Optimization Temperature Programming of Microwave-Assisted Synthesis ZnO Nanoneedle Arrays for Optical and Surface-Enhanced Raman Scattering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of ZnO Seed Layer
2.2. Fabrication of ZnO Nanoneedle Arrays
2.3. Characteristics
2.4. SERS Testing
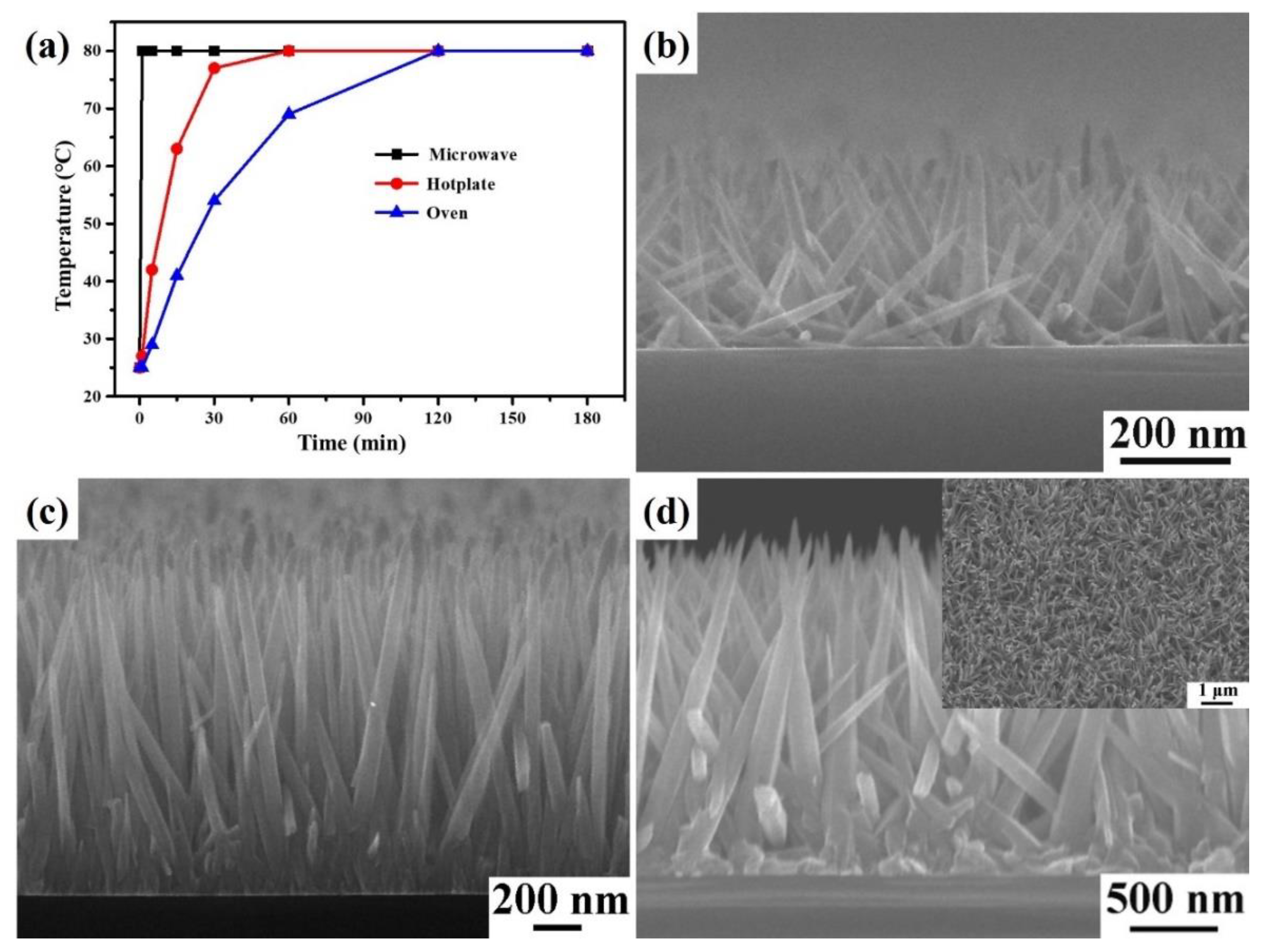
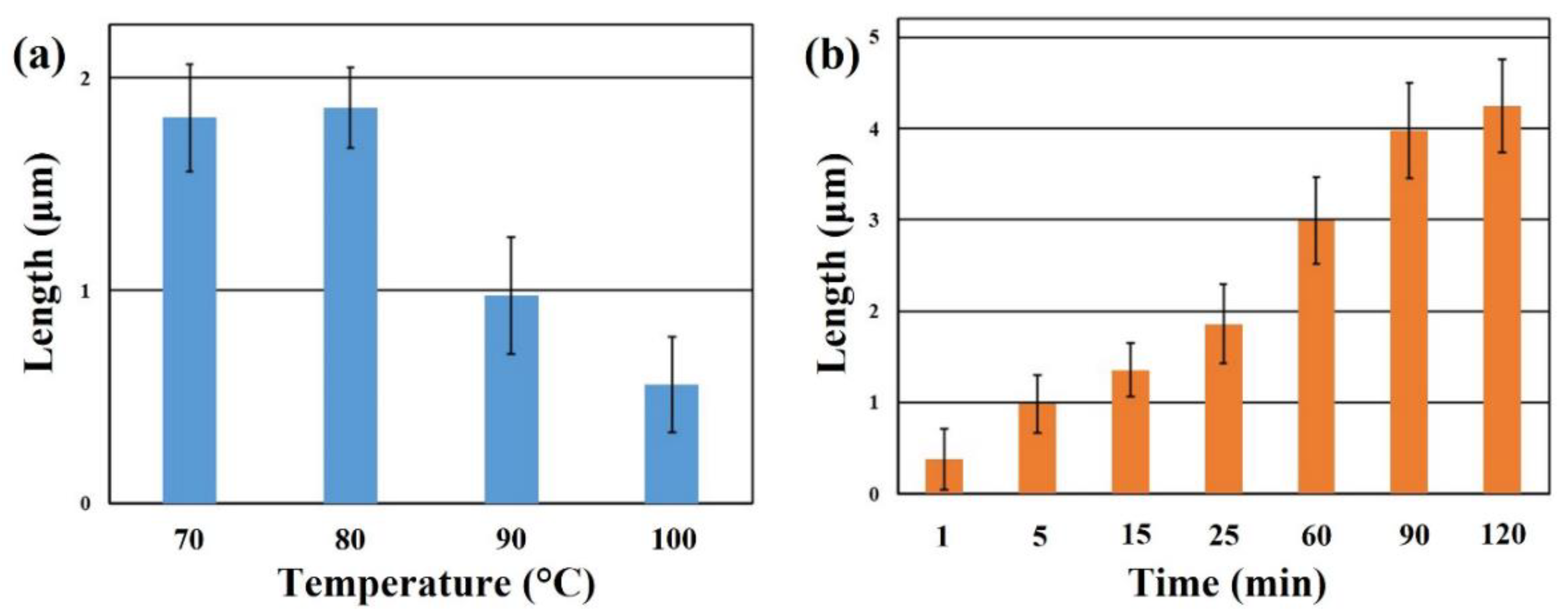
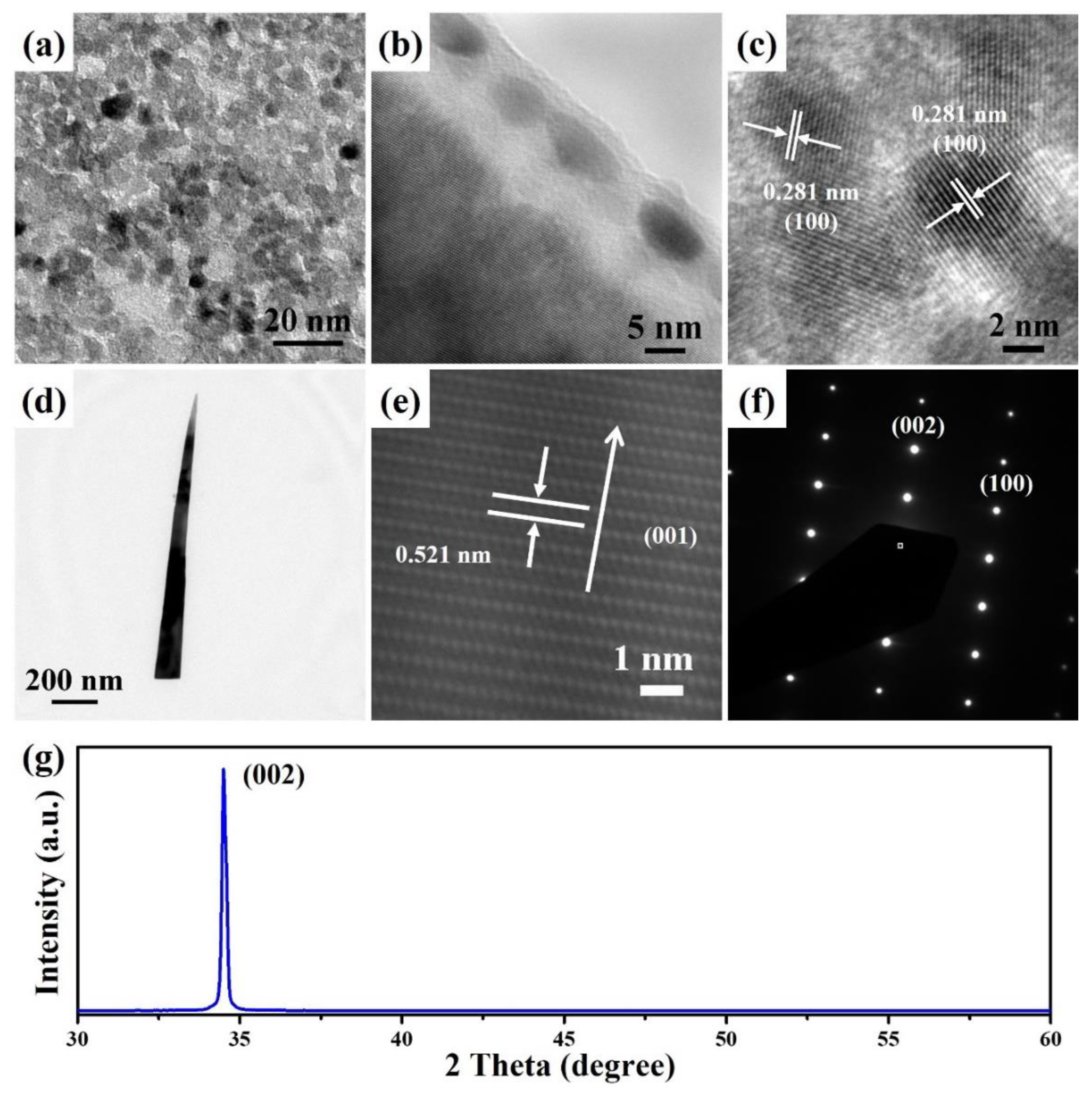
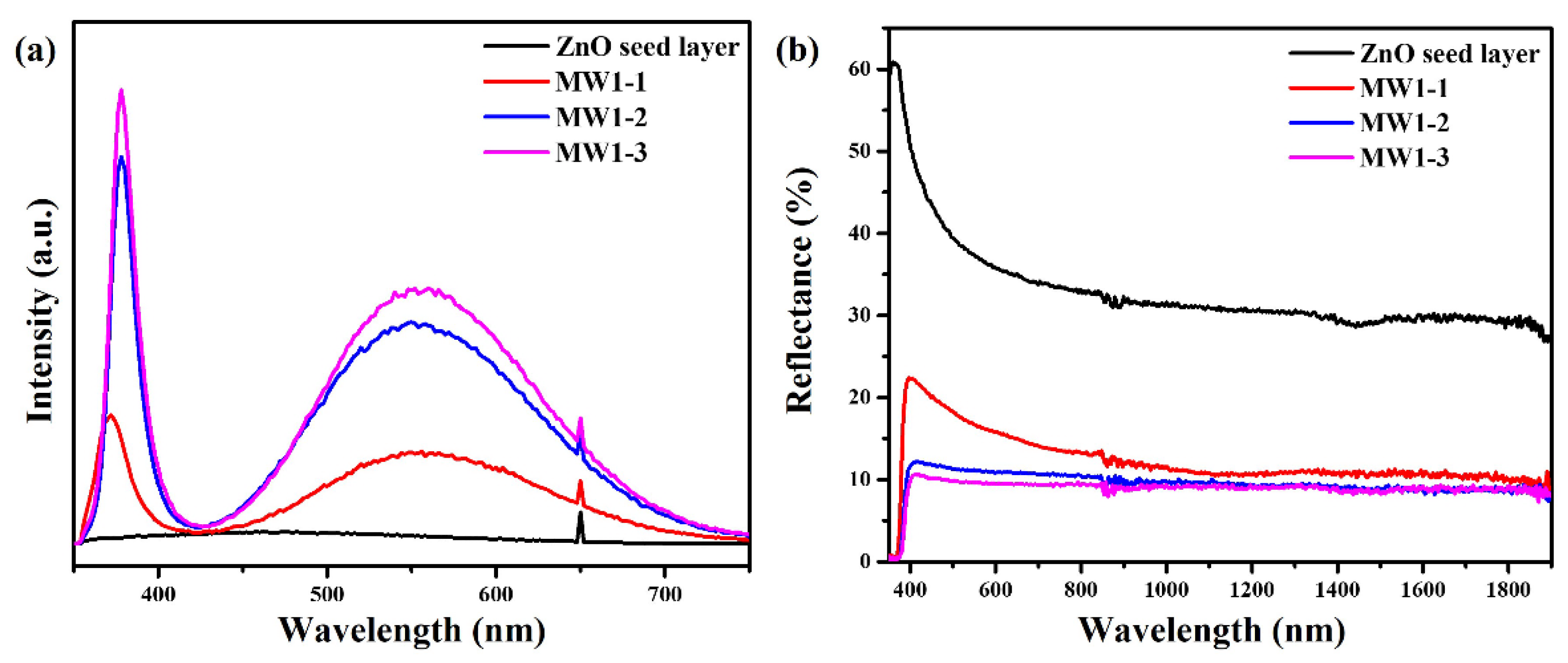
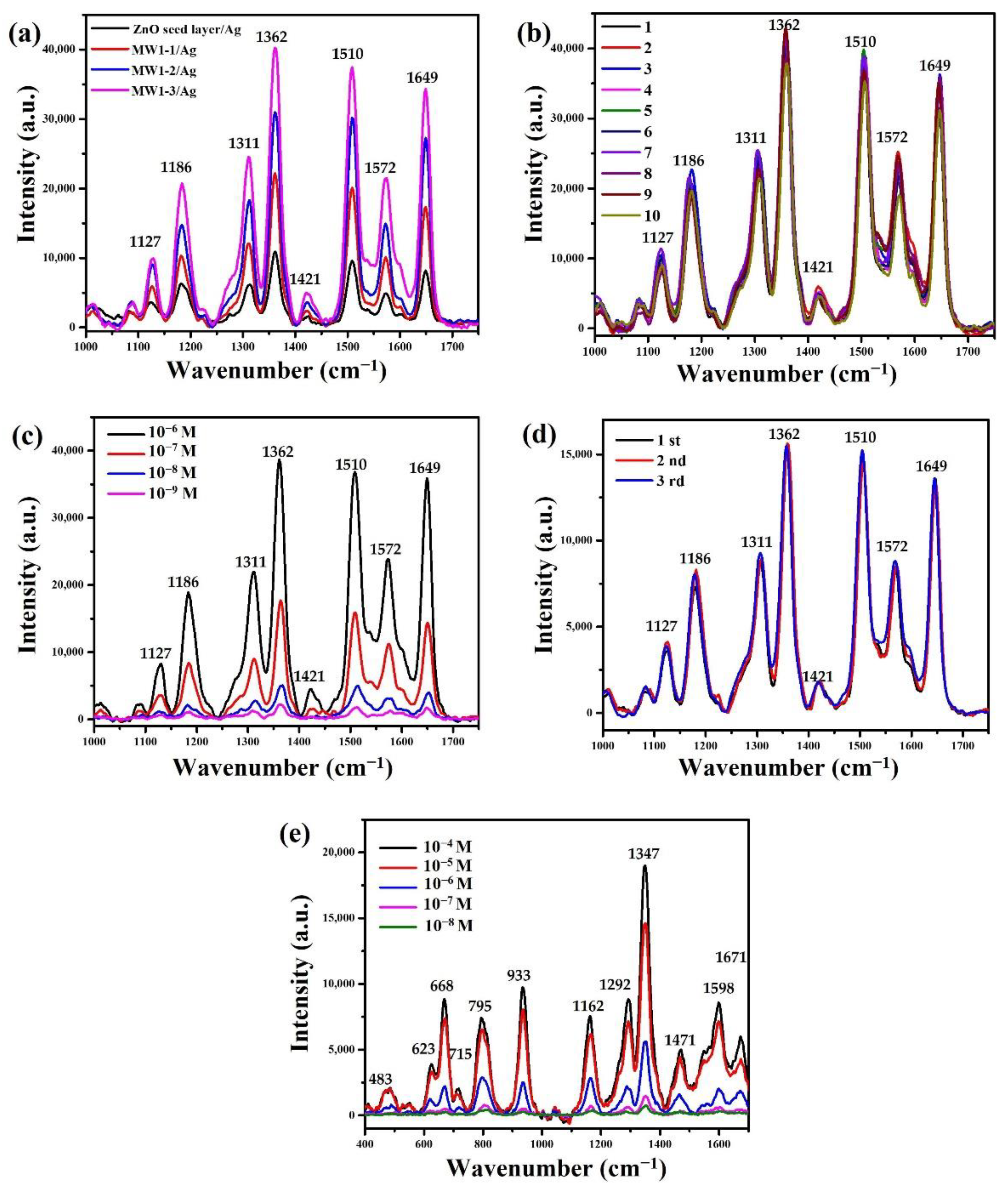
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Tsuji, M. Microwave-Assisted Synthesis of Metallic Nanomaterials in Liquid Phase. ChemistrySelect 2017, 2, 805–819. [Google Scholar] [CrossRef]
- Zaker, A.; Chen, Z.; Wang, X.; Zhang, Q. Microwave-assisted pyrolysis of sewage sludge: A review. Fuel Process. Technol. 2019, 187, 84–104. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, Y.; Huang, K. Advances in Microwave-Assisted Production of Reduced Graphene Oxide. Front. Chem. 2019, 7, 755. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Varma, R.S. Microwave-Assisted Organic Synthesis and Transformations using Benign Reaction Media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.K.; Gawande, M.B.; Zboril, R.; Varma, R.S. Microwave-assisted synthesis—Catalytic applications in aqueous media. Coord. Chem. Rev. 2015, 291, 68–94. [Google Scholar] [CrossRef]
- Onwudiwe, D.C. Microwave-assisted synthesis of PbS nanostructures. Heliyon 2019, 5, e01413. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P.; Savu, R.; Moshkalev, S.A. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: A review. Mater. Today Chem. 2019, 12, 282–314. [Google Scholar] [CrossRef]
- Díaz de Greñu, B.; de los Reyes, R.; Costero, A.M.; Amorós, P.; Ros-Lis, J.V. Recent Progress of Microwave-Assisted Synthesis of Silica Materials. Nanomaterials 2020, 10, 1092. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: A review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Budarin, V.L.; Shuttleworth, P.S.; De bruyn, M.; Farmer, T.J.; Gronnow, M.J.; Pfaltzgraff, L.; Macquarrie, D.J.; Clark, J.H. The potential of microwave technology for the recovery, synthesis and manufacturing of chemicals from bio-wastes. Catal. Today 2015, 239, 80–89. [Google Scholar] [CrossRef]
- Patel, K.K.; Purohit, R. Improved shape memory and mechanical properties of microwave-induced shape memory polymer/MWCNTs composites. Mater. Today Commun. 2019, 20, 100579. [Google Scholar] [CrossRef]
- Mostafa, M.H.; Elsawy, M.A.; Darwish, M.S.A.; Hussein, L.I.; Abdaleem, A.H. Microwave-Assisted preparation of Chitosan/ZnO nanocomposite and its application in dye removal. Mater. Chem. Phys. 2020, 248, 122914. [Google Scholar] [CrossRef]
- Liu, F.-K.; Huang, P.-W.; Chang, Y.-C.; Ko, F.-H.; Chu, T.-C. Combining Optical Lithography with Rapid Microwave Heating for the Selective Growth of Au/Ag Bimetallic Core/Shell Structures on Patterned Silicon Wafers. Langmuir 2005, 21, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Hamoud, G.A. Antibacterial and Immunomodulatory Potentials of Biosynthesized Ag, Au, Ag-Au Bimetallic Alloy Nanoparticles Using the Asparagus racemosus Root Extract. Nanomaterials 2020, 10, 2453. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kwon, Y.J.; Mirzaei, A.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S. Synthesis of zinc oxide semiconductors-graphene nanocomposites by microwave irradiation for application to gas sensors. Sens. Actuators B Chem. 2017, 249, 590–601. [Google Scholar] [CrossRef]
- Faraji, S.; Ani, F.N. Microwave-assisted synthesis of metal oxide/hydroxide composite electrodes for high power supercapacitors—A review. J. Power Sources 2014, 263, 338–360. [Google Scholar] [CrossRef]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Guo, S.; Dai, Q.; Wang, Z.; Yao, H. Rapid microwave irradiation synthesis of carbon nanotubes on graphite surface and its application on asphalt reinforcement. Compos. Part B Eng. 2017, 124, 134–143. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Advances in Microwave Synthesis of Nanoporous Materials. Adv. Mater. 2021, 33, 2103477. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Pazirofteh, M.; Dehghani, M.; Asghari, M.; Rezakazemi, M.; Valderrama, C.; Cortina, J.-L. Application of ZnO nanostructures in ceramic and polymeric membranes for water and wastewater technologies: A review. Chem. Eng. J. 2020, 391, 123475. [Google Scholar] [CrossRef]
- Chou, C.-M.; Chang, T.-T.; Chen, C.-Y.; Chang, Y.-C. Constructing Er-Doped ZnO/CuS/Au Core-Shell Nanowires with Enhanced Photocatalytic and SERS Properties. Catalysts 2021, 11, 1347. [Google Scholar] [CrossRef]
- Consonni, V.; Briscoe, J.; Kärber, E.; Li, X.; Cossuet, T. ZnO nanowires for solar cells: A comprehensive review. Nanotechnology 2019, 30, 362001. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Man Ching Ng, A. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar] [CrossRef]
- Medina Cruz, D.; Mostafavi, E.; Vernet-Crua, A.; Barabadi, H.; Shah, V.; Cholula-Díaz, J.L.; Guisbiers, G.; Webster, T.J. Green nanotechnology-based zinc oxide (ZnO) nanomaterials for biomedical applications: A review. J. Phys. Mater. 2020, 3, 034005. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, C.-M.; Guo, J.-Y. Fabrication of novel ZnO nanoporous films for efficient photocatalytic applications. J. Photochem. Photobiol. A 2018, 356, 340–346. [Google Scholar] [CrossRef]
- Liu, L.; Hong, K.; Ge, X.; Liu, D.; Xu, M. Controllable and Rapid Synthesis of Long ZnO Nanowire Arrays for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 15551–15555. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hsu, C.-C.; Wu, S.-H.; Chuang, K.-W.; Chen, Y.-F. Fabrication of Cu-doped ZnO nanoneedles on different substrate via wet chemical approach: Structural characterization and photocatalytic performance. Appl. Surf. Sci. 2018, 447, 213–221. [Google Scholar] [CrossRef]
- Rana, A.U.H.S.; Kang, M.; Kim, H.-S. Microwave-assisted Facile and Ultrafast Growth of ZnO Nanostructures and Proposition of Alternative Microwave-assisted Methods to Address Growth Stoppage. Sci. Rep. 2016, 6, 24870. [Google Scholar] [CrossRef] [PubMed]
- Mahpeykar, S.M.; Koohsorkhi, J.; Ghafoori-fard, H. Ultra-fast microwave-assisted hydrothermal synthesis of long vertically aligned ZnO nanowires for dye-sensitized solar cell application. Nanotechnology 2012, 23, 165602. [Google Scholar] [CrossRef]
- Kim, M.K.; Yi, D.K.; Paik, U. Tunable, Flexible Antireflection Layer of ZnO Nanowires Embedded in PDMS. Langmuir 2010, 26, 7552–7554. [Google Scholar] [CrossRef]
- Salman, K.A.; Omar, K.; Hassan, Z. Effective conversion efficiency enhancement of solar cell using ZnO/PS antireflection coating layers. Sol. Energy 2012, 86, 541–547. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Dastjerdi, H.T.; Zhao, J.; Shulenberger, K.E.; Carbonera, C.; Po, R.; Cominetti, A.; Bianchi, G.; Klein, N.D.; Bawendi, M.G.; et al. Light Management in Organic Photovoltaics Processed in Ambient Conditions Using ZnO Nanowire and Antireflection Layer with Nanocone Array. Small 2019, 15, 1900508. [Google Scholar] [CrossRef]
- Lee, J.W.; Ye, B.U.; Kim, D.-Y.; Kim, J.K.; Heo, J.; Jeong, H.Y.; Kim, M.H.; Choi, W.J.; Baik, J.M. ZnO Nanowire-Based Antireflective Coatings with Double-Nanotextured Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 1375–1379. [Google Scholar] [CrossRef]
- Huang, J.-H.; Chen, C.-Y.; Lai, Y.-F.; Shih, Y.-I.; Lin, Y.-C.; He, J.-H.; Liu, C.-P. Large-Area Oblique-Aligned ZnO Nanowires through a Continuously Bent Columnar Buffer: Growth, Microstructure, and Antireflection. Cryst. Growth Des. 2010, 10, 3297–3301. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Ruby, D.S.; Peters, D.W.; McKenzie, B.B.; Hsu, J.W.P. ZnO Nanostructures as Efficient Antireflection Layers in Solar Cells. Nano Lett. 2008, 8, 1501–1505. [Google Scholar] [CrossRef]
- Chang, Y.-C. Low temperature and large-scale growth of ZnO nanoneedle arrays with enhanced optical and surface-enhanced Raman scattering properties. Sens. Actuators B Chem. 2016, 225, 498–503. [Google Scholar] [CrossRef]
- Rovisco, A.; Morais, M.; Branquinho, R.; Fortunato, E.; Martins, R.; Barquinha, P. Microwave-Assisted Synthesis of Zn2SnO4 Nanostructures for Photodegradation of Rhodamine B under UV and Sunlight. Nanomaterials 2022, 12, 2119. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.-H.; Lo, W.-J.; Chang, Y.-C.; Guo, J.-Y.; Chen, C.-M. ZnO nanowires coated stainless steel meshes as hierarchical photocatalysts for catalytic photodegradation of four kinds of organic pollutants. J. Alloy. Compd. 2016, 678, 137–146. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Yang, W.-C.; Chang, C.-M.; Hsu, P.-C.; Chen, L.-J. Controlled Growth of ZnO Nanopagoda Arrays with Varied Lamination and Apex Angles. Cryst. Growth Des. 2009, 9, 3161–3167. [Google Scholar] [CrossRef]
- Jacobsson, T.J.; Viarbitskaya, S.; Mukhtar, E.; Edvinsson, T. A size dependent discontinuous decay rate for the exciton emission in ZnO quantum dots. Phys. Chem. Chem. Phys. 2014, 16, 13849–13857. [Google Scholar] [CrossRef]
- He, X.N.; Gao, Y.; Mahjouri-Samani, M.; Black, P.N.; Allen, J.; Mitchell, M.; Xiong, W.; Zhou, Y.S.; Jiang, L.; Lu, Y.F. Surface-enhanced Raman spectroscopy using gold-coated horizontally aligned carbon nanotubes. Nanotechnology 2012, 23, 205702. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-H.; Chuang, K.-W.; Chang, Y.-C. Ag/Ga-doped ZnO/pyramidal silicon as a multifunctional surface-enhanced Raman scattering substrate. J. Alloy. Compd. 2022, 893, 162288. [Google Scholar] [CrossRef]
- Skarp, K.-P.; Shams, A.; Montelin, H.; Lagerbäck, P.; Tängdén, T. Synergistic and bactericidal activities of mecillinam, amoxicillin and clavulanic acid combinations against extended-spectrum β-lactamase (ESBL)-producing Escherichia coli in 24-h time–kill experiments. Int. J. Antimicrob. Agents 2019, 53, 74–79. [Google Scholar] [CrossRef]
- Belkadi, A.; Meliani, M.F.; Mokhtar, A.; Djelad, A.; Abdelkrim, S.; Kebir-Medjhouda, Z.A.; Bengueddach, A.; Sassi, M. Amoxicillin loaded bentonite, advanced low-cost antibacterial and environmentally friendly materials. J. Mol. Struct. 2022, 1270, 133880. [Google Scholar] [CrossRef]
- Kibar, G.; Topal, A.E.; Dana, A.; Tuncel, A. Newly designed silver coated-magnetic, monodisperse polymeric microbeads as SERS substrate for low-level detection of amoxicillin. J. Mol. Struct. 2016, 1119, 133–138. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Wu, S.-H. Bi-functional Al-doped ZnO@SnO2 heteronanowires as efficient substrates for improving photocatalytic and SERS performance. J. Ind. Eng. Chem. 2019, 76, 333–343. [Google Scholar] [CrossRef]


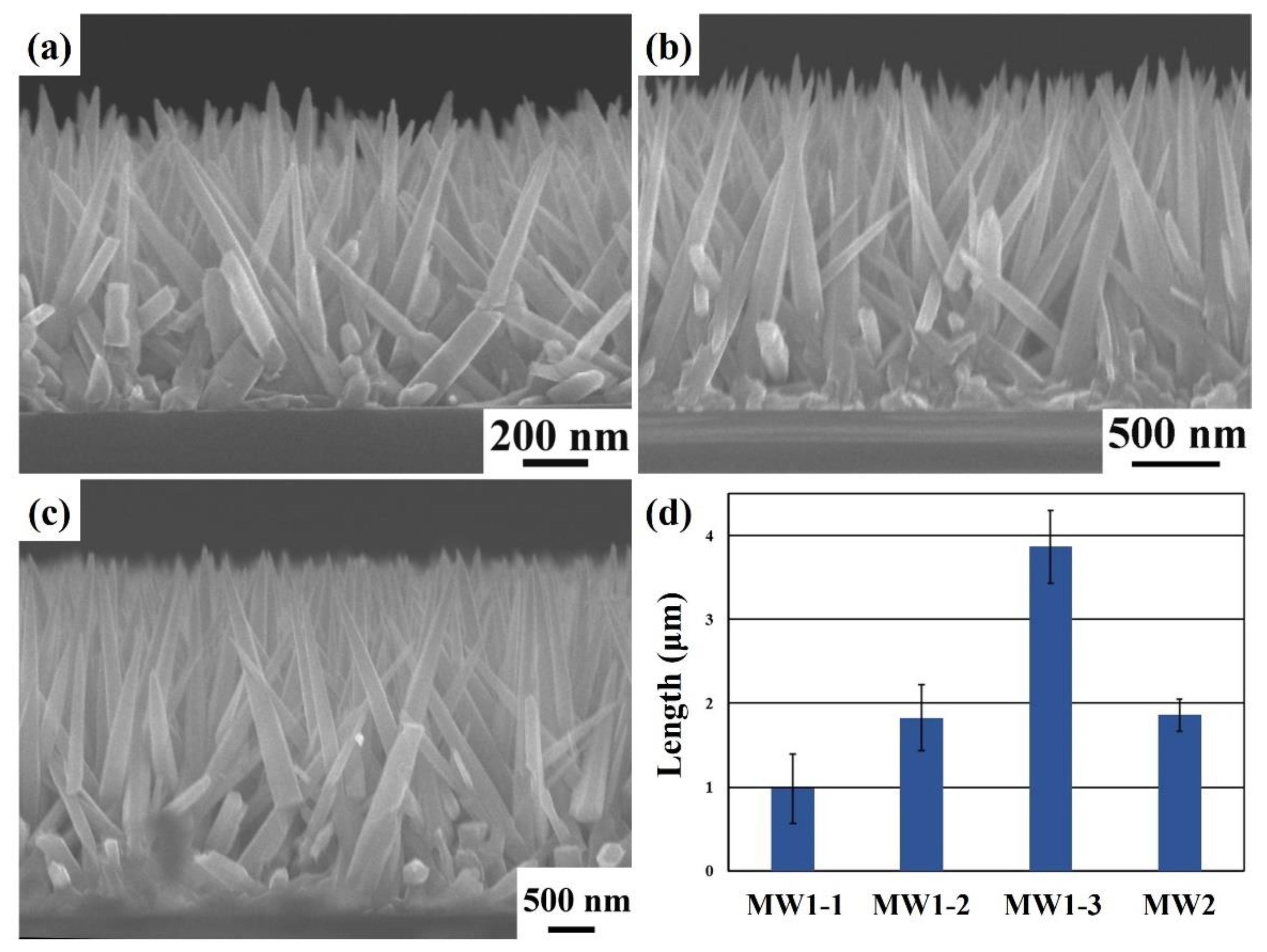
| Sample | Heating Time (min) | Holding Time (min) | Heating Time (min) | Holding Time (min) | Heating Time (min) | Holding Time (min) |
|---|---|---|---|---|---|---|
| MW1-1 | 1 (80 °C) | 4 | ||||
| MW1-2 | 1 (80 °C) | 4 | 1 (90 °C) | 9 | ||
| MW1-3 | 1 (80 °C) | 4 | 1 (90 °C) | 9 | 1 (100 °C) | 9 |
| MW2 | 1 (80 °C) | 25 | ||||
| MW3 | 1 (80 °C) | 4 | 1 (100 °C) | 9 | 1 (120 °C) | 9 |
| MW4 | 1 (80 °C) | 4 | 1 (90 °C) | 14 | 1 (100 °C) | 4 |
| MW5 | 1 (80 °C) | 4 | 1 (90 °C) | 4 | 1 (100 °C) | 14 |
| MW6 | 1 (80 °C) | 4 | 1 (85 °C) | 4 | 1 (90 °C) | 4 |
| 1 (95 °C) | 4 | 1 (100 °C) | 4 | |||
| MW7 | 1 (80 °C) | 4 | 1 (90 °C) | 4 | 1 (100 °C) | 4 |
| 1 (110 °C) | 4 | 1 (120 °C) | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-H.; Chang, Y.-C.; Lee, C.-I.; Lin, Y.-R.; Ko, F.-H. Optimization Temperature Programming of Microwave-Assisted Synthesis ZnO Nanoneedle Arrays for Optical and Surface-Enhanced Raman Scattering Applications. Nanomaterials 2022, 12, 3989. https://doi.org/10.3390/nano12223989
Chang T-H, Chang Y-C, Lee C-I, Lin Y-R, Ko F-H. Optimization Temperature Programming of Microwave-Assisted Synthesis ZnO Nanoneedle Arrays for Optical and Surface-Enhanced Raman Scattering Applications. Nanomaterials. 2022; 12(22):3989. https://doi.org/10.3390/nano12223989
Chicago/Turabian StyleChang, Tung-Hao, Yu-Cheng Chang, Chung-I Lee, Ying-Ru Lin, and Fu-Hsiang Ko. 2022. "Optimization Temperature Programming of Microwave-Assisted Synthesis ZnO Nanoneedle Arrays for Optical and Surface-Enhanced Raman Scattering Applications" Nanomaterials 12, no. 22: 3989. https://doi.org/10.3390/nano12223989
APA StyleChang, T.-H., Chang, Y.-C., Lee, C.-I., Lin, Y.-R., & Ko, F.-H. (2022). Optimization Temperature Programming of Microwave-Assisted Synthesis ZnO Nanoneedle Arrays for Optical and Surface-Enhanced Raman Scattering Applications. Nanomaterials, 12(22), 3989. https://doi.org/10.3390/nano12223989








