Nanomaterials for Cortisol Sensing
Abstract
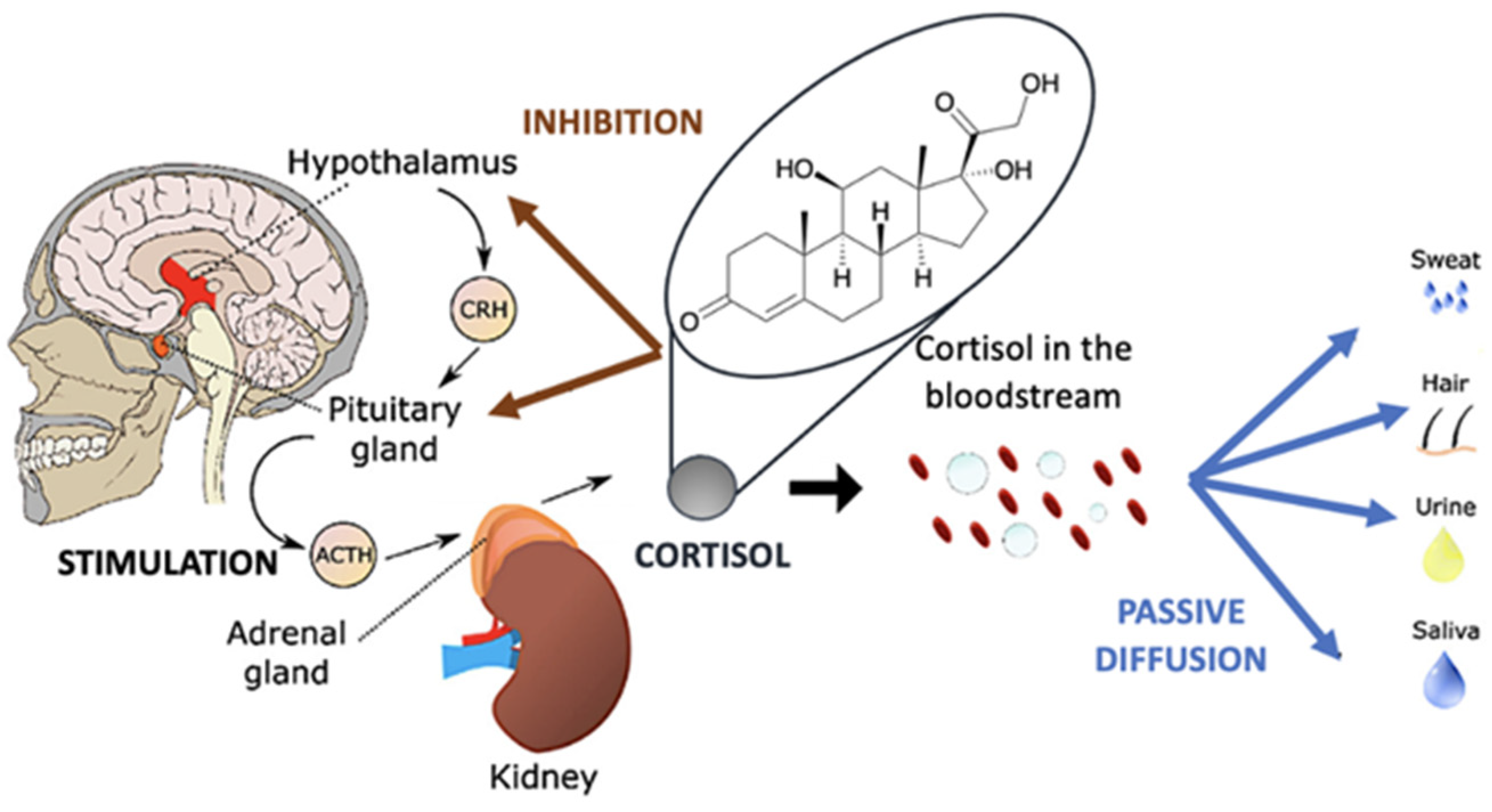
1. Introduction
2. Nanomaterials for Sensing
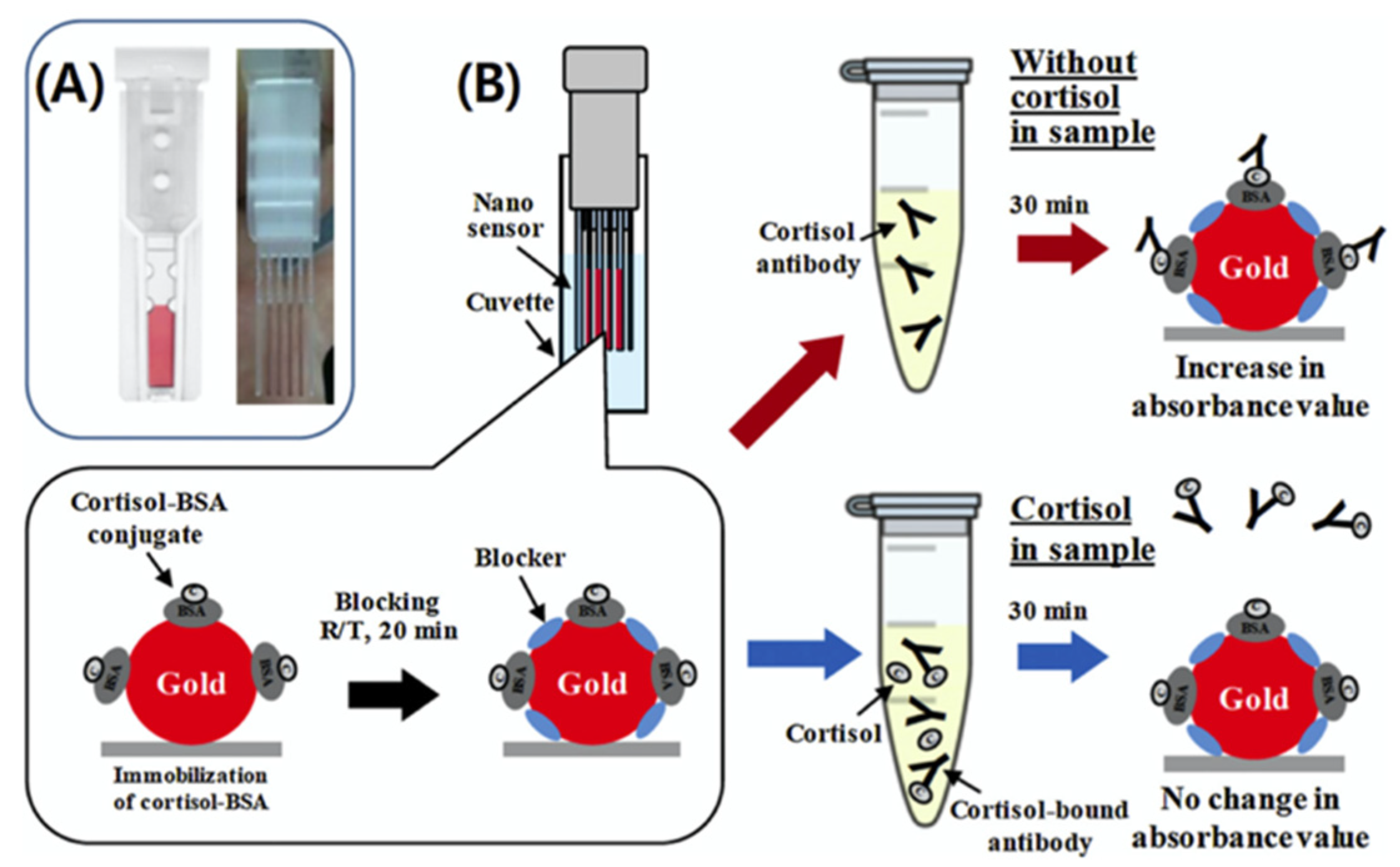
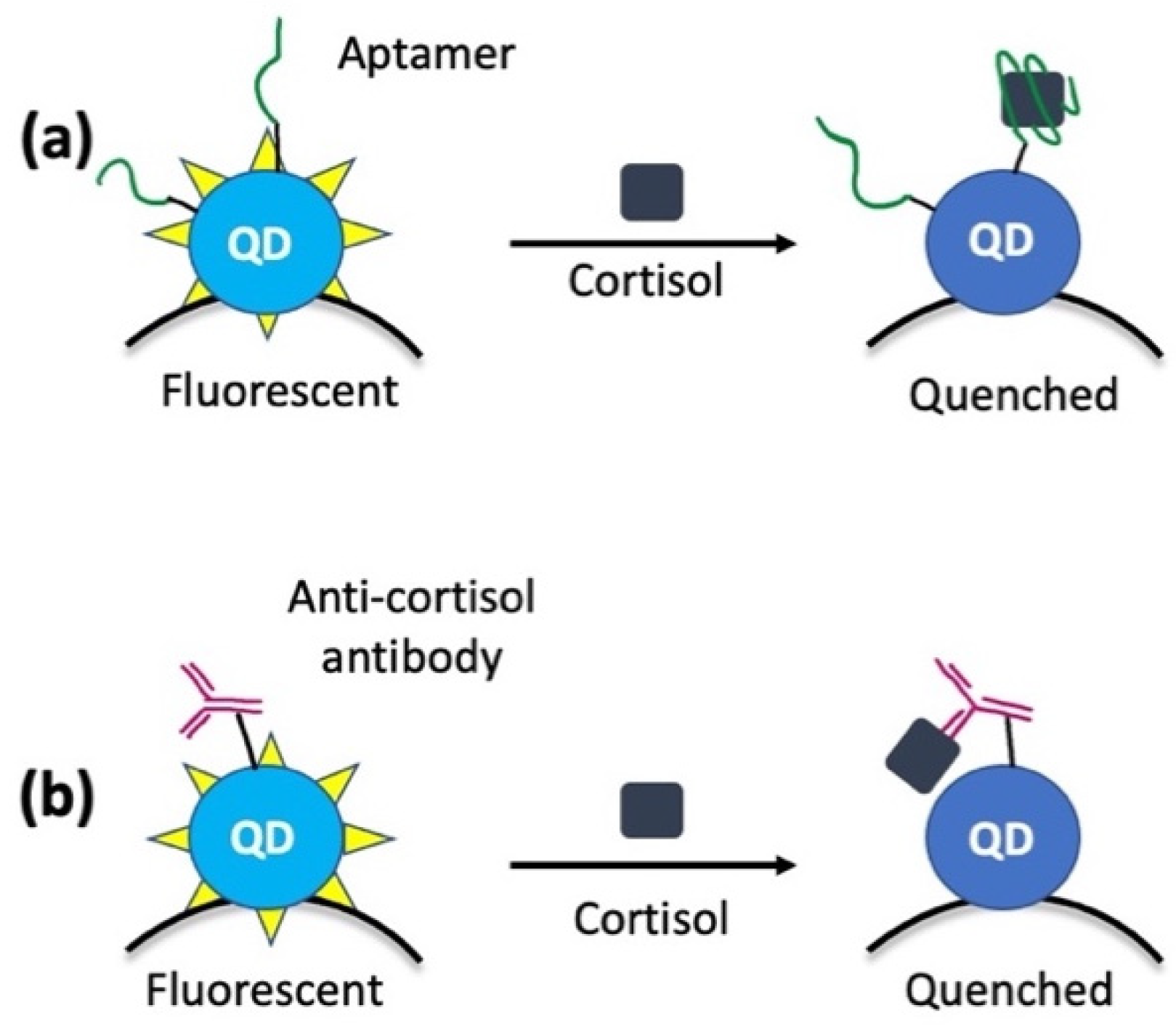
3. Optical Nanosensors
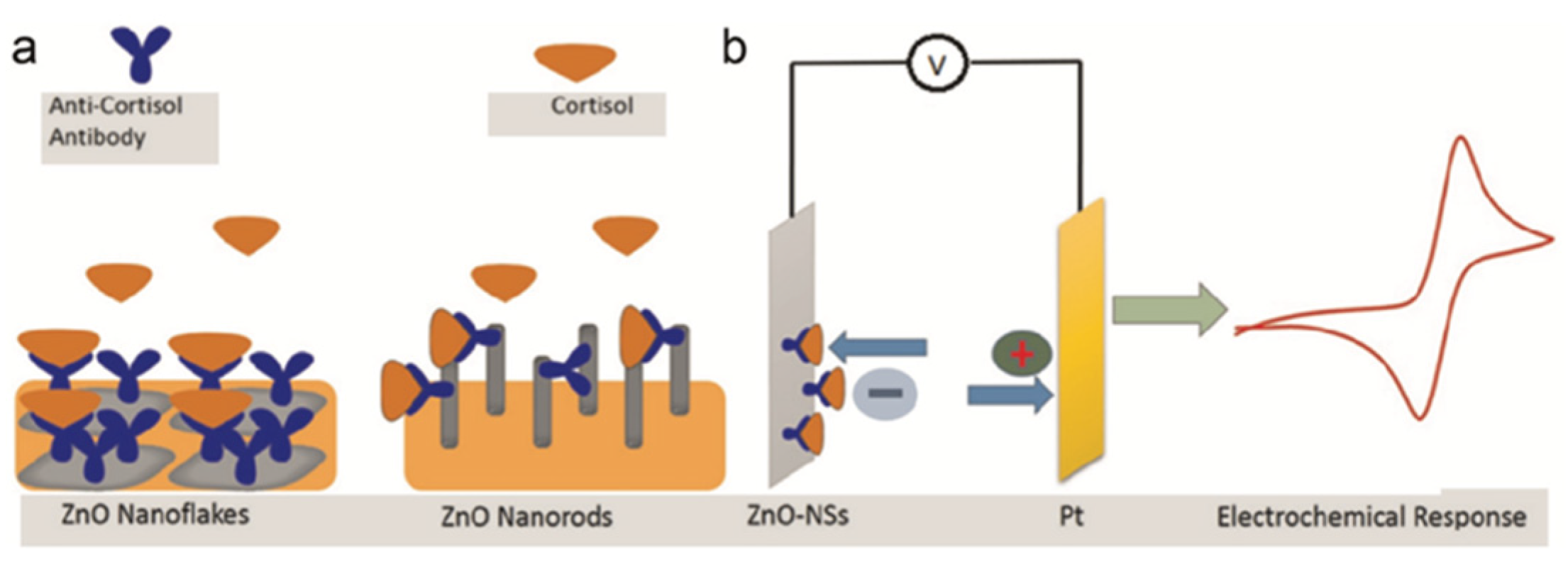
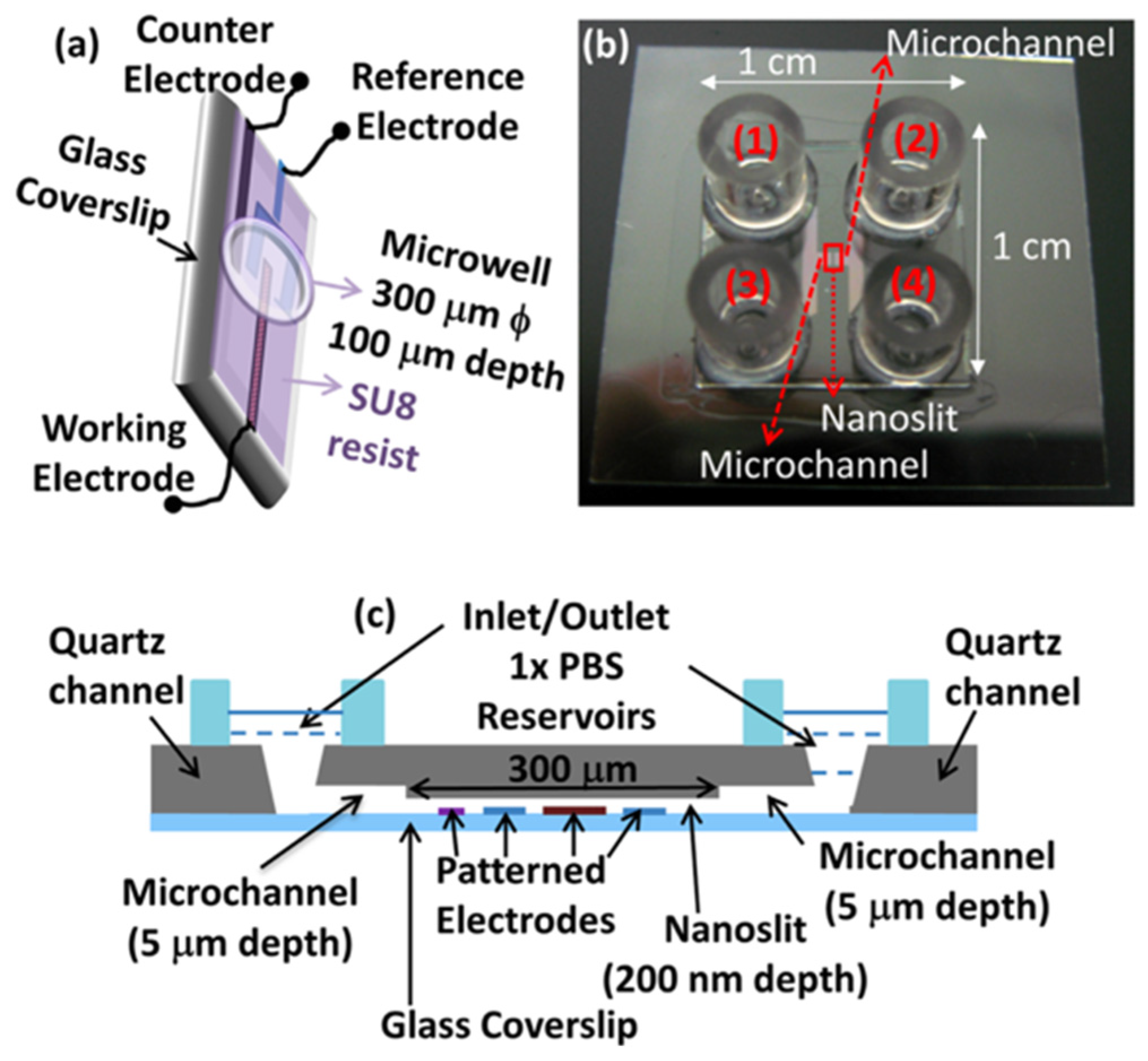
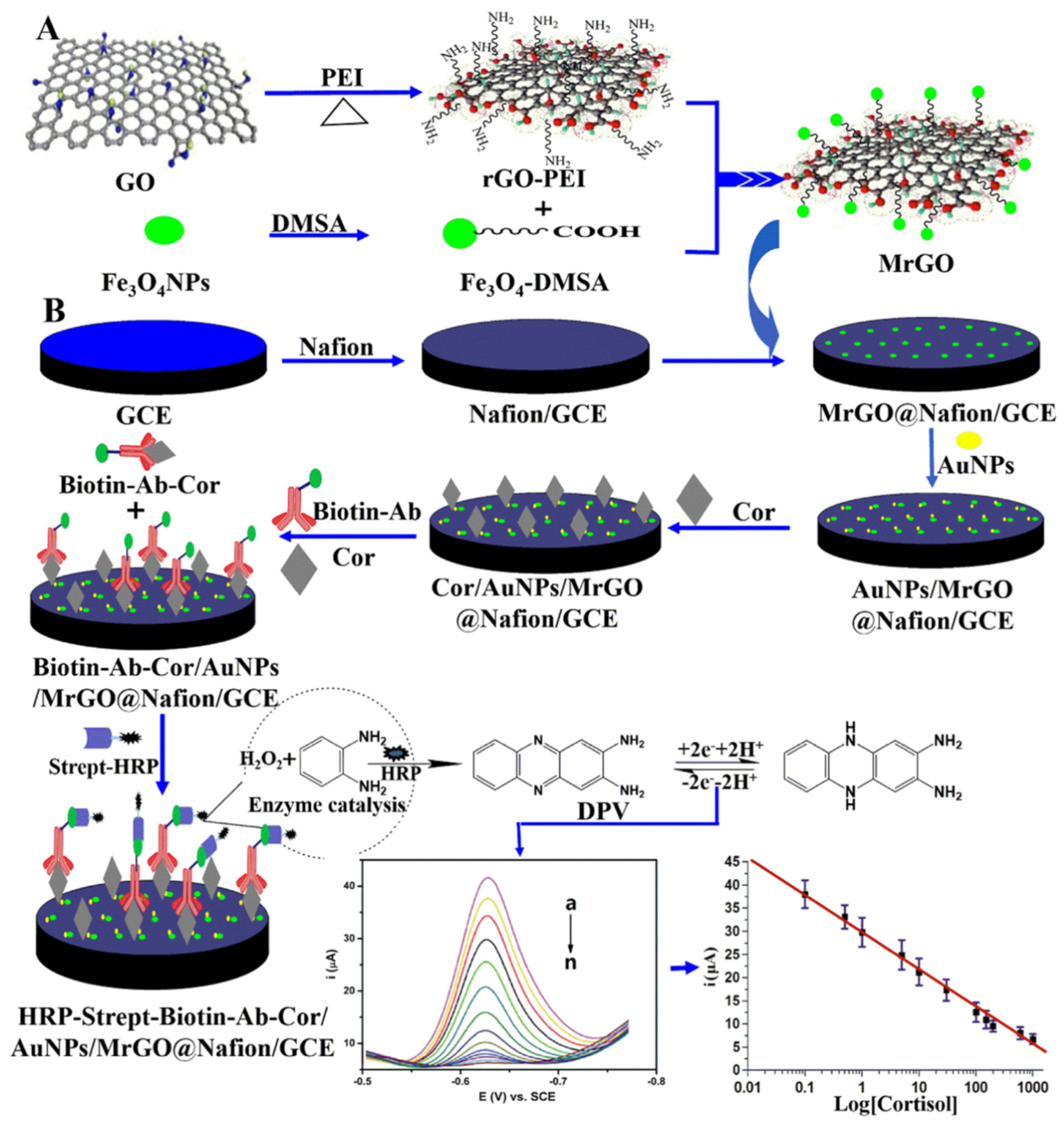
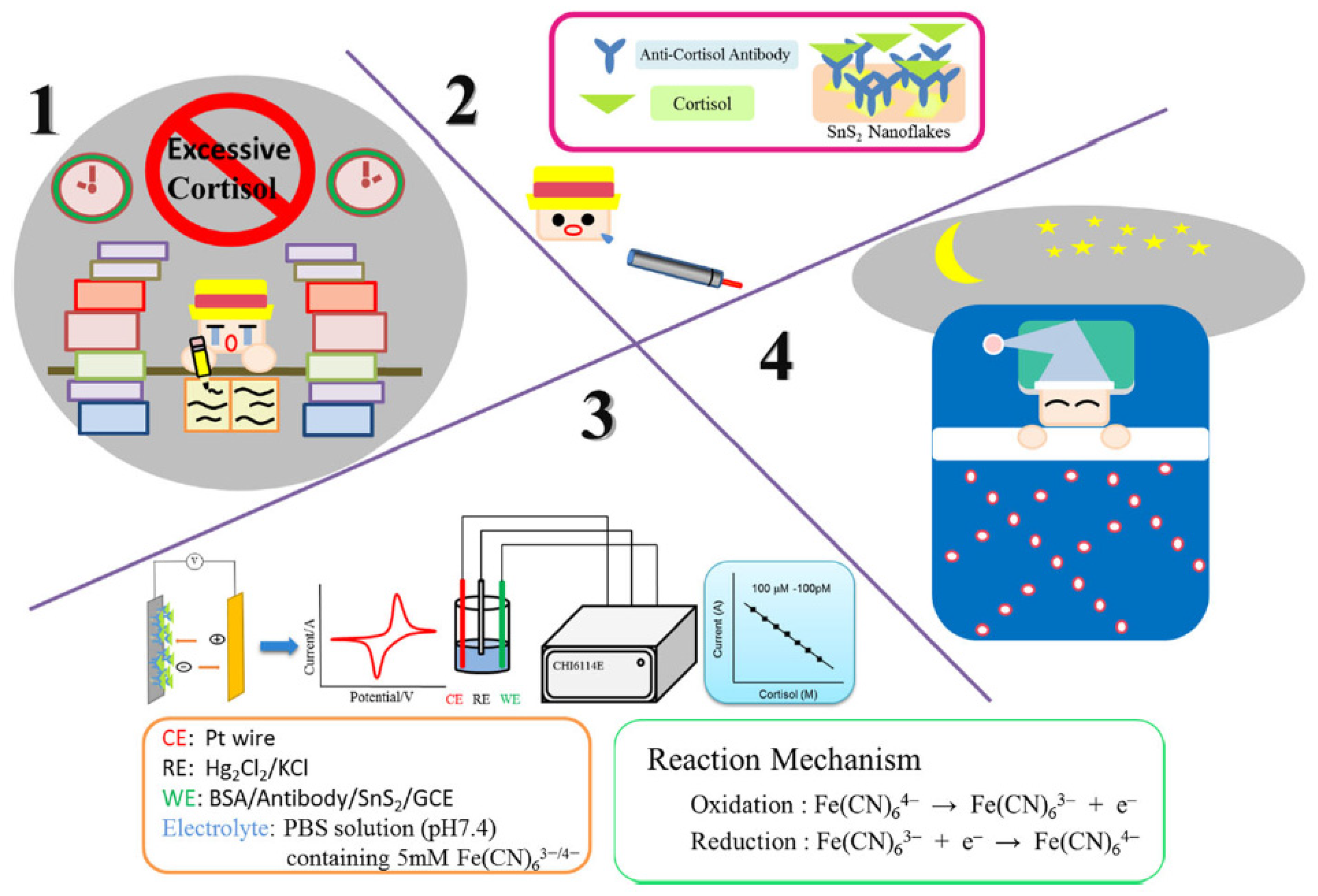
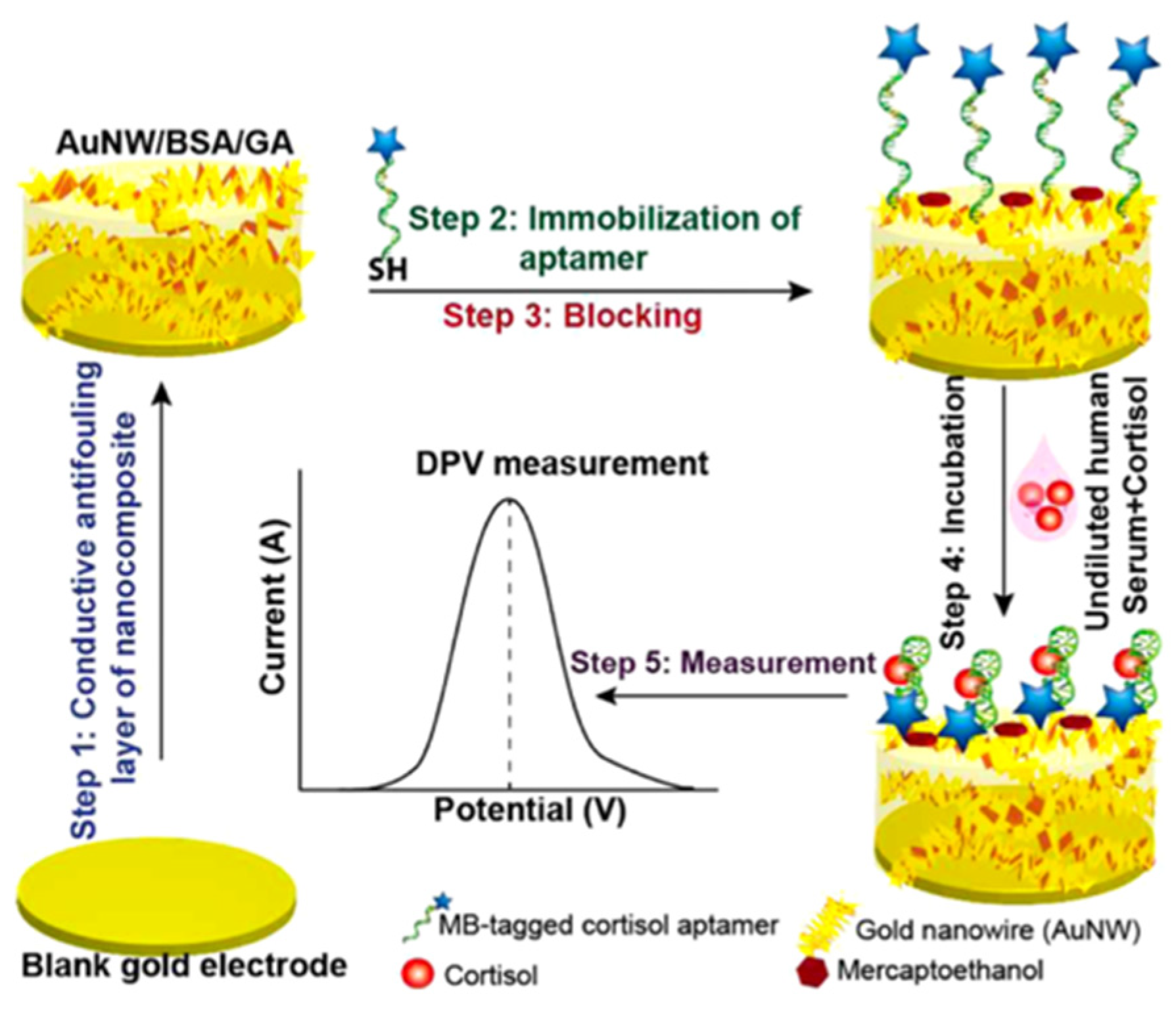
4. Electrochemical Nanosensors
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243. [Google Scholar] [CrossRef] [PubMed]
- Sawyers, C.; Sheerin, C.; Eastman, M.; Burchett, J.; Howell, P.; Neigh, G.; Amstadter, A.B.; Hettema, J.; Roberson-Nay, R. Genetic, and Environmental Influences on Cortisol Reactivity to a Psychosocial Stressor in Adolescents and Young Adults. Psychoneuroendocrinology 2021, 127, 105195. [Google Scholar] [CrossRef] [PubMed]
- Quin, H.Y.; Cheng, C.W.; Tang, X.D.; Bian, Z.X. Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 14126. [Google Scholar] [CrossRef]
- Zefferino, R.; Gioia, S.D.; Conese, M. Molecular links between endocrine, nervous and immune system during chronic stress. Brain Behav. 2020, 1, e01960. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.-C.; Meng, L.-B.; Hao, M.-L.; Zhang, Y.-M.; Gong, T.; Guo, Z.-G. Chronic stress: A critical risk factor for atherosclerosis. J. Int. Med. Res. 2019, 47, 1429. [Google Scholar] [CrossRef]
- Charney, D.S. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. Am. J. Psychiatry 2004, 161, 195–216. [Google Scholar] [CrossRef]
- Djuric, Z.; Bird, C.E.; Furumoto-Dawson, A.; Rauscher, G.H.; Ruffin, M.T.; Stowe, I.V.R.P.; Tucker, K.L.; Masi, C.M. Biomarkers of Psychological Stress in Health Disparities Research. Open Biomarkers J. 2008, 1, 7–19. [Google Scholar] [CrossRef]
- Pradhan, T.; Jung, H.S.; Jang, J.H.; Kim, T.W.; Kang, C.; Kim, J.S. Chemical sensing of neurotransmitters. Chem. Soc. Rev. 2014, 43, 4684–4713. [Google Scholar] [CrossRef]
- Taves, M.D.; Gomez-Sanchez, C.E.; Soma, K.K. Extra-adrenal glucocorticoids and mineralocorticoids: Evidence for local synthesis, regulation, and function. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E11–E24. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–887. [Google Scholar] [CrossRef]
- Gallagher, T.F.; Yoshida, K.; Roffwarg, H.D.; Fukushima, D.K.; Weitzman, E.D.; Hellman, L. ACTH and cortisol secretory patterns in man. J. Clin. Endocrinol. Metab. 1973, 36, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Debono, M. Replication of cortisol circadian rhythm: New advances in hydrocortisone replacement therapy. Ther. Adv. Endocrinol. Metabol. 2010, 1, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Clow, A.; Hucklebridge, F.; Stalder, T.; Evans, P.; Thorn, L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010, 35, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sephton, S.E.; Lush, E.; Dedert, E.A.; Floyd, A.R.; Rebholz, W.N.; Dhabhar, F.S.; Spiegel, D.; Salmon, P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav. Immun. 2013, 30, S163–S170. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Zagoory-Sharon, O.; Feldman, R.; Lewis, J.G.; Weller, A. Measuring cortisol in human psychobiological studies. Physiol. Behav. 2007, 90, 43–53. [Google Scholar] [CrossRef]
- Khelifa, L.; Hu, Y.; Jiang, N.; Yetisen, A.K. Lateral flow assays for hormone detection. Lab Chip 2022, 22, 2451–2475. [Google Scholar] [CrossRef]
- Zea, M.; Bellagambi, F.G.; Halima, H.B.; Zine, N.; Jaffrezi-Renault, N.; Villa, R.; Gabriela, G.; Errachid, A. Electrochemical sensors for cortisol detections: Almost there. Trends Analyt. Chem. 2020, 132, 116058. [Google Scholar] [CrossRef]
- Xing, S.; Jiang, J.; Pan, T. Interfacial microfluidic transport on micropatterned superhydrophobic textile. Lab Chip 2013, 13, 1937–1947. [Google Scholar] [CrossRef]
- Bennett, A.; Hayssen, V. Hair as a biological indicator of drug use, drug abuse or chronic exposure to environmental toxicants. Int. J. Toxicol. 2006, 25, 143–163. [Google Scholar] [CrossRef]
- Venugopal, M.; Feuvrel, K.E.; Mongin, D.; Bambot, S.; Faupel, M.; Panangadan, A.; Talukder, A.; Pidva, R. Clinical evaluation of a novel interstitial fluid sensor system for remote continuous alcohol monitoring. Sens. J. IEEE 2008, 8, 71–80. [Google Scholar] [CrossRef]
- Kaushik, A.; Yndart, A.; Jayant, R.D.; Sagar, V.; Atluri, V.; Bhansali, S.; Nair, M. Electrochemical sensing method for point-of-care cortisol detection in human immunodeficiency virus-infected patients. Int. J. Nanomed. 2015, 10, 677–685. [Google Scholar] [CrossRef]
- Kim, H.T.; Jin, E.; Lee, M.H. Portable Chemiluminescence-Based Lateral Flow Assay Platform for the Detection of Cortisol in Human Serum. Biosensors 2021, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z. Capillary Electrophoresis-Based Immunoassay for the Determination of Brevetoxin-B in Shellfish using Electrochemical Detection. J. Chromatograp. Sci. 2013, 51, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Baraket, A.; Lee, M.; Zine, N.; Sigaud, M.; Yaakoubi, N.; Trivella, M.G.; Zabala, M.; Bausells, J.; Jaffrezic-Renault, N.; Errachid, A. Diazonium modified gold microelectrodes onto polyimide substrates for impedimetric cytokine detection with an integrated Ag/AgCl reference electrode. Sens. Actuator B Chem. 2013, 189, 165–172. [Google Scholar] [CrossRef]
- Stefan, R.I.; Van, J.S.; Aboul-Enein, H.Y. Design and Use of Electrochemical Sensors in Enantioselective High Throughput Screening of Drugs. A Minireview. Comb. Chem. High Throughput Screen 2000, 3, 445–454. [Google Scholar] [CrossRef]
- Lee, S.; Hwan, S.L.; Hye, J.S.; Seol, A.K.; Jimyeong, P.; Hyu, C.K.; Hyogyeong, K.; Hyung, J.K.; Yun, T.K.; Kyoung, R.L.; et al. Simultaneous Determination of Cortisol and Cortisone from Human Serum by Liquid Chromatography-Tandem Mass Spectrometry. J. Anal. Methods Chem. 2014, 2014, 787483. [Google Scholar] [CrossRef]
- Baid, S.K.; Sinaii, N.; Wade, M.; Rubino, D.; Nieman, L.K. Radioimmunoassay and Tandem Mass Spectrometry Measurement of Bedtime Salivary Cortisol Levels: A Comparison of Assays to Establish Hypercortisolism. J. Clin. Endocrinol. Metab. 2007, 92, 3102–3107. [Google Scholar] [CrossRef]
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart electronic yarns and wearable fabrics for human biomonitoring made by carbon nanotube coating with polyelectrolytes. Nano Lett. 2018, 8, 4151–4157. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectr. 2014, 53, 499–512. [Google Scholar] [CrossRef]
- Abidin, A.S.Z.; Rahim, R.A.; Arshad, M.K.M.; Nabilah, M.F.F.; Voon, C.H.; Tang, T.-H.; Citartan, M. Current and Potential Developments of Cortisol Aptasensing towards Point-of-Care Diagnostics (POTC). Sensors 2017, 17, 1180. [Google Scholar] [CrossRef] [PubMed]
- Tuccitto, N.; Fichera, L.; Ruffino, R.; Cantaro, V.; Sfuncia, G.; Nicotra, G.; Sfrazzetto, G.T.; Li-Destri, G.; Valenti, A.; Licciardello, A.; et al. Carbon Quantum Dots as Fluorescence Nanochemosensors for Selective Detection of Amino Acids. ACS Appl. Nano Mater. 2021, 4, 6250–6256. [Google Scholar] [CrossRef]
- Santonocito, R.; Tuccitto, N.; Cantaro, V.; Carbonaro, A.B.; Pappalardo, A.; Greco, V.; Buccilli, V.; Maida, P.; Zavattaro, D.; Sfuncia, G.; et al. Smartphone-Assisted Sensing of Trinitrotoluene by Optical Array. ACS Omega ASAP 2022, 7, 37122–37132. [Google Scholar] [CrossRef] [PubMed]
- Tuccitto, N.; Spitaleri, L.; Destri, G.L.; Pappalardo, A.; Gulino, A.; Sfrazzetto, G.T. Supramolecular Sensing of a Chemical Warfare Agents Simulant by Functionalized Carbon Nanoparticles. Molecules 2020, 25, 5731. [Google Scholar] [CrossRef] [PubMed]
- Tuccitto, N.; Riela, L.; Zammataro, A.; Spitaleri, L.; Destri, G.L.; Sfuncia, G.; Nicotra, G.; Pappalardo, A.; Capizzi, G.; Sfrazzetto, G.T. Functionalized Carbon Nanoparticle-Based Sensors for Chemical Warfare Agents. ACS Appl. Nano Mater. 2020, 3, 8182–8191. [Google Scholar] [CrossRef]
- Qin, G.; Zhao, S.; Huang, Y.; Jiang, J.; Liu, Y.-M. A sensitive gold nanoparticles sensing platform based on resonance energy transfer for chemiluminescence light on detection of biomolecules. Biosens. Bioelectr. 2013, 46, 119–123. [Google Scholar] [CrossRef]
- Chen, S.; Liu, M.-X.; Yu, Y.-L.; Wang, J.-H. Room-temperature synthesis of fluorescent carbon-based nanoparticles and their application in multidimensional sensing. Sens. Actuators B Chem. 2019, 288, 749–756. [Google Scholar] [CrossRef]
- Santonocito, R.; Intravaia, M.; Caruso, I.M.; Pappalardo, A.; Sfrazzetto, G.T.; Tuccitto, N. Fluorescence sensing by carbon nanoparticles. Nanoscale Adv. 2022, 4, 1926–1948. [Google Scholar] [CrossRef]
- Testa, C.; Zammataro, A.; Pappalardo, A.; Sfrazzetto, G.T. Catalysis with carbon nanoparticles. RSC Adv. 2019, 9, 27659–27664. [Google Scholar] [CrossRef]
- Yadav, S.; Raman, A.P.S.; Meena, H.; Goswami, A.G.; Kumar, V.B.; Jain, P.; Kumar, G.; Sagar, M.; Rana, D.K.; Bahadur, I.; et al. An Update on Graphene Oxide: Applications and Toxicity. ACS Omega 2022, 7, 35387–35445. [Google Scholar] [CrossRef]
- Jafari-Nodoushan, H.; Mojtabavi, S.; Faramarzi, M.A.; Samadi, N. Organic-inorganic hybrid nanoflowers: The known, the unknown, and the future. Adv. Colloid Interface Sci. 2022, 309, 102780. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, T.; Chen, L.; Chen, Y.; Bo-Ru, Y.; Luo, Y.; Gui-Shi, L. Self-assembly, alignment, and patterning of metal nanowires. Nanoscale Horiz. 2022, 7, 1299–1339. [Google Scholar] [CrossRef] [PubMed]
- Aspoukeh, P.K.; Barzinjy, A.A.; Hamad, S.M. Synthesis, Properties and uses of ZnO nanorods: A mini review. Int. Nano Lett. 2022, 12, 153–168. [Google Scholar] [CrossRef]
- Jiao, M.-Z.; Chen, X.-Y.; Hu, K.-X.; Qian, D.-Y.; Zhao, X.-H.; Ding, E.-J. Recent developments of nanomaterials-based conductive type methane sensors. Rare Met. 2021, 40, 1515–1527. [Google Scholar] [CrossRef]
- Walcarius, A.; Minteer, S.D.; Wang, J.; Lin, Y.; Merkoçi, A. Nanomaterials for bio-functionalized electrodes: Recent trends. J. Mater. Chem. B 2013, 1, 4878–4908. [Google Scholar] [CrossRef]
- Jeon, J.; Uthaman, S.; Lee, J.; Hwanga, H.; Kim, G.; Yoo, P.J.; Hammock, B.D.; Kim, C.S.; Park, Y.-S.; Park, I.-K. In-direct localized surface plasmon resonance (LSPR)-based nanosensors for highly sensitive and rapid detection of cortisol. Sens. Actuators B Chem. 2018, 266, 710–716. [Google Scholar] [CrossRef]
- Stevens, R.C.; Soelberg, S.D.; Near, S.; Furlong, C.E. Detection of cortisol in saliva with a flow-filtered, portable surface plasmon resonance biosensor system. Anal. Chem. 2008, 80, 6747–6751. [Google Scholar] [CrossRef]
- Tahara, Y.; Huang, Z.; Kiritoshi, T.; Onodera, T.; Toko, K. Development of indirect competitive immuno-assay method using SPR detection for rapid and highly sensitive measurement of salivary cortisol levels. Front. Bioeng. Biotechnol. 2014, 2, 15. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, B.; Tanyi, E.K.; Yeasmin, S.; Cheng, L.-J. Label-Free Sensitive Detection of Steroid Hormone Cortisol Based on Target-Induced Fluorescence Quenching of Quantum Dots. Langmuir 2020, 36, 7781–7788. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef]
- Liu, Y.; Kannegulla, A.; Wu, B.; Cheng, L.-J. Quantum dot fullerene-based molecular beacon nanosensors for rapid, highly sensitive nucleic acid detection. ACS Appl. Mater. Interfaces 2018, 10, 18524–18531. [Google Scholar] [CrossRef] [PubMed]
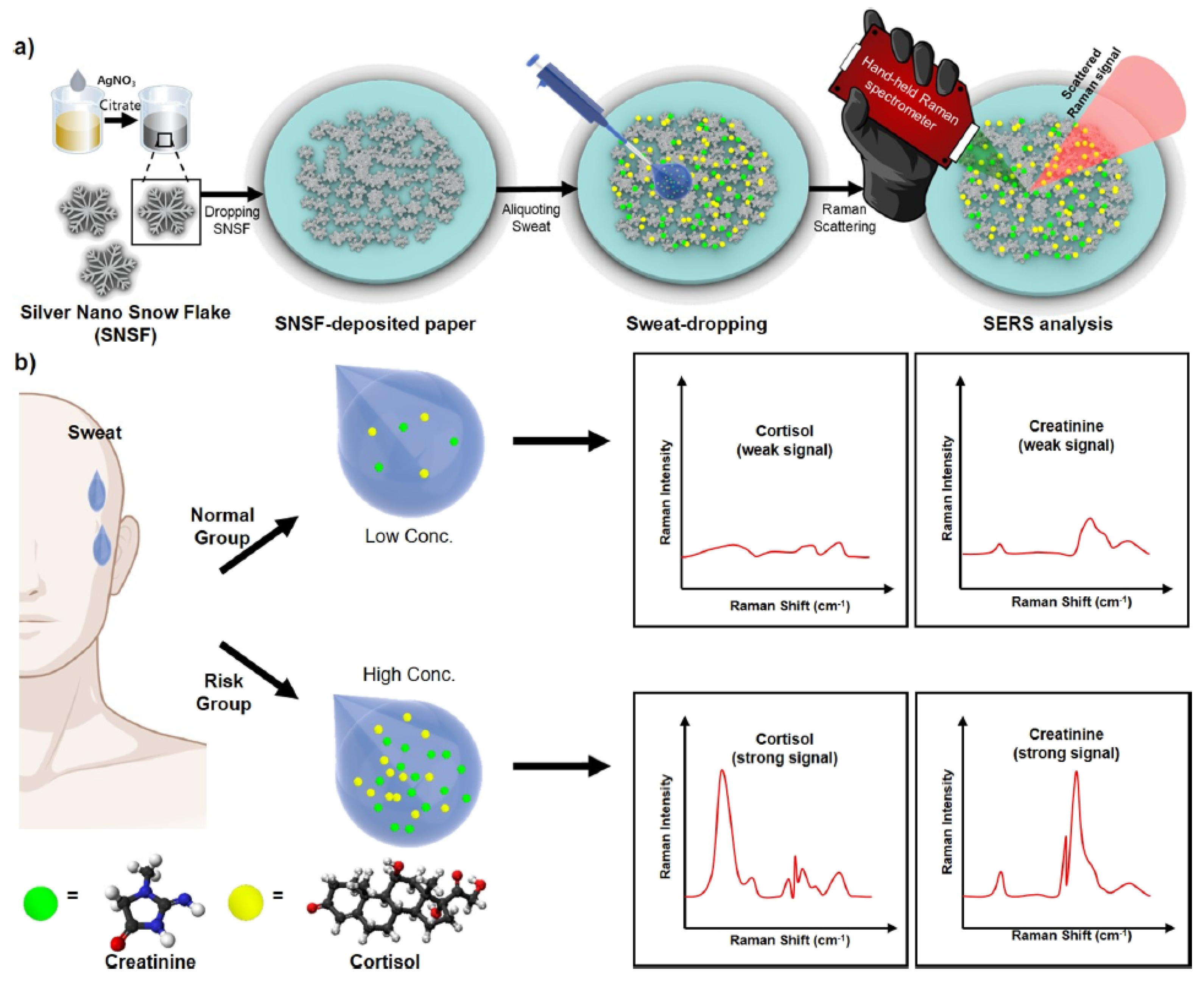
- Kim, H.S.; Kim, H.J.; Lee, J.; Lee, T.; Yun, J.; Lee, G.; Hong, Y. Hand-Held Raman Spectrometer-Based Dual Detection of Creatinine and Cortisol in Human Sweat Using Silver Nanoflakes. Anal. Chem. 2021, 93, 14996–15004. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Leis, A.; Rivera-Arreba, I.; Sanchez-Cortes, S. Morphological tuning of plasmonic silver nanostars by controlling the nanoparticle growth mechanism: Application in the SERS detection of the amyloid marker Congo Red. Colloid Surf. Physicochem. Eng. Asp. 2017, 535, 49–60. [Google Scholar] [CrossRef]
- Barfidokht, A.; Mishra, R.K.; Seenivasan, R.; Shuyang, L.; Lee, J.H.; Wang, J.; Hall, D.A. Wearable electrochemical glove-based sensor for rapid and on-site detection of fentanyl. Sens. Actuators B Chem. 2019, 296, 126422. [Google Scholar] [CrossRef] [PubMed]
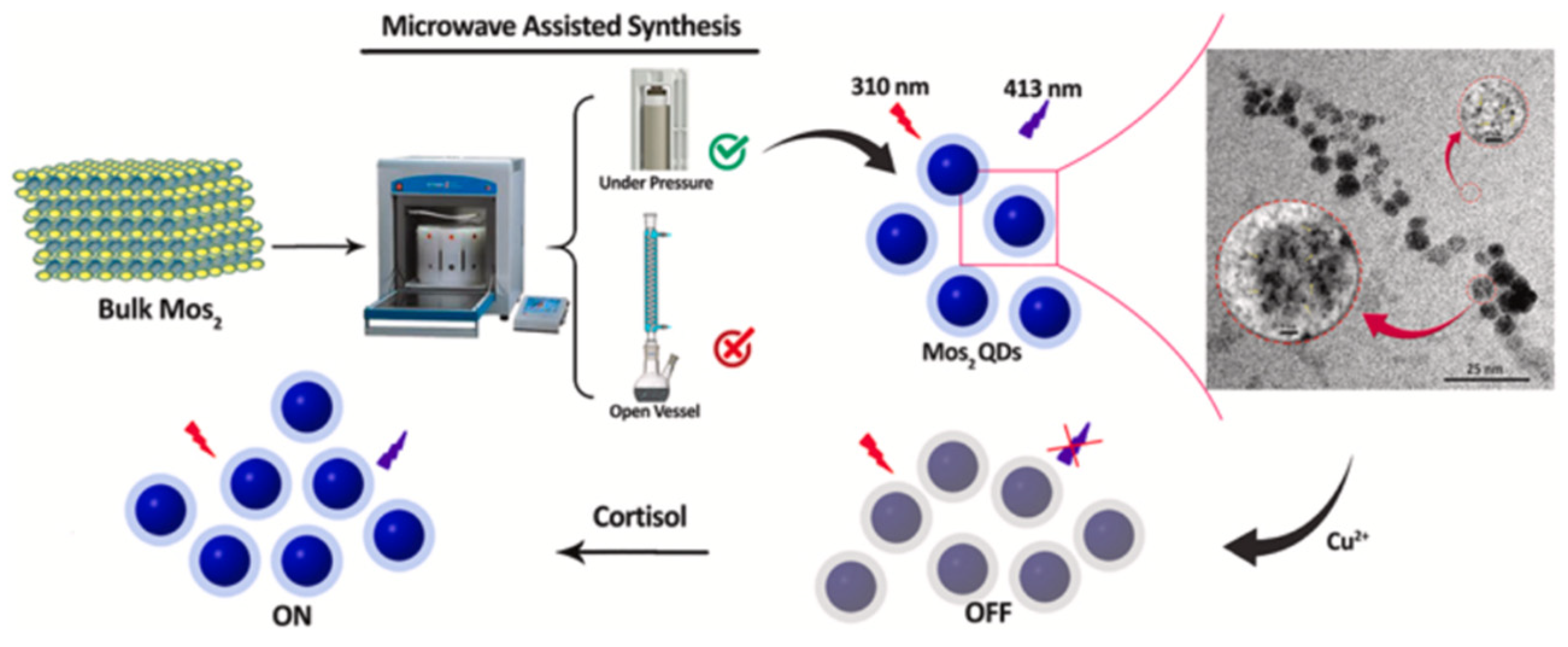
- Mohammad-Andashti, P.; Ramezani, Z.; Zare-Shahabadi, V.; Torabi, P. Rapid and green synthesis of highly luminescent MoS2 quantum dots via microwave exfoliation of MoS2 powder and its application as a fluorescence probe for cortisol detection in human saliva. Colloids Surf. A Physicochem. Eng. Asp. 2022, 64, 129048. [Google Scholar] [CrossRef]
- Yilmaz, G.; Saylan, Y.; Göktürk, I.; Yılmaz, F.; Denizli, A. Selective Amplification of Plasmonic Sensor Signal for Cortisol Detection Using Gold Nanoparticles. Biosensors 2022, 12, 482. [Google Scholar] [CrossRef] [PubMed]
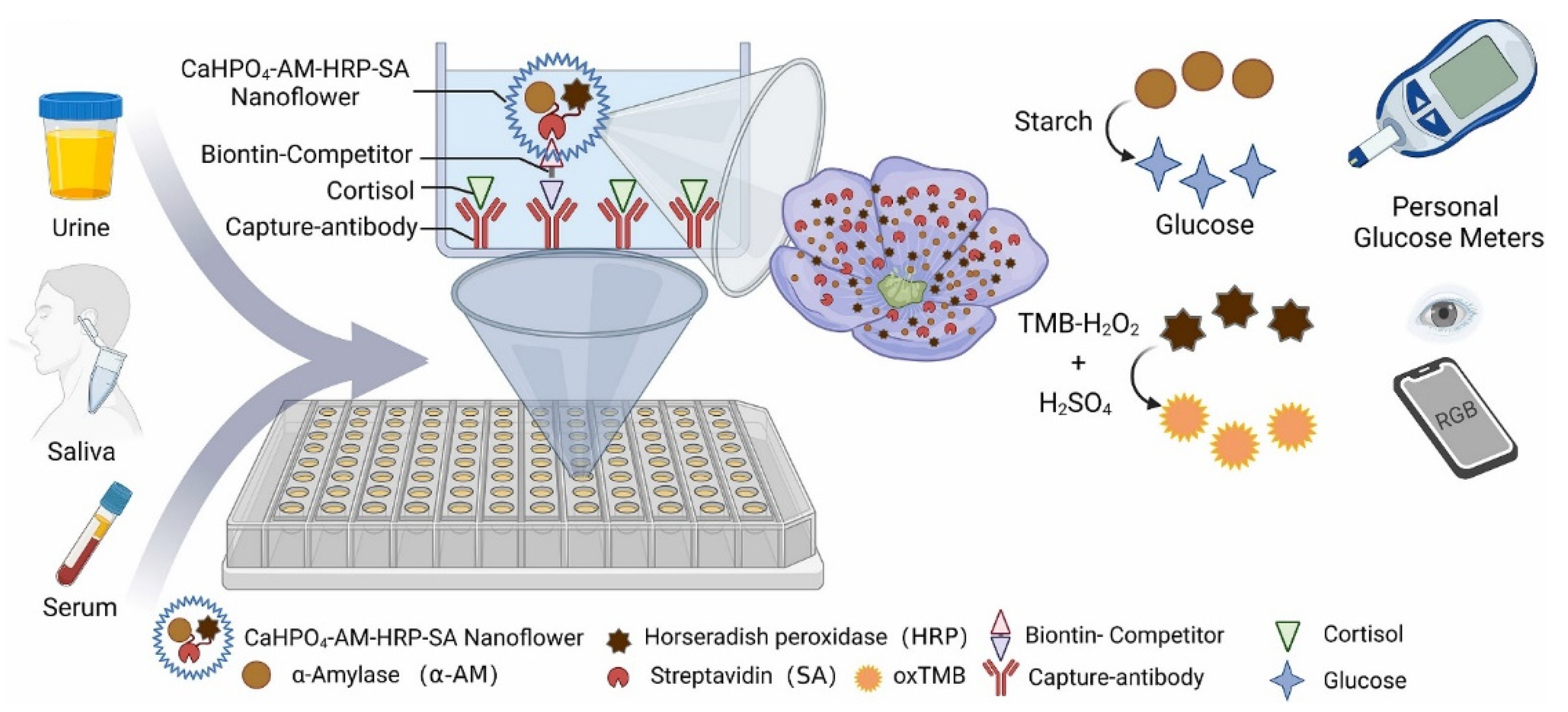
- Yang, S.; Dai, F.; Lu, L.; Yin, M.; Xue, L.; Feng, W.; Li, B.; Jiao, J.; Chen, Q. All-in-one calcium nanoflowers for dual outputs biosensor: A simultaneous strategy for depression drug evaluation and non-invasive stress assessment. Biosens. Bioelectr. 2022, 216, 114655. [Google Scholar] [CrossRef]
- Singh, N.K.; Arya, S.K.; Estrela, P.; Goswami, P. Capacitive malaria aptasensor using Plasmodium falciparum glutamate dehydrogenase as target antigen in undiluted human serum. Biosens. Bioelectr. 2018, 117, 246–252. [Google Scholar] [CrossRef]
- Sun, A.C.; Hall, D.A. Point-of-Care Smartphone-based Electrochemical Biosensing. Electroanalysis 2019, 31, 2–16. [Google Scholar] [CrossRef]
- Venkatesh, A.G.; Brickner, H.; Looney, D.; Hall, D.A.; Aronoff, S.E. Clinical detection of Hepatitis C viral infection by yeast-secreted HCV-core: Gold-binding-peptide. Biosens. Bioelectr. 2018, 119, 230–236. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics0. Biosens. Bioelectr. 2006, 21, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
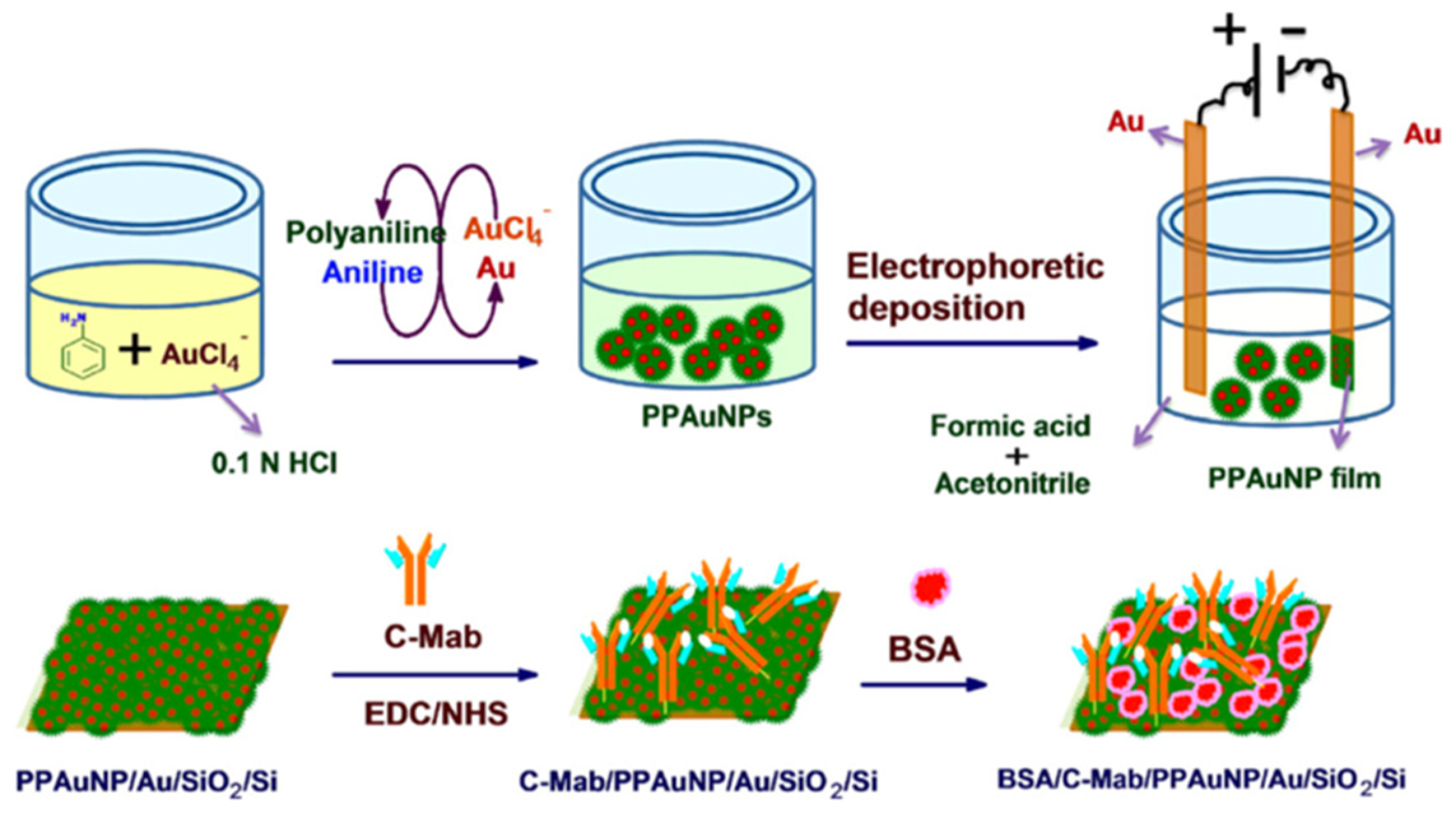
- Arya, S.K.; Dey, A.; Bhansali, S. Polyaniline protected gold nanoparticles based mediator and label free electrochemical cortisol biosensor. Biosens. Bioelectr. 2011, 28, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Vabbina, P.K.; Kaushik, A.; Pokhrel, N.; Bhansali, S.; Pala, N. Electrochemical cortisol immunosensors based on sonochemically synthesized zinc oxide 1D nanorods and 2D nanoflakes. Biosens. Bioelectr. 2015, 63, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Moore, J.A.; Chávez, J.L.; Hagen, J.A.; Loughnane, N.K.; Chou, C.-F.; Swami, N.S. Aptamer-functionalized nanoparticles for surface immobilization-free electrochemical detection of cortisol in a microfluidic device. Biosens. Bioelectr. 2016, 78, 244–252. [Google Scholar] [CrossRef]
- Nijhuis, J.; Schmidt, S.; Tran, N.N.; Hessel, V. Microfluidics and Macrofluidics in Space: ISS-Proven Fluidic Transport and Handling Concepts Front. Sp. Technol. 2022, 2, 779696. [Google Scholar] [CrossRef]
- Sun, B.; Gou, Y.; Ma, Y.; Zheng, X.; Bai, R.; Abdelmoaty, A.A.A.; Hu, F. Investigate electrochemical immunosensor of cortisol based on gold nanoparticles/magnetic functionalized reduced graphene oxide Author links open overlay panel. Biosens. Bioelectr. 2017, 88, 55–62. [Google Scholar] [CrossRef]
- Liu, X.; Hsu, S.P.C.; Liu, W.-C.; Wang, Y.-M.; Liu, X.; Lo, C.-S.; Lin, Y.-C.; Nabilla, S.C.; Li, Z.; Hong, Y.; et al. Salivary Electrochemical Cortisol Biosensor Based on Tin Disulfide Nanoflakes. Nanoscale Res. Lett. 2019, 14, 14–189. [Google Scholar] [CrossRef]
- Wang, C.; Tang, K.; Yang, Q.; Qian, Y. Raman scattering, far infrared spectrum and photoluminescence of SnS2 nanocrystallites. Chem. Phys. Lett. 2002, 357, 371–375. [Google Scholar] [CrossRef]
- Klinghammer, S.; Voitsekhivska, T.; Licciardello, N.; Kim, K.; Baek, C.-K.; Cho, H.; Wolter, K.-J.; Kirschbaum, C.; Baraban, L.; Cuniberti, G. Nanosensor-Based Real-Time Monitoring of Stress Biomarkers in Human Saliva Using a Portable Measurement System. ACS Sens. 2020, 5, 4081–4091. [Google Scholar] [CrossRef]
- Tran, D.P.; Winter, M.A.; Wolfrum, B.; Stockmann, R.; Yang, C.-T.; Pourhassan-Moghaddam, M.; Offenhaüsser, A.; Thierry, B. Toward Intraoperative Detection of Disseminated Tumor Cells in Lymph Nodes with Silicon Nanowire Field Effect Transistors. ACS Nano 2016, 10, 2357–2364. [Google Scholar] [CrossRef]
- Martin, J.A.; Chávez, J.L.; Chushak, Y.; Chapleau, R.R.; Hagen, J.; Kelley-Loughnane, N. Tunable Stringency Aptamer Selection and Gold Nanoparticle Assay for Detection of Cortisol. Anal. Bioanal. Chem. 2014, 406, 4637–4647. [Google Scholar] [CrossRef] [PubMed]
- Madhu, S.; Anthuuvan, A.J.; Ramasamy, S.; Manickam, P.; Bhansali, S.; Nagamony, P.; Chinnuswamy, V. ZnO Nanorod Integrated Flexible Carbon Fibers for Sweat Cortisol Detection. ACS Appl. Electron. Mater. 2020, 2, 499–509. [Google Scholar] [CrossRef]
- Rison, S.; Rajeev, R.; Bhat, V.S.; Mathews, A.T.; Varghese, A.; Hegde, G. Non-enzymatic electrochemical determination of salivary cortisol using ZnO-graphene nanocomposites. RSC Adv. 2021, 11, 37877. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, A.; Mujawar, M.A.; Manickam, P.; Bhansali, S. Plasma-Induced Enhancement in Electronic Properties of Gold Nanoparticles: Application in Electrochemical Biosensing of Cortisol. ACS Appl. Electron. Mater. 2021, 3, 230–237. [Google Scholar] [CrossRef]
- Singh, N.K.; Chung, S.; Sveiven, M.; Hall, D.A. Cortisol Detection in Undiluted Human Serum Using a Sensitive Electrochemical Structure-Switching Aptamer over an Antifouling Nanocomposite Layer. ACS Omega 2021, 6, 27888–27897. [Google Scholar] [CrossRef]
- Madhu, S.; Ramasamy, S.; Magudeeswaran, V.; Manickam, P.; Nagamony, P.; Chinnuswamy, V. SnO2 nanoflakes deposited carbon yarn-based electrochemical immunosensor towards cortisol measurement. J. Nanostruct. Chem. 2022, in press. [Google Scholar] [CrossRef]
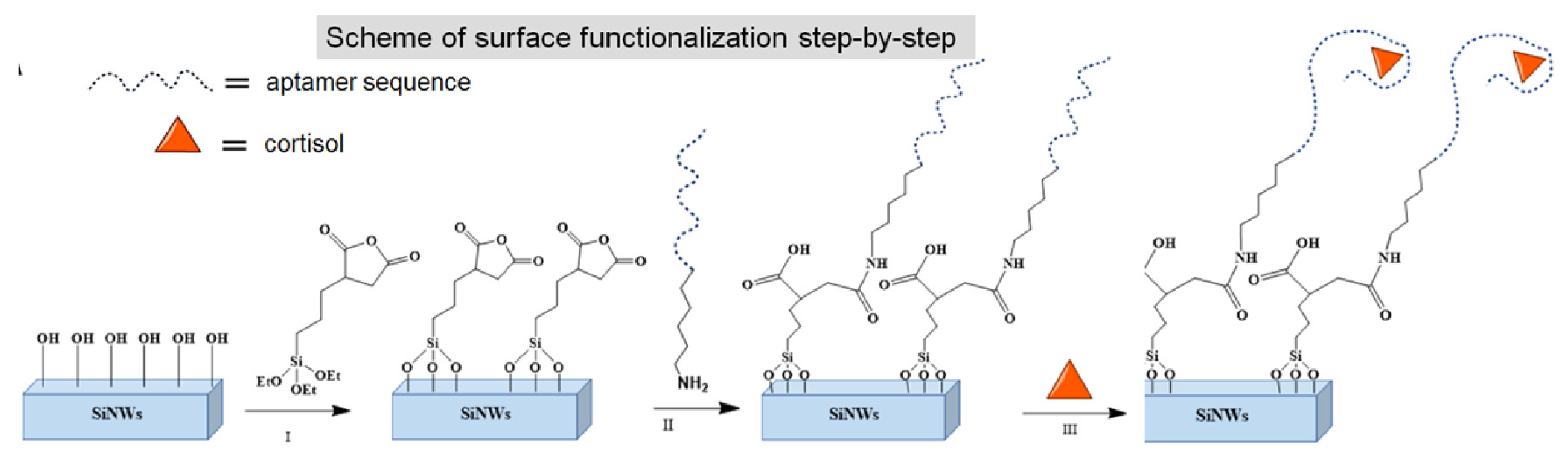
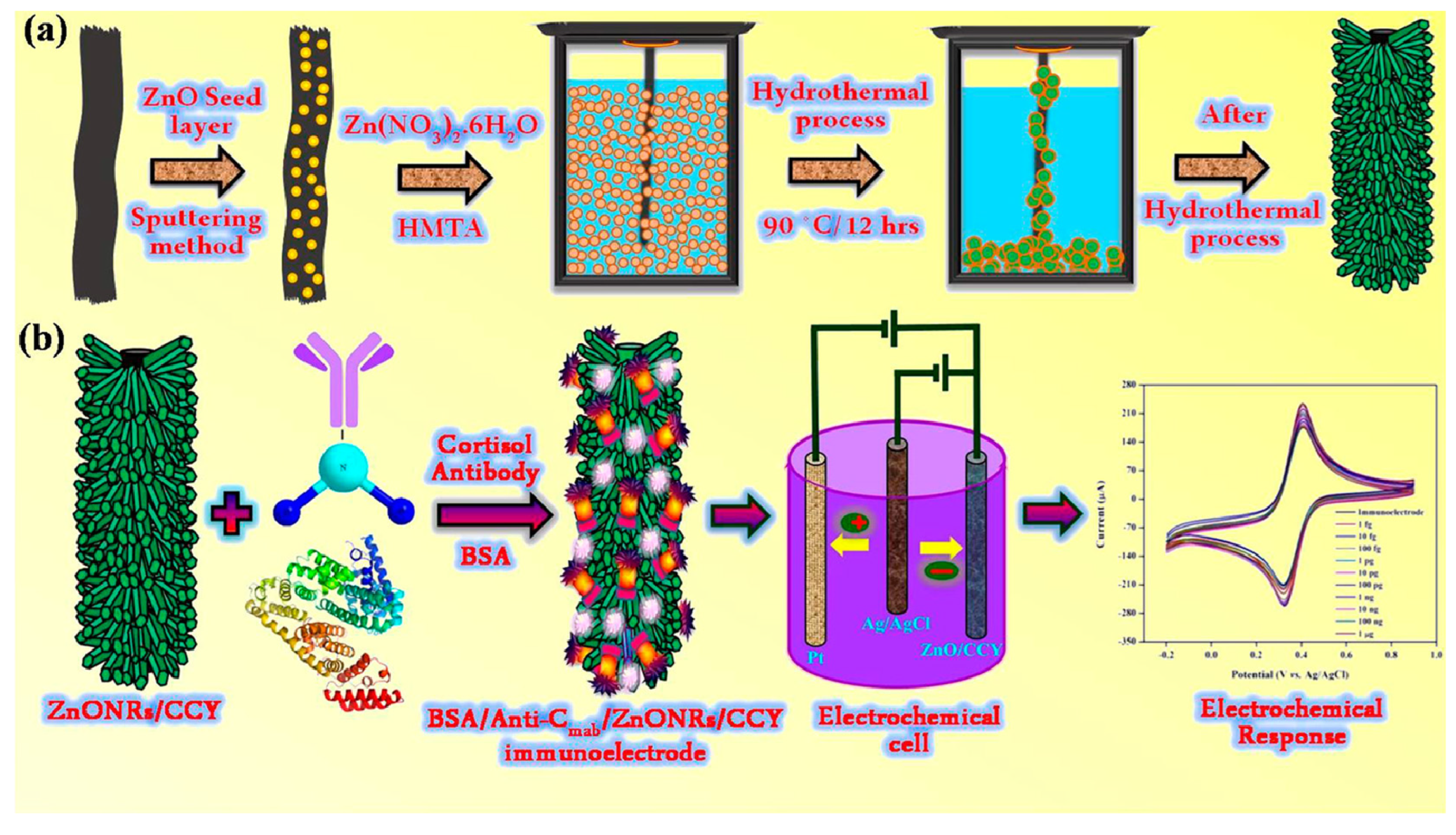
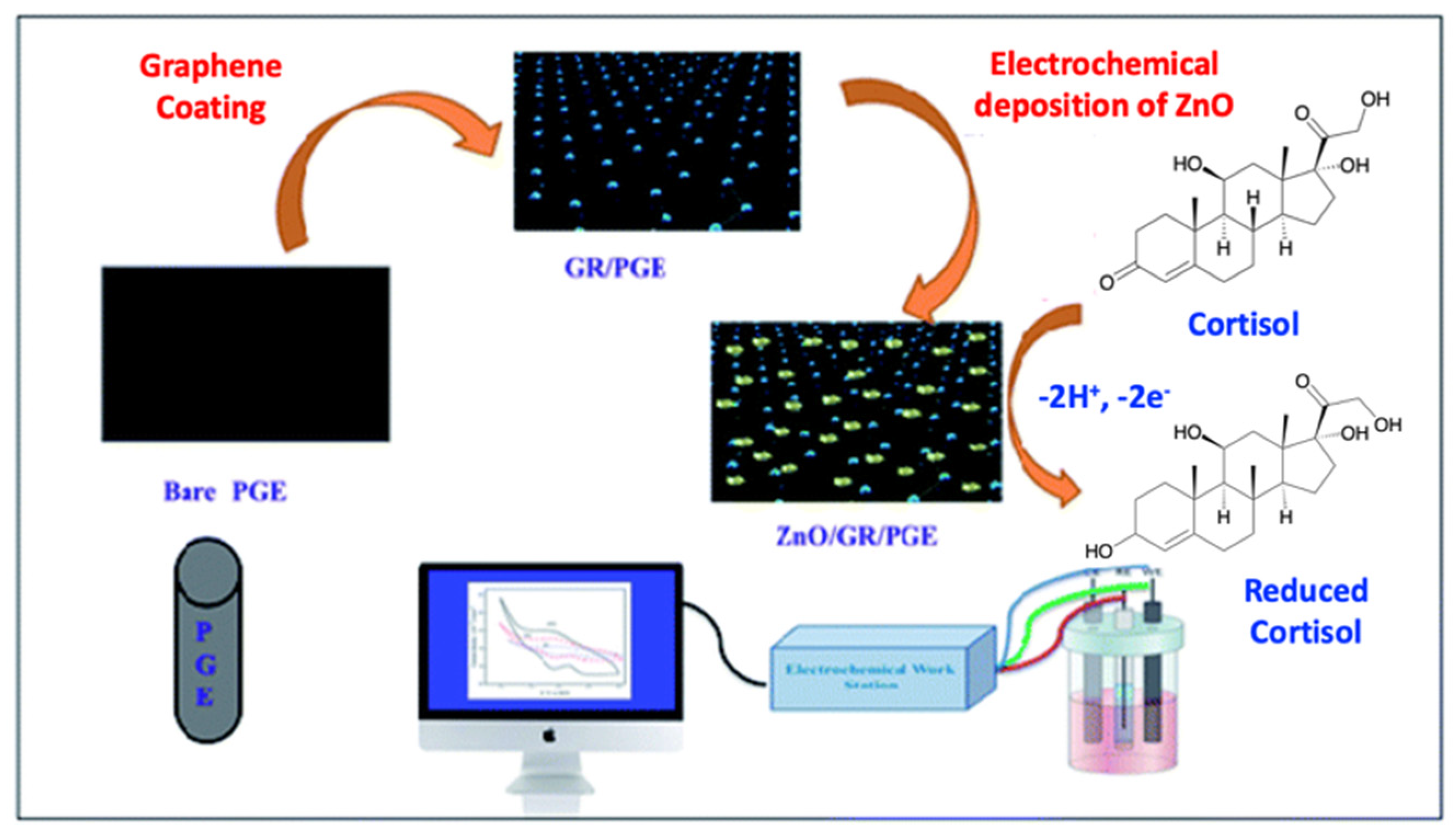
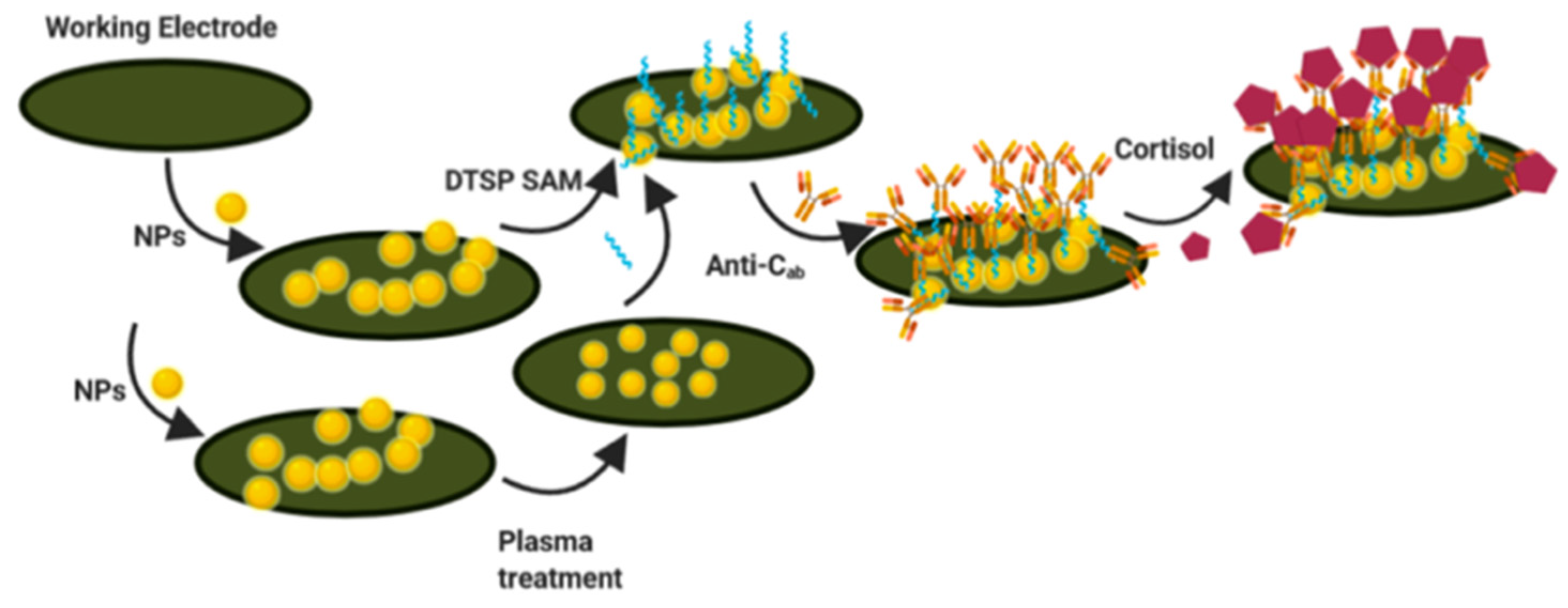
| Sample | Cortisol Concentrations |
|---|---|
| Blood | 25 mg/mL (morning), 2 mg/mL (midnight) |
| Urine a | 21,458–149,696 ng/24 h 44,000–140,000 ng/24 h |
| Saliva | 1–12 ng/mL (morning), 0.1–3 ng/mL (evening) |
| Sweat | 8–142 ng/mL |
| Hair | 18–153 pg/mg |
| Interstitial fluid (ISF) | 12–34 ng/mL (morning), 9–13 ng/mL (evening) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trusso Sfrazzetto, G.; Santonocito, R. Nanomaterials for Cortisol Sensing. Nanomaterials 2022, 12, 3790. https://doi.org/10.3390/nano12213790
Trusso Sfrazzetto G, Santonocito R. Nanomaterials for Cortisol Sensing. Nanomaterials. 2022; 12(21):3790. https://doi.org/10.3390/nano12213790
Chicago/Turabian StyleTrusso Sfrazzetto, Giuseppe, and Rossella Santonocito. 2022. "Nanomaterials for Cortisol Sensing" Nanomaterials 12, no. 21: 3790. https://doi.org/10.3390/nano12213790
APA StyleTrusso Sfrazzetto, G., & Santonocito, R. (2022). Nanomaterials for Cortisol Sensing. Nanomaterials, 12(21), 3790. https://doi.org/10.3390/nano12213790