Extraction of Metal Ions by Interfacially Active Janus Nanoparticles Supported by Wax Colloidosomes Obtained from Pickering Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PS Seed Nanoparticles
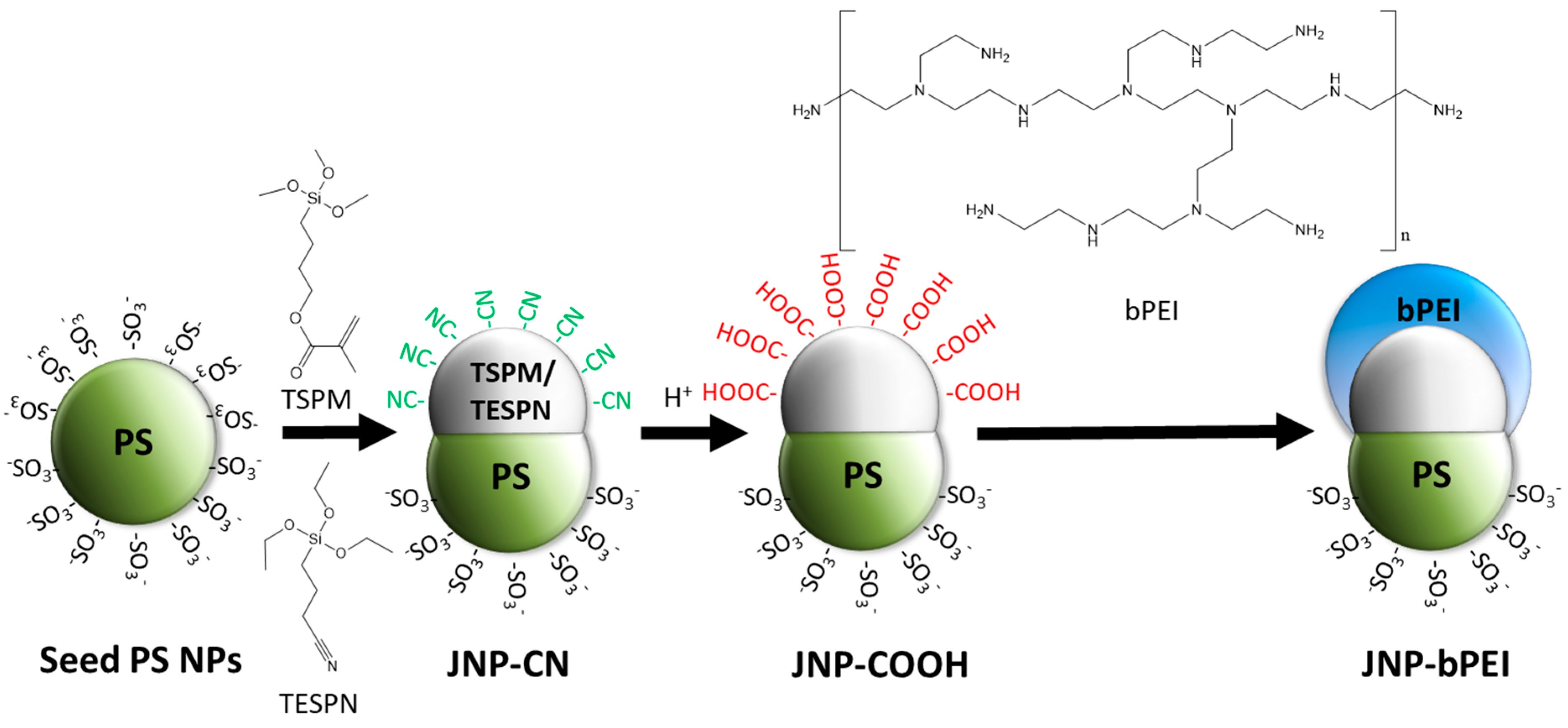
2.3. Synthesis and Surface Modification of Janus Nanoparticles
2.4. Grafting of bPEI to the Janus Nanoparticles
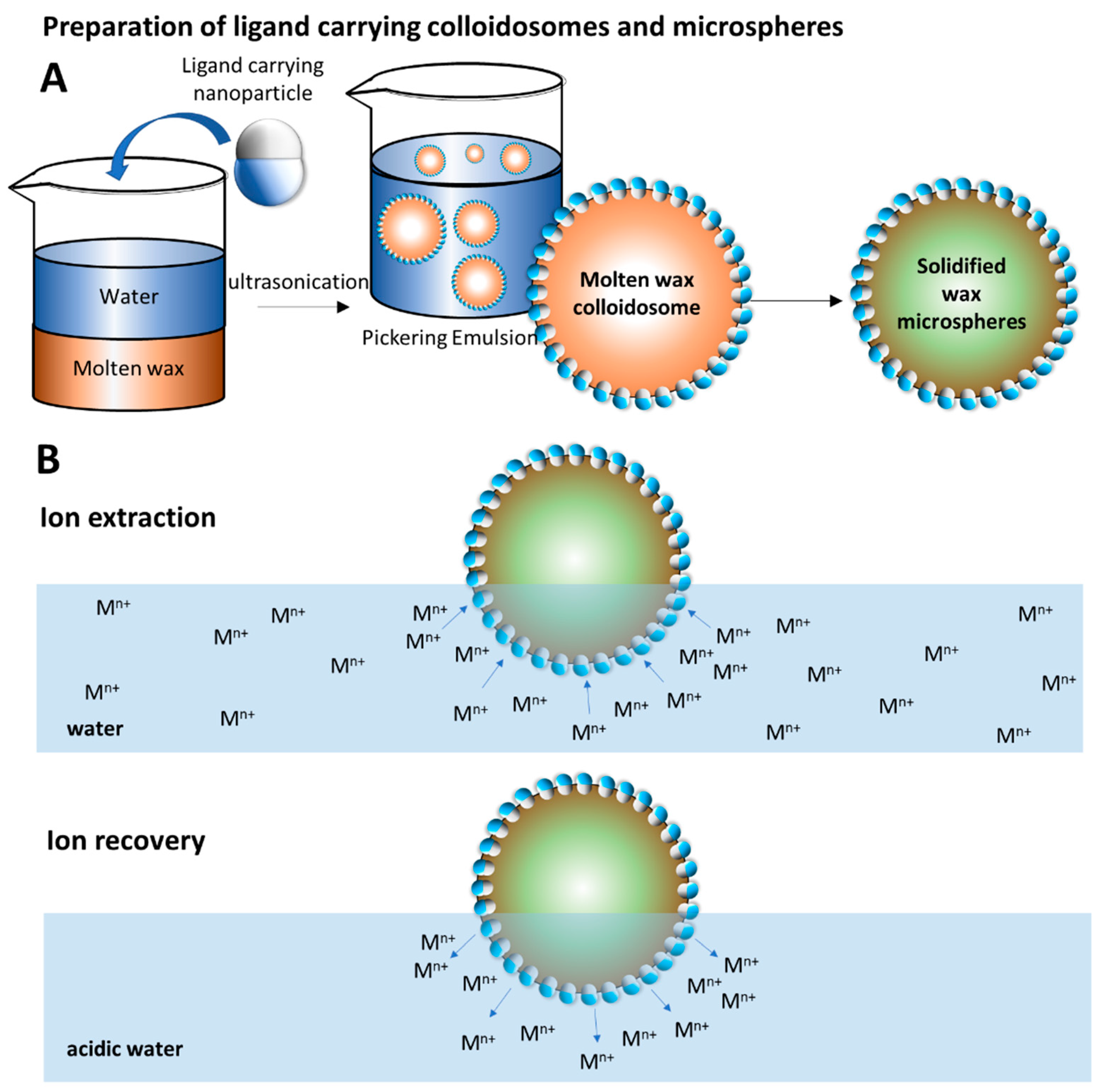
2.5. Preparation of Wax Colloidosomes
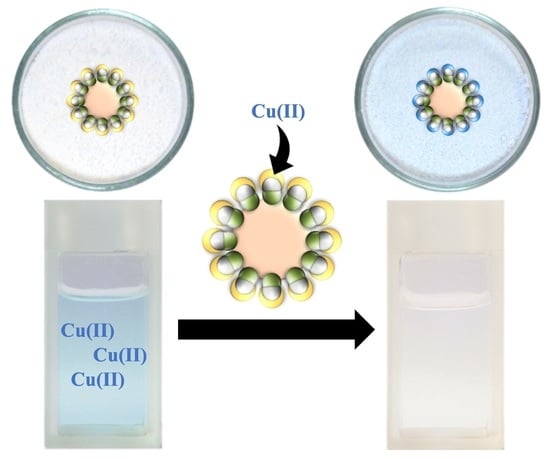
2.6. Metal Ion Extraction and Recovery
2.7. Statistical Analysis of the Data
3. Results and Discussions
3.1. Synthesis and Functionalization of Janus Nanoparticles (JNPs)
3.2. Selective Grafting of bPEI on One Janus Lobe
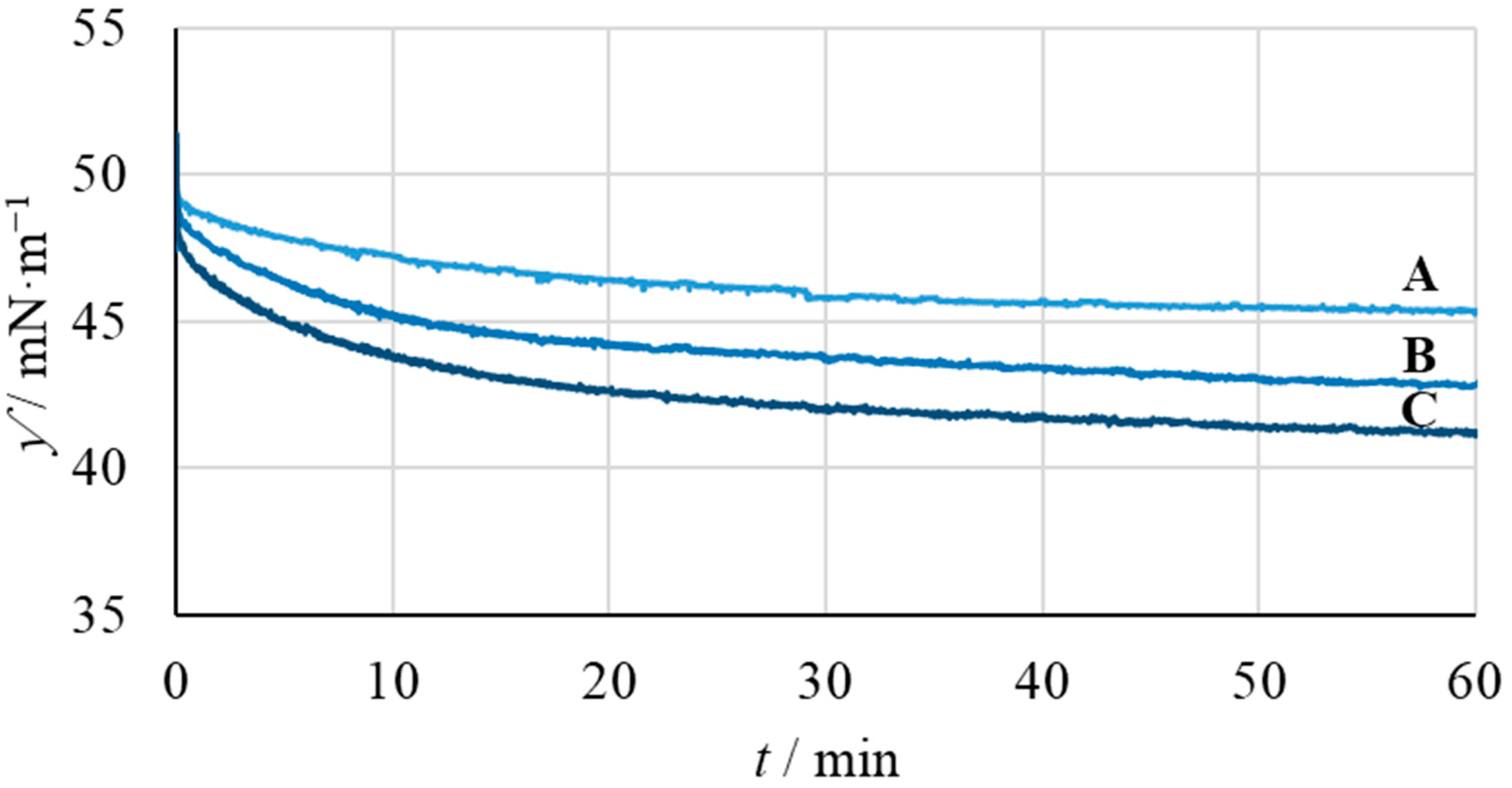
3.3. Interfacial Activity of JNP-bPEI Homologous Series
3.4. Preparation of Wax Colloidosomes
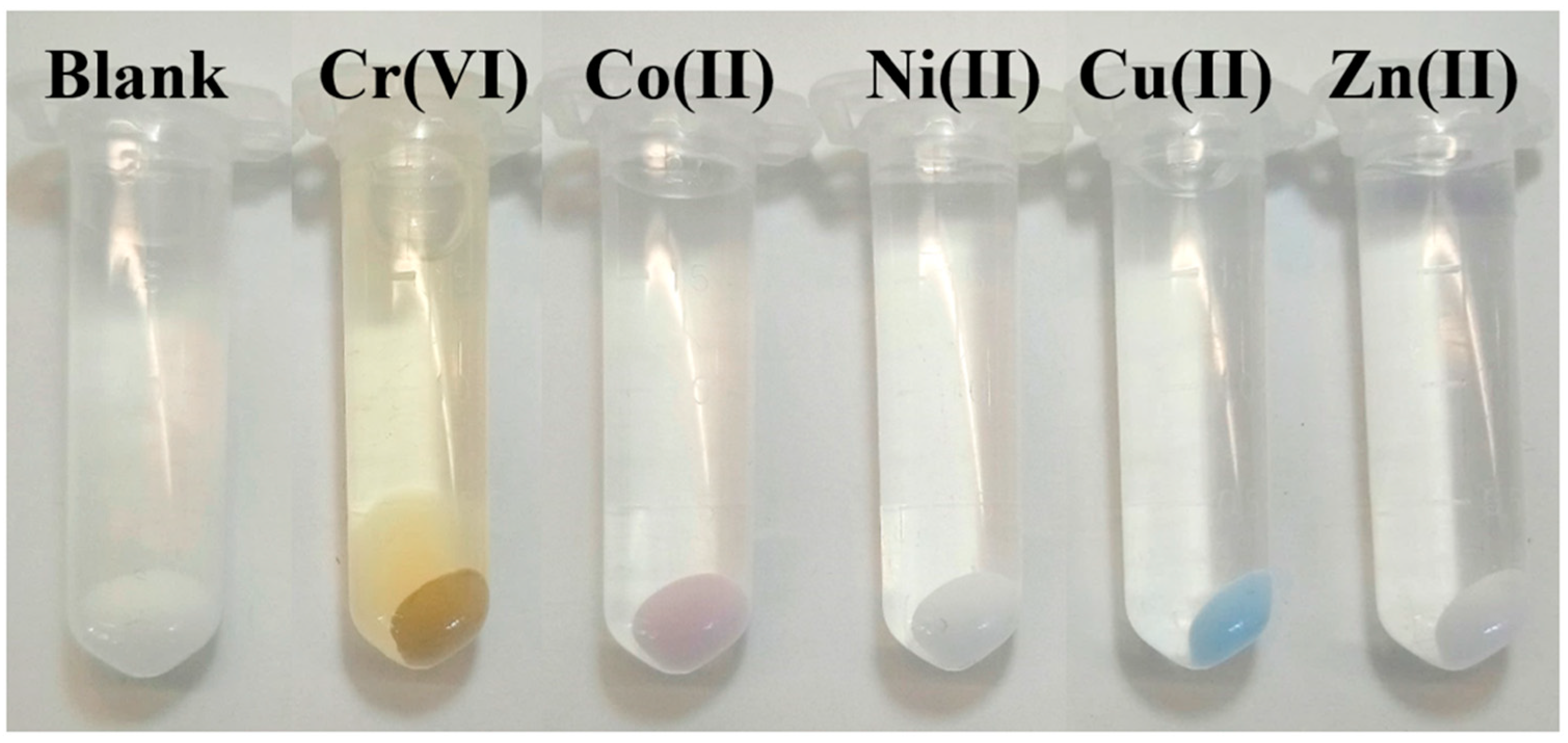
3.5. Extraction of Metal Ions by JNPs
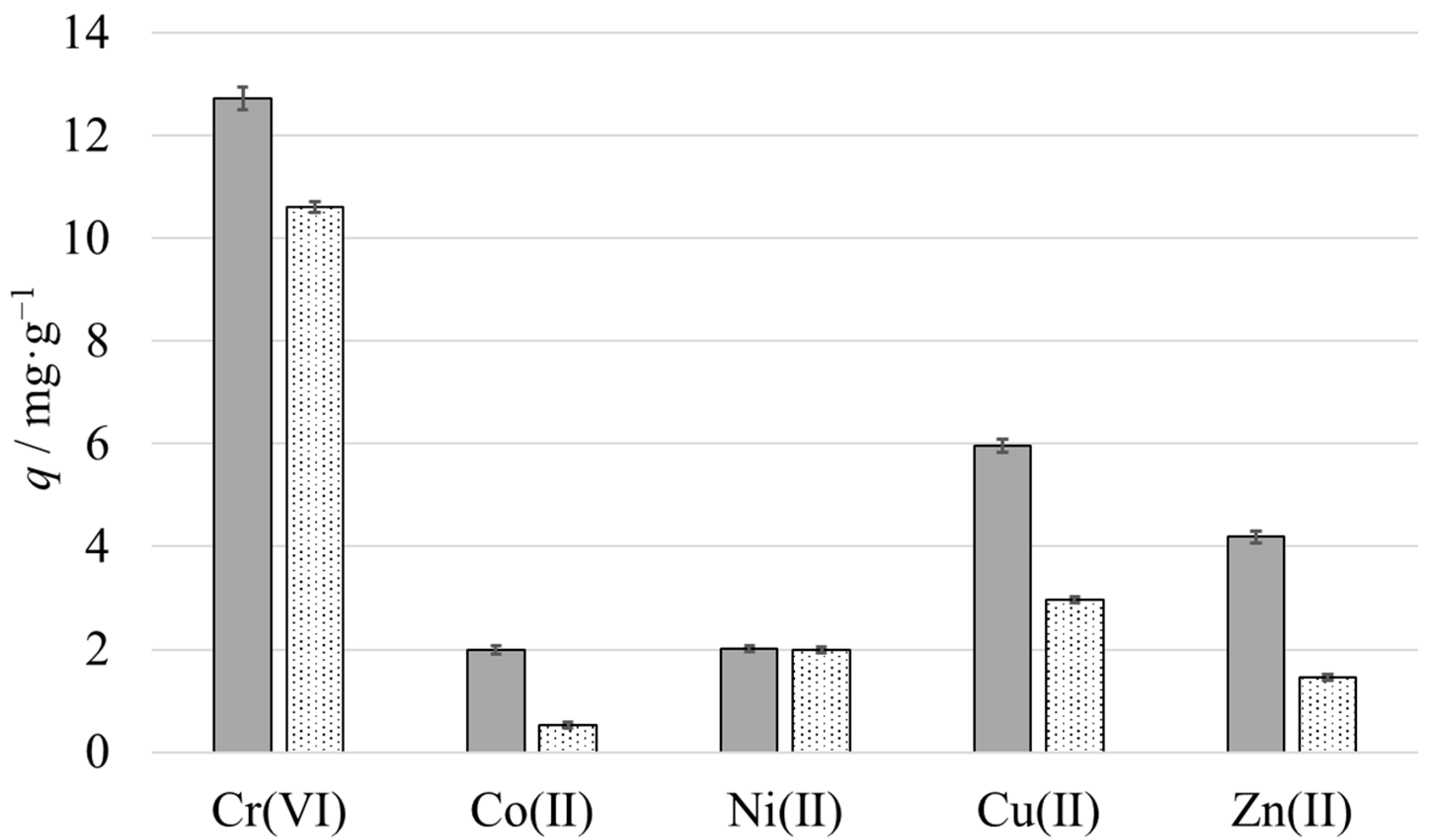
3.6. Metal Ion Extraction Performance in the Homologous Series of JNPs
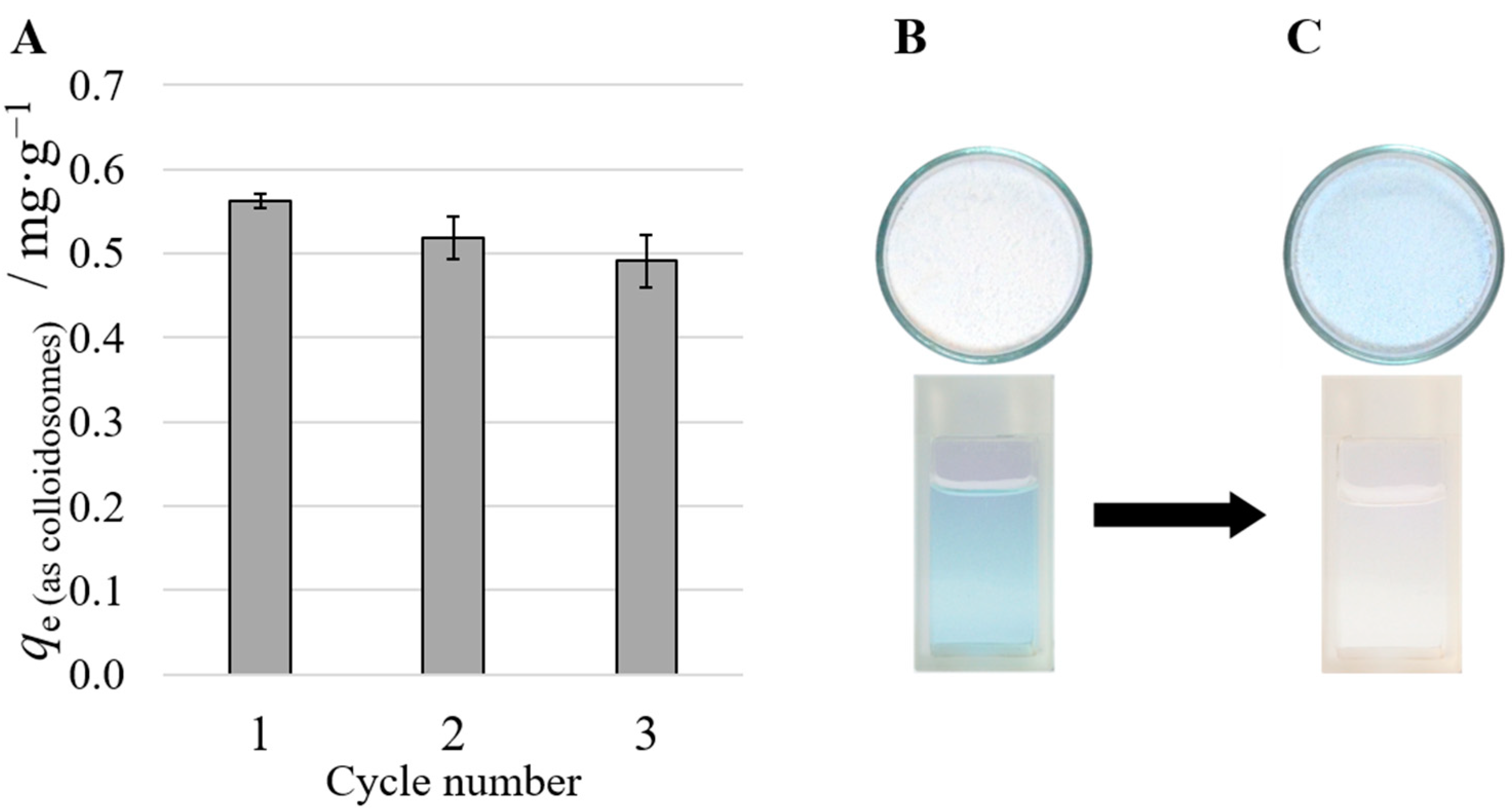
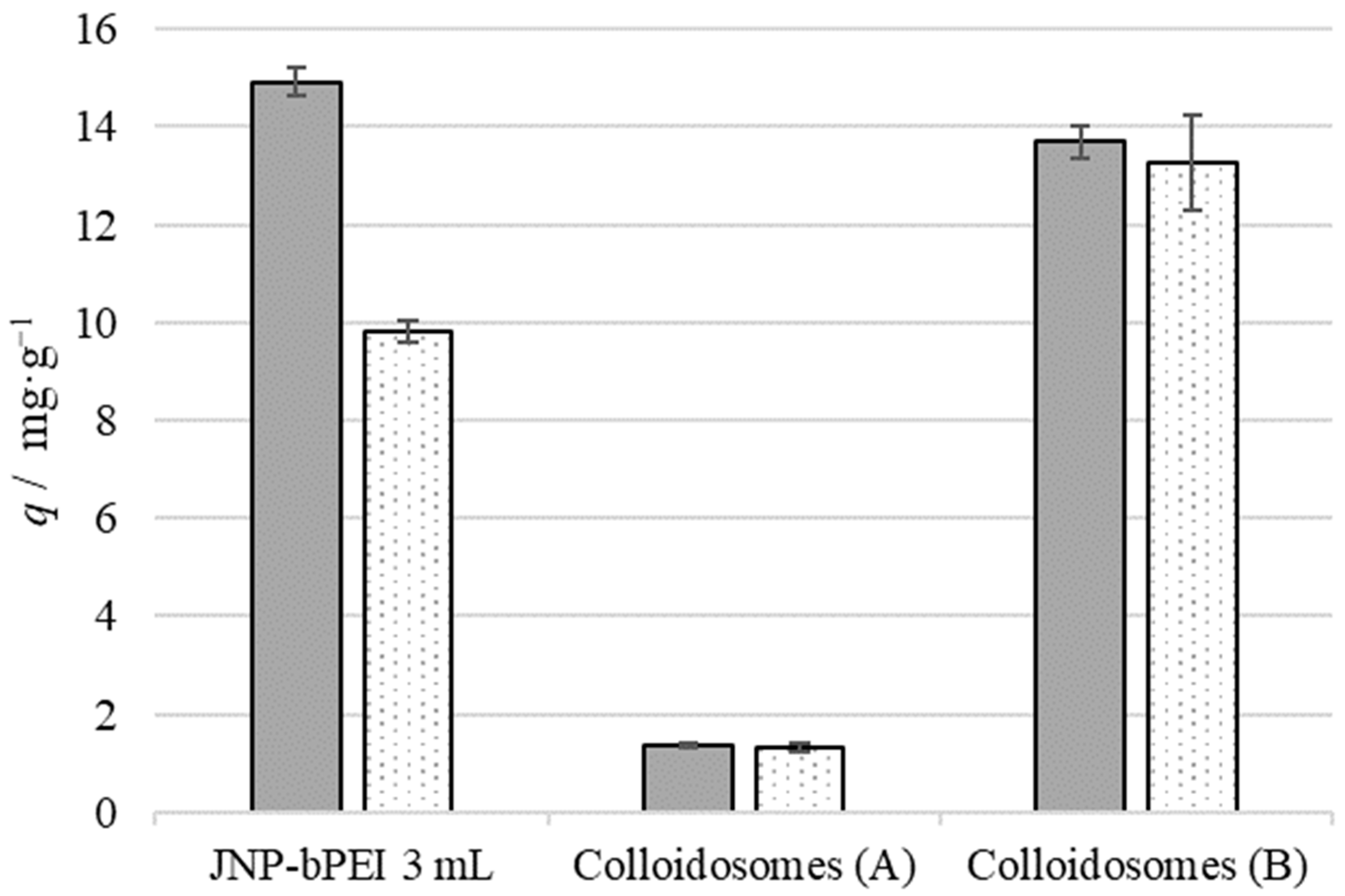
3.7. Metal Ion Extraction by JNPs Supported by Wax Colloidosomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Sulaiman, R.N.R.; Othman, N.; Amin, N.A.S. Emulsion Liquid Membrane Stability in the Extraction of Ionized Nanosilver from Wash Water. J. Ind. Eng. Chem. 2014, 20, 3243–3250. [Google Scholar] [CrossRef]
- Kocherginsky, N.M.; Yang, Q.; Seelam, L. Recent Advances in Supported Liquid Membrane Technology. Sep. Purif. Technol. 2007, 53, 171–177. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Feng, S.; Li, H.; Wan, Y.; Zhang, X. Recent Development of Ionic Liquid Membranes. Green Energy Environ. 2016, 1, 43–61. [Google Scholar] [CrossRef]
- Fontàs, C.; Tayeb, R.; Dhahbi, M.; Gaudichet, E.; Thominette, F.; Roy, P.; Steenkeste, K.; Fontaine-Aupart, M.-P.; Tingry, S.; Tronel-Peyroz, E. Polymer Inclusion Membranes: The Concept of Fixed Sites Membrane Revised. J. Membr. Sci. 2007, 290, 62–72. [Google Scholar] [CrossRef]
- Nghiem, L.; Mornane, P.; Potter, I.; Perera, J.; Cattrall, R.; Kolev, S. Extraction and Transport of Metal Ions and Small Organic Compounds Using Polymer Inclusion Membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Ghaemi, N.; Daraei, P. Enhancement in Copper Ion Removal by PPy@Al2O3 Polymeric Nanocomposite Membrane. J. Ind. Eng. Chem. 2016, 40, 26–33. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Kusumastuti, A.; Derek, C.J.C.; Ooi, B.S. Emulsion Liquid Membrane for Heavy Metal Removal: An Overview on Emulsion Stabilization and Destabilization. Chem. Eng. J. 2011, 171, 870–882. [Google Scholar] [CrossRef]
- Wu, D.; Honciuc, A. Design of Janus Nanoparticles with PH-Triggered Switchable Amphiphilicity for Interfacial Applications. ACS Appl. Nano Mater. 2018, 1, 471–482. [Google Scholar] [CrossRef]
- Wu, D.; Chew, J.W.; Honciuc, A. Polarity Reversal in Homologous Series of Surfactant-Free Janus Nanoparticles: Toward the Next Generation of Amphiphiles. Langmuir 2016, 32, 6376–6386. [Google Scholar] [CrossRef]
- Honciuc, A. Amphiphilic Janus Particles at Interfaces. In Flowing Matter; Toschi, F., Sega, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 95–136. ISBN 978-3-030-23369-3. [Google Scholar]
- Honciuc, A. Chemistry of Functional Materials Surfaces and Interfaces: Fundamentals and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-821059-8. [Google Scholar]
- Honciuc, A.; Negru, O.-I. Role of Surface Energy of Nanoparticle Stabilizers in the Synthesis of Microspheres via Pickering Emulsion Polymerization. Nanomaterials 2022, 12, 995. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.L.; Williams, M.; Armes, S.P. Colloidosomes: Synthesis, Properties and Applications. J. Colloid Interface Sci. 2015, 447, 217–228. [Google Scholar] [CrossRef]
- Mihali, V.; Honciuc, A. Self-Assembly of Strongly Amphiphilic Janus Nanoparticles into Freestanding Membranes. Adv. Mater. Interfaces 2021, 9, 2101713. [Google Scholar] [CrossRef]
- Csikós, R.; Kestzhelyi, S.; Mózes, G. Paraffin Products; Mózes, G., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Zeppieri, S.; Rodríguez, J.; de Ramos, A.L.L. Interfacial Tension of Alkane + Water Systems. J. Chem. Eng. Data 2001, 46, 1086–1088. [Google Scholar] [CrossRef]
- Wu, D.; Binks, B.P.; Honciuc, A. Modeling the Interfacial Energy of Surfactant-Free Amphiphilic Janus Nanoparticles from Phase Inversion in Pickering Emulsions. Langmuir 2018, 34, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Granick, S. Janus Balance of Amphiphilic Colloidal Particles. J. Chem. Phys. 2007, 127, 161102. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Z.; Wu, D.; Lee, H.T.; Wang, H.; Honciuc, A.; Chew, J.W. Synthesis of Ligand-Carrying Polymeric Nanoparticles for Use in Extraction and Recovery of Metal Ions. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 533, 179–186. [Google Scholar] [CrossRef]
- Mishra, V.; Balomajumder, C.; Agarwal, V.K. Biosorption of Zn (II) onto the Surface of Non-Living Biomasses: A Comparative Study of Adsorbent Particle Size and Removal Capacity of Three Different Biomasses. Water Air Soil Pollut. 2010, 211, 489–500. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, G.; Liu, Z.; Meng, M.; Jiang, Y.; Ni, L.; Guo, W.; Liu, F. Preparation of Core–Shell Ion Imprinted Nanoparticles via Photoinitiated Polymerization at Ambient Temperature for Dynamic Removal of Cobalt in Aqueous Solution. RSC Adv. 2015, 5, 85691–85704. [Google Scholar] [CrossRef]
- Adibmehr, Z.; Faghihian, H. Preparation of Highly Selective Magnetic Cobalt Ion-Imprinted Polymer Based on Functionalized SBA-15 for Removal Co2+ from Aqueous Solutions. J. Environ. Heal. Sci. Eng. 2019, 17, 1213–1225. [Google Scholar] [CrossRef]
- Gao, B.; Gao, Y.; Li, Y. Preparation and Chelation Adsorption Property of Composite Chelating Material Poly (Amidoxime)/SiO2 towards Heavy Metal Ions. Chem. Eng. J. 2010, 158, 542–549. [Google Scholar] [CrossRef]
- Saraji, M.; Yousefi, H. Selective Solid-Phase Extraction of Ni (II) by an Ion-Imprinted Polymer from Water Samples. J. Hazard. Mater. 2009, 167, 1152–1157. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauli, O.; Honciuc, A. Extraction of Metal Ions by Interfacially Active Janus Nanoparticles Supported by Wax Colloidosomes Obtained from Pickering Emulsions. Nanomaterials 2022, 12, 3738. https://doi.org/10.3390/nano12213738
Pauli O, Honciuc A. Extraction of Metal Ions by Interfacially Active Janus Nanoparticles Supported by Wax Colloidosomes Obtained from Pickering Emulsions. Nanomaterials. 2022; 12(21):3738. https://doi.org/10.3390/nano12213738
Chicago/Turabian StylePauli, Oliver, and Andrei Honciuc. 2022. "Extraction of Metal Ions by Interfacially Active Janus Nanoparticles Supported by Wax Colloidosomes Obtained from Pickering Emulsions" Nanomaterials 12, no. 21: 3738. https://doi.org/10.3390/nano12213738
APA StylePauli, O., & Honciuc, A. (2022). Extraction of Metal Ions by Interfacially Active Janus Nanoparticles Supported by Wax Colloidosomes Obtained from Pickering Emulsions. Nanomaterials, 12(21), 3738. https://doi.org/10.3390/nano12213738