Encapsulation of Imazalil in HKUST-1 with Versatile Antimicrobial Activity
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials
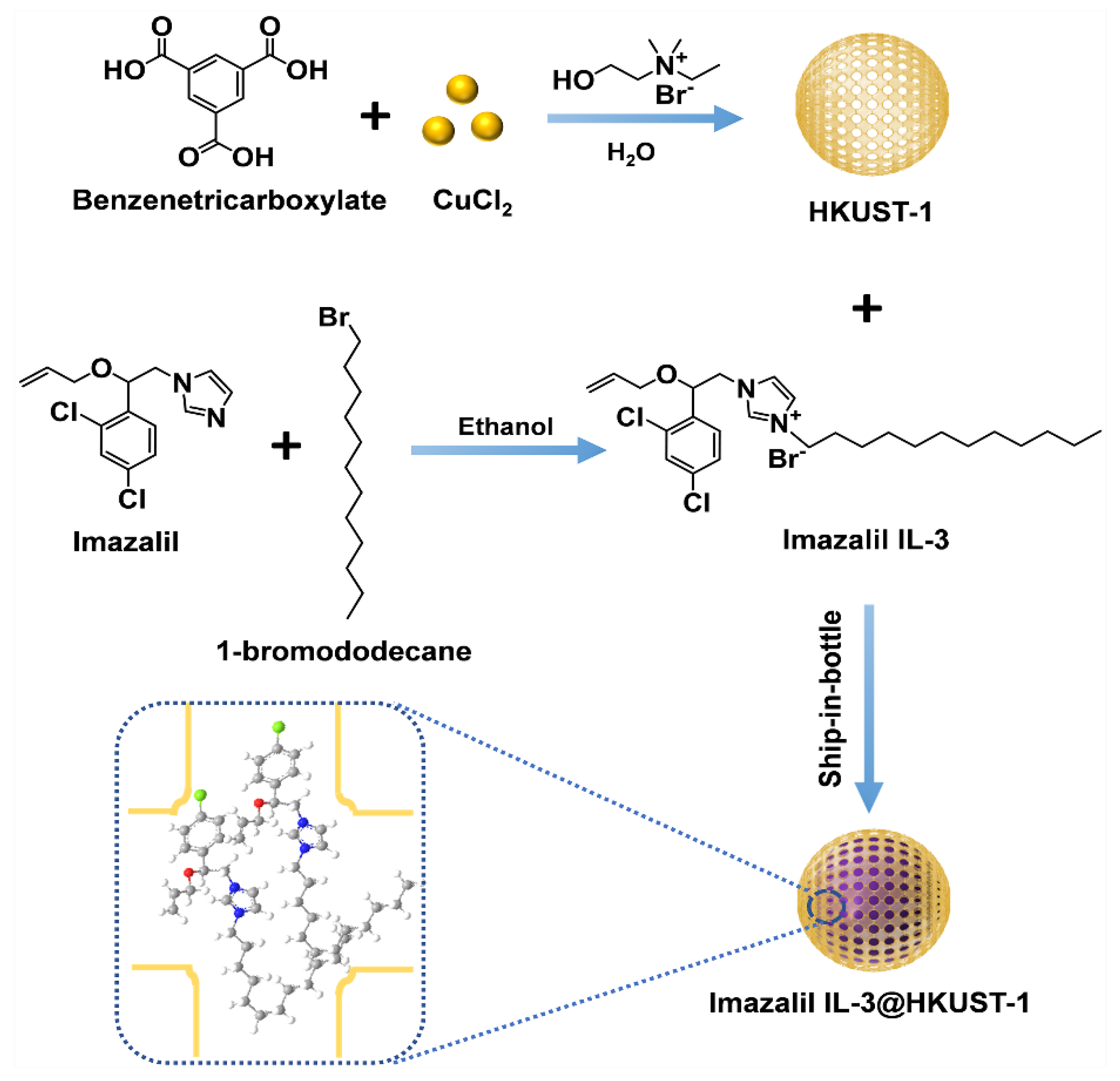
2.2. Synthesis Imazalil IL-3@HKUST-1
2.2.1. Synthesis of N-ethyl-2-hydroxy-N,N-dimethylethanaminium Bromide
2.2.2. Synthesis of Imazalil Ionic Liquid-3 (Imazalil IL-3)
2.2.3. Synthesis of HKUST-1
2.2.4. Synthesis of Imazalil IL-3@HKUST-1
2.3. Characterization
2.4. Antibacterial Activity
2.5. Antifungal Activity
2.6. Safety to Plants
2.7. Drug Release from Imazalil IL-3@HKUST-1
2.8. Statistical Analysis
3. Results and Discussion
3.1. Fabrication and Characterization of Imazalil IL-3@HKUST-1
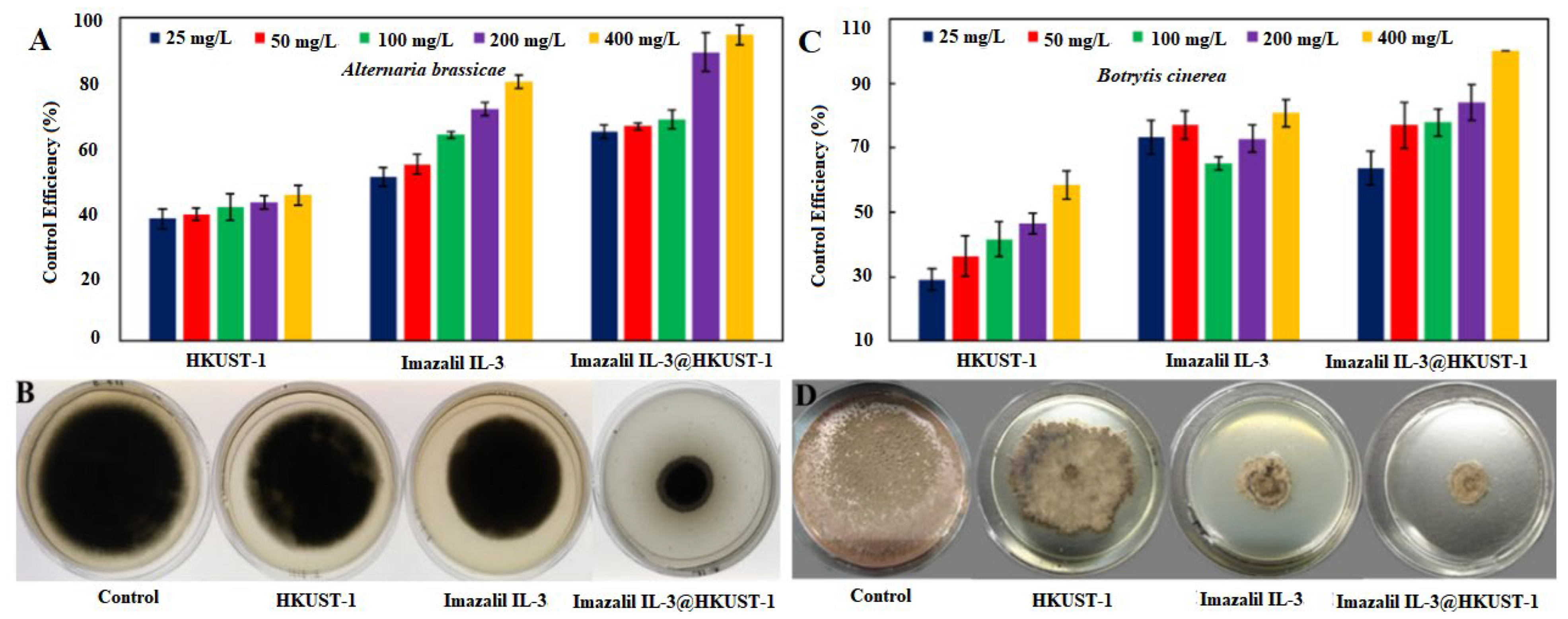
3.2. In Vitro Bactericidal Activity Test
3.3. In Vitro Fungicidal Activity Test
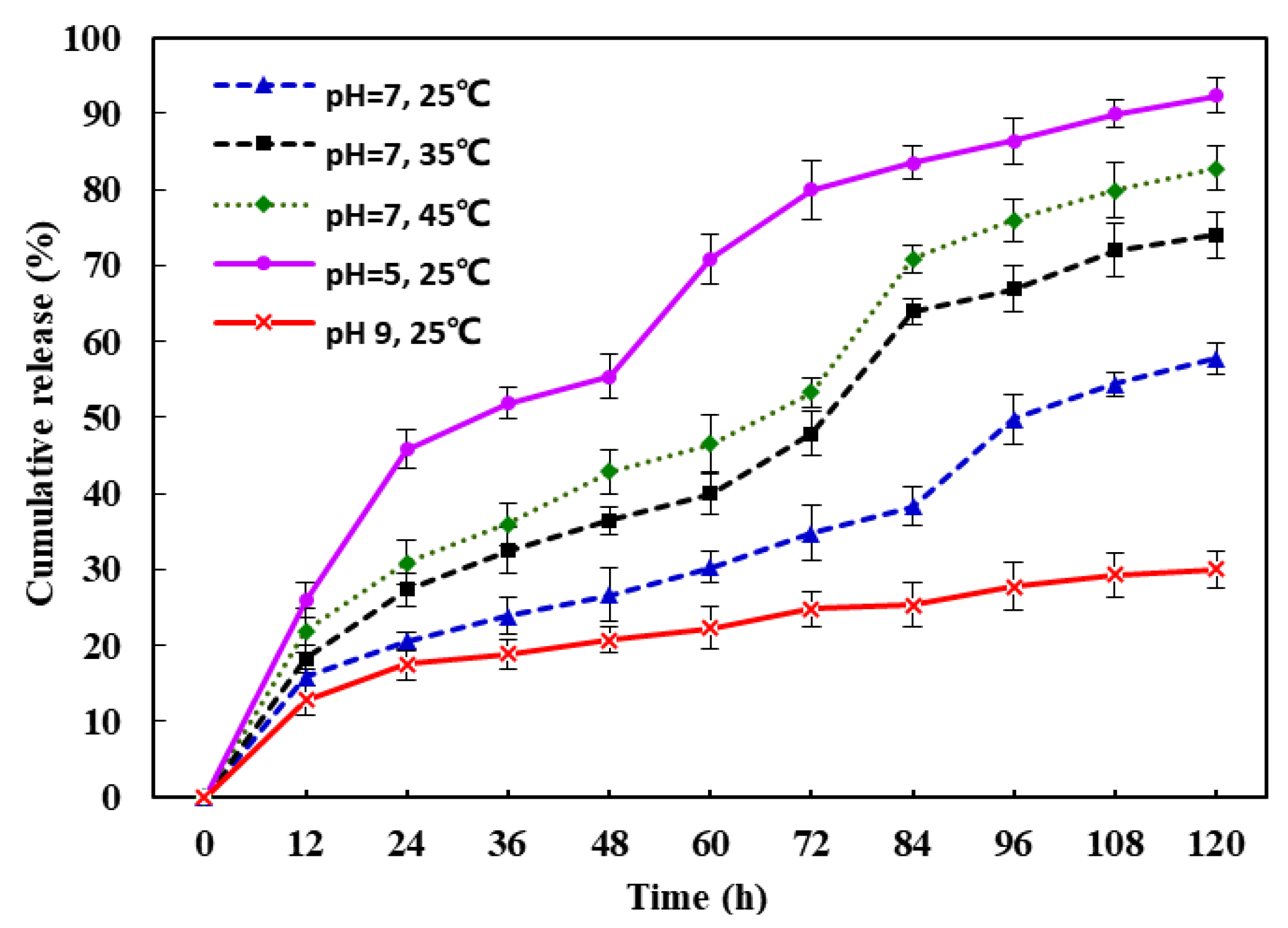
3.4. In Vitro Drug Release Testing
3.5. Mathematical Model
3.6. Safety Evaluation of Imazalil IL-3@HKUST-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aiking, H.; de Boer, J. The next protein transition. Trends Food Sci. Technol. 2020, 105, 515–522. [Google Scholar] [CrossRef]
- Liang, Y.; Song, J.; Dong, H.; Huo, Z.; Gao, Y.; Zhou, Z.; Tian, Y.; Li, Y.; Cao, Y. Fabrication of pH-responsive nanoparticles for high efficiency pyraclostrobin delivery and reducing environmental impact. Sci. Total Environ. 2021, 787, 147422. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Brooks, B.W.; Zeng, E.Y.; You, J. Comparative mammalian hazards of neonicotinoid insecticides among exposure durations. Environ. Int. 2019, 125, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Fan, C.; Dong, H.; Zhang, W.; Tang, G.; Yang, J.; Jiang, N.; Cao, Y. Preparation of MSNs-chitosan@prochloraz nanoparticles for reducing toxicity and improving release properties of prochloraz. ACS Sustain. Chem. Eng. 2018, 6, 10211–10220. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Krisnawati, D.I.; Kristanto, H.; Jazidie, A.; Nuh, M.; Cheng, T.-M.; Kuo, T.-R. Nanomaterials for the photothermal killing of bacteria. Nanomaterials 2020, 10, 1123. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Y.; Zhou, Z.; Yang, J.; Tian, Y.; Niu, J.; Tang, G.; Tang, J.; Chen, X.; Li, Y.; et al. Metal-organic framework nanohybrid carrier for precise pesticide delivery and pest management. Chem. Eng. J. 2021, 422, 130143. [Google Scholar] [CrossRef]
- Zhuang, J.; Young, A.P.; Tsung, C.-K. Integration of biomolecules with metal–organic frameworks. Small 2017, 13, 1700880. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Ferey, G.; Morris, R.E.; Serre, C. Metal–organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Huang, G.; Chen, J.; Tang, X.; Xiong, D.; Liu, Z.; Wu, J.; Sun, W.-Y.; Lin, B. Facile method to prepare a novel biological HKUST-1@CMCS with macroscopic shape control for the long-acting and sustained release. ACS Appl. Mater. Interfaces 2019, 11, 10389–10398. [Google Scholar] [CrossRef]
- Moussa, Z.; Hmadeh, M.; Abiad, M.G.; Dib, O.H.; Patra, D. Encapsulation of curcumin in cyclodextrin-metal organic frameworks: Dissociation of loaded CD-MOFs enhances stability of curcumin. Food Chem. 2016, 212, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Oktawiec, J.; Taylor, M.K.; Hudson, M.R.; Rodriguez, J.; Bachman, J.E.; Gonzalez, M.I.; Cervellino, A.; Guagliardi, A.; Brown, C.M. Methane storage in flexible metal–organic frameworks with intrinsic thermal management. Nature 2015, 527, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Upreti, S.; Singh, J.; Ghosh, M.P.; Basu, T. Rapid electrochemical quantification for in vitro release trait of ophthalmic drug loaded within mucoadhesive metal organic framework (MOF). ChemistrySelect 2021, 6, 3006–3012. [Google Scholar]
- Zhang, X.; Tang, X.; Zhao, C.; Yuan, Z.; Zhang, D.; Zhao, H.; Yang, N.; Guo, K.; He, Y.; He, Y.; et al. A pH-responsive MOF for site-specific delivery of fungicide to control citrus disease of Botrytis cinerea. Chem. Eng. J. 2022, 431, 133351. [Google Scholar] [CrossRef]
- Yang, S.; Tang, R.; Dai, Y.; Wang, T.; Zeng, Z.; Zhang, L. Fabrication of cellulose acetate membrane with advanced ultrafiltration performances and antibacterial properties by blending with HKUST-1@LCNFs. Sep. Purif. Technol. 2021, 279, 119524. [Google Scholar] [CrossRef]
- Gautam, S.; Singhal, J.; Lee, H.K.; Chae, K.H. Drug delivery of paracetamol by metal-organic frameworks (HKUST-1): Improvised synthesis and investigations. Mater. Today Chem. 2022, 23, 100647. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, T.; Zhao, S.; Rong, H.; Tian, Y.; Zhu, G. Uniform and stable immobilization of metal-organic frameworks into chitosan matrix for enhanced tetracycline removal from water. Chem. Eng. J. 2020, 382, 122893. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Qiu, L.-G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.-Y.; Jiang, X. Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure: An efficient and environmentally friendly method. Mater. Lett. 2009, 63, 78–80. [Google Scholar] [CrossRef]
- Marqus, S.; Ahmed, H.; Rezk, A.R.; Huynh, T.; Lawrie, A.; Nguyen, D.; Ehrnst, Y.; Dekiwadia, C.; Yeo, L.Y. Enhanced antimicrobial activity and low phytotoxicity of acoustically synthesized large aspect ratio Cu-BTC metal–organic frameworks with exposed metal sites. ACS Appl. Mater. Interfaces 2021, 13, 58309–58318. [Google Scholar] [CrossRef]
- Celis-Arias, V.; Loera-Serna, S.; Beltrán, H.I.; Álvarez-Zeferino, J.C.; Garrido, E.; Ruiz-Ramos, R. The fungicide effect of HKUST-1 on Aspergillus niger, Fusarium solani and Penicillium chrysogenum. New J. Chem. 2018, 42, 5570–5579. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Q.-W.; Zhuang, C.; Tang, P.-P.; Lin, N.; Wei, L.-Q. Controlled release of drug molecules in metal–organic framework material HKUST-1. Inorg. Chem. Commun. 2017, 79, 78–81. [Google Scholar]
- Li, R.; Pan, X.; Tao, Y.; Jiang, D.; Chen, Z.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Zheng, Y. Systematic evaluation of chiral fungicide imazalil and its major metabolite R14821 (Imazalil-M): Stability of enantiomers, enantioselective bioactivity, aquatic toxicity, and dissipation in greenhouse vegetables and soil. J. Agric. Food Chem. 2019, 67, 11331–11339. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, M.; Senaeve, D.; Fevery, D.; Spanoghe, P. Influence of adjuvants on the dissipation of fenpropimorph, pyrimethanil, chlorpyrifos and lindane on the solid/gas interface. Chemosphere 2015, 138, 357–363. [Google Scholar] [CrossRef]
- Tang, R.; Tang, T.; Tang, G.; Liang, Y.; Wang, W.; Yang, J.; Niu, J.; Tang, J.; Zhou, Z.; Cao, Y. Pyrimethanil ionic liquids paired with various natural organic acid anions for reducing its adverse impacts on the environment. J. Agric. Food Chem. 2019, 67, 11018–11024. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Hao, L.; Ru, J.; Zhao, Y.; Wang, H.; Bai, G.; Wang, J. The self-assembly of ionic liquids surfactants in ethanolammonium nitrate ionic liquid. J. Mol. Liq. 2018, 254, 130–136. [Google Scholar] [CrossRef]
- Gao, M.R.; Yuan, J.; Antonietti, M. Ionic Liquids and Poly (ionic liquid) s for Morphosynthesis of Inorganic Materials. Chem.-Eur. J. 2017, 23, 5391–5403. [Google Scholar] [CrossRef] [PubMed]
- Wilms, W.; Woźniak-Karczewska, M.; Syguda, A.; Niemczak, M.; Ławniczak, Ł.; Pernak, J.; Rogers, R.D.; Chrzanowski, Ł. Herbicidal ionic liquids: A promising future for old herbicides? Review on synthesis, toxicity, biodegradation, and efficacy studies. J. Agric. Food Chem. 2020, 68, 10456–10488. [Google Scholar] [CrossRef]
- Kaczmarek, D.K.; Gwiazdowska, D.; Marchwińska, K.; Klejdysz, T.; Wojcieszak, M.; Materna, K.; Pernak, J. Amino acid-based dicationic ionic liquids as complex crop protection agents. J. Mol. Liq. 2022, 360, 119357. [Google Scholar] [CrossRef]
- Ten, A.; Zazybin, A.; Zolotareva, D.; Dauletbakov, A.; Rafikova, K.; Yu, V.; Giner, B. Ionic liquids in agrochemistry. Curr. Org. Chem. 2020, 24, 1181–1195. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Jia, H.; Yao, Y.; Song, J.; Dong, H.; Cao, Y.; Zhu, F.; Huo, Z. Pectin functionalized metal-organic frameworks as dual-stimuli-responsive carriers to improve the pesticide targeting and reduce environmental risks. Colloids Surf. B 2022, 219, 112796. [Google Scholar] [CrossRef]
- Karimi Alavijeh, R.; Akhbari, K. Biocompatible MIL-101 (Fe) as a smart carrier with high loading potential and sustained release of curcumin. Inorg. Chem. 2020, 59, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
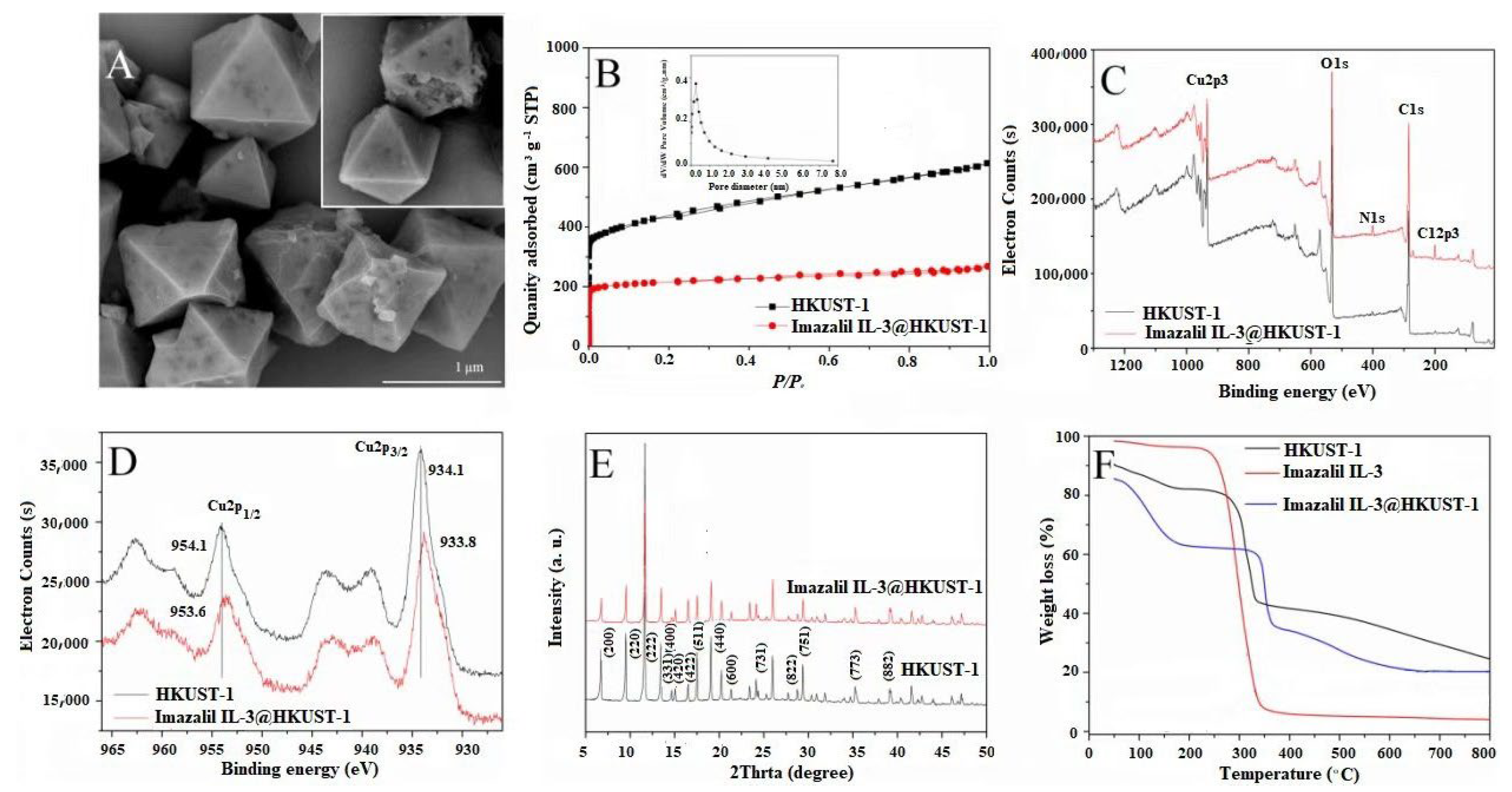
| Composition | HKUST-1 | Imazalil IL-3@HKUST-1 |
|---|---|---|
| C Wt.% | 58.97 | 61.27 |
| O Wt.% | 32.93 | 25.68 |
| N Wt.% | - | 3.38 |
| Cu Wt.% | 7.24 | 5.49 |
| Cl Wt.% | 0.86 | 4.17 |
| Compounds | Ratio | Pseudomonas syringae | Xanthomonas campestris | ||
|---|---|---|---|---|---|
| EC50 (mg L−1) | Co-Toxicity Coefficient | EC50 (mg L−1) | Co-Toxicity Coefficient | ||
| Imazalil IL-3 | 1:0 | 526.0 | \ | 482.3 | \ |
| HKUST-1 | 1:0 | 248.8 | \ | 229.2 | \ |
| Imazalil IL-3@HKUST-1 | 4.07:95.93 | 405.5 | 102.75 | 222.5 | 105.26 |
| Compounds | Ratio | Alternaria brassicae | Botrytis cinerea | ||
|---|---|---|---|---|---|
| EC50 (mg L−1) | Co-Toxicity Coefficient | EC50 (mg L−1) | Co-Toxicity Coefficient | ||
| Imazalil IL-3 | 1:0 | 5.94 | / | 4.036 | / |
| HKUST-1 | 1:0 | 589.35 | / | 516.2 | / |
| Imazalil IL-3@HKUST-1 | 4.07:95.93 | 67.43 | 174.89 | 51.54 | 162.46 |
| Sample | Conditions | k | n | r | T50 (h) |
|---|---|---|---|---|---|
| Imazalil IL-3@HKUST-1 | pH = 5, 25 ℃ | 7.338 | 0.54 | 0.9955 | 34.42 |
| pH = 9, 25 ℃ | 5.262 | 0.36 | 0.9728 | 510.25 | |
| pH = 7, 25 ℃ | 3.345 | 0.57 | 0.9656 | 116.02 | |
| pH = 7, 35 ℃ | 3.605 | 0.63 | 0.9814 | 67.18 | |
| pH = 7, 45 ℃ | 4.492 | 0.60 | 0.9818 | 54.82 |
| Treatment | Dosage (mg/L) | Fresh Weight (g) | Leaf SPAD Value |
|---|---|---|---|
| Imazalil IL-3@HKUST-1 | 300 | 1.34 ± 0.06 a * | 29.65 ± 1.33 a |
| 600 | 1.33 ± 0.04 a | 31.65 ± 1.53 a | |
| 900 | 1.36 ± 0.04 a | 30.78 ± 1.47 a | |
| HKUST-1 | 300 | 1.34 ± 0.02 a | 29.25 ± 1.06 a |
| 600 | 1.34 ± 0.05 a | 29.52 ± 1.23 a | |
| 900 | 1.35 ± 0.06 a | 30.16 ± 1.42 a | |
| Imazalil IL | 300 | 1.29 ± 0.06 a | 29.23 ± 1.65 a |
| 600 | 1.29 ± 0.03 a | 31.26 ± 1.32 a | |
| 900 | 1.30 ± 0.06 a | 30.66 ± 1.18 a | |
| H2O | 1.25 ± 0.09 a | 29.06 ± 1.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; He, Y.; Fan, C.; Zhu, Z.; Zhang, C.; Liu, X.; Qian, K.; Tang, T. Encapsulation of Imazalil in HKUST-1 with Versatile Antimicrobial Activity. Nanomaterials 2022, 12, 3879. https://doi.org/10.3390/nano12213879
Dong H, He Y, Fan C, Zhu Z, Zhang C, Liu X, Qian K, Tang T. Encapsulation of Imazalil in HKUST-1 with Versatile Antimicrobial Activity. Nanomaterials. 2022; 12(21):3879. https://doi.org/10.3390/nano12213879
Chicago/Turabian StyleDong, Hongqiang, Yuke He, Chen Fan, Zhongqiang Zhu, Chunrong Zhang, Xinju Liu, Kun Qian, and Tao Tang. 2022. "Encapsulation of Imazalil in HKUST-1 with Versatile Antimicrobial Activity" Nanomaterials 12, no. 21: 3879. https://doi.org/10.3390/nano12213879
APA StyleDong, H., He, Y., Fan, C., Zhu, Z., Zhang, C., Liu, X., Qian, K., & Tang, T. (2022). Encapsulation of Imazalil in HKUST-1 with Versatile Antimicrobial Activity. Nanomaterials, 12(21), 3879. https://doi.org/10.3390/nano12213879






