LSPR Tunable Ag@PDMS SERS Substrate for High Sensitivity and Uniformity Detection of Dye Molecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
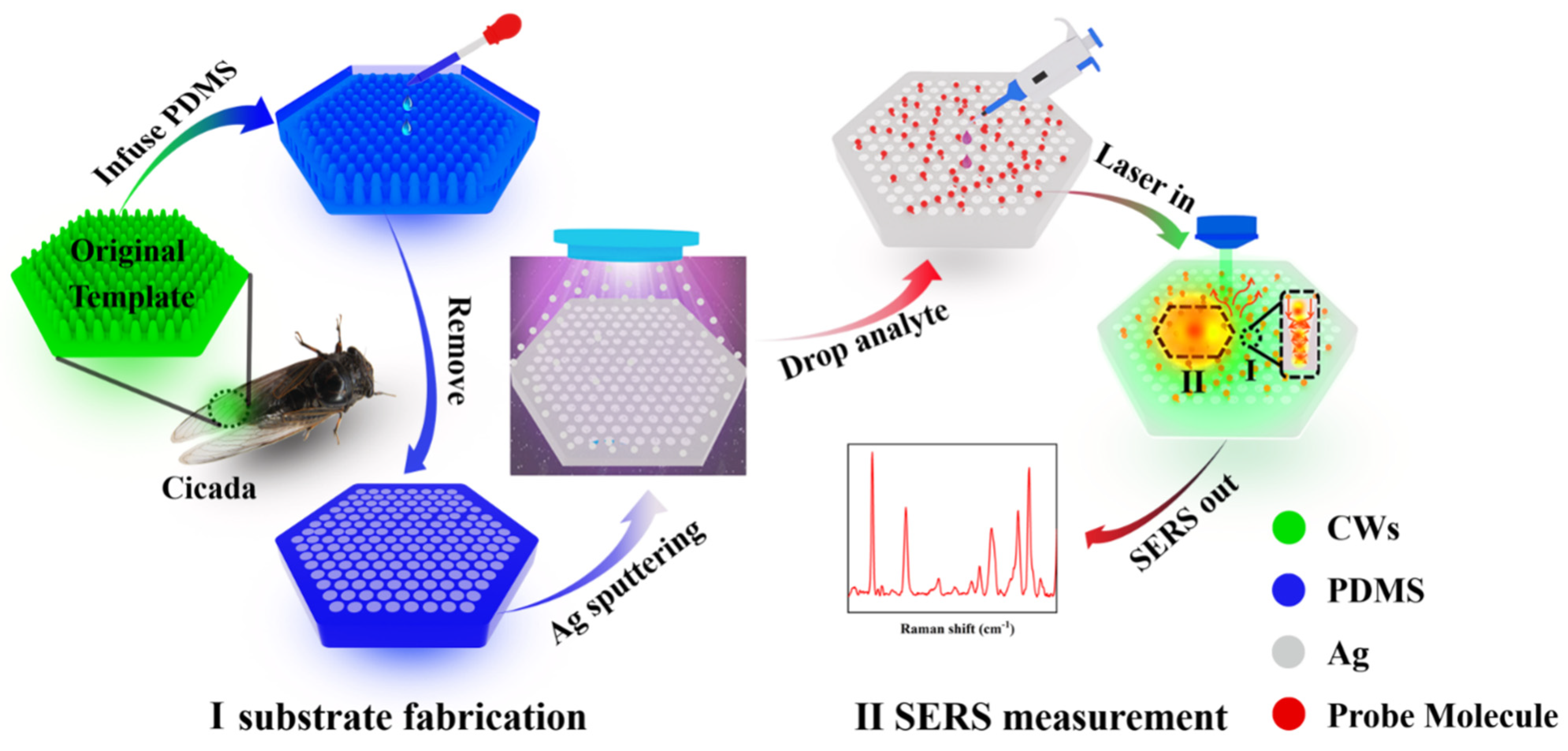
2.2. Fabrication of Ag@PDMS Substrate
2.3. SERS Measurements and Enhancement Factor Calculation
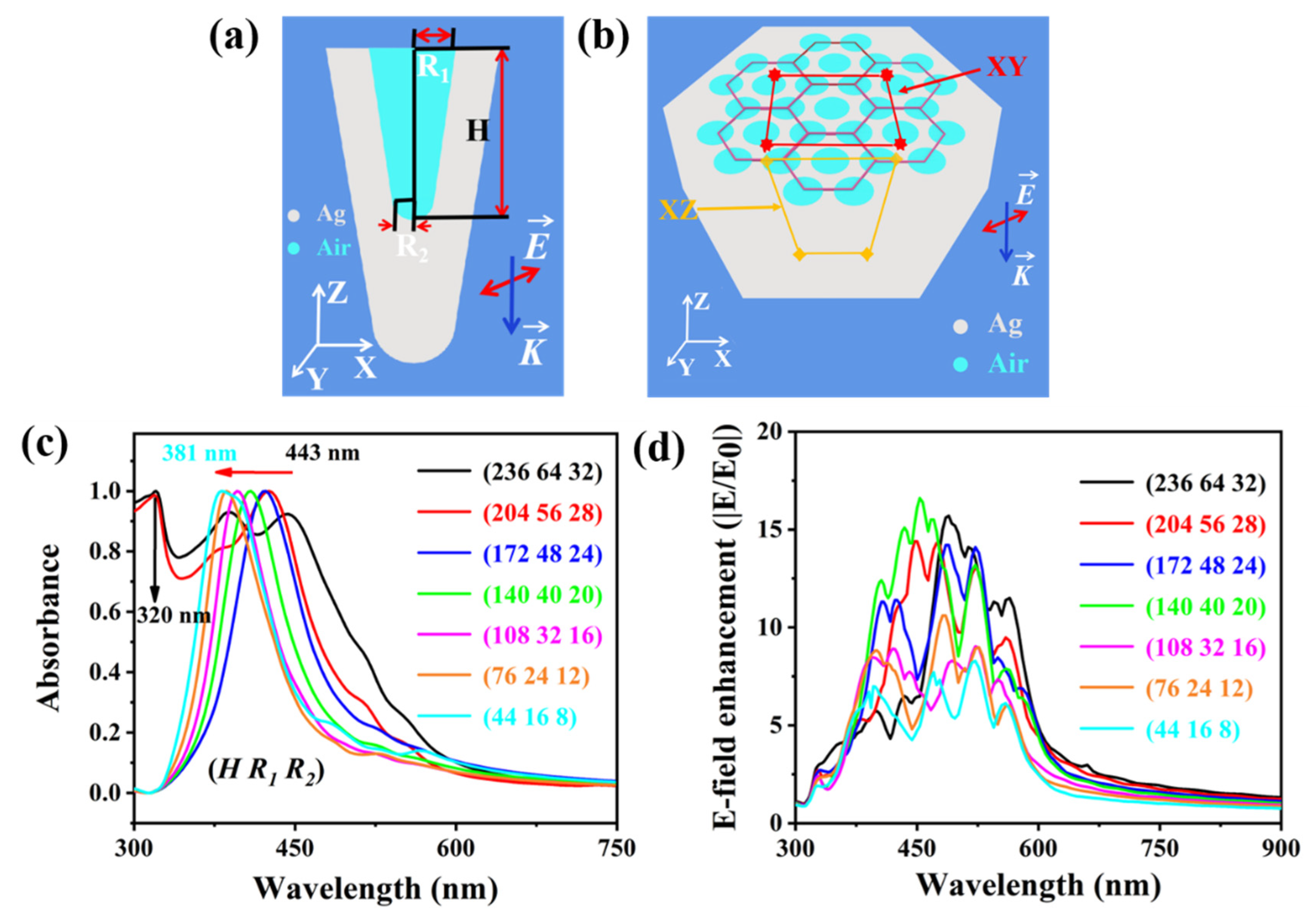
2.4. Theoretical Simulations of an Optical Cavity Unit Based on Ag@PDMS Substrate
3. Results and Discussion
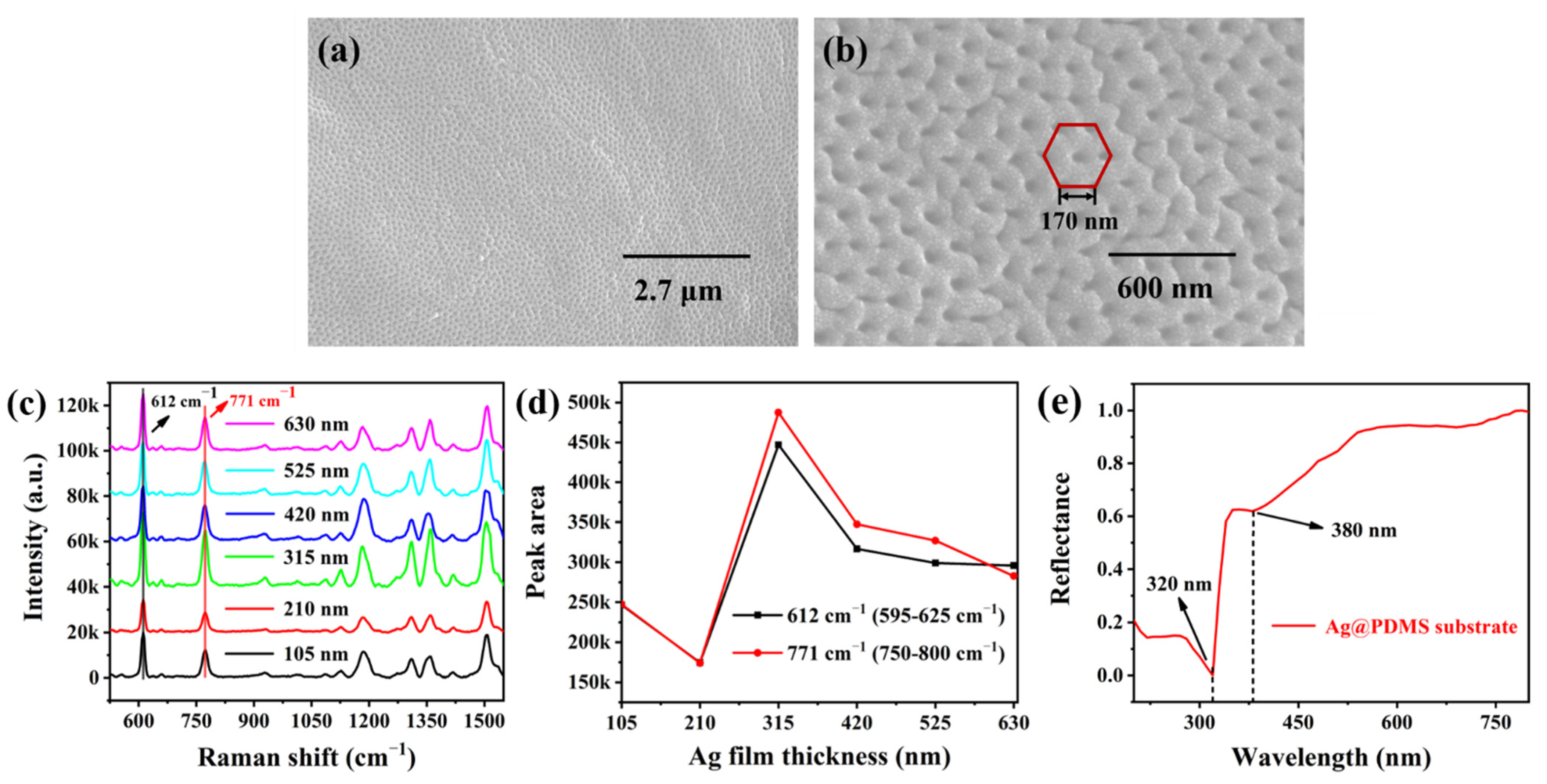
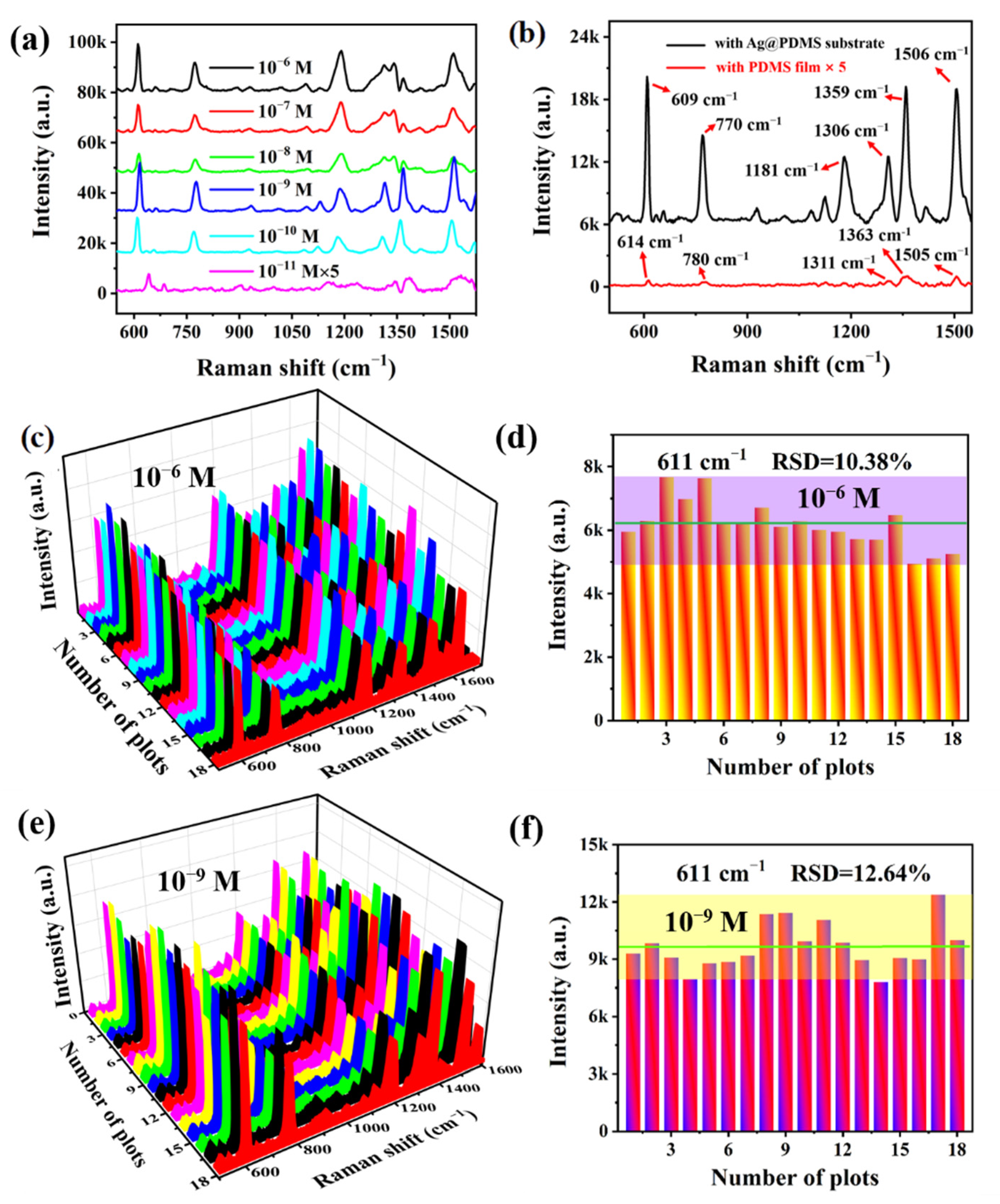
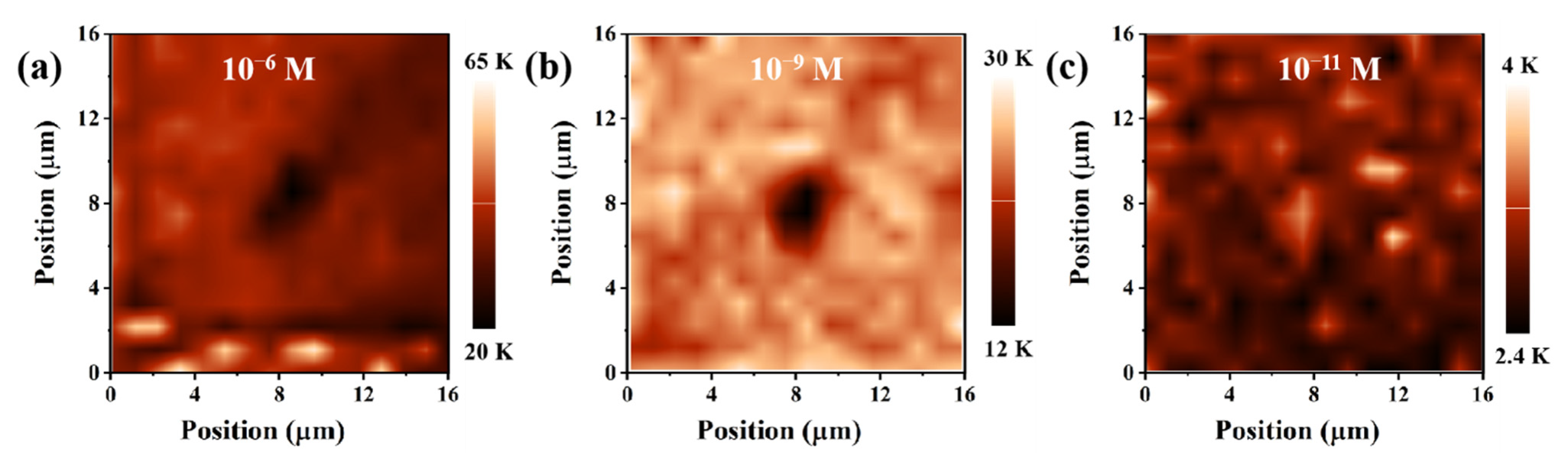
3.1. Characterization and Optimization of the Ag@PDMS Substrate
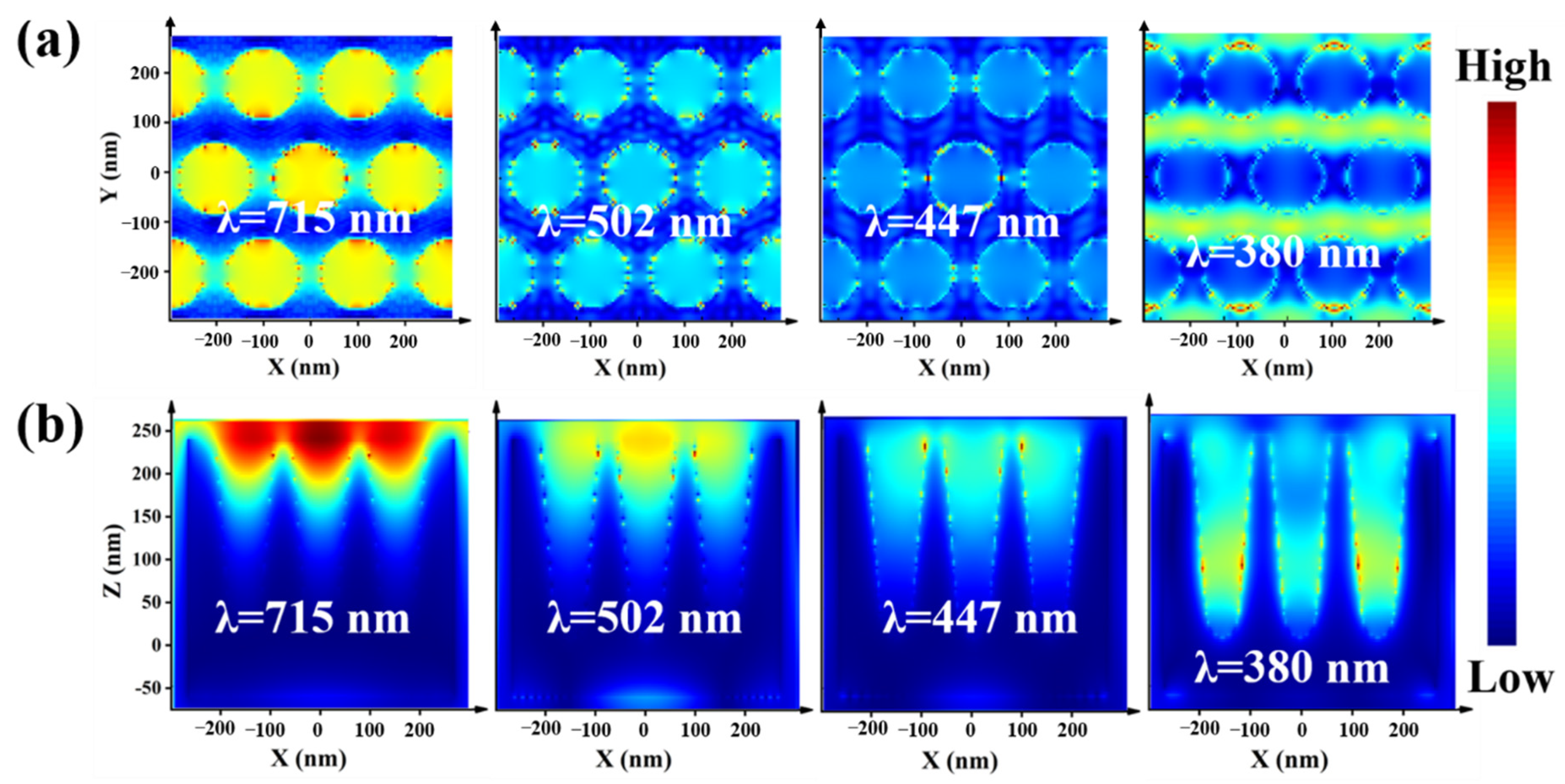
3.2. Theoretical Simulations of an Optical Cavity Unit Based on Ag@PDMS Substrate
3.3. SERS Performances of the Ag@PDMS Substrate
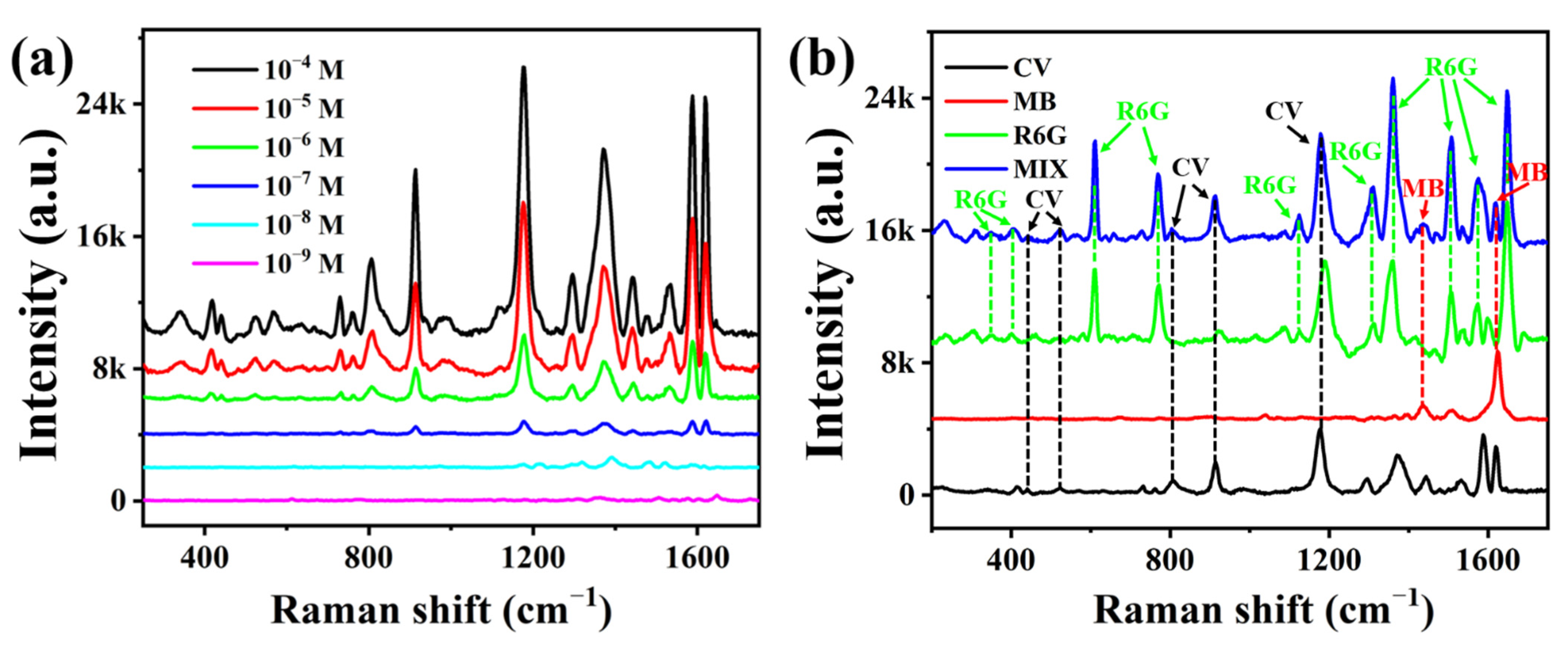
3.4. Practical Application of the Ag@PDMS SERS Substrate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Pu, H.; Wei, Q. Polymer multilayers enabled stable and flexible Au@Ag nanoparticle array for nondestructive SERS detection of pesticide residues. Talanta 2021, 223, 121782. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, D.; Lin, J.; Wu, X.; Xu, X.; Akakuru, O.U.; Zhang, H.; Zhang, Z.; Xie, Y.; Wu, A.; et al. Ultrahigh SERS activity of the TiO2@Ag nanostructure leveraged for accurately detecting CTCs in peripheral blood. Biomater. Sci. 2022, 10, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Iarossi, M.; de Fazio, A.F.; Huang, J.A.; de Angelis, F. Label-Free Optical Analysis of Biomolecules in Solid-State Nanopores: Toward Single-Molecule Protein Sequencing. ACS Photonics 2022, 9, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Ge, C.; Li, S.; Chen, L.; Wang, L.; Xu, Y. In Situ Detection of Endotoxin in Bacteriostatic Process by SERS Chip Integrated Array Microchambers within Bioscaffold Nanostructures and SERS Tags. ACS Appl. Mater. Interfaces 2020, 12, 28985–28992. [Google Scholar] [PubMed]
- Qi, X.; Ye, Y.; Wang, H.; Zhao, B.; Xu, L.; Zhang, Y.; Wang, X.; Zhou, N. An ultrasensitive and dual-recognition SERS biosensor based on Fe3O4@Au-Teicoplanin and aptamer functionalized Au@Ag nanoparticles for detection of Staphylococcus aureus. Talanta 2022, 250, 123648. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.-C.; Hu, S.; Yan, S.; Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2020, 2, 253–271. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguie, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Tan, X.; Lu, Z.; Han, H. Cauliflower-Inspired 3D SERS Substrate for Multiple Mycotoxins Detection. Anal. Chem. 2019, 91, 3885–3892. [Google Scholar] [CrossRef]
- Hussain, A.; Pu, H.; Hu, B.; Sun, D.W. Au@Ag-TGANPs based SERS for facile screening of thiabendazole and ferbam in liquid milk. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 245, 118908. [Google Scholar] [CrossRef]
- Yaseen, T.; Pu, H.; Sun, D.W. Fabrication of silver-coated gold nanoparticles to simultaneously detect multi-class insecticide residues in peach with SERS technique. Talanta 2019, 196, 537–545. [Google Scholar] [CrossRef]
- Nair, S.; Gomez-Cruz, J.; Ascanio, G.; Docoslis, A.; Sabat, R.G.; Escobedo, C. Cicada Wing Inspired Template-Stripped SERS Active 3D Metallic Nanostructures for the Detection of Toxic Substances. Sensors 2021, 21, 1699. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.M.; White, I.M. A critical review of flexible and porous SERS sensors for analytical chemistry at the point-of-sample. Anal. Chim. Acta 2019, 1060, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Aybeke, E.N.; Lacroute, Y.; Elie-Caille, C.; Bouhelier, A.; Bourillot, E.; Lesniewska, E. Homogeneous large-scale crystalline nanoparticle-covered substrate with high SERS performance. Nanotechnology 2015, 26, 245302. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, R.E.; Aybeke, E.N.; Bourillot, E.; Lacroute, Y.; Lesniewska, E.; Adam, P.M.; Bijeon, J.L. Fabrication of Annealed Gold Nanostructures on Pre-Treated Glow-Discharge Cleaned Glasses and Their Used for Localized Surface Plasmon Resonance (LSPR) and Surface Enhanced Raman Spectroscopy (SERS) Detection of Adsorbed (Bio)molecules. Sensors 2017, 17, 236. [Google Scholar] [CrossRef]
- Ma, Y.; Du, Y.; Chen, Y.; Gu, C.; Jiang, T.; Wei, G.; Zhou, J. Intrinsic Raman signal of polymer matrix induced quantitative multiphase SERS analysis based on stretched PDMS film with anchored Ag nanoparticles/Au nanowires. Chem. Eng. J. 2020, 381, 122710. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Pu, H.; Wei, Q.; Huang, L. Stable, Flexible, and High-Performance SERS Chip Enabled by a Ternary Film-Packaged Plasmonic Nanoparticle Array. ACS Appl. Mater. Interfaces 2019, 11, 29177–29186. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, J.; Zhang, Y.; Zhao, Z.; Gu, C.; Chen, D.; Zhou, J.; Jiang, T. Surfactant–free synthesis of flower–like Au NPs/Au island hybrid substrate for quantitative SERS detection of pesticide residues on fruit. J. Alloys Compd. 2022, 918, 165706. [Google Scholar] [CrossRef]
- Jia, P.; Shi, H.; Niu, Y.; Xu, C.; Sun, X. Enhanced Optical Forces and Tunable LSPR of Ag Triangular Nanoplates for Plasmonic Tweezers. Plasmonics 2021, 17, 551–558. [Google Scholar] [CrossRef]
- Sergides, M.; Truong, V.G.; Chormaic, S.N. Highly tunable plasmonic nanoring arrays for nanoparticle manipulation and detection. Nanotechnology 2016, 27, 365301. [Google Scholar] [CrossRef]
- Kozhina, E.P.; Andreev, S.N.; Tarakanov, V.P.; Bedin, S.A.; Doludenko, I.M.; Naumov, A.V. Study of Local Fields of Dendrite Nanostructures in Hot Spots Formed on SERS-Active Substrates Produced via Template-Assisted Synthesis. Bull. Russ. Acad. Sci. Phys. 2021, 84, 1465–1468. [Google Scholar] [CrossRef]
- Kozhina, E.P.; Bedin, S.A.; Nechaeva, N.L.; Podoynitsyn, S.N.; Tarakanov, V.P.; Andreev, S.N.; Grigoriev, Y.V.; Naumov, A.V. Ag-Nanowire Bundles with Gap Hot Spots Synthesized in Track-Etched Membranes as Effective SERS-Substrates. Appl. Sci. 2021, 11, 1375. [Google Scholar] [CrossRef]
- Xiang, X.W.S.; Pang, Y.; Ge, C.; Xu, Y.; Chen, L.; Li, S.; Wang, L. Porous Au/AAO: A Simple and Feasible SERS Substrate for Dynamic Monitoring and Mechanism Analysis of DNA Oxidation. Appl. Surf. Sci. 2022, 606, 154842. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, Q.; Feng, L.; Li, X.; Zhu, H.; Miao, H.; Zeng, Z.; Wang, Y.; Li, Y.; Wang, L.; et al. Light-Trapping SERS Substrate with Regular Bioinspired Arrays for Detecting Trace Dyes. ACS Appl. Mater. Interfaces 2021, 13, 11535–11542. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, M.; Sun, X.; Wang, Y.; Shi, G.; Ma, W.; Hou, P. Sandwich-like Ag@Cu@CW SERS substrate with tunable nanogaps and component based on the Plasmonic nanonodule structures for sensitive detection crystal violet and 4-aminothiophenol. Appl. Surf. Sci. 2019, 479, 879–886. [Google Scholar] [CrossRef]
- Takei, H.; Nagata, K.; Frese, N.; Golzhauser, A.; Okamoto, T. Surface-Enhanced Raman Spectroscopy for Molecule Characterization: HIM Investigation into Sources of SERS Activity of Silver-Coated Butterfly Scales. Nanomaterials 2021, 11, 1741. [Google Scholar] [CrossRef]
- Kumar, P.; Khosla, R.; Soni, M.; Deva, D.; Sharma, S.K. A highly sensitive, flexible SERS sensor for malachite green detection based on Ag decorated microstructured PDMS substrate fabricated from Taro leaf as template. Sens. Actuators B Chem. 2017, 246, 477–486. [Google Scholar] [CrossRef]
- Wang, M.; Yan, X.; Shi, G.; Shang, Z.; Zhang, A.; Ma, W. Optical properties of Ag@cicada wing substrate deposited by Ag nanoparticles. Curr. Appl. Phys. 2020, 20, 1253–1262. [Google Scholar] [CrossRef]
- Yan, Z.; Xia, M.; Zhang, P.; Xie, Y.-H. Self-Aligned Trapping and Detecting Molecules Using a Plasmonic Tweezer with an Integrated Electrostatic Cell. Adv. Opt. Mater. 2017, 5, 1600329. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Niu, S.; Zhang, Y.; Han, Z.; Ren, L. Integrated super-hydrophobic and antireflective PDMS bio-templated from nano-conical structures of cicada wings. RSC Adv. 2016, 6, 108974–108980. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Feng, X.; Jiao, Z.; Zhang, J.; Niu, S.; Han, Z.; Ren, L. Rapid Fabrication of Bio-inspired Antireflection Film Replicating From Cicada Wings. J. Bionic Eng. 2020, 17, 34–44. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Jiang, Z.; Shi, Z.; Wang, J.; Du, L. Highly Sensitive and Reproducible SERS Substrates Based on Ordered Micropyramid Array and Silver Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 29222–29229. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, P.; Wang, W.; Zhang, X.; Kuo, H.C.; Xu, H.; Wang, Z.M. High-Q Plasmonic Resonances: Fundamentals and Applications. Adv. Opt. Mater. 2021, 9, 2001520. [Google Scholar] [CrossRef]
- Ghorbanzadeh, M.; Jones, S.; Moravvej-Farshi, M.K.; Gordon, R. Improvement of Sensing and Trapping Efficiency of Double Nanohole Apertures via Enhancing the Wedge Plasmon Polariton Modes with Tapered Cusps. ACS Photonics 2017, 4, 1108–1113. [Google Scholar] [CrossRef]
- Yang, K.-H.; Liu, Y.-C.; Yu, C.-C. Enhancements in intensity and stability of surface-enhanced Raman scattering on optimally electrochemically roughened silver substrates. J. Mater. Chem. 2008, 18, 4849–4855. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ma, S.; Yang, Q.Q.; Li, X.J. Size-dependent SERS detection of R6G by silver nanoparticles immersion-plated on silicon nanoporous pillar array. Appl. Surf. Sci. 2012, 258, 5881–5885. [Google Scholar] [CrossRef]
- Feng, L.; Ma, R.; Wang, Y.; Xu, D.; Xiao, D.; Liu, L.; Lu, N. Silver-coated elevated bowtie nanoantenna arrays: Improving the near-field enhancement of gap cavities for highly active surface-enhanced Raman scattering. Nano Res. 2015, 8, 3715–3724. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Yu, J.; Jiang, S.; Xu, S.; Yang, C.; Liu, Y.J.; Gao, X.; Liu, A.; Man, B. SERS activated platform with three-dimensional hot spots and tunable nanometer gap. Sens. Actuators B Chem. 2018, 258, 163–171. [Google Scholar] [CrossRef]
- Li, J.; Deng, T.S.; Liu, X.; Dolan, J.A.; Scherer, N.F.; Nealey, P.F. Hierarchical Assembly of Plasmonic Nanoparticle Heterodimer Arrays with Tunable Sub-5 nm Nanogaps. Nano Lett. 2019, 19, 4314–4320. [Google Scholar] [CrossRef]
- Zeng, Y.; Gao, R.; Wang, J.; Shih, T.-M.; Sun, G.; Lin, J.; He, Y.; Chen, J.; Zhan, D.; Zhu, J.; et al. Light-Trapped Nanocavities for Ultraviolet Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2021, 125, 17241–17247. [Google Scholar] [CrossRef]
- Petrus, O.; Macko, J.; Orinakova, R.; Orinak, A.; Mudra, E.; Kupkova, M.; Farka, Z.; Pastucha, M.; Socha, V. Detection of organic dyes by surface-enhanced Raman spectroscopy using plasmonic NiAg nanocavity films. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 249, 119322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, Y.; Yang, J.; Li, J.; Feng, Y.; Quan, M.; Yang, Z.; Xiao, S. Synergistic plasmon resonance coupling and light capture in ordered nanoarrays as ultrasensitive and reproducible SERS substrates. Nanoscale 2020, 12, 18056–18066. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Sun, D.W.; Pu, H.; Wei, Q. Fabrication of gold nanorods for SERS detection of thiabendazole in apple. Talanta 2019, 195, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.M.; Clark, S.B.; Johnson, A.S.; Andersen, W.C.; Turnipseed, S.B.; Lohne, J.J.; Burger, R.J.; Ayres, P.R.; Carr, J.R.; Madson, M.R. Analysis of sulfonamides, trimethoprim, fluoroquinolones, quinolones, triphenylmethane dyes and methyltestosterone in fish and shrimp using liquid chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 972, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Bharagava, R.N. Exposure to Crystal Violet, Its Toxic, Genotoxic and Carcinogenic Effects on Environment and Its Degradation and Detoxification for Environmental Safety. Rev. Environ. Contam. Toxicol. 2016, 237, 71–104. [Google Scholar]
- Xi, W.; Shrestha, B.K.; Haes, A.J. Promoting Intra- and Intermolecular Interactions in Surface-Enhanced Raman Scattering. Anal. Chem. 2018, 90, 128–143. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Shi, H.; Jia, P.; Sun, X. LSPR Tunable Ag@PDMS SERS Substrate for High Sensitivity and Uniformity Detection of Dye Molecules. Nanomaterials 2022, 12, 3894. https://doi.org/10.3390/nano12213894
Yan X, Shi H, Jia P, Sun X. LSPR Tunable Ag@PDMS SERS Substrate for High Sensitivity and Uniformity Detection of Dye Molecules. Nanomaterials. 2022; 12(21):3894. https://doi.org/10.3390/nano12213894
Chicago/Turabian StyleYan, Xiaoya, Hongyan Shi, Pengxue Jia, and Xiudong Sun. 2022. "LSPR Tunable Ag@PDMS SERS Substrate for High Sensitivity and Uniformity Detection of Dye Molecules" Nanomaterials 12, no. 21: 3894. https://doi.org/10.3390/nano12213894
APA StyleYan, X., Shi, H., Jia, P., & Sun, X. (2022). LSPR Tunable Ag@PDMS SERS Substrate for High Sensitivity and Uniformity Detection of Dye Molecules. Nanomaterials, 12(21), 3894. https://doi.org/10.3390/nano12213894




