The Role of Nitrogen-Doped TiO2 Supported by Platinum Catalyst Synthesized via Various Mode Preparations for Photocatalytic Enhancement
Abstract
1. Introduction
2. Materials and Methodology
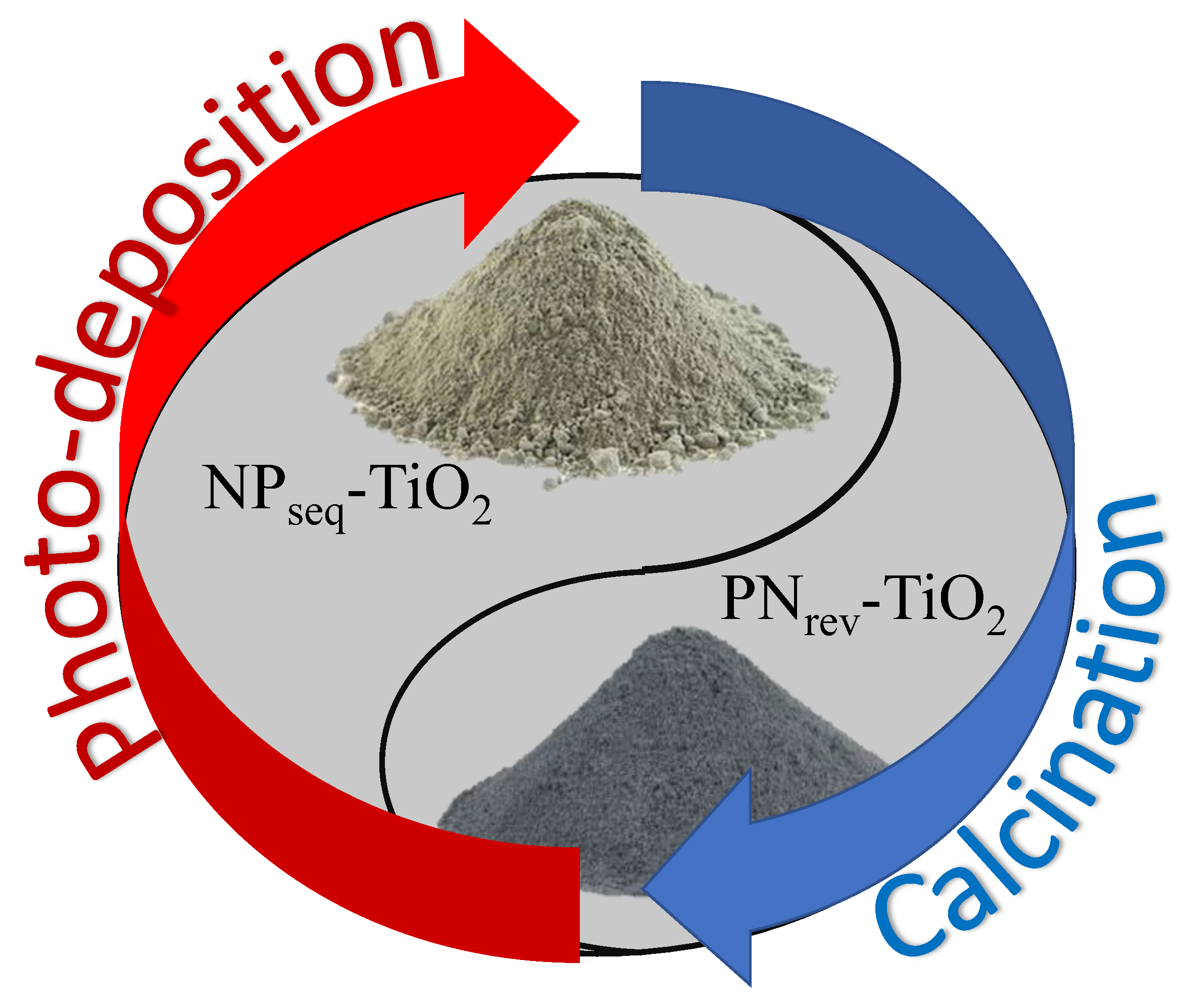
2.1. Synthesis of Photocatalyst
2.2. Photocatalytic Activity
2.3. Characterization
3. Results and Discussion
3.1. Crystallinity
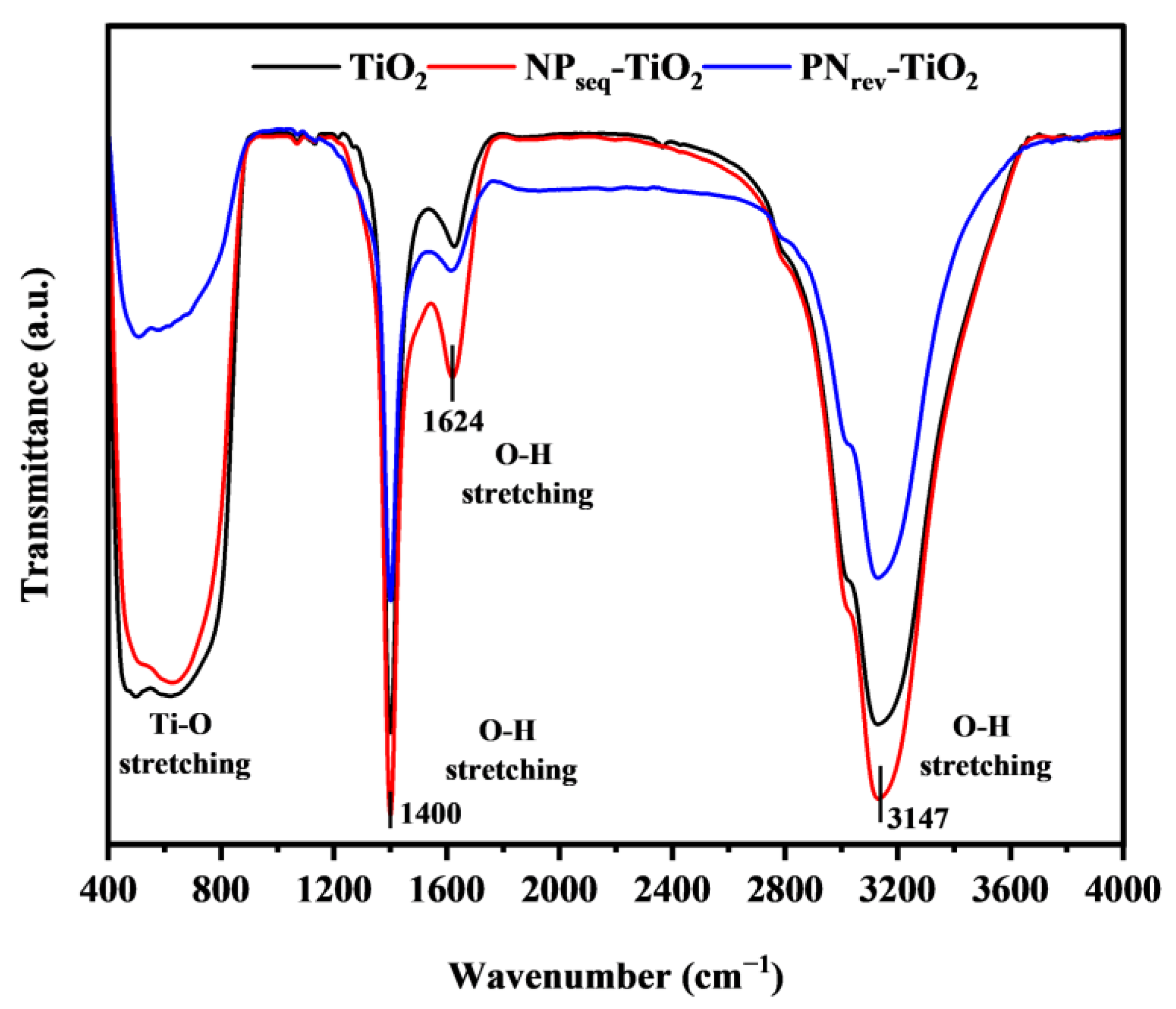
3.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
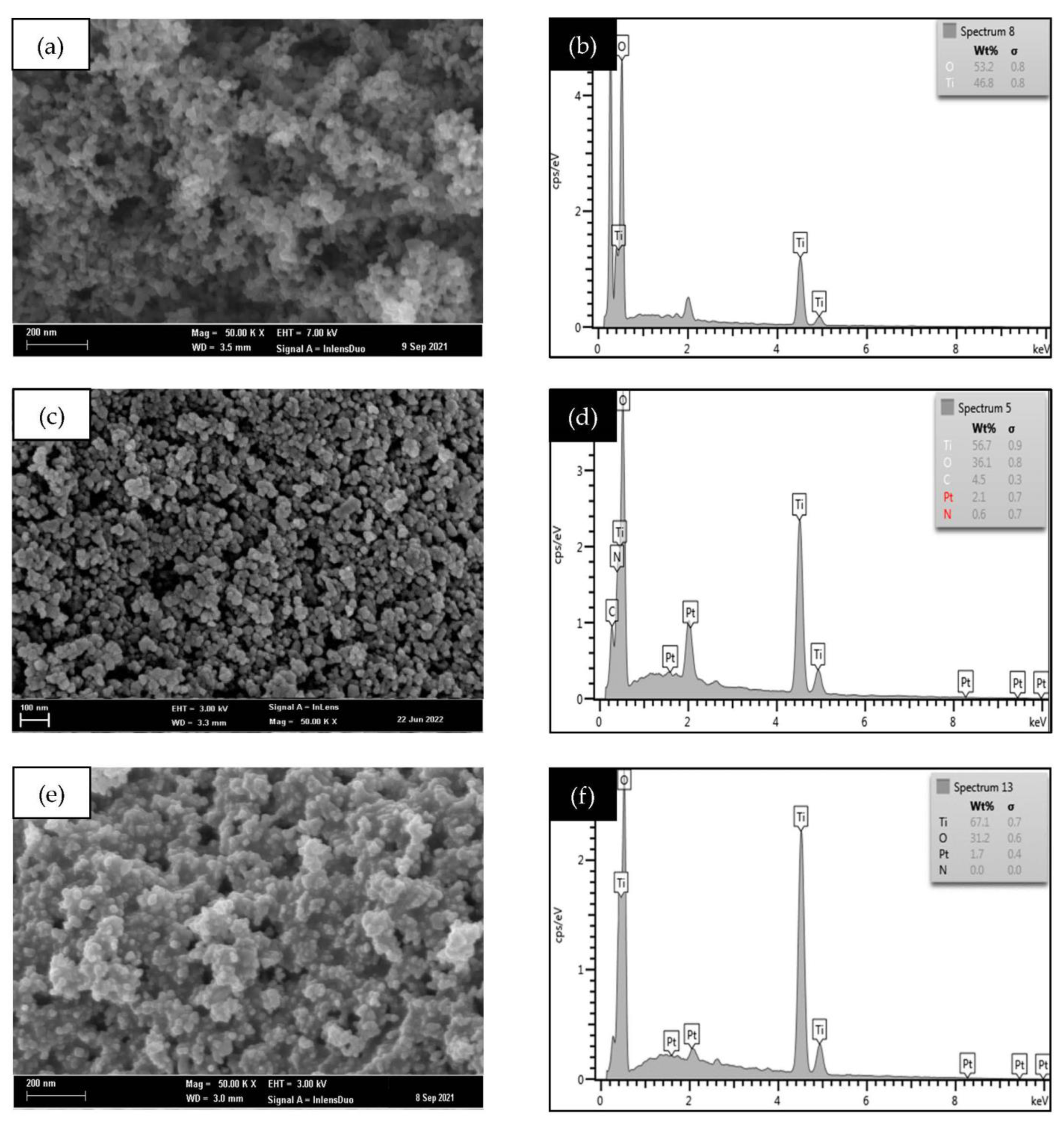
3.3. Morphological and Structural Analysis
3.4. Elemental Analysis (CHNS-O) and (ICP/OES)
3.5. Optical Studies
3.6. Photocatalytic Study
3.7. Chemical Oxygen Demand Analysis (COD)
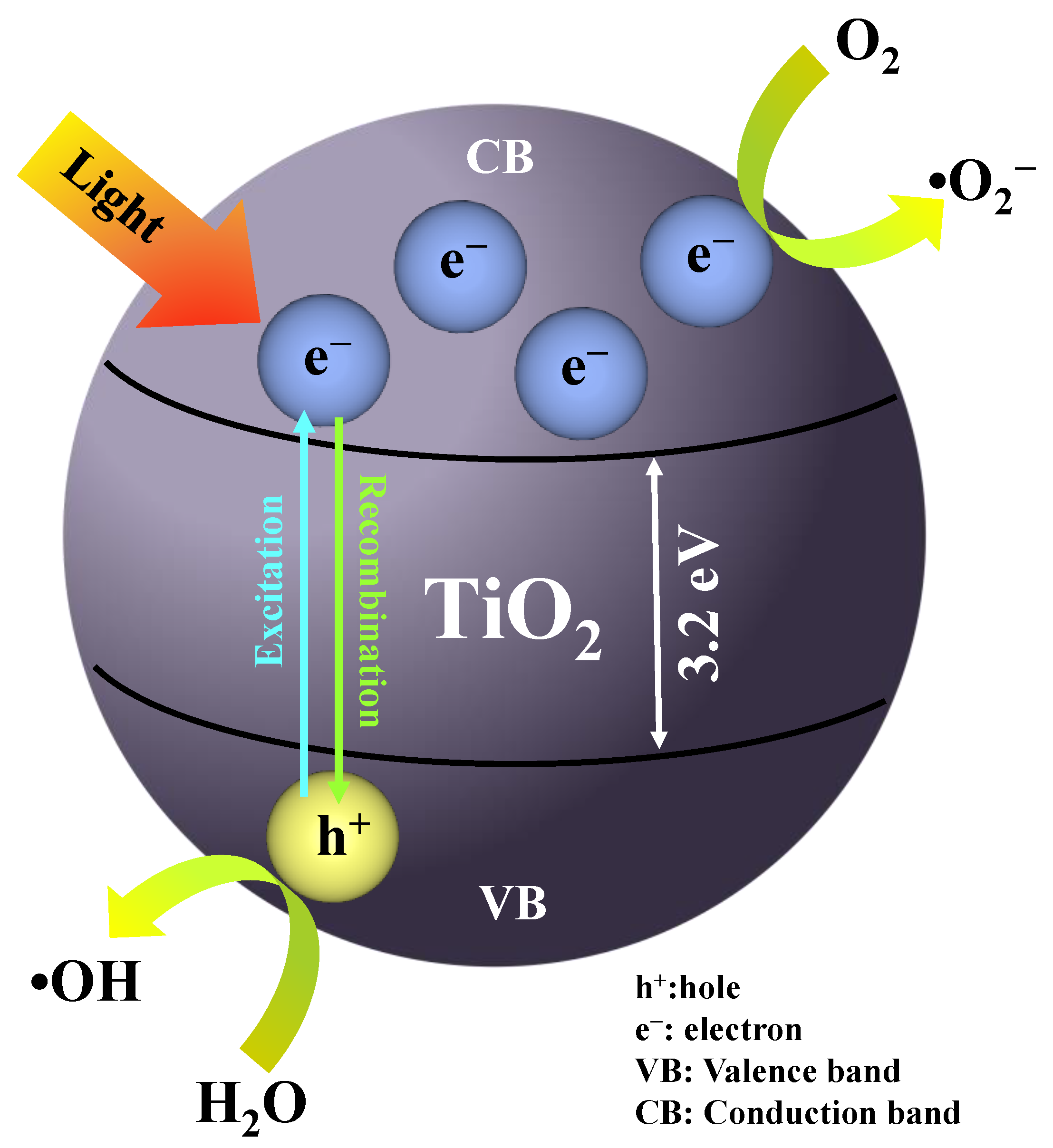
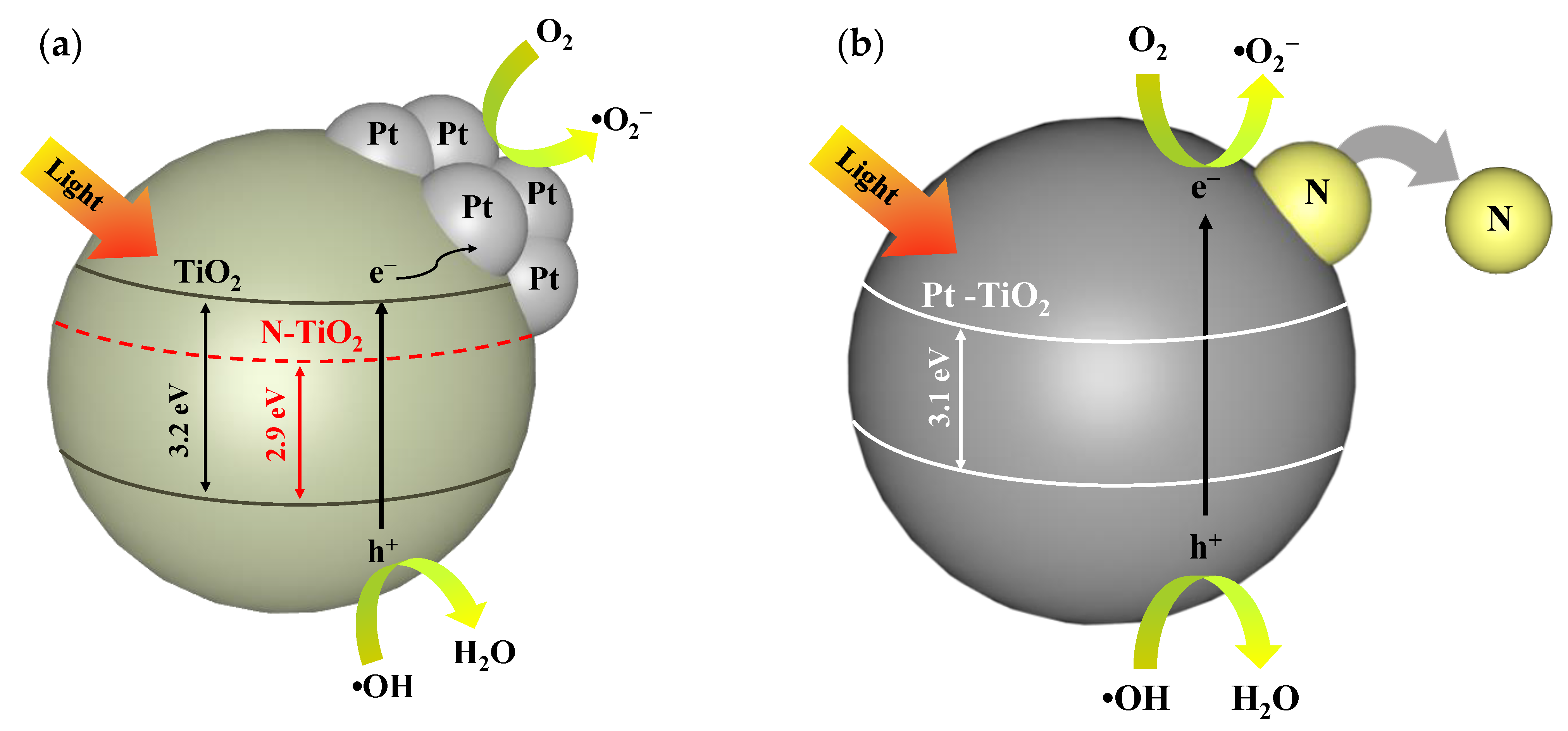
3.8. Mechanism Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, R.; Sharma, V. Advances in the Synthesis and Antimicrobial Applications of Metal Oxide Nanostructures. In Advanced Ceramics for Versatile Interdisciplinary Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 339–369. [Google Scholar]
- Yu, D.; Fang, H.; Qiu, P.; Meng, F.; Liu, H.; Wang, S.; Lv, P.; Cong, X.; Niu, Q.; Li, T. Improving the Performance of ZnS Photocatalyst in Degrading Organic Pollutants by Constructing Composites with Ag2O. Nanomaterials 2021, 11, 1451. [Google Scholar] [CrossRef]
- Murillo-Sierra, J.; Hernández-Ramírez, A.; Hinojosa-Reyes, L.; Guzmán-Mar, J. A review on the development of visible light-responsive WO3-based photocatalysts for environmental applications. Chem. Eng. J. Adv. 2021, 5, 100070. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium Dioxide (TiO₂)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Rosli, M.A.; Hamzah, S.R.; Muhamad, N.A.; Ghani, N.I.; Natar, N.S.; Ikhwan, S.; Nazeri, N.S.; Azami, M.S.; Ishak MA, M.; Ramli, M.Z.; et al. Acid Photo Etching Effect of Epoxidized Natural Rubber (ENR) and Polyvinyl Chloride (PVC) as Polymer Binder. Malays. J. Chem. 2022, 24, 228–239. [Google Scholar]
- Moma, J.; Baloyi, J. Modified Titanium Dioxide for Photocatalytic Applications. In Photocatalysts—Applications and Attributes; Khan, S.B., Akhtar, K., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Ong, W.L.; Wang, J.; Ho, G.W. Structural design of TiO2-based photocatalyst for H2 production and degradation applications. Catal. Sci. Technol. 2015, 5, 4703–4726. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Hussein, F.H.; Shaheed, M.A. Preparation and applications of titanium dioxide and zinc oxide nanoparticles. J. Environ. Anal. Chem. 2015, 2, 2380–2391. [Google Scholar]
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef]
- Gaur, S.S.; Jagadeesan, H. Titanium Dioxide (TiO2) based photocatalysis for textile wastewater treatment–its applications and biosafety–A Review. Int. J. Mech. Eng. 2021, 6, 974–5823. [Google Scholar]
- Park, H.; Kim, H.-I.; Moon, G.-H.; Choi, W. Photoinduced charge transfer processes in solar photocatalysis based on modified TiO2. Energy Environ. Sci. 2016, 9, 411–433. [Google Scholar] [CrossRef]
- Li, B.; Wu, S.; Gao, X. Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review. Nanotechnol. Rev. 2020, 9, 1080–1103. [Google Scholar] [CrossRef]
- Sohn, Y.S.; Smith, Y.R.; Misra, M.; Subramanian, V.R. Electrochemically assisted photocatalytic degradation of methyl orange using anodized titanium dioxide nanotubes. Appl. Catal. B Environ. 2008, 84, 372–378. [Google Scholar] [CrossRef]
- Serpone, N. Is the band gap of pristine TiO2 narrowed by anion-and cation-doping of titanium dioxide in second-generation photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Sohail, M.; Altalhi, T.; Al-Sehemi, A.G.; Taha, T.A.M.; El-Nasser, K.S.; Al-Ghamdi, A.A.; Boukhari, M.; Palamanit, A.; Hayat, A.; Amin, M.A.; et al. Nanostructure engineering via intramolecular construction of carbon nitride as efficient photocatalyst for CO2 reduction. Nanomaterials 2021, 11, 3245. [Google Scholar] [CrossRef]
- Vu, T.A.; Dao, C.D.; Hoang, T.T.; Le, G.H.; Nguyen, K.T.; Dang, P.T.; Tran, H.T.; Nguyen, T.V. Study on photocatalytic activity of TiO2 and non-metal doped TiO2 in Rhodamine B degradation under visible light irradiation. Int. J. Nanotechnol. 2013, 10, 235. [Google Scholar] [CrossRef]
- Sirivallop, A.; Areerob, T.; Chiarakorn, S. Enhanced Visible Light Photocatalytic Activity of N and Ag Doped and Co-Doped TiO2 Synthesized by Using an In-Situ Solvothermal Method for Gas Phase Ammonia Removal. Catalysts 2020, 10, 251. [Google Scholar] [CrossRef]
- Nawawi, W.; Nawi, M. Carbon coated nitrogen doped P25 for the photocatalytic removal of organic pollutants under solar and low energy visible light irradiations. J. Mol. Catal. A Chem. 2014, 383-384, 83–93. [Google Scholar] [CrossRef]
- Azami, M.; Nawawi, W.; Jawad, A.H.; Ishak, M.; Ismail, K. N-doped TiO2 Synthesised via Microwave Induced Photocatalytic on RR4 dye Removal under LED Light Irradiation. Sains Malays. 2017, 46, 1309–1316. [Google Scholar] [CrossRef]
- Potdar, S.B.; Huang, C.-M.; Praveen, B.; Manickam, S.; Sonawane, S.H. Highly Photoactive Titanium Dioxide Supported Platinum Catalyst: Synthesis Using Cleaner Ultrasound Approach. Catalysts 2022, 12, 78. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Trejo-Tzab, R.; Avila-Ortega, A.; Quintana-Owen, P.; Rangel, R.; Álvarez-Lemus, M.A. Platinum-Decorated TiO2: One Step Fast Monometallic Impregnation and Plasma Effect on Nanoparticles. J. Compos. Sci. 2021, 6, 4. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Wang, J.; Gan, Y.; Liu, L.; Ju, M. Microwave-assisted ionic liquid synthesis of Ti3+ self-doped TiO2 hollow nanocrystals with enhanced visible-light photoactivity. Appl. Catal. B Environ. 2016, 191, 94–105. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.; Popov, A. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Natar, N.; Ikhwan, S.; Nazeri, N.; Hamzah, S.; Rosli, M.; Ghani, N.; Ishak, M.; Razak, S.; Azami, M.; Nawawi, W. Preparation of water-base immobilized N doped TiO2 using DSAT technique for photocatalytic degradation of methylene blue dye. Mater. Today Proc. 2022, 66, 4036–4044. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mubarak, N.S.A.; Ishak, M.A.M.; Ismail, K.; Nawawi, W. Kinetics of photocatalytic decolourization of cationic dye using porous TiO2 film. J. Taibah Univ. Sci. 2016, 10, 352–362. [Google Scholar] [CrossRef]
- Avril, L.; Bourgeois, S.; Simon, P.; Domenichini, B.; Zanfoni, N.; Herbst, F.; Imhoff, L. Nanostructured Pt–TiO2 composite thin films obtained by direct liquid injection metal organic chemical vapor deposition: Control of chemical state by X-ray photoelectron spectroscopy. Thin Solid Films 2015, 591, 237–244. [Google Scholar] [CrossRef]
- Huang, H.; Huang, H.; Hu, P.; Ye, X.; Leung, D.Y.C. Removal of Formaldehyde Using Highly Active Pt/TiO2 Catalysts without Irradiation. Int. J. Photoenergy 2013, 2013, 350570. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Scott, K. Study of niobium and tantalum doped titania-supported Pt electrocatalysts for methanol oxidation and oxygen reduction reactions. Catal. Sci. Technol. 2014, 4, 3891–3898. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Weng, C.-H.; Hsu, H.-J.; Lin, Y.-H.; Shiesh, C.-C. The Synergistic Effect of Nitrogen Dopant and Calcination Temperature on the Visible-Light-Induced Photoactivity of N-Doped TiO2. Int. J. Photoenergy 2013, 2013, 268723. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Singh, P.K. Synthesis and characterization of nano crystalline nitrogen doped titanium dioxide. Mater. Today: Proc. 2021, 45, 3666–3669. [Google Scholar] [CrossRef]
- Asif, M.; Zafar, M.; Akhter, P.; Hussain, M.; Umer, A.; Razzaq, A.; Kim, W.-Y. Effect of Urea Addition on Anatase Phase Enrichment and Nitrogen Doping of TiO2 for Photocatalytic Abatement of Methylene Blue. Appl. Sci. 2021, 11, 8264. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Hossain, M.K.; Pervez, M.F.; Uddin, M.J.; Tayyaba, S.; Mia, M.N.H.; Bashar, M.S.; Jewel, M.K.H.; Haque, M.A.S.; Hakim, M.A.; Khan, M.A. Influence of natural dye adsorption on the structural, morphological and optical properties of TiO2 based photoanode of dye-sensitized solar cell. Mater. Sci. 2018, 36, 93–101. [Google Scholar] [CrossRef]
- Saber, N.B.; Mezni, A.; Alrooqi, A.; Altalhi, T. Enhancement of photocatalytic activity using ternary Au@ Pt-TiO2/nano-GO heterojunction for environmental remediation and clean energy production. Mater. Res. Express 2021, 8, 045014. [Google Scholar] [CrossRef]
- Song, M.; Li, M.; Guo, Y.; Huang, X.; Wang, S.; Ren, L.; Li, L.; Wu, Y. Facile fabrication of ordered assembled TiO2/g-C3N4 nanosheets with enhanced photocatalytic activity. Ceram. Int. 2021, 47, 15594–15603. [Google Scholar] [CrossRef]
- Raj, K.; Viswanathan, B. Effect of surface area, pore volume and particle size of P25 titania on the phase transformation of anatase to rutile. Indian J. Chem. 2009, 48, 1378–1382. [Google Scholar]
- Samsudin, E.M.; Hamid, S.B.A.; Juan, J.C.; Basirun, W.J.; Kandjani, A.E.; Bhargava, S.K. Controlled nitrogen insertion in titanium dioxide for optimal photocatalytic degradation of atrazine. RSC Adv. 2015, 5, 44041–44052. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Wan, J. Enhanced Photocatalytic Activity of Nitrogen Doped TiO2 Anatase Nano-Particle under Simulated Sunlight Irradiation. Energy Procedia 2012, 16, 598–605. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Khairy, M.; Zakaria, W. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Chetibi, L.; Busko, T.; Kulish, N.P.; Hamana, D.; Chaieb, S.; Achour, S. Photoluminescence properties of TiO2 nanofibers. J. Nanoparticle Res. 2017, 19, 129. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Nawawi, W.I.; Nawi, M.A.; Ishak, M.A.M. Effect of Carbon and Nitrogen Modified TiO2 on Photoluminescence Property and Photocatalytic activity. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2015; Volume 754, pp. 1197–1201. [Google Scholar]
- Mei, P.; Henderson, M.; Kassiba, A.; Gibaud, A. EPR study of nitrogen-doped mesoporous TiO2 powders. J. Phys. Chem. Solids 2010, 71, 1–6. [Google Scholar] [CrossRef]
- Zhang, Z.; Long, J.; Xie, X.; Lin, H.; Zhou, Y.; Yuan, R.; Dai, W.; Ding, Z.; Wang, X.; Fu, X. Probing the Electronic Structure and Photoactivation Process of Nitrogen-Doped TiO2 Using DRS, PL, and EPR. ChemPhysChem 2012, 13, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, M.; Peng, F.; Sun, X.; Han, J. Fabrication of g-C3N4 nanosheets anchored with controllable CdS nanoparticles for enhanced visible-light photocatalytic performance. Front. Chem. 2021, 9, 746031. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.M.; Ivanova, I.; Hussein, F.H.; Bahnemann, D.W. Role of Platinum Deposited on TiO2 in Photocatalytic Methanol Oxidation and Dehydrogenation Reactions. Int. J. Photoenergy 2014, 2014, 503516. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Y.; Zhou, B.; Wei, Z.; Zhu, Z.; Han, L.; Zhang, P.; Hu, Y. Preparation and photocatalytic performance of silver-modified and nitrogen-doped TiO2 nanomaterials with oxygen vacancies. New J. Chem. 2021, 45, 4694–4704. [Google Scholar] [CrossRef]
- Foo, C.; Li, Y.; Lebedev, K.; Chen, T.; Day, S.; Tang, C.; Tsang, S.C.E. Characterisation of oxygen defects and nitrogen impurities in TiO2 photocatalysts using variable-temperature X-ray powder diffraction. Nat. Commun. 2021, 12, 661. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen doped titanium dioxide (N-TiO2): Synopsis of synthesis methodologies, doping mechanisms, property evaluation and visible light photocatalytic applications. Photochem 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Wang, G.H.; Xu, L.; Zhang, J.; Yin, T.T.; Han, D.Y. Enhanced photocatalytic activity of TiO2 powders (P25) via calcination treatment. Inter. J. Phot. 2012, 2012, 265760. [Google Scholar]
- Mezni, A.; Saber, N.B.; Bukhari, A.; Ibrahim, M.M.; Al-Talhi, H.; Alshehri, N.A.; Wazir, S.A.; El-Sheshtawy, H.S.; Altalhi, T. Plasmonic hybrid platinum-titania nanocomposites as highly active photocatalysts: Self-cleaning of cotton fiber under solar light. J. Mater. Res. Technol. 2020, 9, 1447–1456. [Google Scholar] [CrossRef]
- Hardin, I.R. 8—Chemical Treatment of Textile Dye Effluent. In Environmental Aspects of Textile Dyeing; CRC Press: Boca Raton, FL, USA, 2007; pp. 191–211. [Google Scholar]
- Wang, C. plasmonic and cocatalyst nanoparticles on N–TiO2 for visible-light-driven catalytic organic synthesis. Nanomaterials 2019, 3, 391. [Google Scholar] [CrossRef]
- Cybula, A.; Priebe, J.B.; Pohl, M.-M.; Sobczak, J.W.; Schneider, M.; Zielińska-Jurek, A.; Brückner, A.; Zaleska, A. The effect of calcination temperature on structure and photocatalytic properties of Au/Pd nanoparticles supported on TiO2. Appl. Catal. B Environ. 2014, 152–153, 202–211. [Google Scholar] [CrossRef]

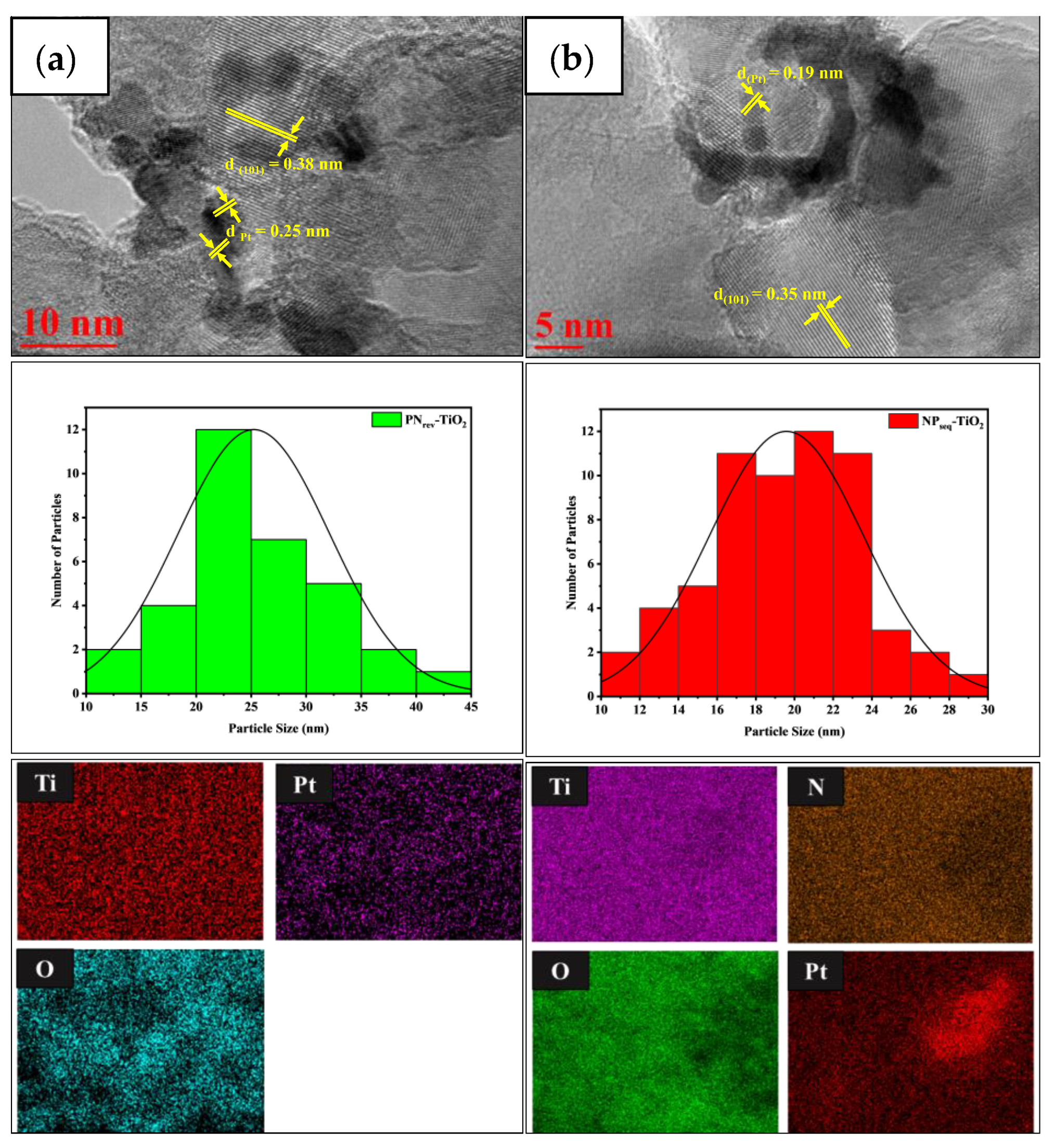
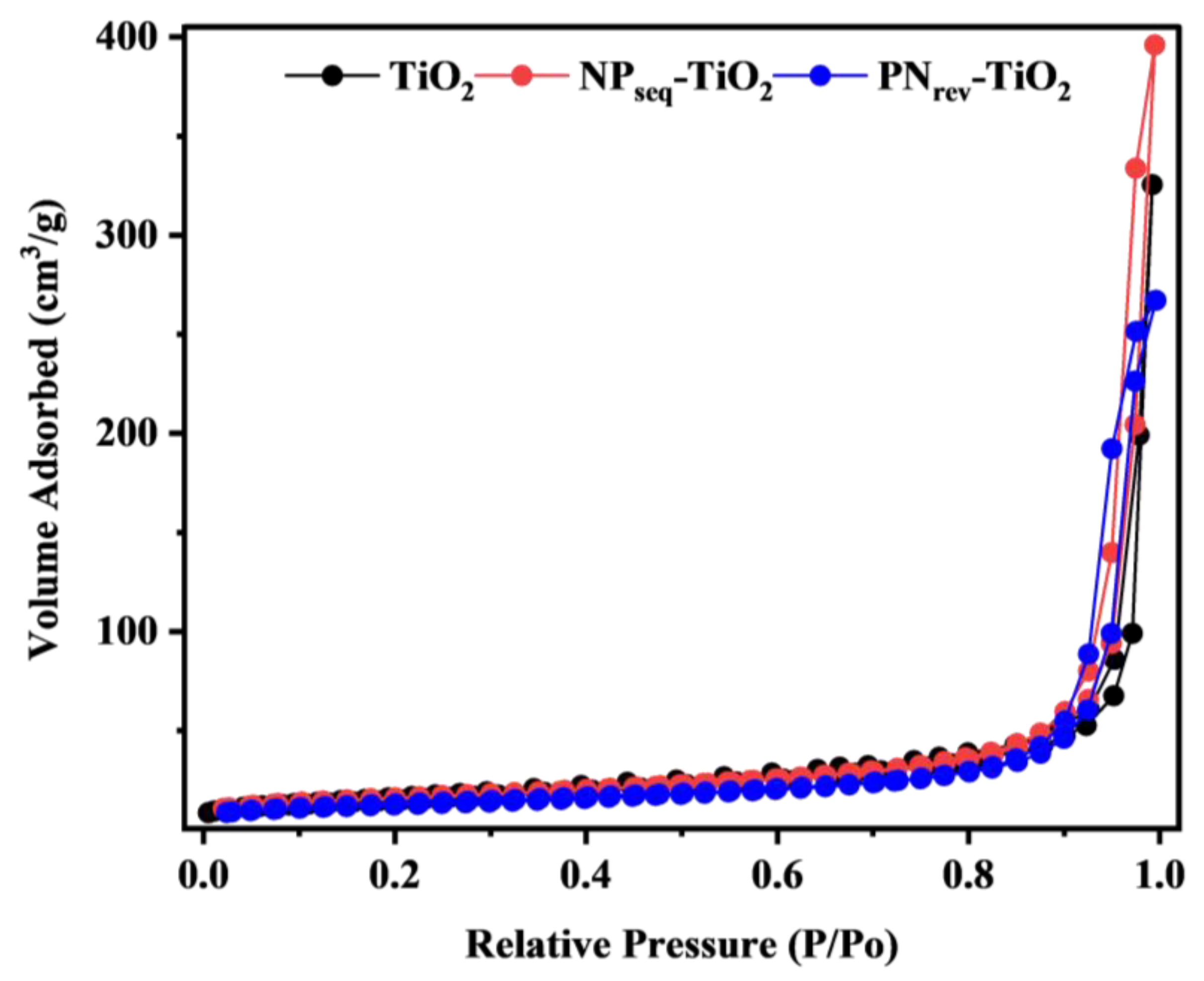
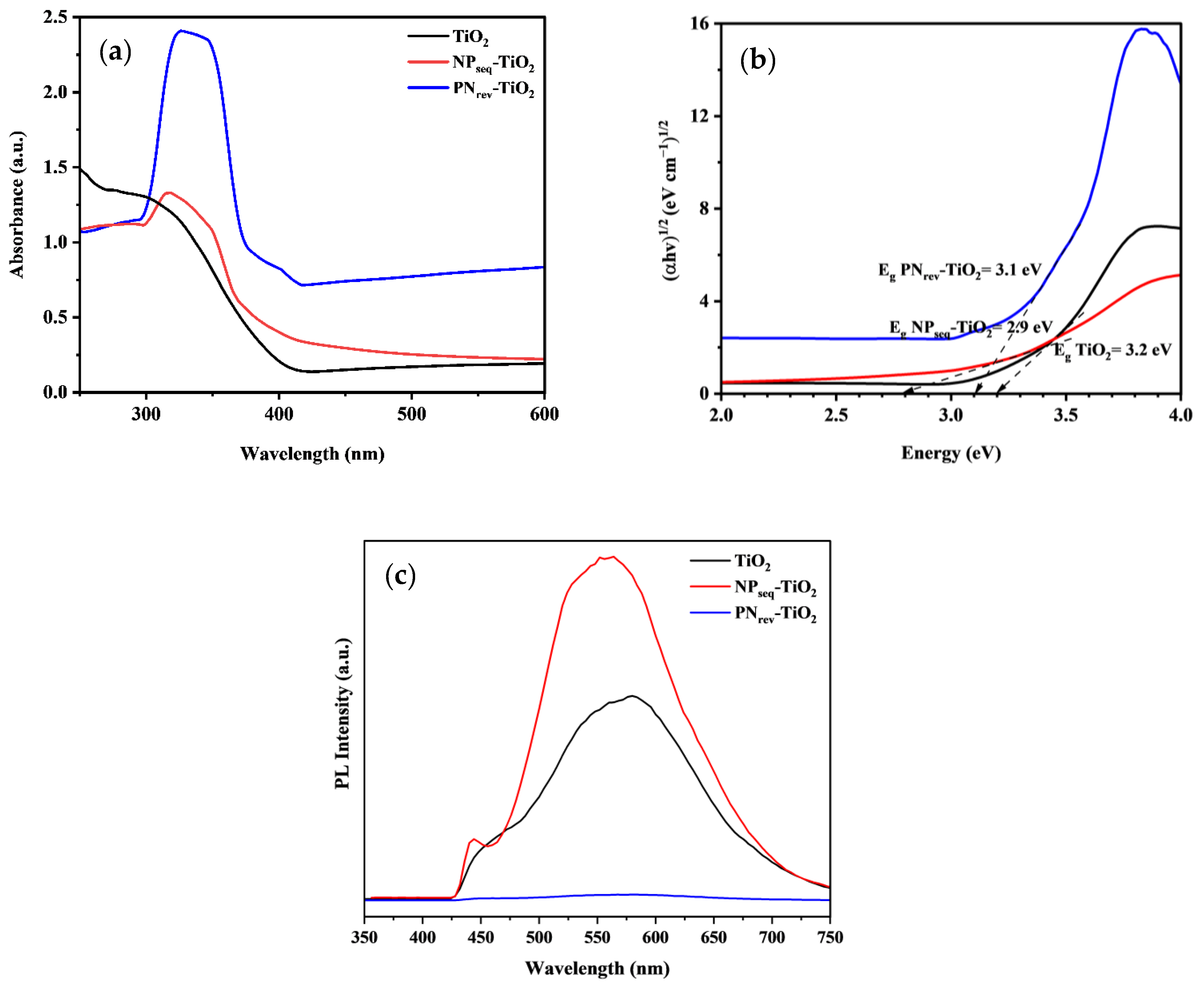
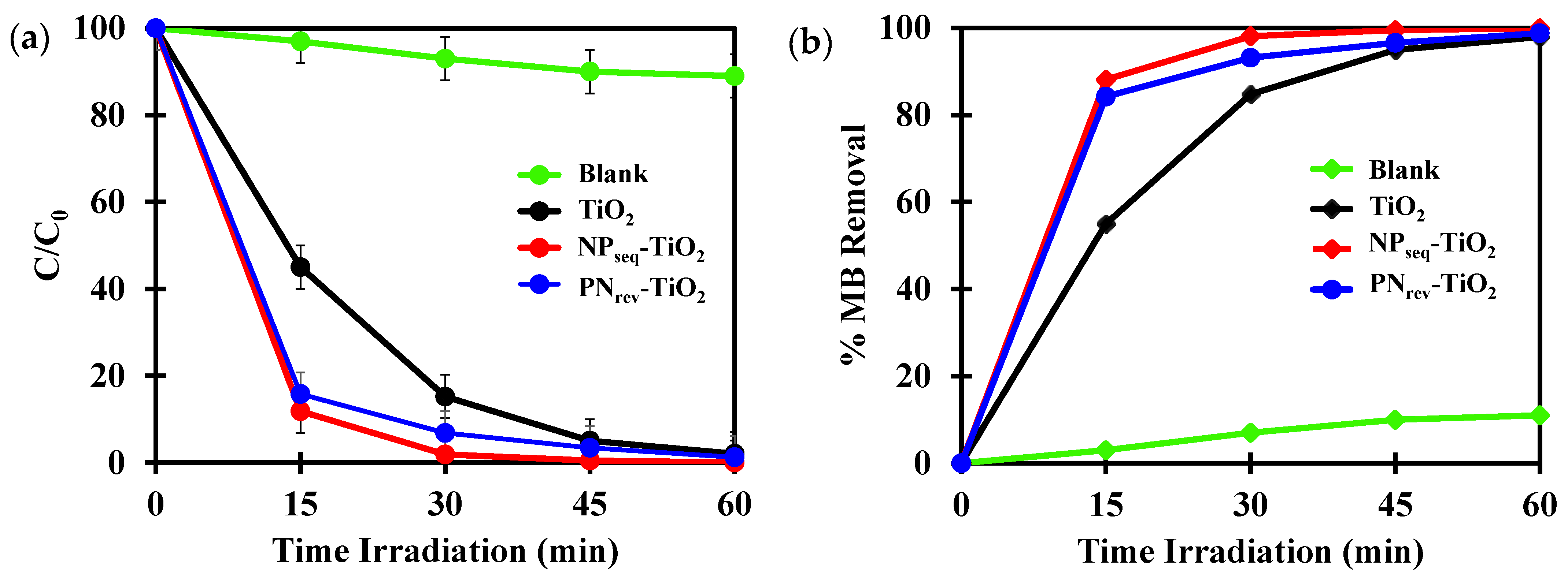
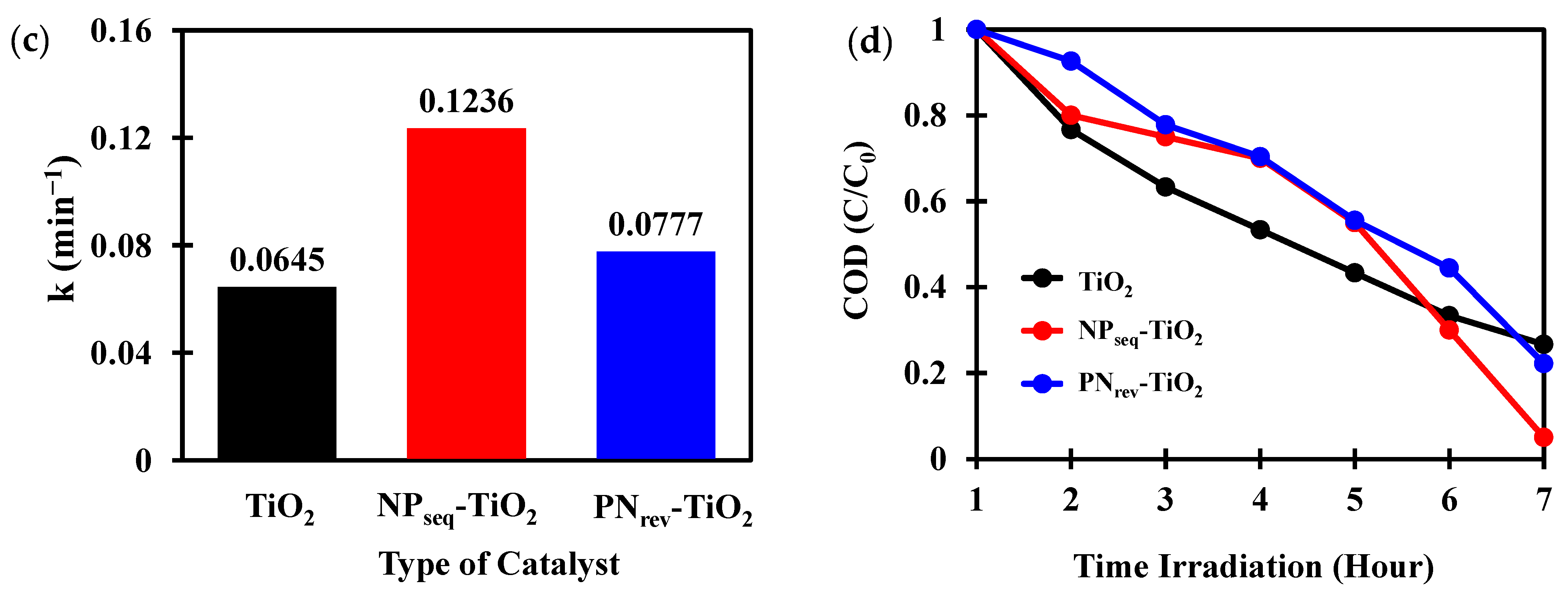
| Sample | CI (%) a | DXRD (nm) b | SBET (m2 g−1) c | V (cm3 g−1) d | L (nm) e | Eg (eV) f |
|---|---|---|---|---|---|---|
| TiO2 | 81.33 | 35.43 | 54 | 0.50 | 37.45 | 3.20 |
| NPseq-TiO2 | 80.81 | 32.86 | 57 | 0.61 | 43.43 | 2.90 |
| PNrev-TiO2 | 79.99 | 40.96 | 25 | 0.41 | 67.3 | 3.10 |
| Sample | Sample Preparation | |||||||
|---|---|---|---|---|---|---|---|---|
| CH₄N₂O (g) | H2PtCl6 (wt. %) | N a (wt. %) | N b (wt. %) | Pt a (wt. %) | Pt c (wt. %) | k (min−1) | R2 | |
| TiO2 | - | - | - | - | - | - | 0.0645 | 0.9986 |
| NPseq-TiO2 | 1.0 | 2.0 | 0.60 | 1.58 | 2.10 | 2.10 | 0.1236 | 0.9980 |
| PNrev-TiO2 | 1.0 | 2.0 | - | - | 1.70 | 2.20 | 0.0777 | 0.9839 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natar, N.S.; Ghani, N.I.A.; Hamzah, S.R.; Rosli, M.A.; Muhamad, N.A.; Azami, M.S.; Ishak, M.A.M.; Razak, S.; Nawawi, W.I. The Role of Nitrogen-Doped TiO2 Supported by Platinum Catalyst Synthesized via Various Mode Preparations for Photocatalytic Enhancement. Nanomaterials 2022, 12, 3998. https://doi.org/10.3390/nano12223998
Natar NS, Ghani NIA, Hamzah SR, Rosli MA, Muhamad NA, Azami MS, Ishak MAM, Razak S, Nawawi WI. The Role of Nitrogen-Doped TiO2 Supported by Platinum Catalyst Synthesized via Various Mode Preparations for Photocatalytic Enhancement. Nanomaterials. 2022; 12(22):3998. https://doi.org/10.3390/nano12223998
Chicago/Turabian StyleNatar, Nadiah Sabihah, Nureel Imanina Abdul Ghani, Siti Raihan Hamzah, Muhammad Afiq Rosli, Nur Aien Muhamad, Mohammad Saifulddin Azami, Mohd Azlan Mohd Ishak, Sharin Razak, and Wan Izhan Nawawi. 2022. "The Role of Nitrogen-Doped TiO2 Supported by Platinum Catalyst Synthesized via Various Mode Preparations for Photocatalytic Enhancement" Nanomaterials 12, no. 22: 3998. https://doi.org/10.3390/nano12223998
APA StyleNatar, N. S., Ghani, N. I. A., Hamzah, S. R., Rosli, M. A., Muhamad, N. A., Azami, M. S., Ishak, M. A. M., Razak, S., & Nawawi, W. I. (2022). The Role of Nitrogen-Doped TiO2 Supported by Platinum Catalyst Synthesized via Various Mode Preparations for Photocatalytic Enhancement. Nanomaterials, 12(22), 3998. https://doi.org/10.3390/nano12223998







