Carbon Nanofibers-Sheathed Graphite Rod Anode and Hydrophobic Cathode for Improved Performance Industrial Wastewater-Driven Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anodes Preparation
2.3. Cathodes Modification
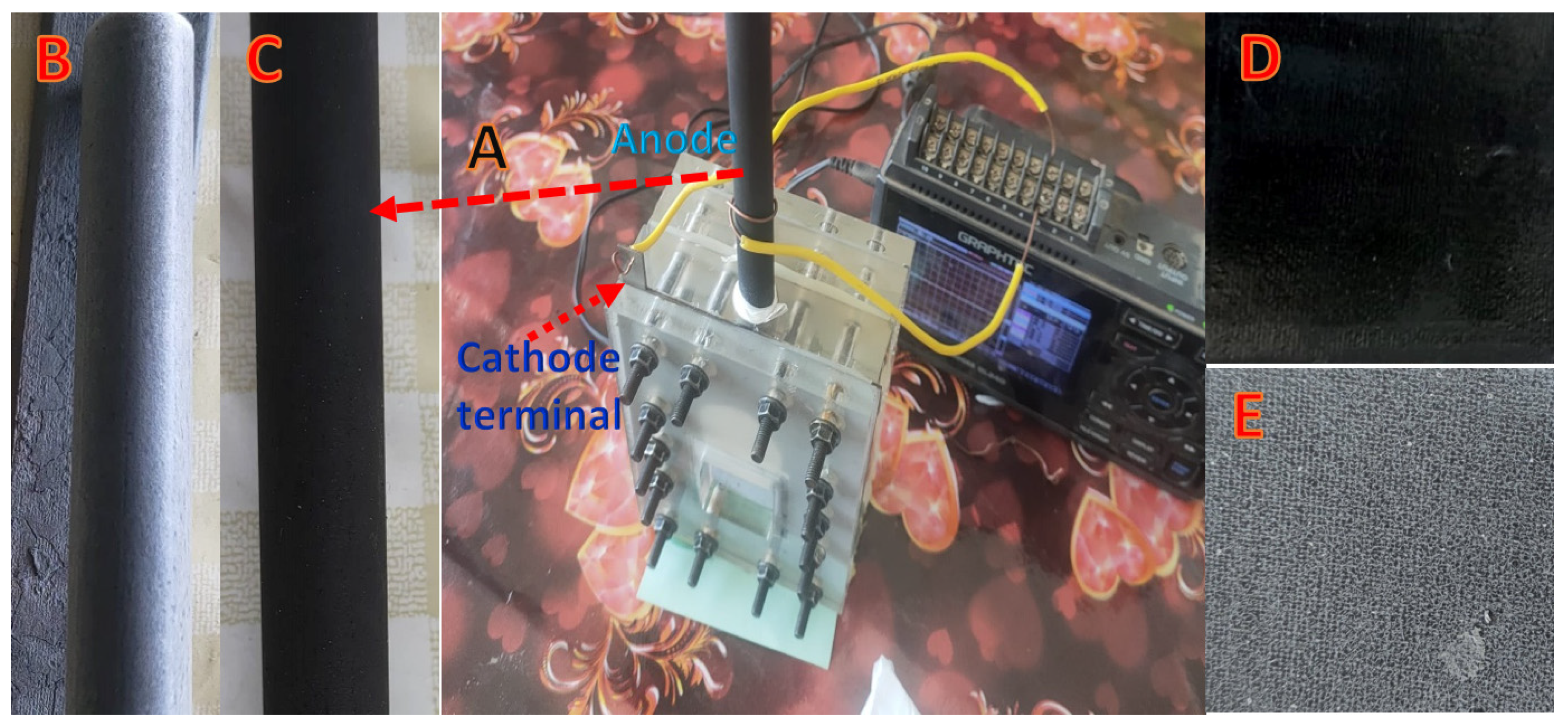
2.4. MFC Construction and Operation
2.5. Characterization
3. Results
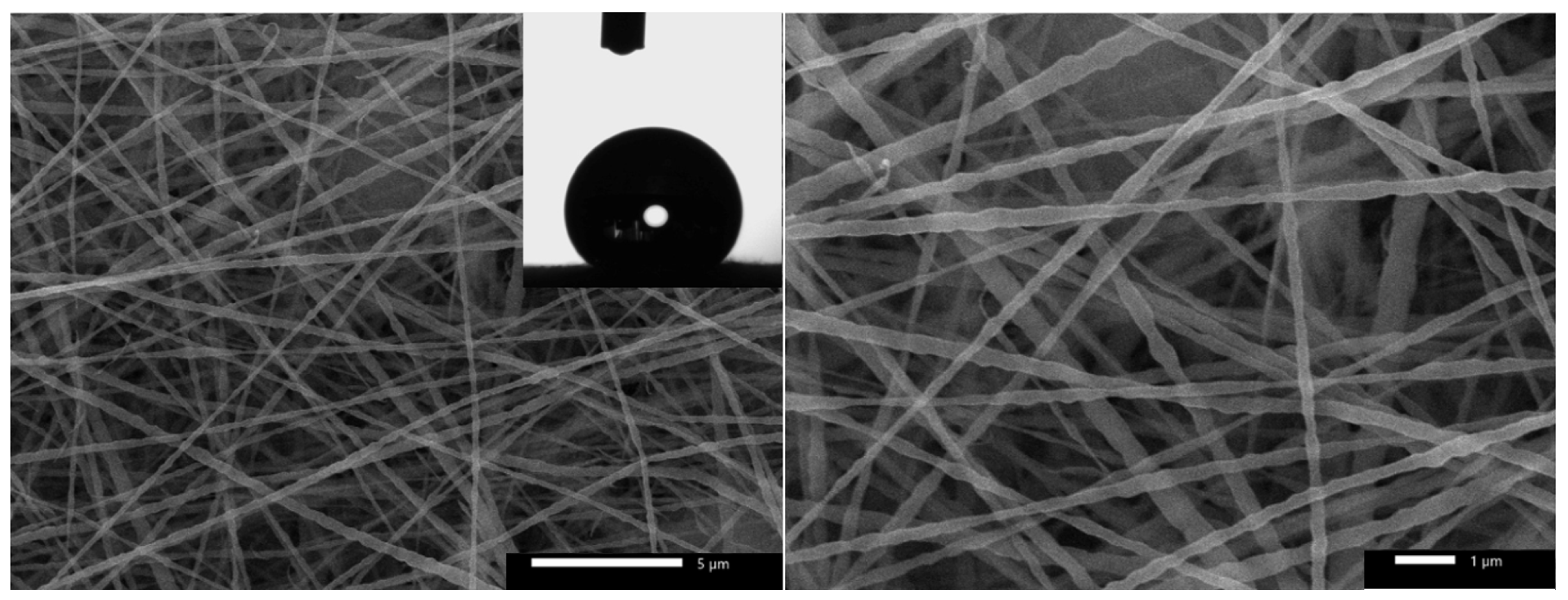
3.1. Hydrophobic Cathode Development
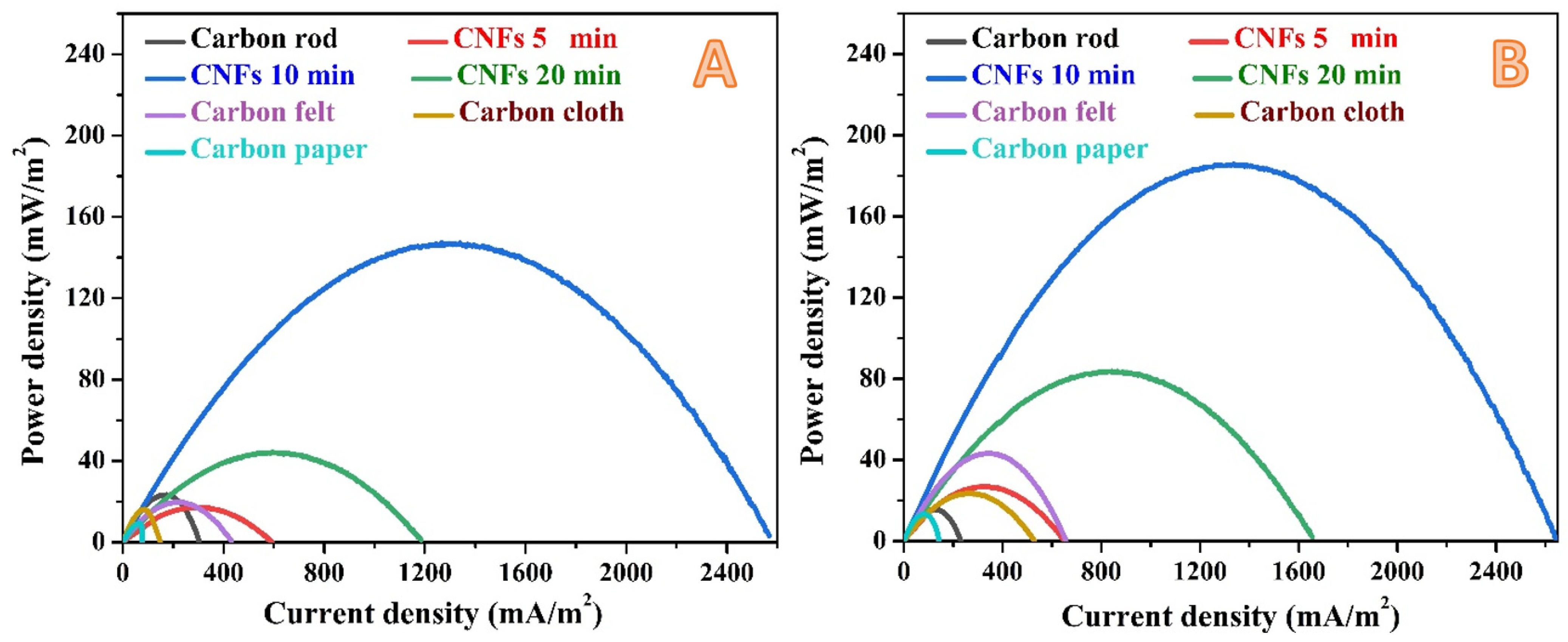
3.2. Performance of the Assembled MFCs
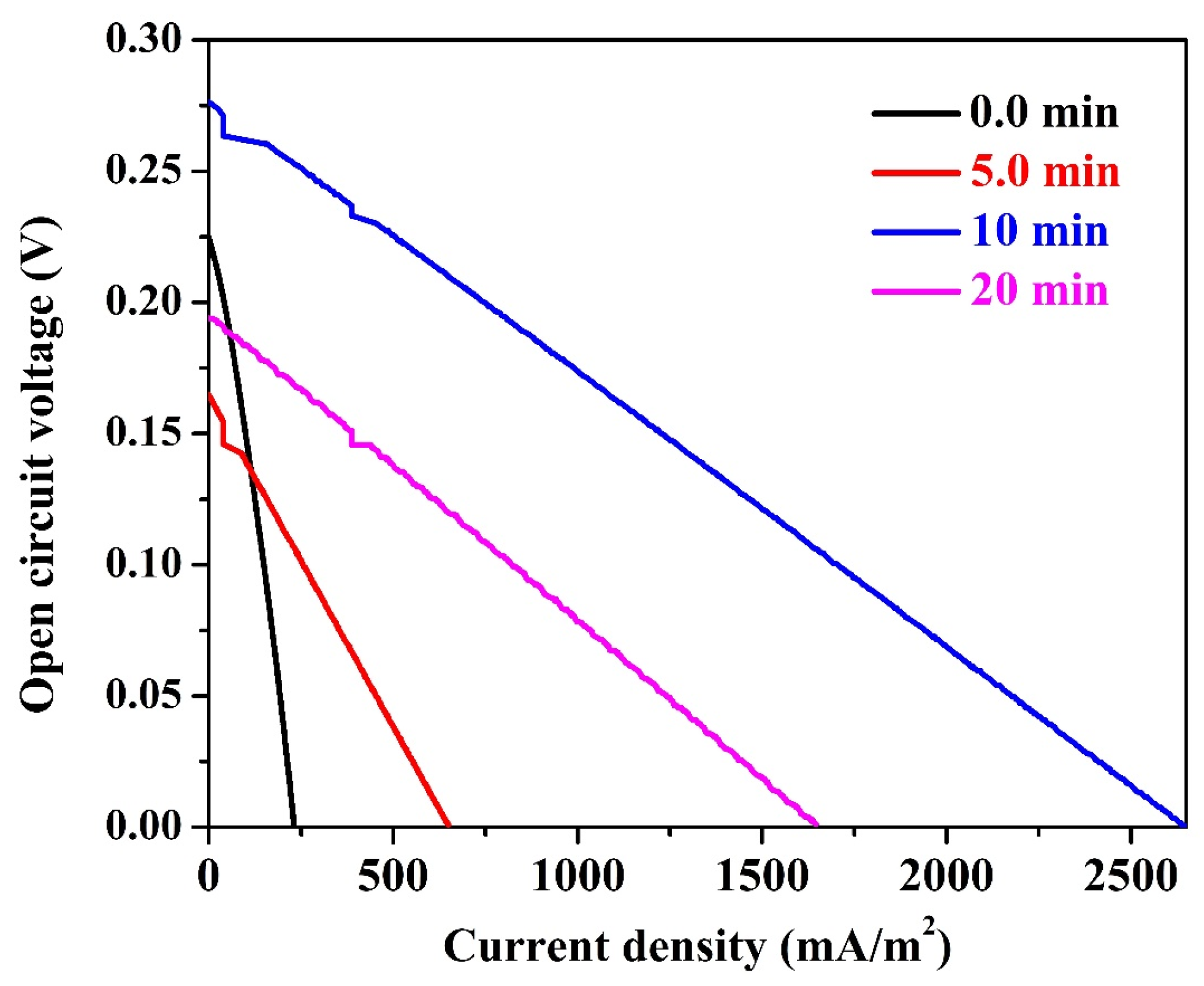
- Despite little improvement in the generated power and current densities upon increasing the working time to one day, carbon paper still has the least anode performance.
- The graphite rode sheathed by carbon nanofiber layer at electrospinning time of 10 min still keeps its distinction by generating power and current densities of 185 ± 7.4 mW/m2 and 2640 ± 26 mA/m2, respectively. These results represent around 25% increase, in term of power, compared to 2 h working time, and 800% compared to the best commercial anode (carbon felt) at the same working time (1 day). However, upon working time increase, the increase in the current density does not match the power density increasing fashion; a little increase in the current density was observed compared to 2 h working time. This finding can be attribute to mass transfer limitation of reaching the organic molecules to the attached microorganisms amongst the carbon nanofiber layers. In other words, increasing the time led to increase the number of the microorganisms in the biofilm, however, as the current represents the liberated electrons due to organic compounds metabolism at the anode surface, the slight change in the current density is attributed to a mass transfer limitation process.
- Among all investigated anodes, only the naked graphite rod showed lower performance upon time increase; the power and current densities decreased from 23 ± 0.4 mW/m2 and 303 ± 7 mA/m2 (at 2 h) to 15 ± 0.7 mW/m2 and 228 ± 6 mA/m2 (at 1 day), respectively. This finding might reflect low adhesion force between the attached microorganisms and the anode surface which results in a high releasing rate.
- There is an observable enhancement in the generated power from the 5-min and 20-min anodes. However, the performance of the later was almost doubled due to increasing the working time to 1 day which represents more attaching of the microorganisms at this anode compared the thin carbon nanofiber layer one (5-min).
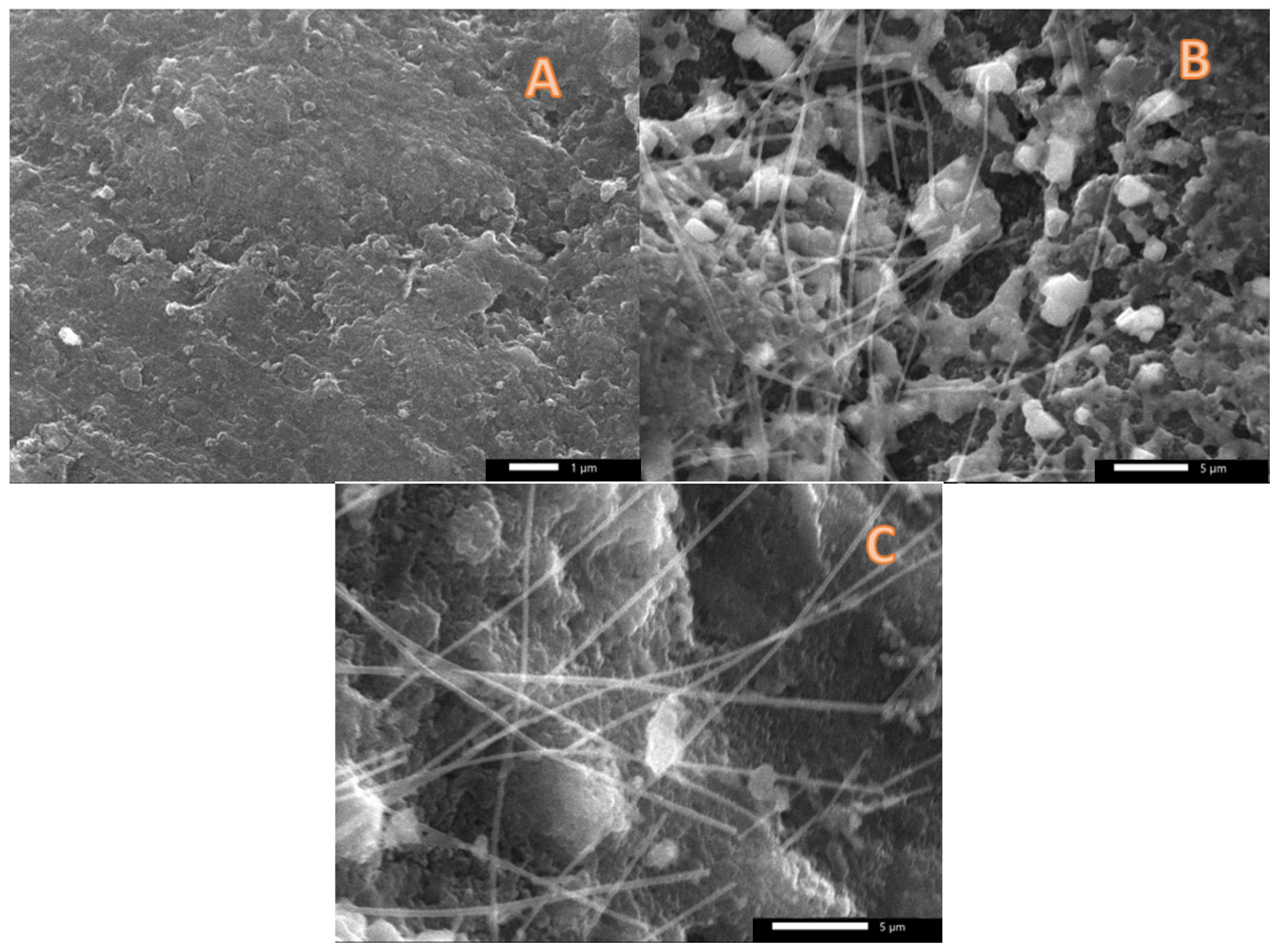
3.3. Used Anodes Morphology
3.4. Cell Electrical Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fadali, O.A.; Mahmoud, M.S.; Abdelraheem, O.H.; Mohammed, S.G. Evaluation of the hydrodynamics generated by agitation and electromagnetic field during the electrocoagulation of oil/water emulsion. J. Water Process Eng. 2018, 25, 182–189. [Google Scholar] [CrossRef]
- Rui, J.; Deng, N.; Zhao, Y.; Tao, C.; Zhou, J.; Zhao, Z.; Huang, X. Activation of persulfate via Mn doped Mg/Al layered double hydroxide for effective degradation of organics: Insights from chemical and structural variability of catalyst. Chemosphere 2022, 302, 134849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, N.; Fan, Z.; Hu, Z.-T.; Fan, L.; Zhou, J.; Huang, X. On-site H2O2 electro-generation process combined with ultraviolet: A promising approach for odorous compounds purification in drinking water system. Chem. Eng. J. 2022, 430, 132829. [Google Scholar] [CrossRef]
- Sophonsiri, C.; Morgenroth, E. Chemical composition associated with different particle size fractions in municipal, industrial, and agricultural wastewaters. Chemosphere 2004, 55, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Kakavandi, B.; Ahmadi, M. Efficient treatment of saline recalcitrant petrochemical wastewater using heterogeneous UV-assisted sono-Fenton process. Ultrason. Sonochem. 2019, 56, 25–36. [Google Scholar] [CrossRef]
- Fito, J.; Tefera, N.; Van Hulle, S.W.H. Sugarcane biorefineries wastewater: Bioremediation technologies for environmental sustainability. Chem. Biol. Technol. Agric. 2019, 6, 6. [Google Scholar] [CrossRef]
- Ingaramo, A.; Heluane, H.; Colombo, M.; Cesca, M. Water and wastewater eco-efficiency indicators for the sugar cane industry. J. Clean. Prod. 2009, 17, 487–495. [Google Scholar] [CrossRef]
- Solomon, S.K. Environmental pollution and its management in sugar industry in India: An appraisal. Sugar Tech 2005, 7, 77–81. [Google Scholar] [CrossRef]
- Sahu, O.; Chaudhari, P. Electrochemical treatment of sugar industry wastewater: COD and color removal. J. Electroanal. Chem. 2015, 739, 122–129. [Google Scholar] [CrossRef]
- Chandrakant, M.; Kedar, R. Physico-chemical analysis and microbial degradation of spent wash from sugar industries. Res. J. Chem. Sci. 2013, 3, 53–56. [Google Scholar]
- Ameta, R.; Kumar, A.; Punjabi, P.; Ameta, S.C. Advanced oxidation processes: Basics and applications. In Wastewater Treatment: Advanced Processes and Technologies; IWA Publishing: London UK, 2012; p. 46. [Google Scholar]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Tan, B.H.; Teng, T.T.; Omar, A.M. Removal of dyes and industrial dye wastes by magnesium chloride. Water Res. 2000, 34, 597–601. [Google Scholar] [CrossRef]
- Kasmi, M. Biological Processes as Promoting Way for Both Treatment and Valorization of Dairy Industry Effluents. Waste Biomass Valorization 2018, 9, 195–209. [Google Scholar] [CrossRef]
- Liang, C.-Z.; Sun, S.-P.; Li, F.-Y.; Ong, Y.-K.; Chung, T.-S. Treatment of highly concentrated wastewater containing multiple synthetic dyes by a combined process of coagulation/flocculation and nanofiltration. J. Membr. Sci. 2014, 469, 306–315. [Google Scholar] [CrossRef]
- Oehmen, A.; Vergel, D.; Fradinho, J.; Reis, M.A.; Crespo, J.G.; Velizarov, S. Mercury removal from water streams through the ion exchange membrane bioreactor concept. J. Hazard. Mater. 2014, 264, 65–70. [Google Scholar] [CrossRef]
- Abdullah, N.; Yuzir, A.; Curtis, T.P.; Yahya, A.; Ujang, Z. Characterization of aerobic granular sludge treating high strength agro-based wastewater at different volumetric loadings. Bioresour. Technol. 2013, 127, 181–187. [Google Scholar] [CrossRef]
- Sahu, O.; Rao, D.G.; Gopal, R.; Tiwari, A.; Pal, D. Treatment of wastewater from sugarcane process industry by electrochemical and chemical process: Aluminum (metal and salt). J. Water Process Eng. 2017, 17, 50–62. [Google Scholar] [CrossRef]
- Logan, B. Biologically extracting energy from wastewater: Biohydrogen production and microbial fuel cells. Environ. Sci. Technol. 2004, 38, 160–167. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Amen, M.T.; Ali, R.H.; Nassar, M.M.; Fadali, O.A.; Ali, M.A.; Kim, H.Y. Carbon Nanofiber Double Active Layer and Co-Incorporation as New Anode Modification Strategies for Power-Enhanced Microbial Fuel Cells. Polymers 2022, 14, 1542. [Google Scholar] [CrossRef]
- Fadzli, F.S.; Bhawani, S.A.; Mohammad, R.E.A. Microbial Fuel Cell: Recent Developments in Organic Substrate Use and Bacterial Electrode Interaction. J. Chem. 2021, 2021, 4570388. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Roh, S.-H. Carbon Nanofiber/Polypyrrole Nanocomposite as Anode Material in Microbial Fuel Cells. J. Nanosci. Nanotechnol. 2017, 17, 5830–5833. [Google Scholar] [CrossRef]
- Jia, Y.; Ma, D.; Wang, X. Electrochemical preparation and application of PANI/MWNT and PPy/MWNT composite anodes for anaerobic fluidized bed microbial fuel cell. 3 Biotech 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Guo, Q.; Wang, X.; Yue, X. Electricity Generation from Wastewater Using an Anaerobic Fluidized Bed Microbial Fuel Cell. Ind. Eng. Chem. Res. 2011, 50, 12225–12232. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Abdelkareem, M.A.; Park, M.; Lee, J.; Kim, T.; Ojha, G.P.; Pant, B.; Park, S.-J.; Kim, H.; Barakat, N.A. Investigating the effect of membrane layers on the cathode potential of air-cathode microbial fuel cells. Int. J. Hydrogen Energy 2017, 42, 24308–24318. [Google Scholar] [CrossRef]
- Amen, M.T.; Barakat, N.A.; Jamal, M.A.H.M.; Hong, S.-T.; Mohamed, I.M.; Salama, A. Anolyte in-situ functionalized carbon nanotubes electrons transport network as novel strategy for enhanced performance microbial fuel cells. Appl. Energy 2018, 228, 167–175. [Google Scholar] [CrossRef]
- Lovley, D.R. Microbial fuel cells: Novel microbial physiologies and engineering approaches. Curr. Opin. Biotechnol. 2006, 17, 327–332. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Choi, T.H.; Won, Y.-B.; Lee, J.-W.; Shin, D.W.; Lee, Y.M.; Kim, M.; Park, H.B. Electrochemical performance of microbial fuel cells based on disulfonated poly(arylene ether sulfone) membranes. J. Power Sources 2012, 220, 269–279. [Google Scholar] [CrossRef]
- Saito, T.; Roberts, T.H.; Long, T.E.; Logan, B.E.; Hickner, M.A. Neutral hydrophilic cathode catalyst binders for microbial fuel cells. Energy Environ. Sci. 2011, 4, 928–934. [Google Scholar] [CrossRef]
- Pocaznoi, D.; Calmet, A.; Etcheverry, L.; Erable, B.; Bergel, A. Stainless steel is a promising electrode material for anodes of microbial fuel cells. Energy Environ. Sci. 2012, 5, 9645–9652. [Google Scholar] [CrossRef]
- Kim, J.R.; Cheng, S.; Oh, S.-E.; Logan, B.E. Power Generation Using Different Cation, Anion, and Ultrafiltration Membranes in Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar] [CrossRef]
- Fan, Y.; Han, S.-K.; Liu, H. Improved performance of CEA microbial fuel cells with increased reactor size. Energy Environ. Sci. 2012, 5, 8273–8280. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Obaid, M.; Sayed, E.T.; Liu, Y.; Lee, J.; Park, M.; Barakat, N.A.M.; Kim, H.Y. Electricity generation from real industrial wastewater using a single-chamber air cathode microbial fuel cell with an activated carbon anode. Bioprocess Biosyst. Eng. 2017, 40, 1151–1161. [Google Scholar] [CrossRef]
- Amen, M.T.; Yasin, A.S.; Hegazy, M.I.; Jamal, M.A.H.M.; Hong, S.-T.; Barakat, N.A.M. Rainwater-driven microbial fuel cells for power generation in remote areas. R. Soc. Open Sci. 2021, 8, 210996. [Google Scholar] [CrossRef]
- Amen, M.T.; Kim, H.Y.; Barakat, N.A.M. Three-dimensional carbon nanofiber-based anode for high generated current and power from air-cathode micro-sized MFC. RSC Adv. 2022, 12, 15486–15492. [Google Scholar] [CrossRef]
- Sanchez, J.-L.; Pinto, D.; Laberty-Robert, C. Electrospun carbon fibers for microbial fuel cells: A novel bioanode design applied to wastewater treatment. Electrochim. Acta 2021, 373, 137864. [Google Scholar] [CrossRef]
- Cai, T.; Huang, M.; Huang, Y.; Zheng, W. Enhanced performance of microbial fuel cells by electrospinning carbon nanofibers hybrid carbon nanotubes composite anode. Int. J. Hydrogen Energy 2019, 44, 3088–3098. [Google Scholar] [CrossRef]
- Fontananova, E.; Bahattab, M.; Aljlil, S.; Alowairdy, M.; Rinaldi, G.; Vuono, D.; Nagy, J.; Drioli, E.; Di Profio, G. From hydrophobic to hydrophilic polyvinylidenefluoride (PVDF) membranes by gaining new insight into material’s propertie. RSC Adv. 2015, 5, 56219–56231. [Google Scholar] [CrossRef]
- Barakat, N.A.; Kanjwal, M.A.; Sheikh, F.A.; Kim, H.Y. Spider-net within the N6, PVA and PU electrospun nanofiber mats using salt addition: Novel strategy in the electrospinning process. Polymer 2009, 50, 4389–4396. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Hoogers, G. Fuel Cell Technology Handbook; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Larminie, J.; Dicks, A.; McDonald, M.S. Fuel Cell Systems Explained; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Mohamed, H.O.; Obaid, M.; Yasin, A.S.; Kim, J.H.; Barakat, N.A.M. Electrodepositing technique for improving the performance of crystalline and amorphous carbonaceous anodes for MFCs. RSC Adv. 2016, 6, 111657–111665. [Google Scholar] [CrossRef]

| pH | COD (mg/L) | Na+ (mg/L) | Ca+2 (mg/L) | Total P (mg/L) | SO4−2 (mg/L) | K+ (mg/L) | TDS (mg/L) | Conductivity (µS/cm) |
|---|---|---|---|---|---|---|---|---|
| 6.5 ± 0.5 | 4000 ± 70 | 360 ± 25 | 33 ± 2 | 18 ± 1.5 | 28 ± 2.5 | 350 ± 15 | 1760 ± 55 | 3600 ± 95 |
| Carbon Paper | Carbon Cloth | Carbon Felt | Graphite Rod | CNFs 5 min | CNFs 10 min | CNFs 20 min | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h | 1 day | 2 h | 1 day | 2 h | 1 day | 2 h | 1 day | 2 h | 1 day | 2 h | 1 day | 2 h | 1 day | |
| P.D (mW/m2) | 9 ± 0.3 | 13 ± 0.3 | 16 ± 0.3 | 43 ± 1.4 | 19 ± 0.7 | 23 ± 0.7 | 23 ± 0.4 | 15 ± 0.7 | 17 ± 0.1 | 27 ± 0.9 | 147 ± 4.5 | 185 ± 7.4 | 44 ± 1.5 | 83 ± 4.3 |
| C.D (mA/m2) | 77 ± 3 | 141 ± 7 | 149 ± 5 | 525 ± 10 | 431 ± 9 | 650 ± 11 | 303 ± 7 | 228 ± 6 | 591 ± 9 | 645 ± 10 | 2565 ± 24 | 2640 ± 26 | 1185 ± 9 | 1650 ± 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, N.A.M.; Ali, R.H.; Kim, H.Y.; Nassar, M.M.; Fadali, O.A.; Tolba, G.M.K.; Moustafa, H.M.; Ali, M.A. Carbon Nanofibers-Sheathed Graphite Rod Anode and Hydrophobic Cathode for Improved Performance Industrial Wastewater-Driven Microbial Fuel Cells. Nanomaterials 2022, 12, 3961. https://doi.org/10.3390/nano12223961
Barakat NAM, Ali RH, Kim HY, Nassar MM, Fadali OA, Tolba GMK, Moustafa HM, Ali MA. Carbon Nanofibers-Sheathed Graphite Rod Anode and Hydrophobic Cathode for Improved Performance Industrial Wastewater-Driven Microbial Fuel Cells. Nanomaterials. 2022; 12(22):3961. https://doi.org/10.3390/nano12223961
Chicago/Turabian StyleBarakat, Nasser A. M., Rasha H. Ali, Hak Yong Kim, Mamdouh M. Nassar, Olfat A. Fadali, Gehan M. K. Tolba, Hager M. Moustafa, and Marwa A. Ali. 2022. "Carbon Nanofibers-Sheathed Graphite Rod Anode and Hydrophobic Cathode for Improved Performance Industrial Wastewater-Driven Microbial Fuel Cells" Nanomaterials 12, no. 22: 3961. https://doi.org/10.3390/nano12223961
APA StyleBarakat, N. A. M., Ali, R. H., Kim, H. Y., Nassar, M. M., Fadali, O. A., Tolba, G. M. K., Moustafa, H. M., & Ali, M. A. (2022). Carbon Nanofibers-Sheathed Graphite Rod Anode and Hydrophobic Cathode for Improved Performance Industrial Wastewater-Driven Microbial Fuel Cells. Nanomaterials, 12(22), 3961. https://doi.org/10.3390/nano12223961








