Encapsulation of Luminescent Gold Nanoclusters into Synthetic Vesicles
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
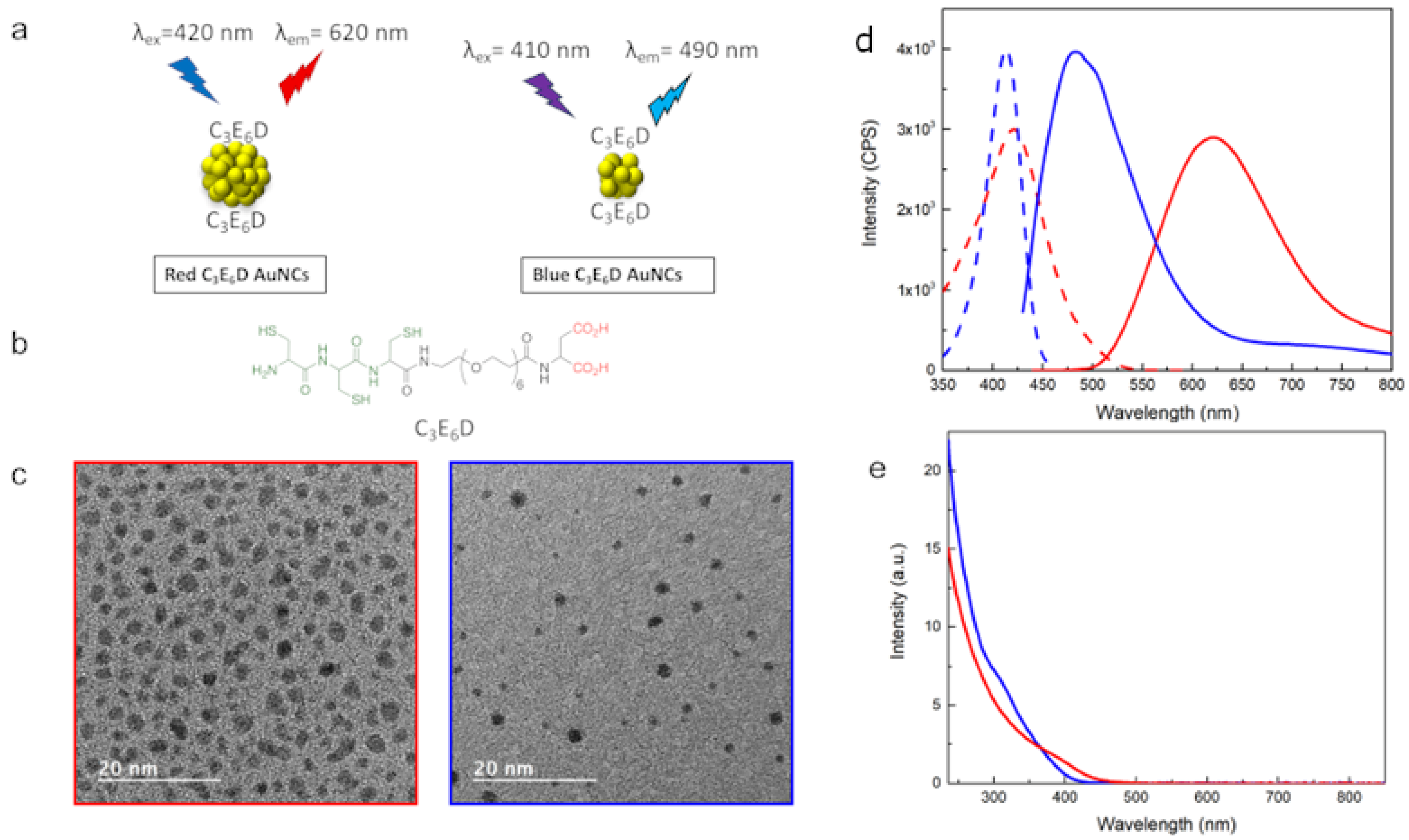
3.1. Synthesis and Characterization of the Red and Blue Gold Nanoclusters (Red and Blue NCs)
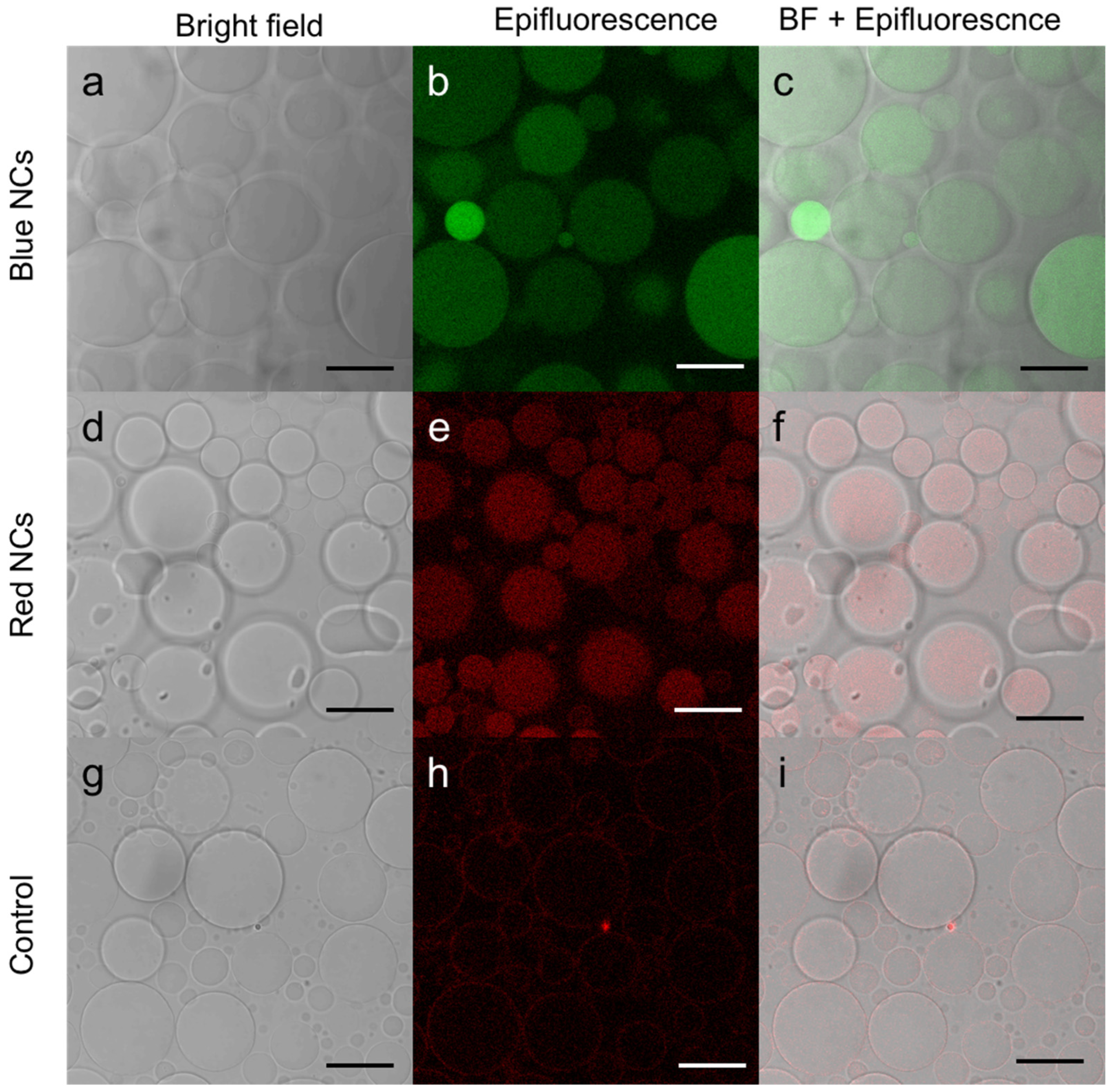
3.2. Red and Blue NCs’ Encapsulation into Giant Unilamellar Vesicles (GUVs)
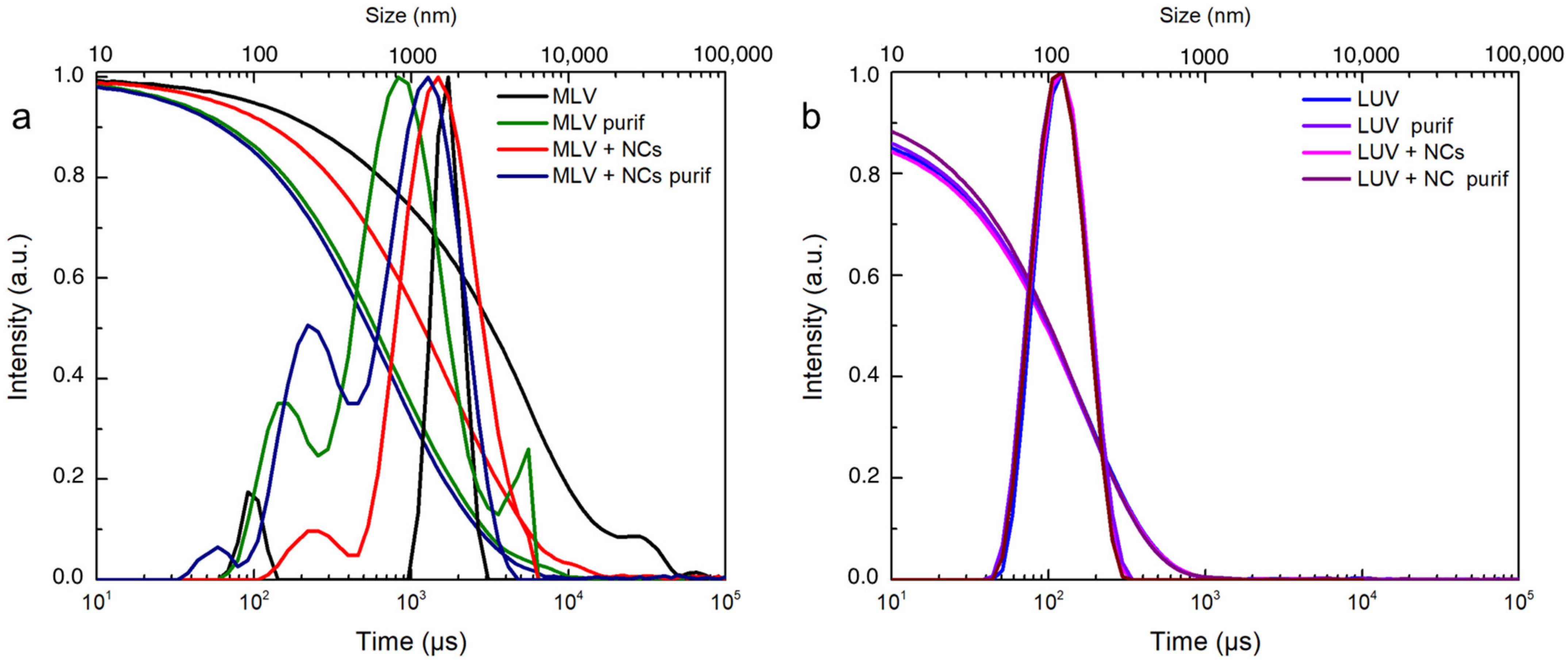
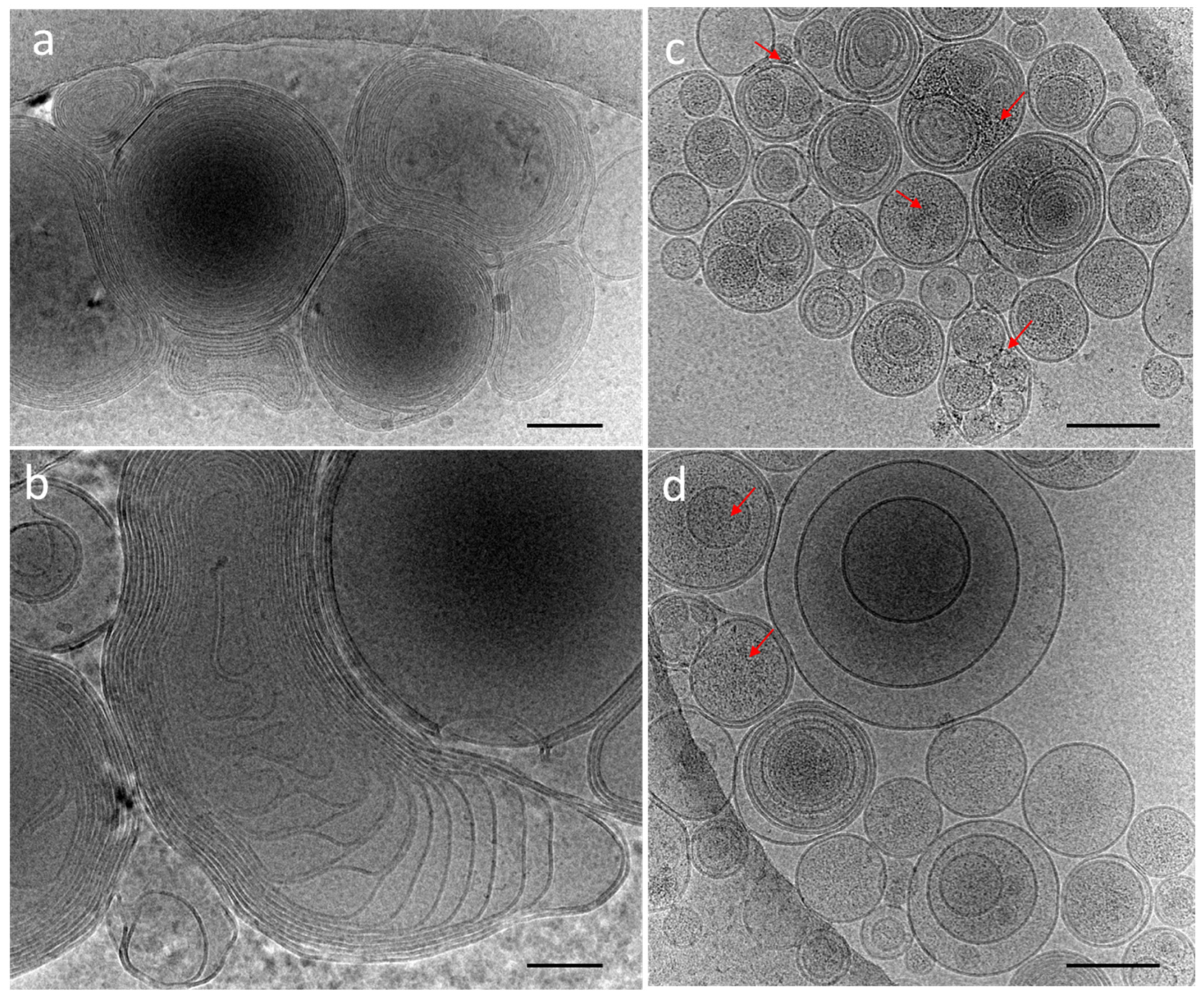
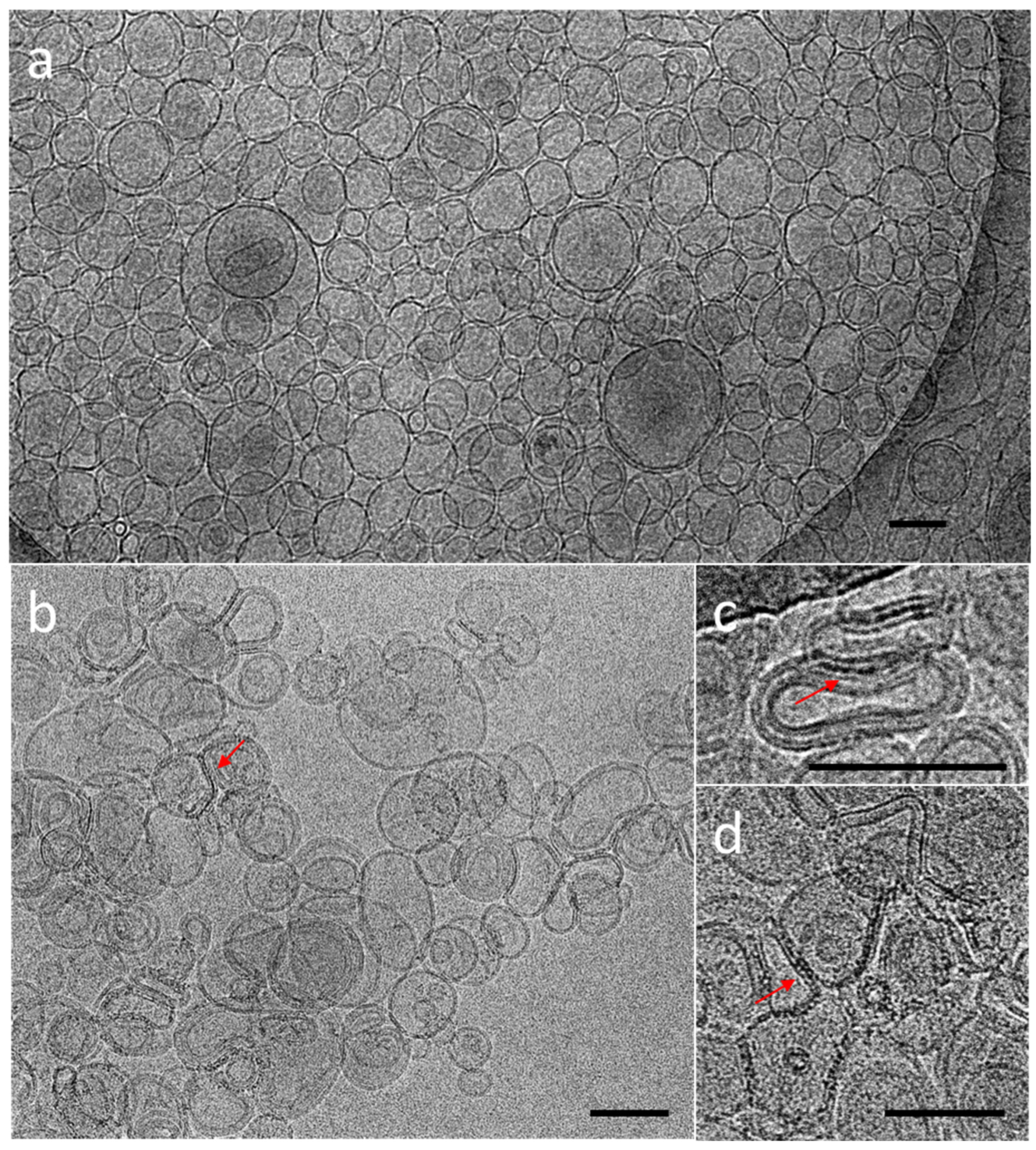
3.3. Red and Blue NCs’ Encapsulation into Multilamellar (MLVs) and Unilamellar (LUVs) Vesicles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Ye, J.; Jiang, H.; Gao, S.; Ge, W.; Chen, Y.; Liu, C.; Amatore, C.; Wang, X. Simultaneous and Multisite Tumor Rapid-Target Bioimaging through in Vivo Biosynthesis of Fluorescent Gold Nanoclusters. RSC Adv. 2014, 4, 37790–37795. [Google Scholar] [CrossRef]
- Nonappa Luminescent Gold Nanoclusters for Bioimaging Applications. Beilstein J. Nanotechnol. 2020, 11, 533–546. [CrossRef]
- Zhang, X.-D.; Luo, Z.; Chen, J.; Song, S.; Yuan, X.; Shen, X.; Wang, H.; Sun, Y.; Gao, K.; Zhang, L.; et al. Ultrasmall Glutathione-Protected Gold Nanoclusters as Next Generation Radiotherapy Sensitizers with High Tumor Uptake and High Renal Clearance. Sci. Rep. 2015, 5, srep08669. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Soleimany, A.P.; Dudani, J.S.; Lin, Y.; Najer, A.; Bekdemir, A.; Chen, Q.; Bhatia, S.N.; Stevens, M.M. Renal Clearable Catalytic Gold Nanoclusters for in Vivo Disease Monitoring. Nat. Nanotechnol. 2019, 14, 883–890. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Wu, D.; Shen, X.; Liu, P.-X.; Fan, F.-Y.; Fan, S.-J. In Vivo Renal Clearance, Biodistribution, Toxicity of Gold Nanoclusters. Biomaterials 2012, 33, 4628–4638. [Google Scholar] [CrossRef]
- Liang, G.; Jin, X.; Zhang, S.; Xing, D. RGD Peptide-Modified Fluorescent Gold Nanoclusters as Highly Efficient Tumor-Targeted Radiotherapy Sensitizers. Biomaterials 2017, 144, 95–104. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and Polymersomes: A Comparative Review towards Cell Mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef]
- Kube, S.; Hersch, N.; Naumovska, E.; Gensch, T.; Hendriks, J.; Franzen, A.; Landvogt, L.; Siebrasse, J.-P.; Kubitscheck, U.; Hoffmann, B.; et al. Fusogenic Liposomes as Nanocarriers for the Delivery of Intracellular Proteins. Langmuir 2017, 33, 1051–1059. [Google Scholar] [CrossRef]
- Cavalcanti, R.R.M.; Lira, R.B.; Riske, K.A. Membrane Fusion Biophysical Analysis of Fusogenic Liposomes. Langmuir 2022, 38, 10430–10441. [Google Scholar] [CrossRef]
- Al-Ahmady, Z.S.; Ali-Boucetta, H. Nanomedicine & Nanotoxicology Future Could Be Reshaped Post-COVID-19 Pandemic. Front. Nanotechnol. 2020, 2, 610465. [Google Scholar] [CrossRef]
- Martin, F.J.; MacDonald, R.C. Lipid Vesicle-Cell Interactions. II. Induction of Cell Fusion. J. Cell Biol. 1976, 70, 506–514. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering Hybrid Exosomes by Membrane Fusion with Liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef]
- Kauscher, U.; Penders, J.; Nagelkerke, A.; Holme, M.N.; Nele, V.; Massi, L.; Gopal, S.; Whittaker, T.E.; Stevens, M.M. Gold Nanocluster Extracellular Vesicle Supraparticles: Self-Assembled Nanostructures for Three-Dimensional Uptake Visualization. Langmuir 2020, 36, 3912–3923. [Google Scholar] [CrossRef]
- Beaune, G.; Dubertret, B.; Clément, O.; Vayssettes, C.; Cabuil, V.; Ménager, C. Giant Vesicles Containing Magnetic Nanoparticles and Quantum Dots: Feasibility and Tracking by Fiber Confocal Fluorescence Microscopy. Angew. Chem. Int. Ed. 2007, 46, 5421–5424. [Google Scholar] [CrossRef]
- Arribas Perez, M.; Beales, P.A. Biomimetic Curvature and Tension-Driven Membrane Fusion Induced by Silica Nanoparticles. Langmuir 2021, 37, 13917–13931. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Alsotari, S.; Alkawareek, M.Y.; Abulateefeh, S.R. Facile Hydrophobication of Glutathione-Protected Gold Nanoclusters and Encapsulation into Poly(Lactide-Co-Glycolide) Nanocarriers. Sci. Rep. 2019, 9, 11098. [Google Scholar] [CrossRef]
- Von White, G.; Chen, Y.; Roder-Hanna, J.; Bothun, G.D.; Kitchens, C.L. Structural and Thermal Analysis of Lipid Vesicles Encapsulating Hydrophobic Gold Nanoparticles. ACS Nano 2012, 6, 4678–4685. [Google Scholar] [CrossRef]
- Maity, A.; De, S.K.; Chakraborty, A. Interaction of Aromatic Amino Acid-Functionalized Gold Nanoparticles with Lipid Bilayers: Insight into the Emergence of Novel Lipid Corona Formation. J. Phys. Chem. B 2021, 125, 2113–2123. [Google Scholar] [CrossRef]
- Michel, R.; Gradzielski, M. Experimental Aspects of Colloidal Interactions in Mixed Systems of Liposome and Inorganic Nanoparticle and Their Applications. IJMS 2012, 13, 11610–11642. [Google Scholar] [CrossRef]
- Contini, C.; Hindley, J.W.; Macdonald, T.J.; Barritt, J.D.; Ces, O.; Quirke, N. Size Dependency of Gold Nanoparticles Interacting with Model Membranes. Commun. Chem. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Chiechio, R.M.; Ducarre, S.; Moulin, G.; Dupont, A.; Marets, C.; Even-Hernandez, P.; Artzner, F.; Musumeci, P.; Franzò, G.; Ravel, C.; et al. Luminescent Gold Nanoclusters Interacting with Synthetic and Biological Vesicles. J. Phys. Chem. Lett. 2022, 13, 6935–6943. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Matsushita-Ishiodori, Y.; Hanczyc, M.M.; Wang, A.; Szostak, J.W.; Yomo, T. Using Imaging Flow Cytometry to Quantify and Optimize Giant Vesicle Production by Water-in-Oil Emulsion Transfer Methods. Langmuir 2019, 35, 2375–2382. [Google Scholar] [CrossRef]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of Large Unilamellar Vesicles by a Rapid Extrusion Procedure. Characterization of Size Distribution, Trapped Volume and Ability to Maintain a Membrane Potential. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1985, 812, 55–65. [Google Scholar] [CrossRef]
- Dubochet, J.; McDowall, A.W. Vitrification of Pure Water for Electron Microscopy. J. Microsc. 1981, 124, 3–4. [Google Scholar] [CrossRef]
- Tanziela, T.; Shaikh, S.; Jiang, H.; Lu, Z.; Wang, X. Efficient Encapsulation of Biocompatible Nanoparticles in Exosomes for Cancer Theranostics. Nano Today 2020, 35, 100964. [Google Scholar] [CrossRef]
- Cantelli, A.; Battistelli, G.; Guidetti, G.; Manzi, J.; Di Giosia, M.; Montalti, M. Luminescent Gold Nanoclusters as Biocompatible Probes for Optical Imaging and Theranostics. Dyes Pigments 2016, 135, 64–79. [Google Scholar] [CrossRef]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Production of Unilamellar Vesicles Using an Inverted Emulsion. Langmuir 2003, 19, 2870–2879. [Google Scholar] [CrossRef]
- Mui, B.L.; Cullis, P.R.; Evans, E.A.; Madden, T.D. Osmotic Properties of Large Unilamellar Vesicles Prepared by Extrusion. Biophys. J. 1993, 64, 443–453. [Google Scholar] [CrossRef]
- Mayer, L.D.; Hope, M.J.; Cullis, P.R. Vesicles of Variable Sizes Produced by a Rapid Extrusion Procedure. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1986, 858, 161–168. [Google Scholar] [CrossRef]
- Chatterjee, A.; Purkayastha, P. The Impact of Lipid Head-Groups in GUVs on Electron Transfer by Surface-Adsorbed Fluorescent Gold Nanoclusters. Mater. Adv. 2021, 2, 1343–1350. [Google Scholar] [CrossRef]
- Ning, B.; Huang, Z.; Youngquist, B.M.; Scott, J.W.; Niu, A.; Bojanowski, C.M.; Zwezdaryk, K.J.; Saba, N.S.; Fan, J.; Yin, X.-M.; et al. Liposome-Mediated Detection of SARS-CoV-2 RNA-Positive Extracellular Vesicles in Plasma. Nat. Nanotechnol. 2021, 16, 1039–1044. [Google Scholar] [CrossRef] [PubMed]


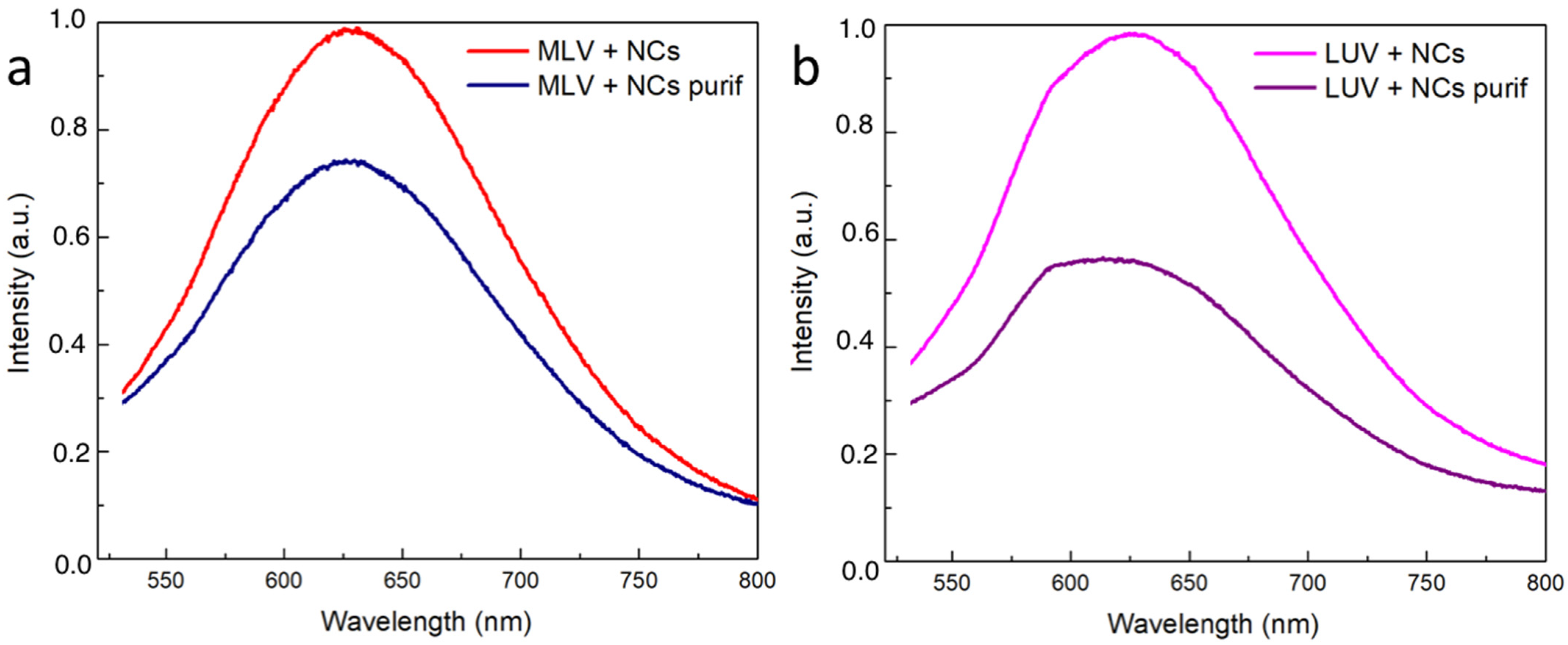
| MLV+NCs | MLV+NCs Purif | LUV+NCs | LUV+NCs Purif |
|---|---|---|---|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiechio, R.M.; Ducarre, S.; Marets, C.; Dupont, A.; Even-Hernandez, P.; Pinson, X.; Dutertre, S.; Artzner, F.; Musumeci, P.; Ravel, C.; et al. Encapsulation of Luminescent Gold Nanoclusters into Synthetic Vesicles. Nanomaterials 2022, 12, 3875. https://doi.org/10.3390/nano12213875
Chiechio RM, Ducarre S, Marets C, Dupont A, Even-Hernandez P, Pinson X, Dutertre S, Artzner F, Musumeci P, Ravel C, et al. Encapsulation of Luminescent Gold Nanoclusters into Synthetic Vesicles. Nanomaterials. 2022; 12(21):3875. https://doi.org/10.3390/nano12213875
Chicago/Turabian StyleChiechio, Regina M., Solène Ducarre, Célia Marets, Aurélien Dupont, Pascale Even-Hernandez, Xavier Pinson, Stéphanie Dutertre, Franck Artzner, Paolo Musumeci, Célia Ravel, and et al. 2022. "Encapsulation of Luminescent Gold Nanoclusters into Synthetic Vesicles" Nanomaterials 12, no. 21: 3875. https://doi.org/10.3390/nano12213875
APA StyleChiechio, R. M., Ducarre, S., Marets, C., Dupont, A., Even-Hernandez, P., Pinson, X., Dutertre, S., Artzner, F., Musumeci, P., Ravel, C., Faro, M. J. L., & Marchi, V. (2022). Encapsulation of Luminescent Gold Nanoclusters into Synthetic Vesicles. Nanomaterials, 12(21), 3875. https://doi.org/10.3390/nano12213875










