The Antimicrobial Effect of Gold Quantum Dots and Femtosecond Laser Irradiation on the Growth Kinetics of Common Infectious Eye Pathogens: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
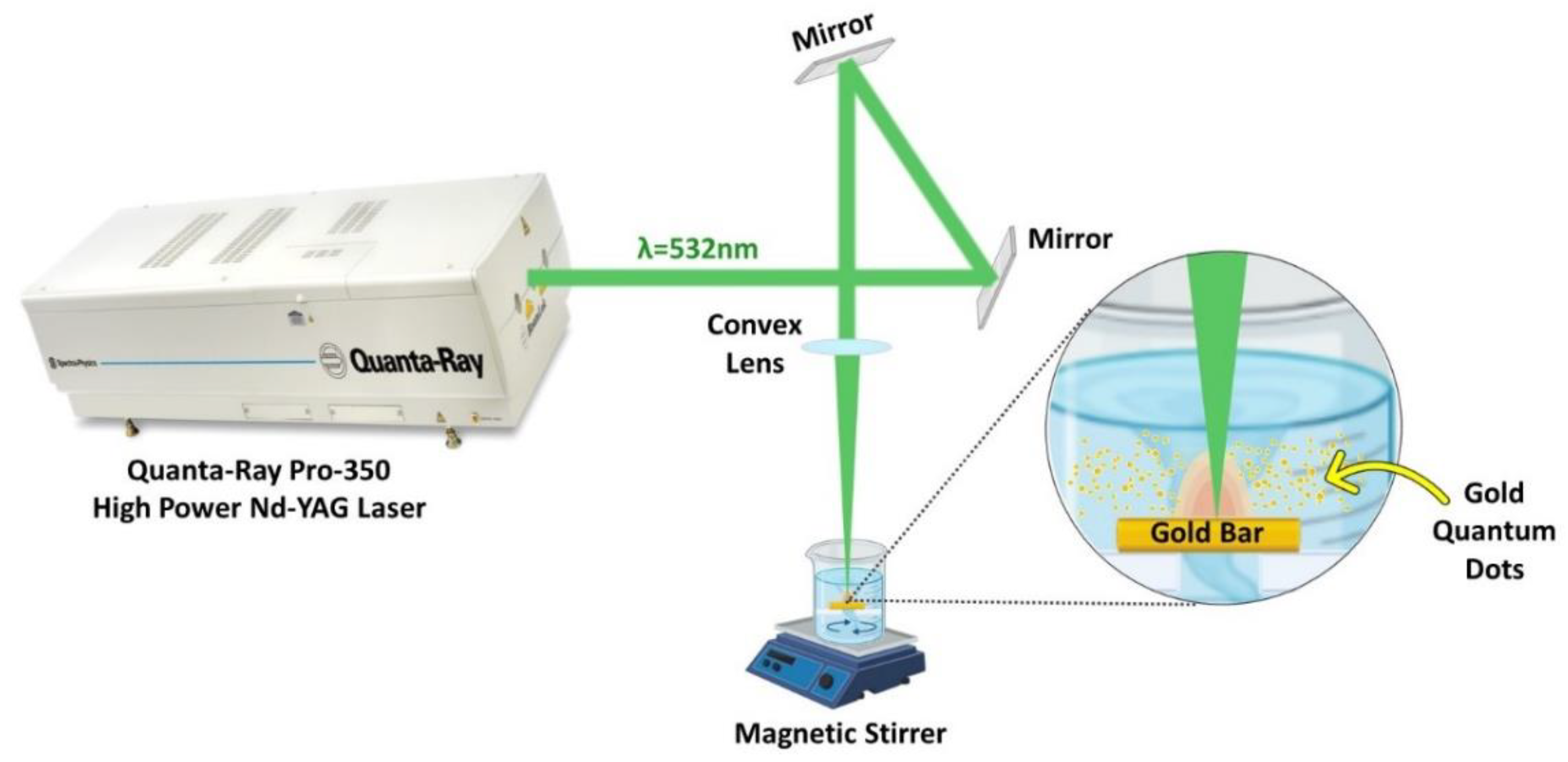
2.1. High-Power Nanosecond Laser System Preparation and Setup for Synthesis of AuQDs
2.2. Characterization of Laser Ablated Au Quantum Dots
2.3. Microorganism and Culture Conditions
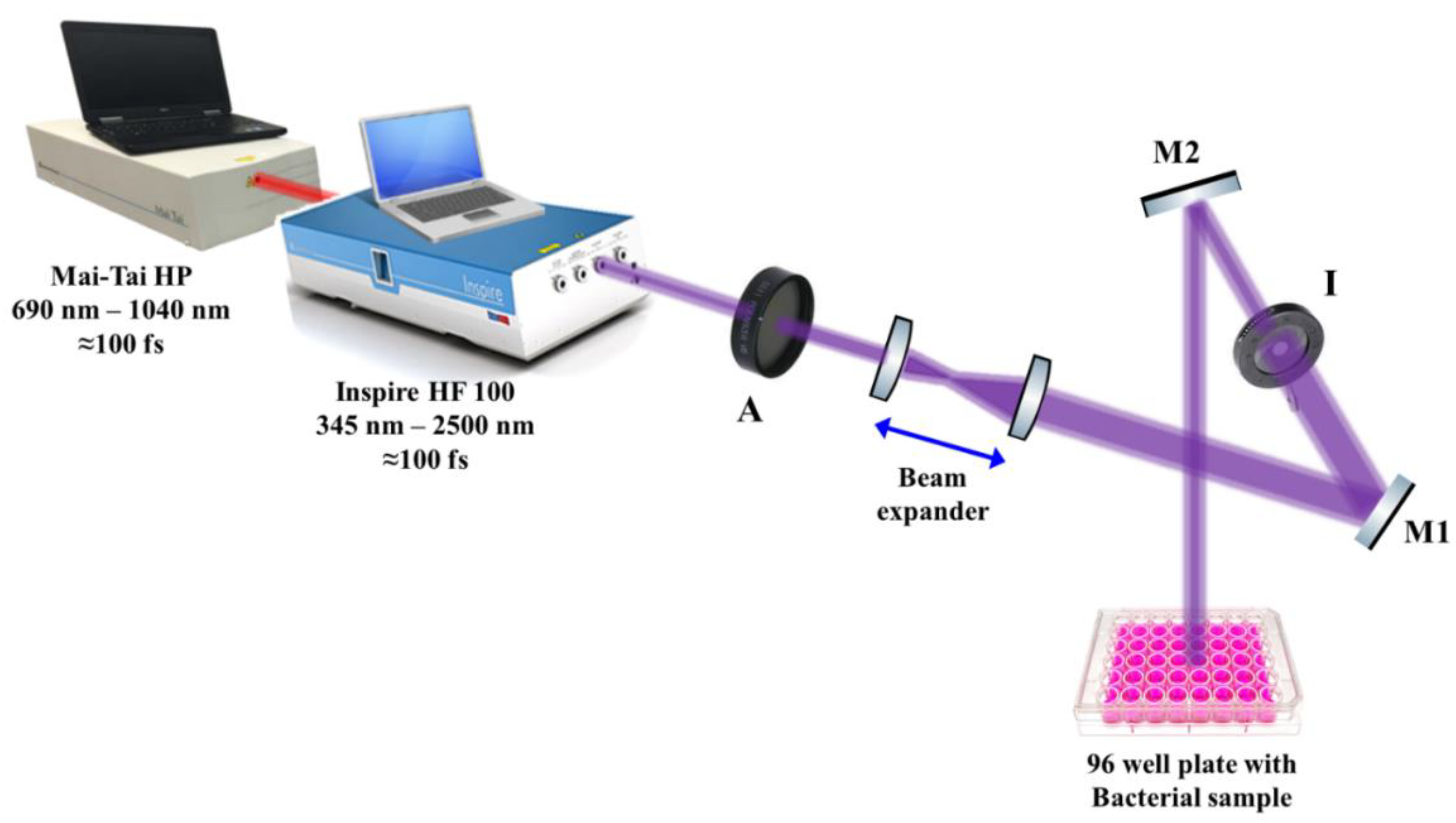
2.4. Femtosecond Laser System Preparation and Setup
2.5. Evaluation of the Growth Kinetics of Gram-Positive and Gram-Negative Bacterial Pathogens after Treatment
2.6. Evaluation of the Growth Kinetics of Candida albicans after Treatment with Laser-Ablated AuQDs and Femtosecond Laser at 400 nm
2.7. Cytotoxicity and Biocompatibility of AuQDs to Adult Retinal Cell Line Using MTT Assay
2.8. In Vitro Wound Scratch Assay of Adult Retinal Cell Line after Treatment with Laser-Ablated AuQDs
2.9. Antioxidant Activities of AuQDs by DPPH Free Radical Scavenging Assay
2.10. Data Analysis
3. Results and Discussion
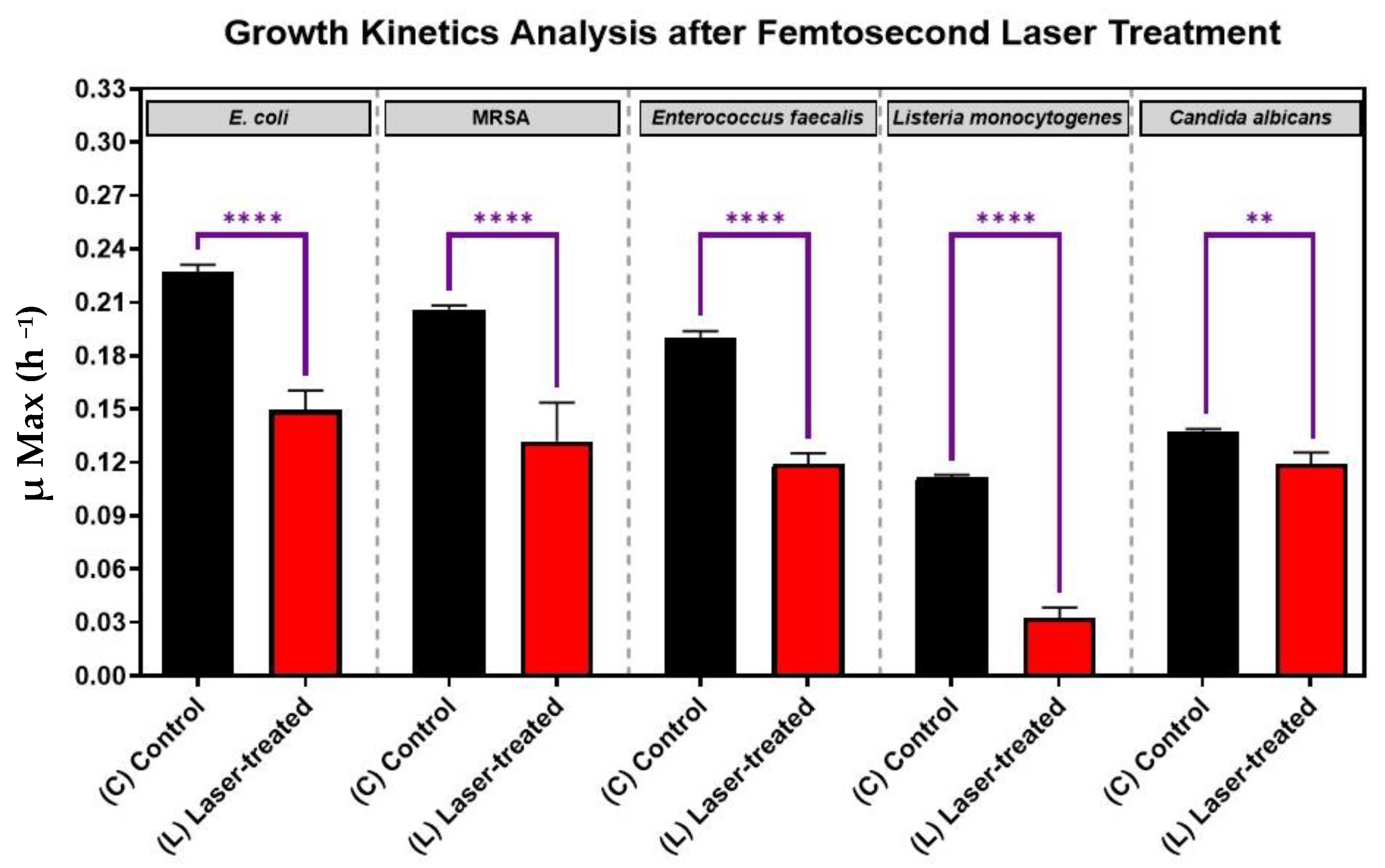
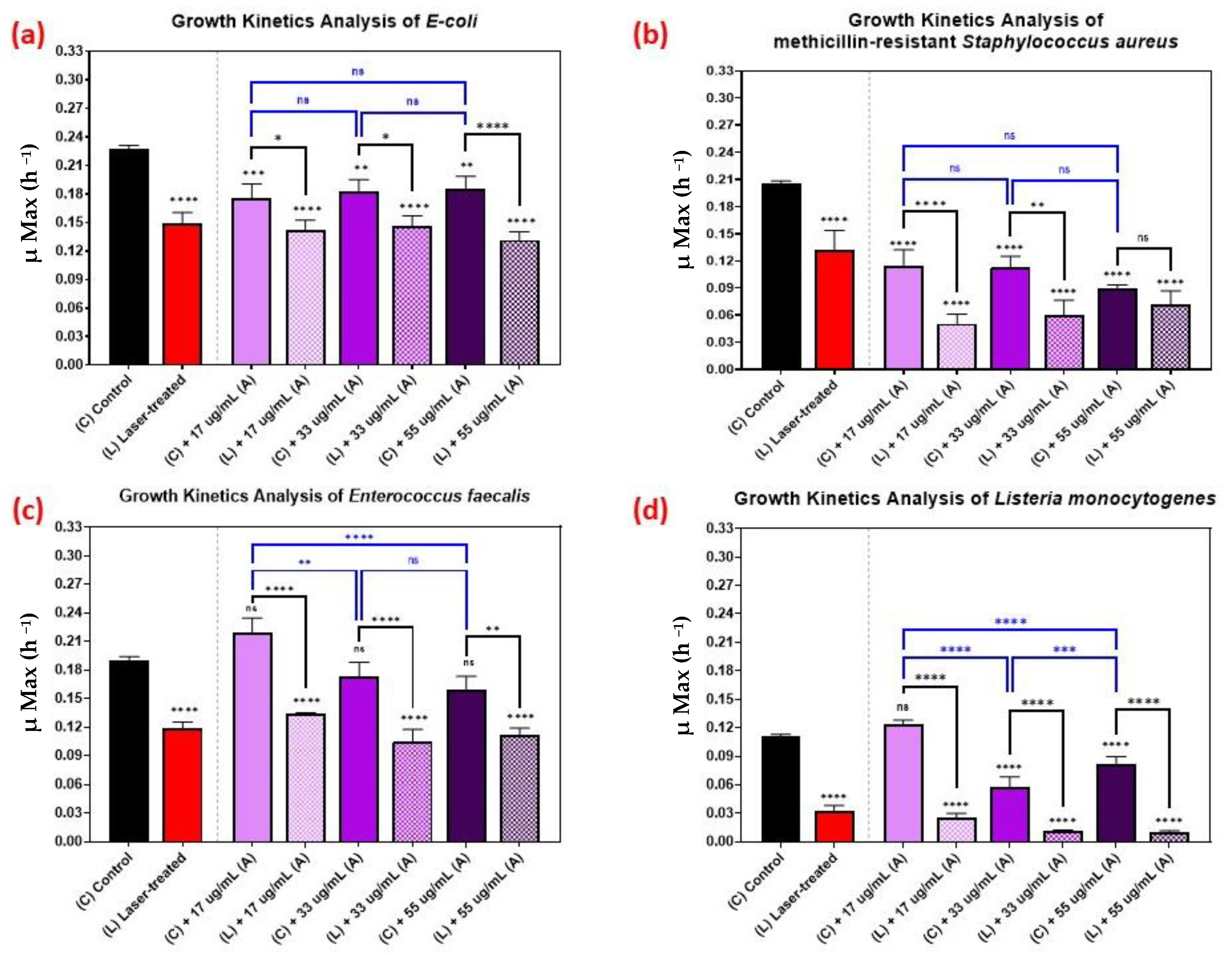
3.1. Growth Kinetics of Femtosecond Laser-Treated Pathogens
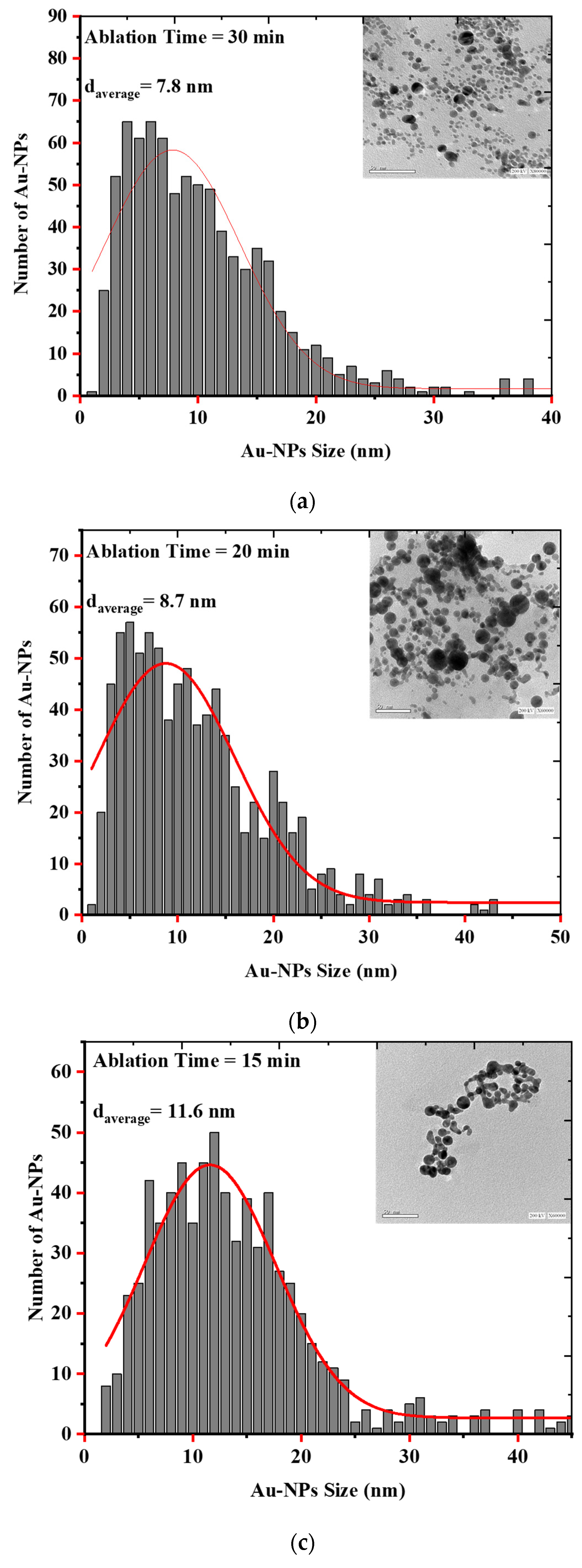
3.2. Synthesis and Characterization of AuQDs for Antimicrobial Testing
3.3. Effect of Nanoparticle Size on the Growth Kinetics of the Microbial Pathogens
3.4. Effect of Nanoparticle Concentration on the Growth Kinetics of the Microbial Pathogens
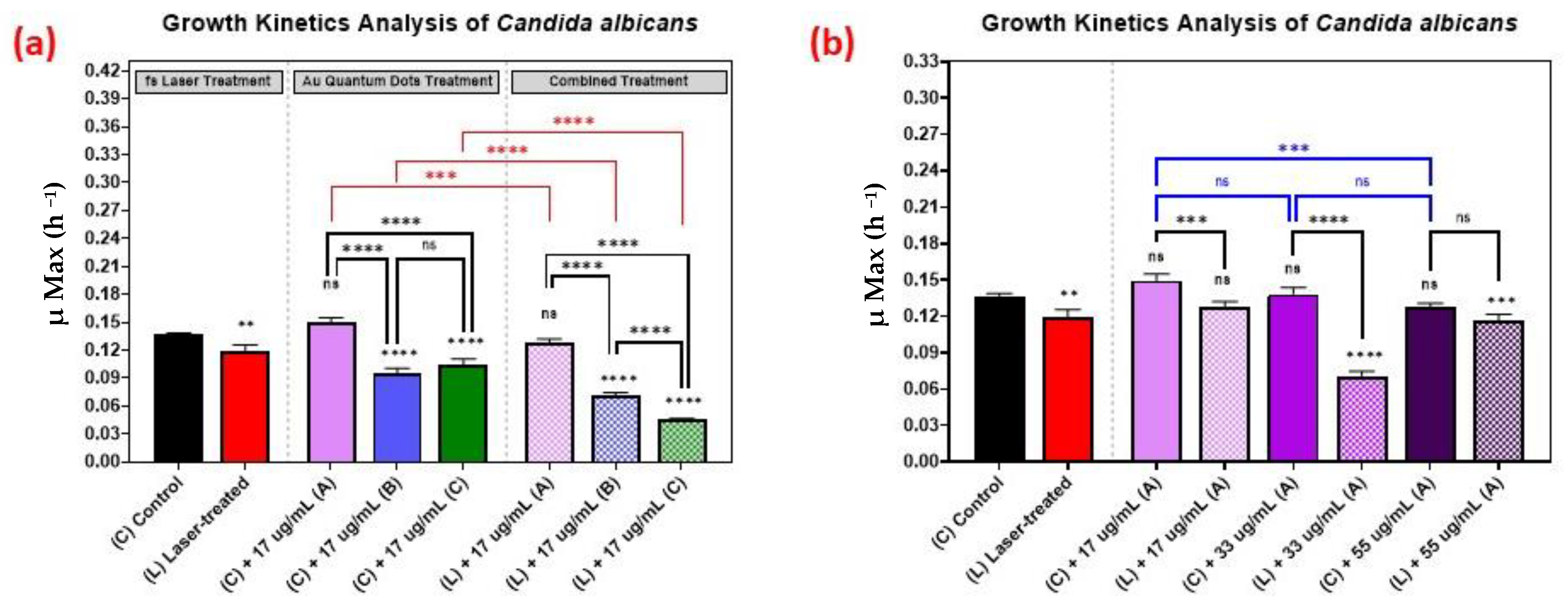
3.5. Growth Kinetics Analysis of Candida albicans
3.6. Cytotoxicity, Biocompatibility, and Antioxidant Ability of AuQDs
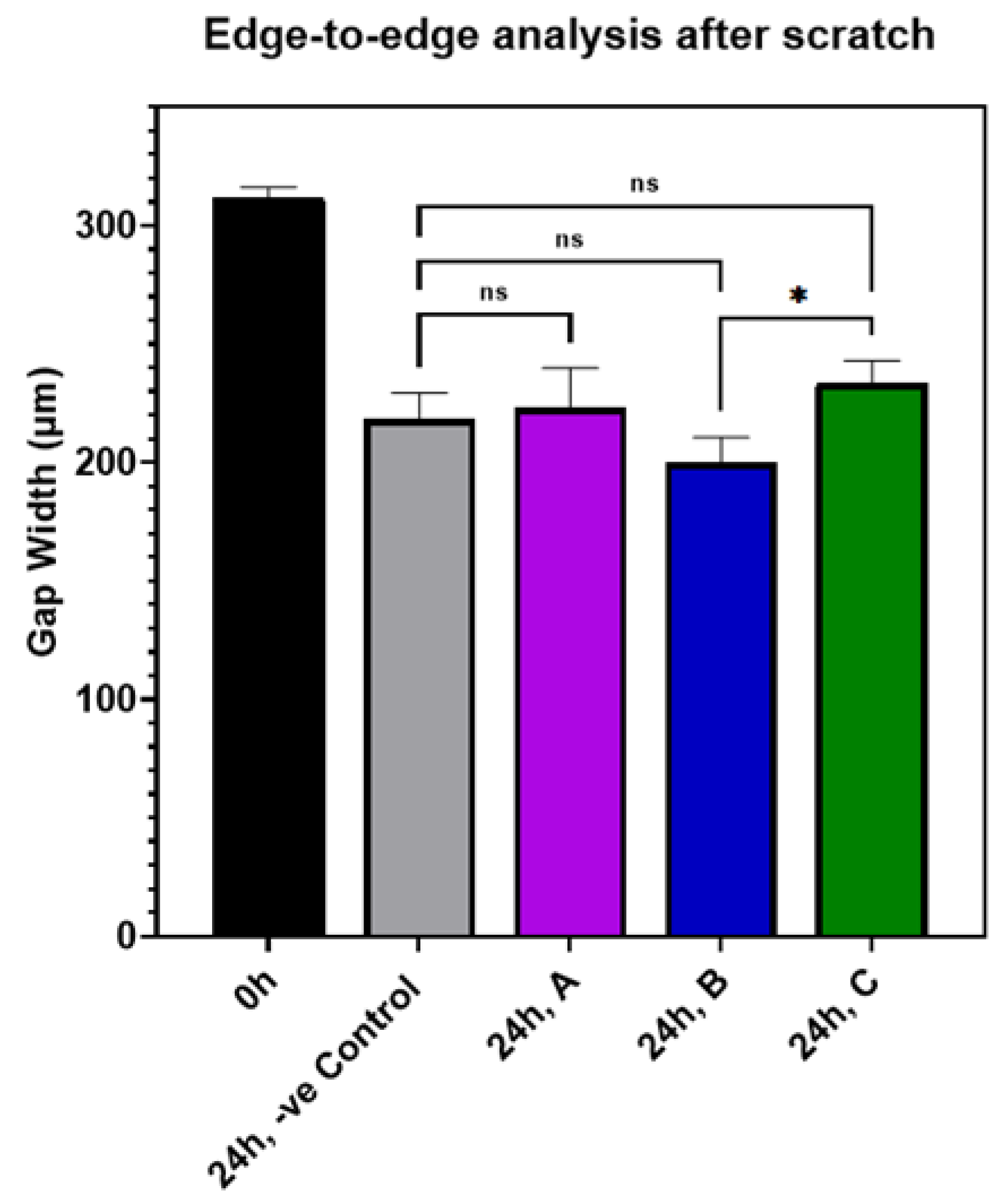
3.7. Effect of AuQDs on Wound Closure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Long, C.; Liu, B.; Xu, C.; Jing, Y.; Yuan, Z.; Lin, X. Causative organisms of post-traumatic endophthalmitis: A 20-year retrospective study. BMC Ophthalmol. 2014, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Teweldemedhin, M.; Saravanan, M.; Gebreyesus, A.; Gebreegziabiher, D. Ocular bacterial infections at Quiha Ophthalmic Hospital, Northern Ethiopia: An evaluation according to the risk factors and the antimicrobial susceptibility of bacterial isolates. BMC Infect. Dis. 2017, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Toshida, H.; Honda, R.; Matsui, A.; Ohta, T.; Asada, Y.; Murakami, A. Prevalence of drug resistance and culture-positive rate among microorganisms isolated from patients with ocular infections over a 4-year period. Clin. Ophthalmol. 2013, 7, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Bertino, J.S., Jr. Impact of antibiotic resistance in the management of ocular infections: The role of current and future antibiotics. Clin. Ophthalmol. 2009, 3, 507–521. [Google Scholar] [CrossRef]
- Teweldemedhin, M.; Gebreyesus, H.; Atsbaha, A.H.; Asgedom, S.W.; Saravanan, M. Bacterial profile of ocular infections: A systematic review. BMC Ophthalmol. 2017, 17, 212. [Google Scholar] [CrossRef]
- Barge, S.; Rothwell, R.; Varandas, R.; Agrelos, L. Enterococcus faecalis Endogenous Endophthalmitis from Valvular Endocarditis. Case Rep. Ophthalmol. Med. 2013, 2013, 174869. [Google Scholar] [CrossRef]
- Greenwald, M.J.; Wohl, L.G.; Sell, C.H. Metastatic bacterial endophthalmitis: A contemporary reappraisal. Surv. Ophthalmol. 1986, 31, 81–101. [Google Scholar] [CrossRef]
- Okada, A.A.; Johnson, R.P.; Liles, W.C.; D’Amico, D.J.; Baker, A.S. Endogenous bacterial endophthalmitis: Report of a ten-year retrospective study. Ophthalmology 1994, 101, 832–838. [Google Scholar] [CrossRef]
- Vaziri, K.; Schwartz, S.G.; Kishor, K.; Flynn, H.W., Jr. Endophthalmitis: State of the art. Clin. Ophthalmol. 2015, 9, 95–108. [Google Scholar] [CrossRef]
- Kresloff, M.S.; Castellarin, A.A.; Zarbin, M.A. Endophthalmitis. Surv. Ophthalmol. 1998, 43, 193–224. [Google Scholar] [CrossRef]
- Khosravi, A.D.; Mehdinejad, M.; Heidari, M. Bacteriological findings in patients with ocular infection and antibiotic susceptibility patterns of isolated pathogens. Singap. Med. J. 2007, 48, 741–743. [Google Scholar]
- Hemavathi; Sarmah, P.; Shenoy, P. Profile of microbial isolates in ophthalmic infections and antibiotic susceptibility of the bacterial isolates: A study in an eye care hospital, bangalore. J. Clin. Diagn. Res. JCDR 2014, 8, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and antibacterial properties of gold nanoparticles: A review. Environ. Chem. Lett. 2021, 19, 167–187. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, L. Nanomaterials arising amid antibiotic resistance. Nat. Rev. Microbiol. 2021, 19, 5–6. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Galdiero, E.; Falanga, A.; Carotenuto, R.; de Alteriis, E.; Guida, M. Toxicity Effects of Functionalized Quantum Dots, Gold and Polystyrene Nanoparticles on Target Aquatic Biological Models: A Review. Molecules 2017, 22, 1439. [Google Scholar] [CrossRef] [PubMed]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef]
- Natan, M.; Banin, E. From Nano to Micro: Using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol. Rev. 2017, 41, 302–322. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, B. Nanoparticle-based photodynamic therapy: New trends in wound healing applications. Mater. Today Adv. 2020, 6, 100049. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.P.; Wang, L.; Benicewicz, B.C.; Decho, A.W. Inorganic nanoparticles engineered to attack bacteria. Chem. Soc. Rev. 2015, 44, 7787–7807. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, V.; Mukherjee, S.; Mukherjee, S.; Singh, S.P.; Prasad, T. ZnO Quantum Dots: Broad Spectrum Microbicidal Agent against Multidrug Resistant Pathogens E. coli and C. albicans. Front. Nanotechnol. 2020, 2, 576342. [Google Scholar]
- Gupta, A.; Moyano, D.F.; Parnsubsakul, A.; Papadopoulos, A.; Wang, L.S.; Landis, R.F.; Das, R.; Rotello, V.M. Ultrastable and Biofunctionalizable Gold Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14096–14101. [Google Scholar] [CrossRef]
- Carrière, M. What About Toxicity and Ecotoxicity of Gold Nanoparticles? In Gold Nanoparticles for Physics, Chemistry and Biology; World Scientific: Singapore, 2012; pp. 333–353. [Google Scholar]
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the Next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kang, H.W. Engineering pharmaceutical nanocarriers for photodynamic therapy on wound healing: Review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110110. [Google Scholar] [CrossRef]
- Pajerski, W.; Ochonska, D.; Brzychczy-Wloch, M.; Indyka, P.; Jarosz, M.; Golda-Cepa, M.; Sojka, Z.; Kotarba, A. Attachment efficiency of gold nanoparticles by Gram-positive and Gram-negative bacterial strains governed by surface charges. J. Nanopart. Res. 2019, 21, 186. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Hayden, S.C.; Zhao, G.; Saha, K.; Phillips, R.L.; Li, X.; Miranda, O.R.; Rotello, V.M.; El-Sayed, M.A.; Schmidt-Krey, I.; Bunz, U.H. Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J. Am. Chem. Soc. 2012, 134, 6920–6923. [Google Scholar] [CrossRef] [PubMed]
- Simon-Deckers, A.; Loo, S.; Mayne-L’hermite, M.; Herlin-Boime, N.; Menguy, N.; Reynaud, C.; Gouget, B.; Carrière, M. Size-, composition- and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ. Sci. Technol. 2009, 43, 8423–8429. [Google Scholar] [CrossRef] [PubMed]
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; El-Gendy, A.O.; Hamblin, M.R.; Mohamed, T. The effect of femtosecond laser irradiation on the growth kinetics of Staphylococcus aureus: An in vitro study. J. Photochem. Photobiol. B Biol. 2021, 221, 112240. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, A.O.; Samir, A.; Ahmed, E.; Enwemeka, C.S.; Mohamed, T. The antimicrobial effect of 400 nm femtosecond laser and silver nanoparticles on gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B Biol. 2021, 223, 112300. [Google Scholar] [CrossRef]
- Boly, R.; Lamkami, T.; Lompo, M.; Dubois, J.; Guissou, I. DPPH free radical scavenging activity of two extracts from Agelanthus dodoneifolius (Loranthaceae) leaves. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 29–34. [Google Scholar]
- Ahmed, E.; El-Gendy, A.O.; Moniem Radi, N.A.; Mohamed, T. The bactericidal efficacy of femtosecond laser-based therapy on the most common infectious bacterial pathogens in chronic wounds: An in vitro study. Lasers Med. Sci. 2021, 36, 641–647. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Cassidy, C.M.; Tunney, M.M.; McCarron, P.A.; Donnelly, R.F. Drug delivery strategies for photodynamic antimicrobial chemotherapy: From benchtop to clinical practice. J. Photochem. Photobiol. B Biol. 2009, 95, 71–80. [Google Scholar] [CrossRef]
- ICNIRP Guidelines on Limits of Exposure to Laser Radiation of Wavelengths between 180 nm and 1000 μm. Health Phys. 2013, 105, 271–295. [CrossRef] [PubMed]
- Mocan, L.; Tabaran, F.A.; Mocan, T.; Pop, T.; Mosteanu, O.; Agoston-Coldea, L.; Matea, C.T.; Gonciar, D.; Zdrehus, C.; Iancu, C. Laser thermal ablation of multidrug-resistant bacteria using functionalized gold nanoparticles. Int. J. Nanomed. 2017, 12, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, H.; Yu, Y.; Sun, L.; Liu, S.; Zhang, C.; He, L. Morphology-dependent bactericidal activities of Ag/CeO2 catalysts against Escherichia coli. J. Inorg. Biochem. 2014, 135, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Tamayo, L.A.; Zapata, P.A.; Vejar, N.D.; Azócar, M.I.; Gulppi, M.A.; Zhou, X.; Thompson, G.E.; Rabagliati, F.M.; Páez, M.A. Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 24–31. [Google Scholar] [CrossRef]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Gois, M.M.; Kurachi, C.; Santana, E.J.; Mima, E.G.; Spolidório, D.M.; Pelino, J.E.; Salvador Bagnato, V. Susceptibility of Staphylococcus aureus to porphyrin-mediated photodynamic antimicrobial chemotherapy: An in vitro study. Lasers Med. Sci. 2010, 25, 391–395. [Google Scholar] [CrossRef]
- Hanakova, A.; Bogdanova, K.; Tomankova, K.; Pizova, K.; Malohlava, J.; Binder, S.; Bajgar, R.; Langova, K.; Kolar, M.; Mosinger, J.; et al. The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin. Microbiol. Res. 2014, 169, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Samaneh, R.; Ali, Y.; Mostafa, J.; Mahmud, N.A.; Zohre, R. Laser therapy for wound healing: A review of current techniques and mechanisms of action. Biosci. Biotechnol. Res. Asia 2015, 12, 217–223. [Google Scholar] [CrossRef]
- Medrado, A.R.; Pugliese, L.S.; Reis, S.R.; Andrade, Z.A. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg. Med. 2003, 32, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson, R.S., Jr.; Dutta, P.K.; Waldman, W.J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833–1852. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Leung, Y.H.; Ng, A.M.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.; Guo, M.Y.; Ng, Y.H.; Djurišić, A.B.; et al. Mechanisms of antibacterial activity of MgO: Non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef]
- Palermo, E.F.; Kuroda, K. Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biotechnol. 2010, 87, 1605–1615. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.-G.; Park, H.-J.; Liu, C.-G.; Liu, C.-S.; Meng, X.-H.; Yu, L.-J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Memar, M.Y.; Ghotaslou, R.; Samiei, M.; Adibkia, K. Antimicrobial use of reactive oxygen therapy: Current insights. Infect. Drug Resist. 2018, 11, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Benítez, E.A.; Velázquez-Guadarrama, N.; Durán Figueroa, N.V.; Quezada, H.; Olivares-Trejo, J.J. Antibacterial mechanism of gold nanoparticles on Streptococcus pneumoniae. Met. Integr. Biometal Sci. 2019, 11, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef]
- Gao, Y.-H.; Zhang, N.-C.; Zhong, Y.-W.; Cai, H.-H.; Liu, Y.-L. Preparation and characterization of antibacterial Au/C core–shell composite. Appl. Surf. Sci. 2010, 256, 6580–6585. [Google Scholar] [CrossRef]
- Clark, C., III; Marshall, W.; Thomas, R. Theoretical analysis of multiple-pulse thermal damage thresholds of the retina. J. Laser Appl. 2013, 25, 012005. [Google Scholar] [CrossRef]
- Faktorovich, E.G. Femtodynamics: Optimizing femtosecond laser settings and procedure techniques to optimize outcomes. Int. Ophthalmol. Clin. 2008, 48, 41–50. [Google Scholar] [CrossRef]
- Vogel, A.; Noack, J.; Hüttman, G.; Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 2005, 81, 1015–1047. [Google Scholar] [CrossRef]
- Norouzi, M. Gold Nanoparticles in Glioma Theranostics. Pharmacol. Res. 2020, 156, 104753. [Google Scholar] [CrossRef]
- El-Gendy, A.O.; Nawaf, K.T.; Ahmed, E.; Samir, A.; Hamblin, M.R.; Hassan, M.; Mohamed, T. Preparation of zinc oxide nanoparticles using laser-ablation technique: Retinal epithelial cell (ARPE-19) biocompatibility and antimicrobial activity when activated with femtosecond laser. J. Photochem. Photobiol. B Biol. 2022, 234, 112540. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Ferrari, M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials 2006, 27, 5307–5314. [Google Scholar] [CrossRef] [PubMed]
- Chavanpatil, M.D.; Khdair, A.; Panyam, J. Nanoparticles for cellular drug delivery: Mechanisms and factors influencing delivery. J. Nanosci. Nanotechnol. 2006, 6, 2651–2663. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Causa, F.; Ferrari, M.; Netti, P.A. The effective dispersion of nanovectors within the tumor microvasculature. Ann. Biomed. Eng. 2006, 34, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef]
- Douglass, M.; Bezak, E.; Penfold, S. Monte Carlo investigation of the increased radiation deposition due to gold nanoparticles using kilovoltage and megavoltage photons in a 3D randomized cell model. Med. Phys. 2013, 40, 071710. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, H.; Khoshgard, K.; Akbarzadeh, F. In vitro outlook of gold nanoparticles in photo-thermal therapy: A literature review. Lasers Med. Sci. 2018, 33, 917–926. [Google Scholar] [CrossRef]


| Microorganism | Strain | Culture Conditions | |
|---|---|---|---|
| Culture Media | Temperature (°C) | ||
| Staphylococcus aureus MRSA | ATCC 43300 | BHI broth | 37 |
| Listeria monocytogenes | ATCC 7644 | BHI broth | 30 |
| Enterococcus faecalis | V583 | BHI broth | 37 |
| E. coli | ATCC 6933 | BHI broth | 37 |
| Candida albicans | ATCC 60913 | BHI broth | 30 |
| Negative Control | DMSO | Sample A | Sample B | Sample C | |
|---|---|---|---|---|---|
| Survival % of retinal epithelial cells at 20 µg/mL (Mean ± SD) | 100 ± 0.64 | 99 ± 1.20 | 86.8 ± 1.30 | 83.5 ± 2.90 | 75.8 ± 1.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gendy, A.O.; Obaid, Y.; Ahmed, E.; Enwemeka, C.S.; Hassan, M.; Mohamed, T. The Antimicrobial Effect of Gold Quantum Dots and Femtosecond Laser Irradiation on the Growth Kinetics of Common Infectious Eye Pathogens: An In Vitro Study. Nanomaterials 2022, 12, 3757. https://doi.org/10.3390/nano12213757
El-Gendy AO, Obaid Y, Ahmed E, Enwemeka CS, Hassan M, Mohamed T. The Antimicrobial Effect of Gold Quantum Dots and Femtosecond Laser Irradiation on the Growth Kinetics of Common Infectious Eye Pathogens: An In Vitro Study. Nanomaterials. 2022; 12(21):3757. https://doi.org/10.3390/nano12213757
Chicago/Turabian StyleEl-Gendy, Ahmed O., Yousif Obaid, Esraa Ahmed, Chukuka S. Enwemeka, Mansour Hassan, and Tarek Mohamed. 2022. "The Antimicrobial Effect of Gold Quantum Dots and Femtosecond Laser Irradiation on the Growth Kinetics of Common Infectious Eye Pathogens: An In Vitro Study" Nanomaterials 12, no. 21: 3757. https://doi.org/10.3390/nano12213757
APA StyleEl-Gendy, A. O., Obaid, Y., Ahmed, E., Enwemeka, C. S., Hassan, M., & Mohamed, T. (2022). The Antimicrobial Effect of Gold Quantum Dots and Femtosecond Laser Irradiation on the Growth Kinetics of Common Infectious Eye Pathogens: An In Vitro Study. Nanomaterials, 12(21), 3757. https://doi.org/10.3390/nano12213757









