Nb and Ni Nanoparticles Anchored on N-Doped Carbon Nanofiber Membrane as Self-Supporting Anode for High-Rate Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
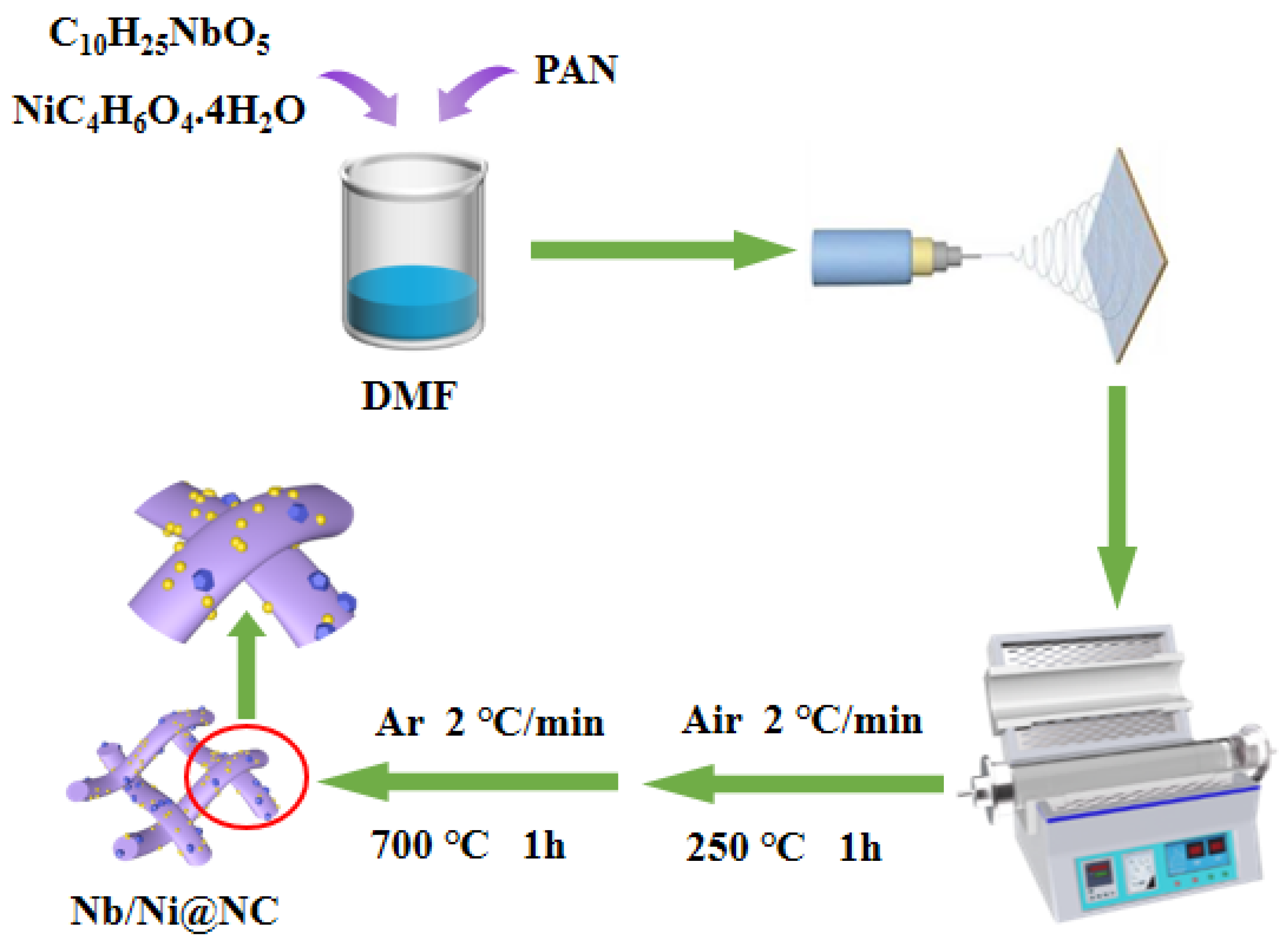
2.1. Material Synthesis
2.2. Characterization
2.3. Electrochemical Measurements
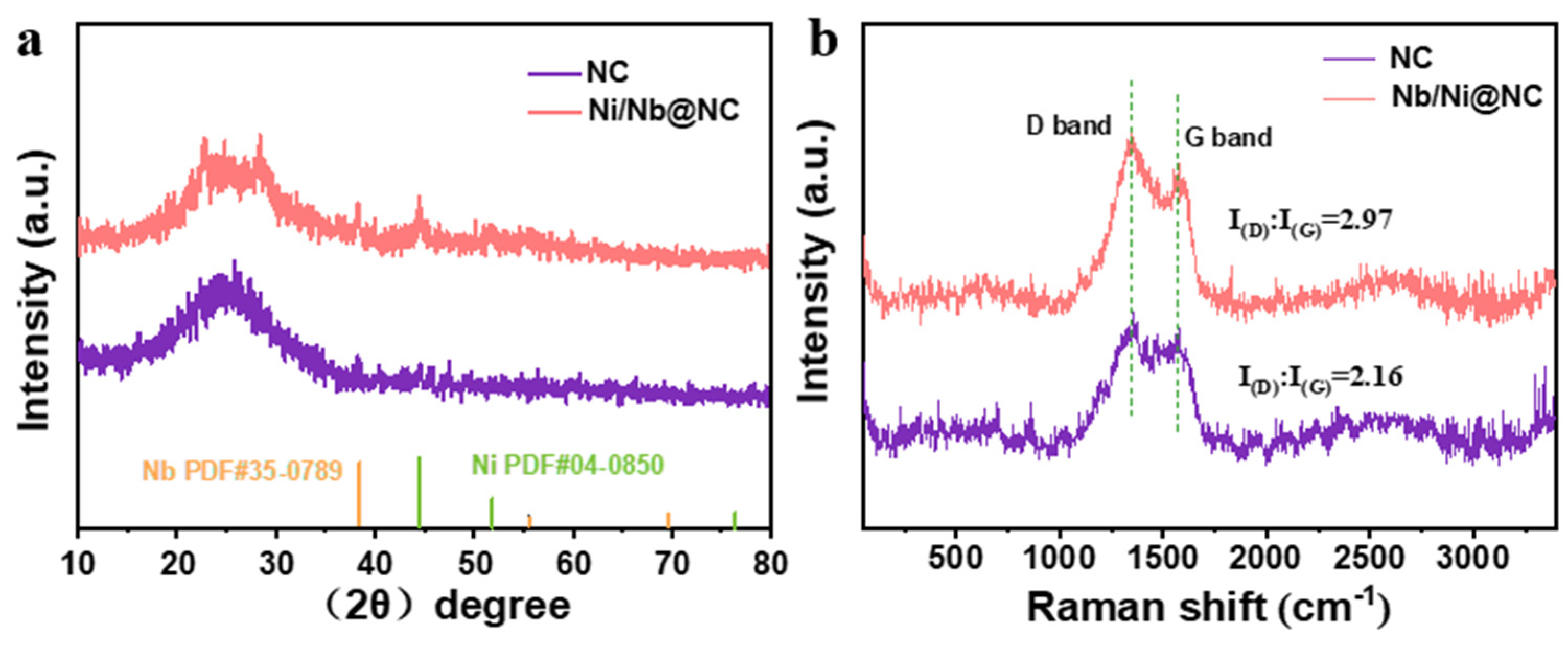
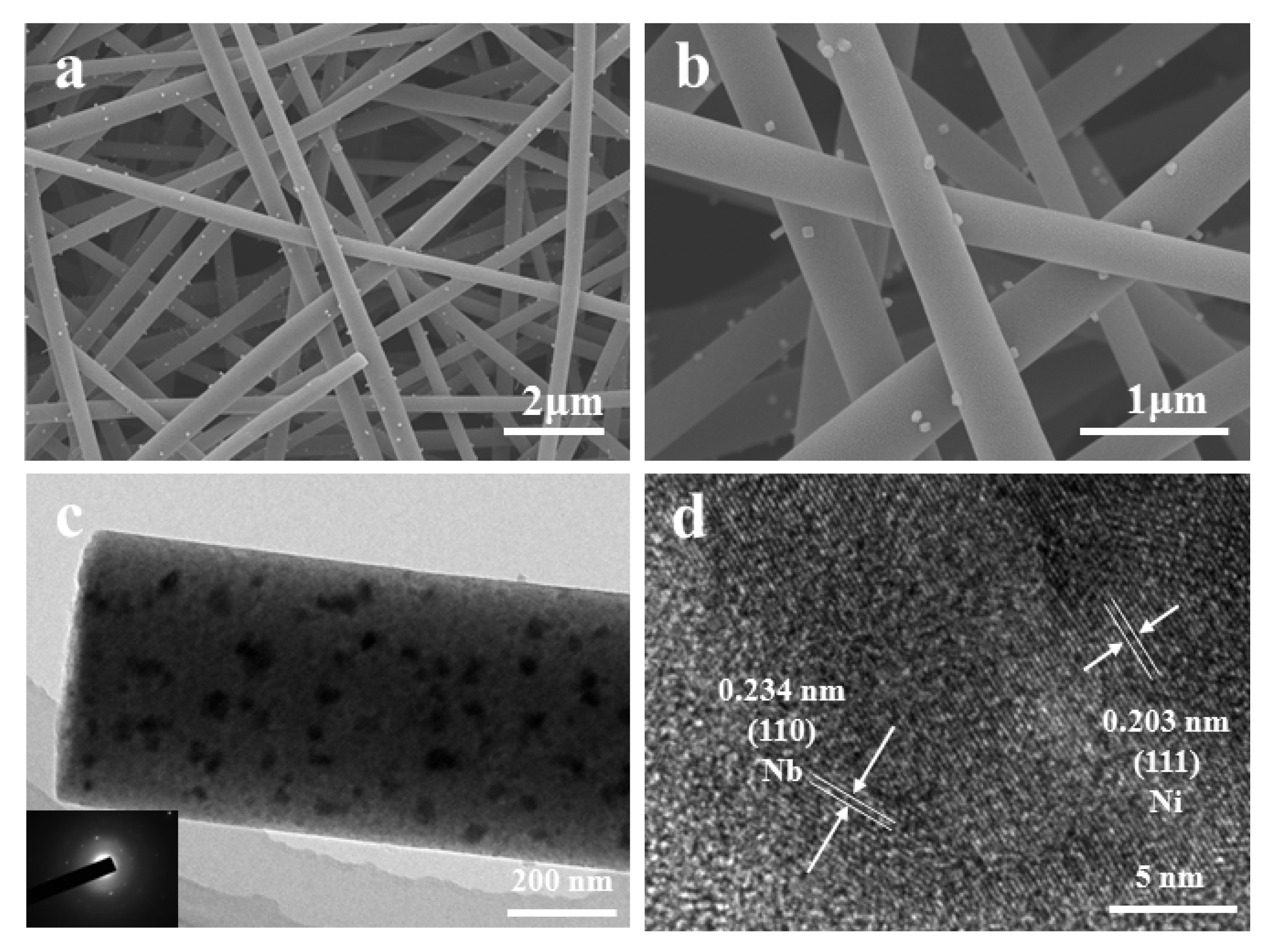
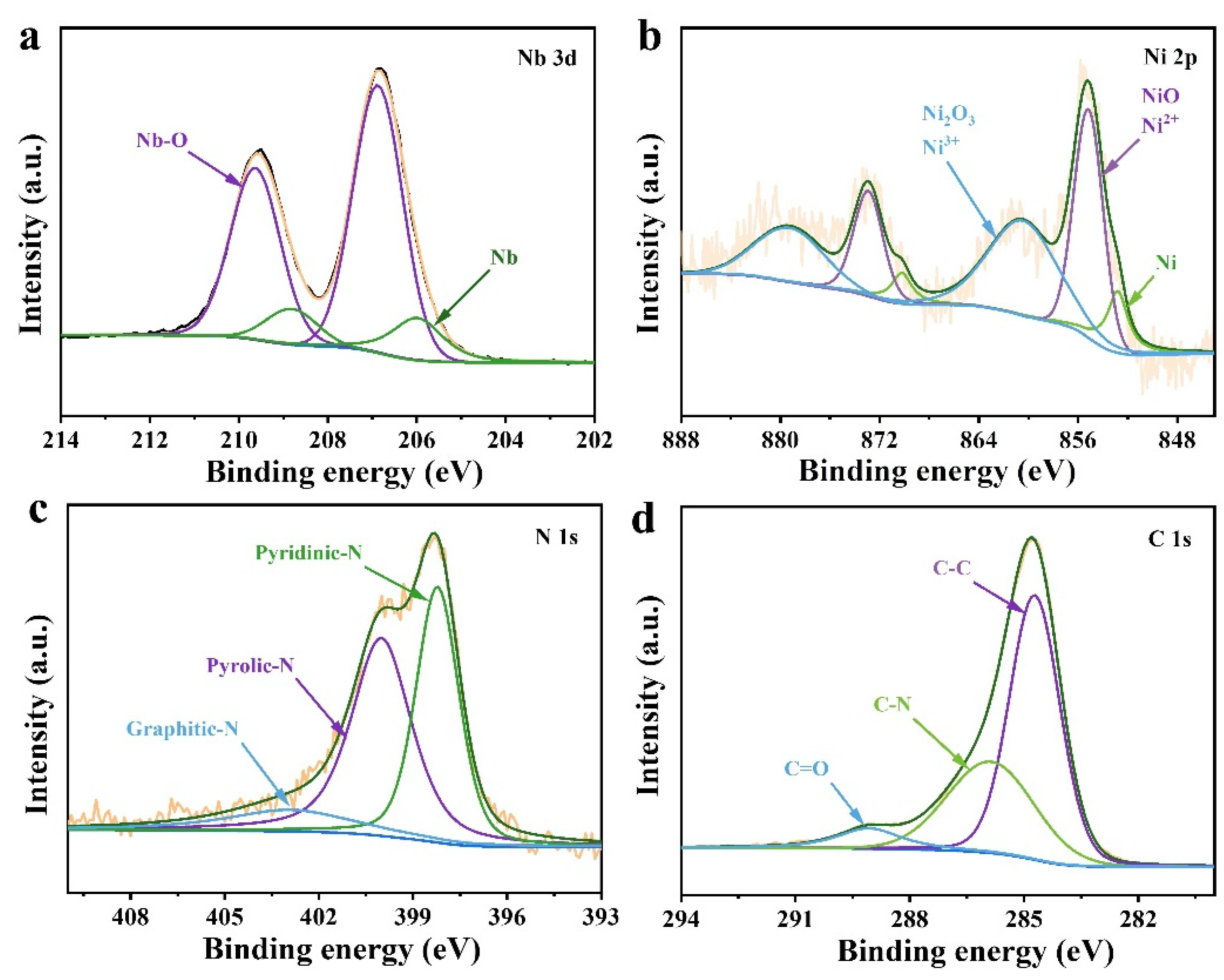
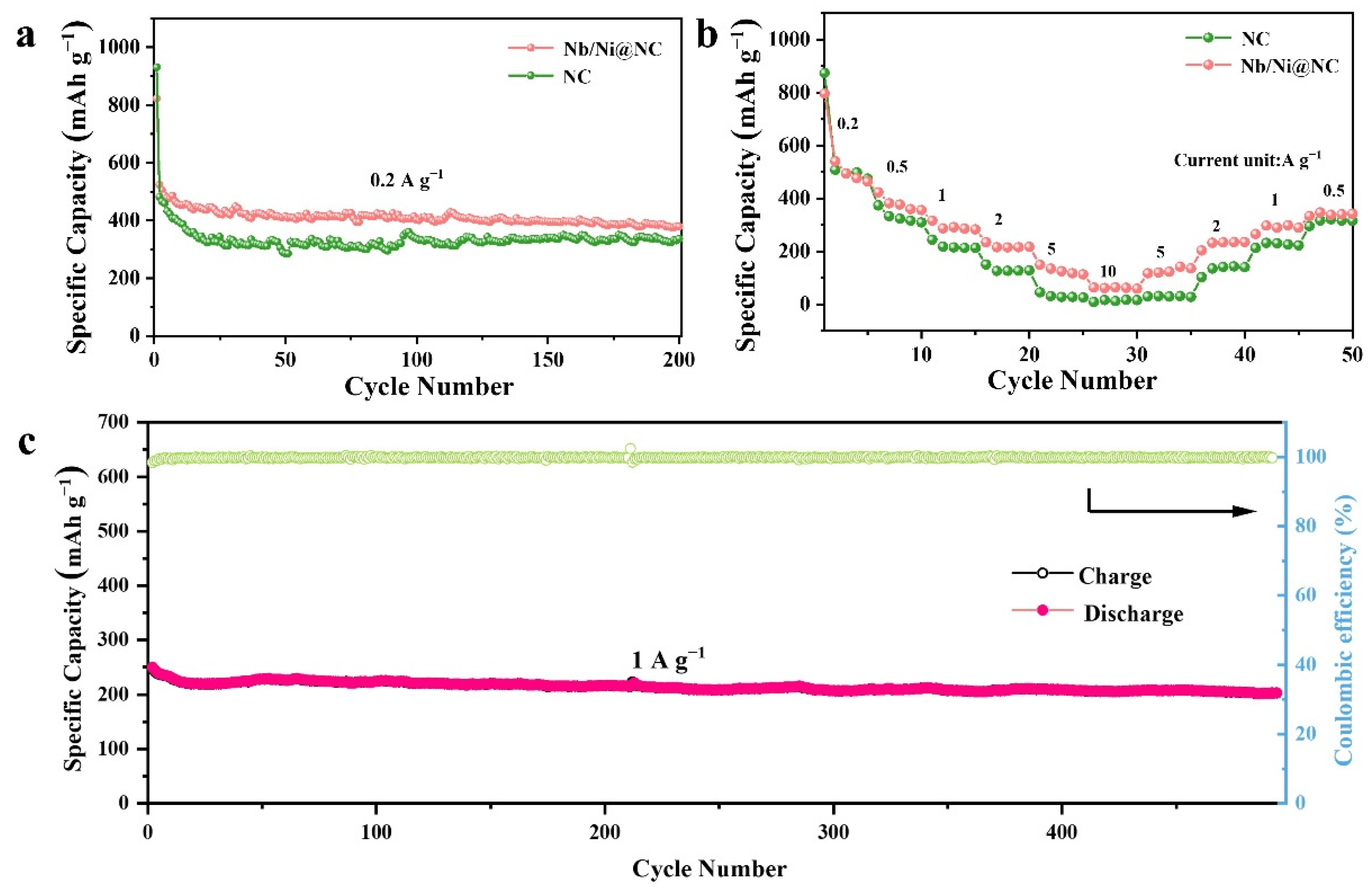
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Evarts, E.C. Lithium batteries: To the limits of lithium. Nature 2015, 526, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Gelinck, G.H.; Huitema, H.E.; Van Veenendaal, E.; Cantatore, E.; Schrijnemakers, L.; van der Puten, J.B.P.H.; Geuns, T.C.T.; Beenhakkers, M.; Giesbers, J.B.; Huisman, B.-H.; et al. Flexible active-matrix displays and shift registers based on solution-processed organic transistors. Nat. Mater. 2004, 3, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef]
- Ko, H.; Kapadia, R.; Takei, K.; Takahashi, T.; Zhang, X.; Javey, A. Multifunctional, flexible electronic systems based on engineered nanostructured materials. Nanotechnology 2012, 23, 344001. [Google Scholar] [CrossRef]
- Kim, D.H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.-S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef]
- Zhou, G.; Li, F.; Cheng H, M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Gwon, H.; Hong, J.; Kim, H.; Seo, D.-H.; Jeon, S.; Kang, K. Recent progress on flexible lithium rechargeable batteries. Energy Environ. Sci. 2014, 7, 538–551. [Google Scholar] [CrossRef]
- Chu, S.; Zhong, Y.; Cai, R.; Zhang, Z.; Wei, S.; Shao, Z. Mesoporous and Nanostructured TiO2 layer with Ultra-High Loading on Nitrogen-Doped Carbon Foams as Flexible and Free-Standing Electrodes for Lithium-Ion Batteries. Small 2016, 12, 6724–6734. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.M.; Khau, B.V.; Davies, G.; Hertzberg, B.; Steingart, D.A.; Arias, A.C. A high areal capacity flexible lithium-ion battery with a strain-compliant design. Adv. Energy Mater. 2015, 5, 1401389. [Google Scholar] [CrossRef]
- Pu, X.; Li, L.; Song, H.; Du, C.; Zhao, Z.; Jiang, C.; Cao, G.; Hu, W.; Wang, Z.L. A self-charging power unit by integration of a textile triboelectric nanogenerator and a flexible lithium-ion battery for wearable electronics. Adv. Mater. 2015, 27, 2472–2478. [Google Scholar] [CrossRef] [PubMed]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 5678. [Google Scholar] [CrossRef]
- Pan, P.; Zhang, M.; Cheng, Z.; Jiang, L.; Mao, J.; Ni, C.; Chen, Q.; Zeng, Y.; Hu, Y.; Fu, K. Garnet ceramic fabric-reinforced flexible composite solid electrolyte derived from silk template for safe and long-term stable All-Solid-State lithium metal batteries. Energy Storage Mater. 2022, 47, 279–287. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Feng, Y.; Tan, R.; Zuo, Y.; Gao, R.; Zhao, Y.; Han, L.; Wang, Z.; Pan, F. Flexible composite solid electrolytefacilitating highly stable “soft contacting” Li-electrolyte interface for solid state lithium-ion batteries. Adv. Energy Mater. 2017, 7, 1701437. [Google Scholar] [CrossRef]
- He, G.; Li, Q.; Shen, Y.; Ding, Y. Flexible Amalgam Film Enables Stable Lithium Metal Anodes with High Capacities. Angew. Chem. Int. Ed. 2019, 58, 18466–18470. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Liao, M.; Wang, B.; Peng, H. Carbon nanomaterials for flexible lithium ion batteries. Carbon 2017, 124, 79–88. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhao, J.; Li, Y. Assembling free-standing and aligned tungstate/MXene fiber for flexible lithium and sodium-ion batteries with efficient pseudocapacitive energy storage. Energy Storage Mater. 2020, 33, 82–87. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Lin, C.; Zhao, X.S. Lithium Titanate Cuboid Arrays Grown on Carbon Fiber Cloth for High-Rate Flexible Lithium-Ion Batteries. Small 2019, 15, e1902183. [Google Scholar] [CrossRef]
- Sang, Z.; Yan, X.; Wen, L.; Su, D.; Zhao, Z.; Liu, Y.; Ji, H.; Liang, J.; Dou, S.X. A graphene-modified flexible SiOC ceramic cloth for high-performance lithium storage. Energy Storage Mater. 2020, 25, 876–884. [Google Scholar] [CrossRef]
- Xiang, M.; Wu, H.; H, L.; Huang, J.; Zheng, Y.; Yang, L.; Jing, P.; Zhang, Y.; Dou, S.; Liu, H. A flexible 3D multifunctional MgO-decorated carbon foam@CNTs hybrid as self-supported cathode for high-performance lithium-sulfur batteries. Adv. Funct. Mater. 2017, 27, 1702573. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zheng, Z.J.; Feng, Y.Q.; Ye, H.; Cao, F.-F.; Guo, Z.-P. Topological design of ultrastrong MXene paper hosted Li enables ultrathin and fully flexible lithium metal batteries. Nano Energy 2020, 74, 104817. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.-Y.; Lu, B.; Song, Y.; Qiao, Y.; Guo, X.; Ao, S.; Zhang, J.; Fang, D.; Bao, Y. Crocodile skin inspired rigid-supple integrated flexible lithium ion batteries with high energy density and bidirectional deformability. Energy Storage Mater. 2022, 47, 149–157. [Google Scholar] [CrossRef]
- Hwang, C.; Song, W.J.; Han, J.G.; Bae, S.; Song, G.; Choi, N.S.; Park, S.; Song, H.-K. Foldable electrode architectures based on silver-nanowire-wound or carbon-nanotube-webbed micrometer-scale fibers of polyethylene terephthalate mats for flexible lithium-ion batteries. Adv. Mater. 2018, 30, 1705445. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Li, K.; Lv, Y.; Xu, T.; Wei, D.; Liu, Z. Highly-safe and ultra-stable all-flexible gel polymer lithium-ion batteries aiming for scalable applications. Adv. Energy Mater. 2020, 10, 1904281. [Google Scholar] [CrossRef]
- Jung, Y.; Jeong, Y.C.; Kim, J.H.; Kim, Y.S.; Kim, T.; Cho, Y.S.; Yang, S.J.; Park, C.R. One step preparation and excellent performance of CNT yarn based flexible micro lithium-ion batteries. Energy Storage Mater. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Woo, S.W.; Jung, H.R.; Yu, H.K.; Kim, K.; Oh, B.H.; Ahn, S.; Lee, S.-Y.; Song, S.-W.; Cho, J.; et al. Cable-type flexible lithium ion battery based on hollow multi-helix electrodes. Adv. Mater. 2012, 24, 5192–5197. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Sun, Q.; Zhang, Y.; Lin, H.; Ren, J.; Lu, X.; Wang, M.; Peng, H. Winding Aligned Carbon Nanotube Composite Yarns into Coaxial Fiber Full Batteries with High Performances. Nano Lett. 2014, 14, 3432–3438. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Karahan, H.E.; Wei, L.; Qian, Q.; Harris, A.T.; Minett, A.I.; Ramakrishna, S.; Ng, A.K.; Chen, Y. Textile energy storage: Structural design concepts, material selection and future perspectives. Energy Storage Mater. 2016, 3, 123–139. [Google Scholar] [CrossRef]
- Chalapat, K.; Chekurov, N.; Jiang, H.; Li, J.; Parviz, B.; Paraoanu, G.S. Self-Organized Origami Structures via Ion-Induced Plastic Strain. Adv. Mater. 2013, 25, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, H.; Liang, H.; Ji, H.; Song, L.; Gao, C.; Xu, H. A Highly Efficient Metal-Free Oxygen Reduction Electrocatalyst Assembled from Carbon Nanotubes and Graphene. Adv. Mater. 2016, 28, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Zhu, L.; Jeong, D.-J.; Chun, K.; Bang, Y.-Y.; Kim, S.-R.; Kim, J.-H.; Oh, S.-K. Transparent flexible heater based on hybrid of carbon nanotubes and silver nanowires. Carbon 2013, 63, 530–536. [Google Scholar] [CrossRef]
- Lota, G.; Fic, K.; Frackowiak, E. Carbon nanotubes and their composites in electrochemical applications. Energy Environ. Sci. 2011, 4, 1592–1605. [Google Scholar] [CrossRef]
- Pan, Z.; Ren, J.; Guan, G.; Fang, X.; Wang, B.; Doo, S.-G.; Son, I.H.; Huang, X.; Peng, H. Synthesizing Nitrogen-Doped Core-Sheath Carbon Nanotube Films for Flexible Lithium Ion Batteries. Adv. Energy Mater. 2016, 6, 1600271. [Google Scholar] [CrossRef]
- Wood, K.N.; O’hayre, R.; Pylypenko, S. Recent progress on nitrogen/carbon structures designed for use in energy and sustainability applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Maiti, U.N.; Lee, W.J.; Lee, J.M.; Oh, Y.; Kim, J.Y.; Kim, J.E.; Shim, J.; Han, T.H.; Kim, S.O. 25th anniversary article: Chemically modified/doped carbon nanotubes & graphene for optimized nanostructures & nanodevices. Adv. Mater. 2014, 26, 40–66. [Google Scholar]
- Cao, Y.; Yu, H.; Tan, J.; Peng, F.; Wang, H.; Li, J.; Zheng, W.; Wong, N.-B. Nitrogen-, phosphorous- and boron-doped carbon nanotubes as catalysts for the aerobic oxidation of cyclohexane. Carbon 2013, 57, 433–442. [Google Scholar] [CrossRef]
- Shi, Q.; Peng, F.; Liao, S.; Wang, H.; Yu, H.; Liu, Z.; Zhang, B.; Su, D. Sulfur and nitrogen co-doped carbon nanotubes for enhancing electrochemical oxygen reduction activity in acidic and alkaline media. J. Mater. Chem. A 2013, 1, 14853–14857. [Google Scholar] [CrossRef]
- Wang, R.; Yang, J.; Chen, X.; Zhao, Y.; Zhao, W.; Qian, G.; Li, S.; Xiao, Y.; Chen, H.; Ye, Y.; et al. Highly Dispersed Cobalt Clusters in Nitrogen-Doped Porous Carbon Enable Multiple Effects for High-Performance Li–S Battery. Adv. Energy Mater. 2020, 10, 1903550. [Google Scholar] [CrossRef]
- Alvar, E.N.; Zhou, B.; Eichhorn, S.H. Carbon-embedded mesoporous Nb-doped TiO2 nanofibers as catalyst support for the oxygen reduction reaction in PEM fuel cells. J. Mater. Chem. A 2016, 4, 6540–6552. [Google Scholar] [CrossRef]
- Chen, Y.M.; Li, X.Y.; Zhou, X.Y.; Yao, H.; Huang, H.; Mai, Y.-W.; Zhou, L. Hollow-tunneled graphitic carbon nanofibers through Ni-diffusion-induced graphitization as high-performance anode materials. Energy Environ. Sci. 2014, 7, 2689–2696. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.C.; Xu, L.; Lin, F.; Cheng, H.-M. Doped Graphene Sheets As Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.H.; Jeong, H.M.; Kim, B.G.; Kang, J.K.; Choi, J.W. Nitrogen-Doped Multiwall Carbon Nanotubes for Lithium Storage with Extremely High Capacity. Nano Lett. 2012, 12, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Cui, J.; Wu, J.; Vajtai, R.; Sun, D.; Ajayan, P.M. Improving the Catalytic Activity of Carbon-Supported Single Atom Catalysts by Polynary Metal or Heteroatom Doping. Small 2020, 16, e1906782. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Tan, X.; Huang, H.; Song, Z.; Sun, Y.; Cui, S.; Wei, Q.; Guo, W.; Li, R.; et al. Implanting CNT Forest onto Carbon Nanosheets as Multifunctional Hosts for High-Performance Lithium Metal Batteries. Small Methods 2019, 3, 1800546. [Google Scholar] [CrossRef]
- Cha, H.; Lee, Y.; Kim, J.; Park, M.; Cho, J. Flexible 3D interlocking lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1801917. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, H.; Peng, Y.; Li, J.; Wang, J.; Hwang, B.-J.; Zhao, J. Realizing high reversible capacity: 3D intertwined CNTs inherently conductive network for CuS as an anode for lithium ion batteries. Chem. Eng. J. 2018, 332, 49–56. [Google Scholar] [CrossRef]
- Wang, F.; Feng, T.; Jin, X.; Zhou, Y.; Xu, Y.; Gao, Y.; Li, H.; Lei, J. Atomic Co/Ni active sites assisted MOF-derived rich nitrogen-doped carbon hollow nanocages for enhanced lithium storage. Chem. Eng. J. 2020, 420, 127583. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, S.; Zhao, S.; Cui, Y.; Lian, J.; Li, G. Nb and Ni Nanoparticles Anchored on N-Doped Carbon Nanofiber Membrane as Self-Supporting Anode for High-Rate Lithium-Ion Batteries. Nanomaterials 2022, 12, 3724. https://doi.org/10.3390/nano12213724
Zhang Y, Zhang S, Zhao S, Cui Y, Lian J, Li G. Nb and Ni Nanoparticles Anchored on N-Doped Carbon Nanofiber Membrane as Self-Supporting Anode for High-Rate Lithium-Ion Batteries. Nanomaterials. 2022; 12(21):3724. https://doi.org/10.3390/nano12213724
Chicago/Turabian StyleZhang, Yezheng, Shan Zhang, Shuo Zhao, Yingxue Cui, Jiabiao Lian, and Guochun Li. 2022. "Nb and Ni Nanoparticles Anchored on N-Doped Carbon Nanofiber Membrane as Self-Supporting Anode for High-Rate Lithium-Ion Batteries" Nanomaterials 12, no. 21: 3724. https://doi.org/10.3390/nano12213724
APA StyleZhang, Y., Zhang, S., Zhao, S., Cui, Y., Lian, J., & Li, G. (2022). Nb and Ni Nanoparticles Anchored on N-Doped Carbon Nanofiber Membrane as Self-Supporting Anode for High-Rate Lithium-Ion Batteries. Nanomaterials, 12(21), 3724. https://doi.org/10.3390/nano12213724






