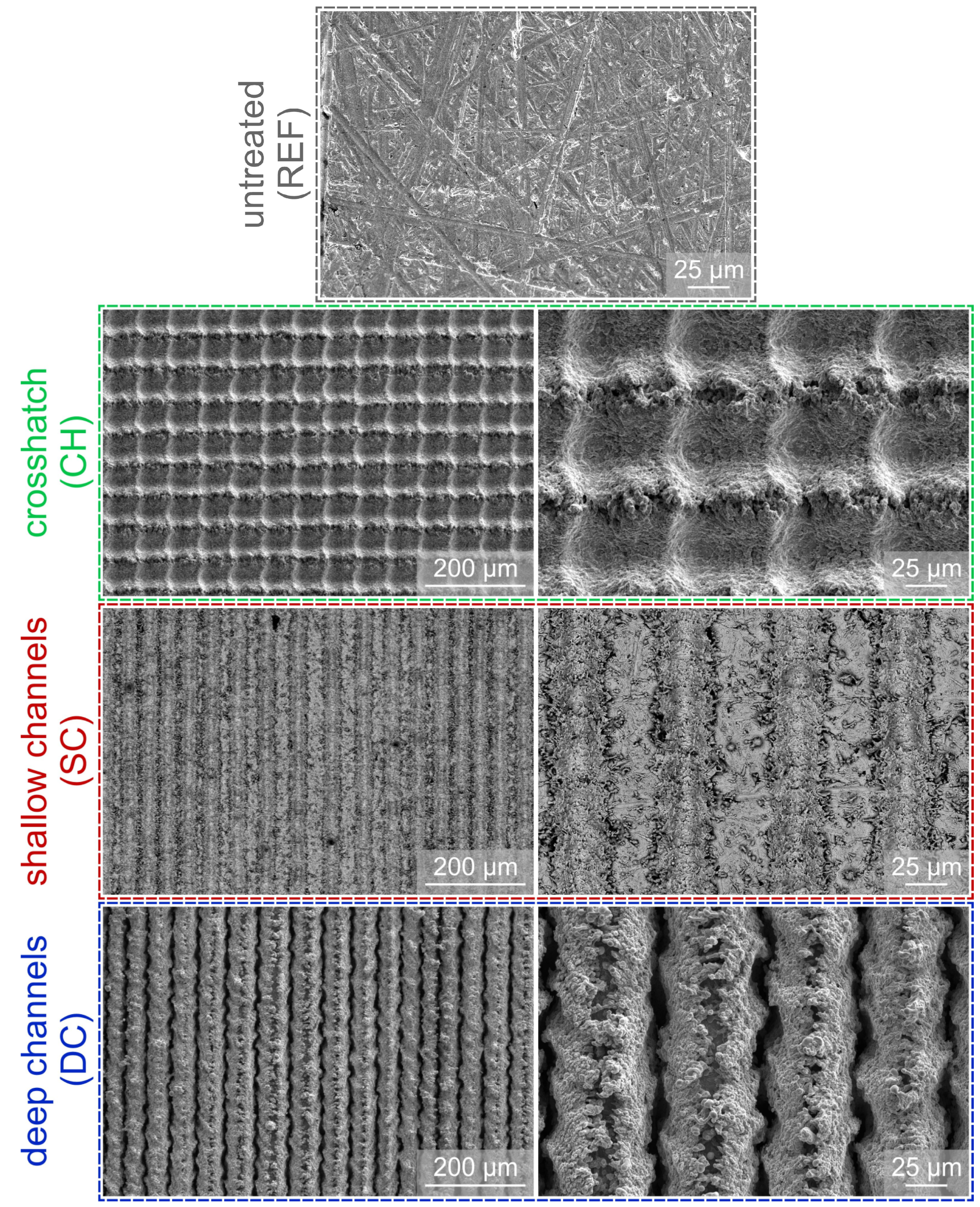
| Sample | Pulse Length (ns) | Pulse Frequency (kHz) | Scanning Speed (mm s−1) | Average Fluence (J cm−2) | Pattern |
|---|---|---|---|---|---|
| CH & CH-H | 40 | 125 | 125 | 48.9 | crosshatch (0° and 90°), spacing Δx = 60 μm |
| SC & SC-H | 30 | 500 | 500 | 12.2 | parallel lines (0°), cyclically variable spacing Δx = {35–65} μm |
| DC & DC-H | 45 | 110 | 110 | 55.6 | parallel lines (0°), cyclically variable spacing Δx = {35–65} μm |
| Sample | Treatment | Description | Contact Angle |
|---|---|---|---|
| REF | none | untreated reference sample | 93° |
| CH | laser texturing | hydrophilic sample with a crosshatch pattern | <1° |
| CH-H | laser texturing + hydrophobization | hydrophobized sample with a crosshatch pattern | 160° |
| SC | laser texturing | hydrophilic sample with a pattern of shallow channels | <1° |
| SC-H | laser texturing + hydrophobization | hydrophobized sample with a pattern of shallow channels | 154° |
| DC | laser texturing | hydrophilic sample with a pattern of deep channels | <1° |
| DC-H | laser texturing + hydrophobization | hydrophobized sample with a pattern of deep channels | 154° |
| Sample | Db @ 50 kW m−2 (mm) | Db @ 100 kW m−2 (mm) |
|---|---|---|
| REF | 1.6 ± 0.3 | 2.4 ± 0.3 |
| CH | 2.0 ± 0.3 | 3.1 ± 0.4 |
| CH-H | 1.2 ± 0.1 | 2.6 ± 0.2 |
| SC | 2.0 ± 0.1 | 2.0 ± 0.3 |
| SC-H | 3.1 ± 0.7 | 1.8 ± 0.2 |
| DC | 1.1 ± 0.1 | 1.6 ± 0.2 |
| DC-H | 0.7 ± 0.1 | 1.0 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Može, M.; Zupančič, M.; Steinbücher, M.; Golobič, I.; Gjerkeš, H. Nanosecond Laser-Textured Copper Surfaces Hydrophobized with Self-Assembled Monolayers for Enhanced Pool Boiling Heat Transfer. Nanomaterials 2022, 12, 4032. https://doi.org/10.3390/nano12224032
Može M, Zupančič M, Steinbücher M, Golobič I, Gjerkeš H. Nanosecond Laser-Textured Copper Surfaces Hydrophobized with Self-Assembled Monolayers for Enhanced Pool Boiling Heat Transfer. Nanomaterials. 2022; 12(22):4032. https://doi.org/10.3390/nano12224032
Chicago/Turabian StyleMože, Matic, Matevž Zupančič, Miha Steinbücher, Iztok Golobič, and Henrik Gjerkeš. 2022. "Nanosecond Laser-Textured Copper Surfaces Hydrophobized with Self-Assembled Monolayers for Enhanced Pool Boiling Heat Transfer" Nanomaterials 12, no. 22: 4032. https://doi.org/10.3390/nano12224032
APA StyleMože, M., Zupančič, M., Steinbücher, M., Golobič, I., & Gjerkeš, H. (2022). Nanosecond Laser-Textured Copper Surfaces Hydrophobized with Self-Assembled Monolayers for Enhanced Pool Boiling Heat Transfer. Nanomaterials, 12(22), 4032. https://doi.org/10.3390/nano12224032









