X-ray Photoelectron Spectroscopy (XPS) Analysis of Ultrafine Au Nanoparticles Supported over Reactively Sputtered TiO2 Films
Abstract
1. Introduction
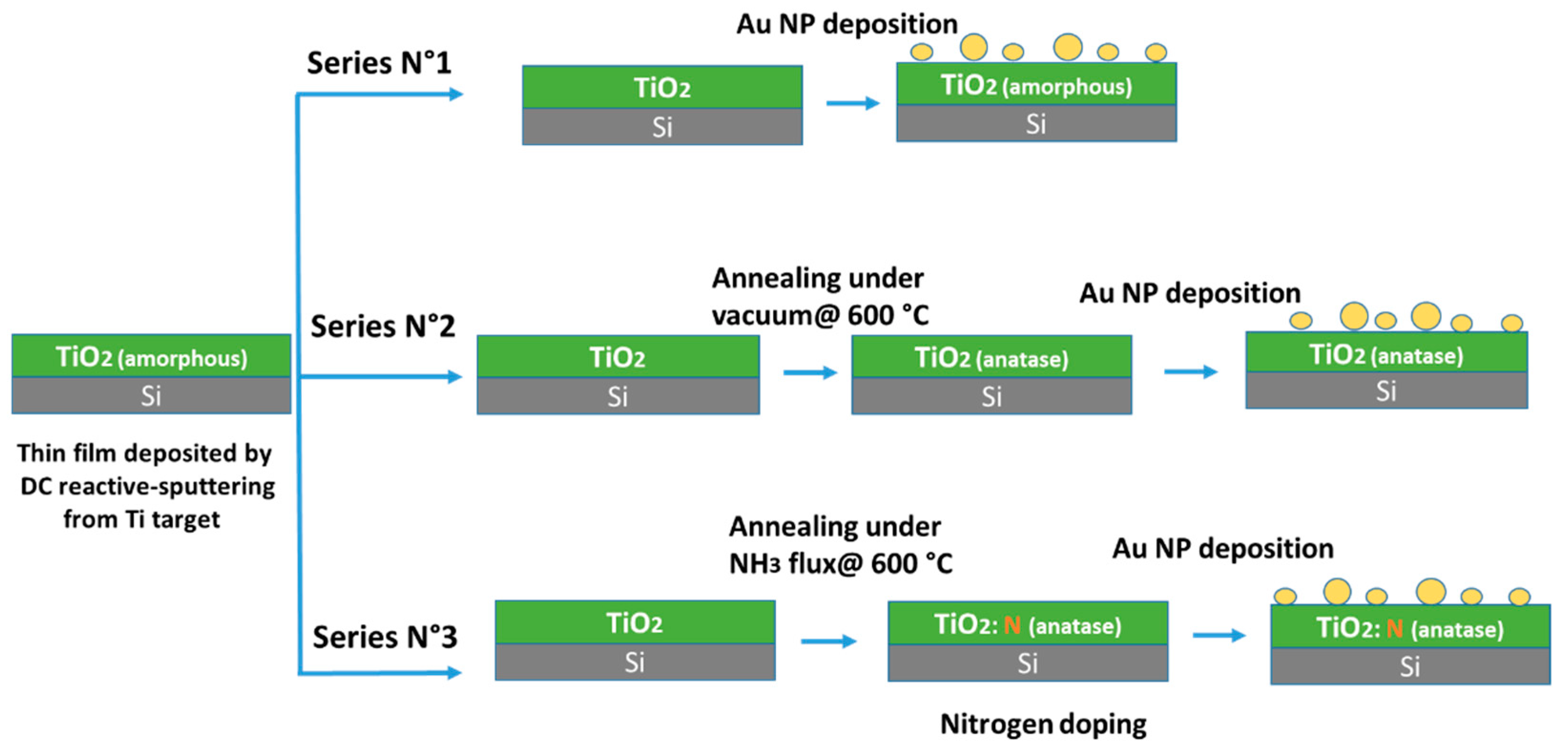
2. Experimental
2.1. Preparation of Titania Films
2.2. Thermal Treatment
2.3. Au NP Deposition
2.4. Materials Characterization
3. Results and Discussion
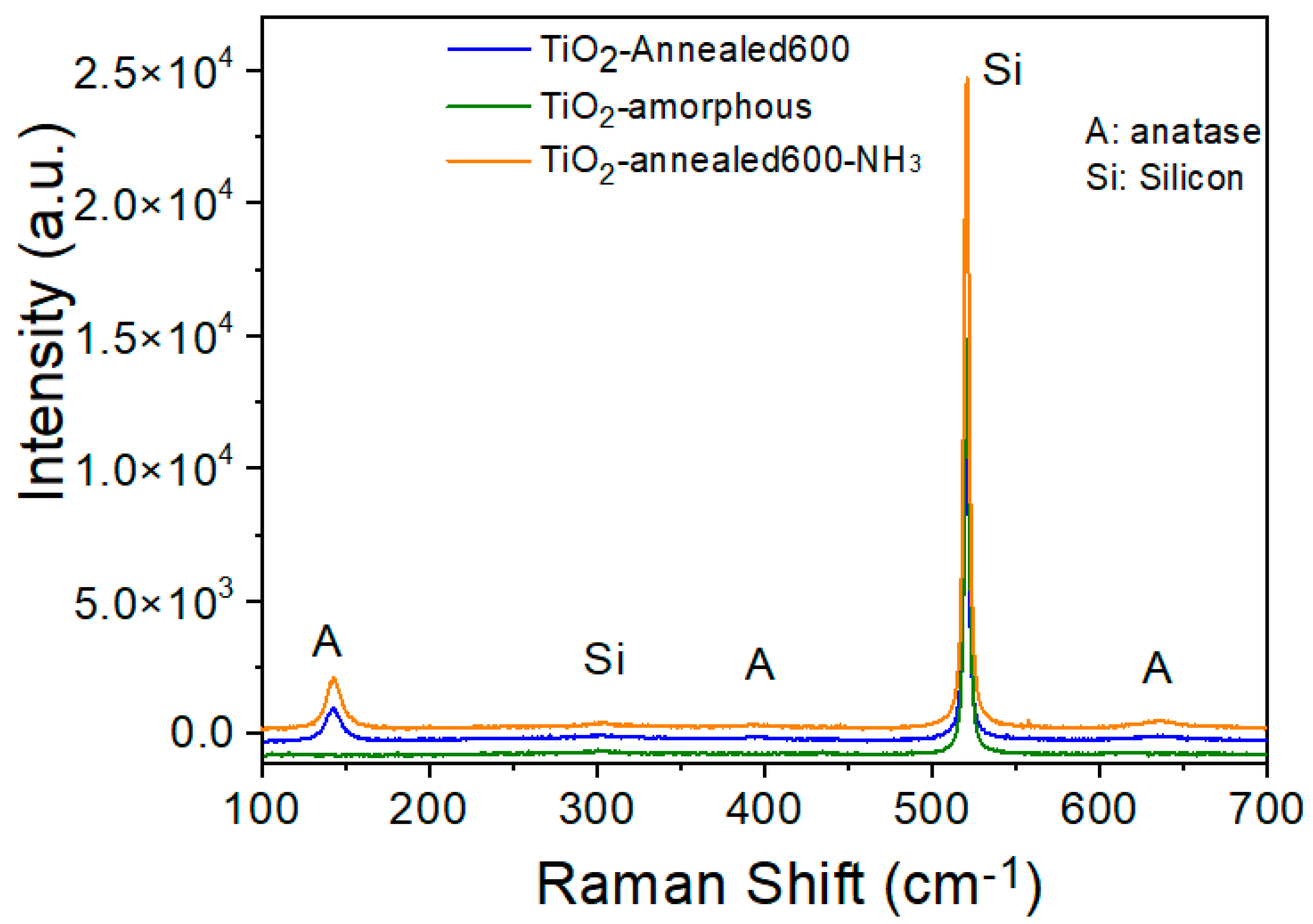
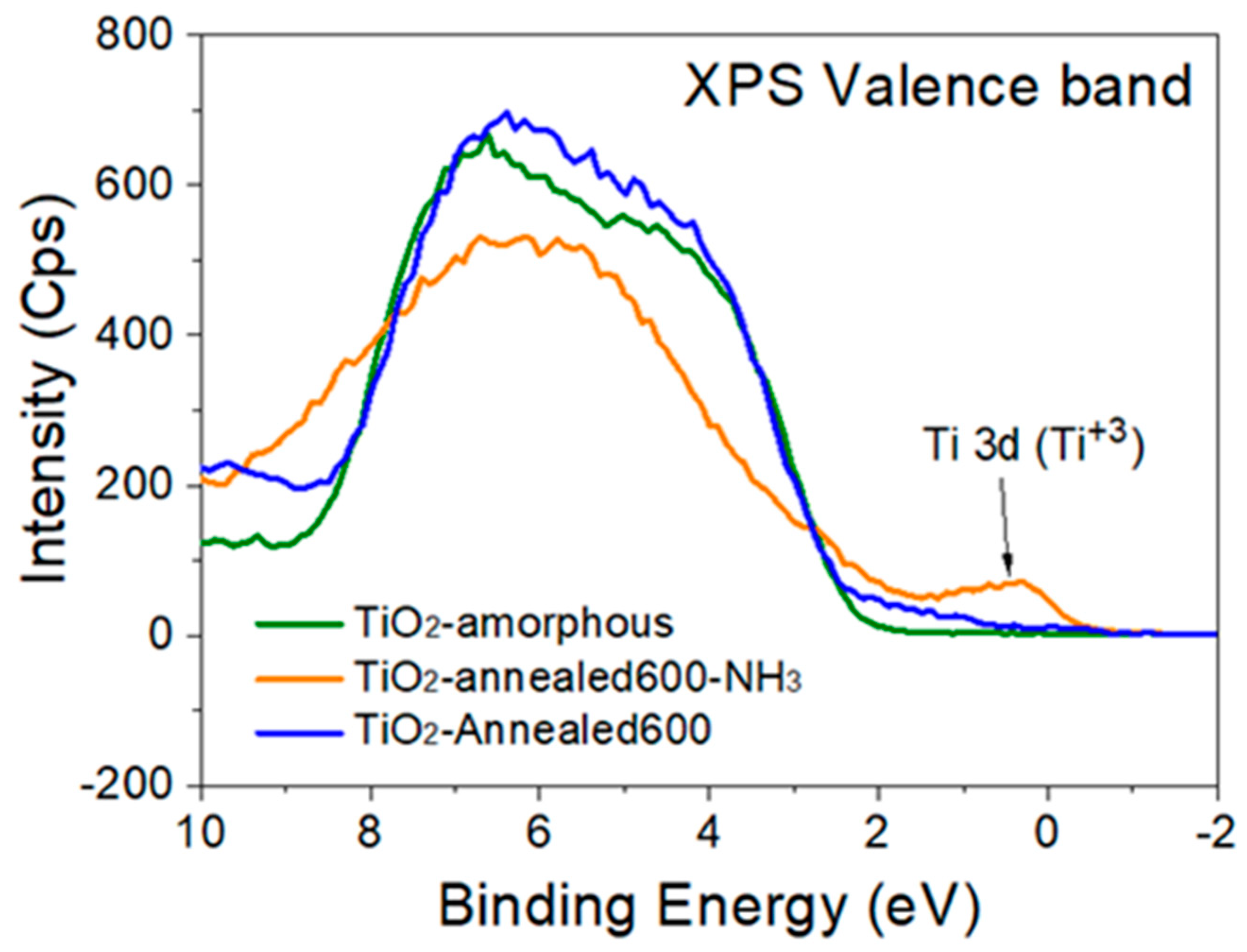
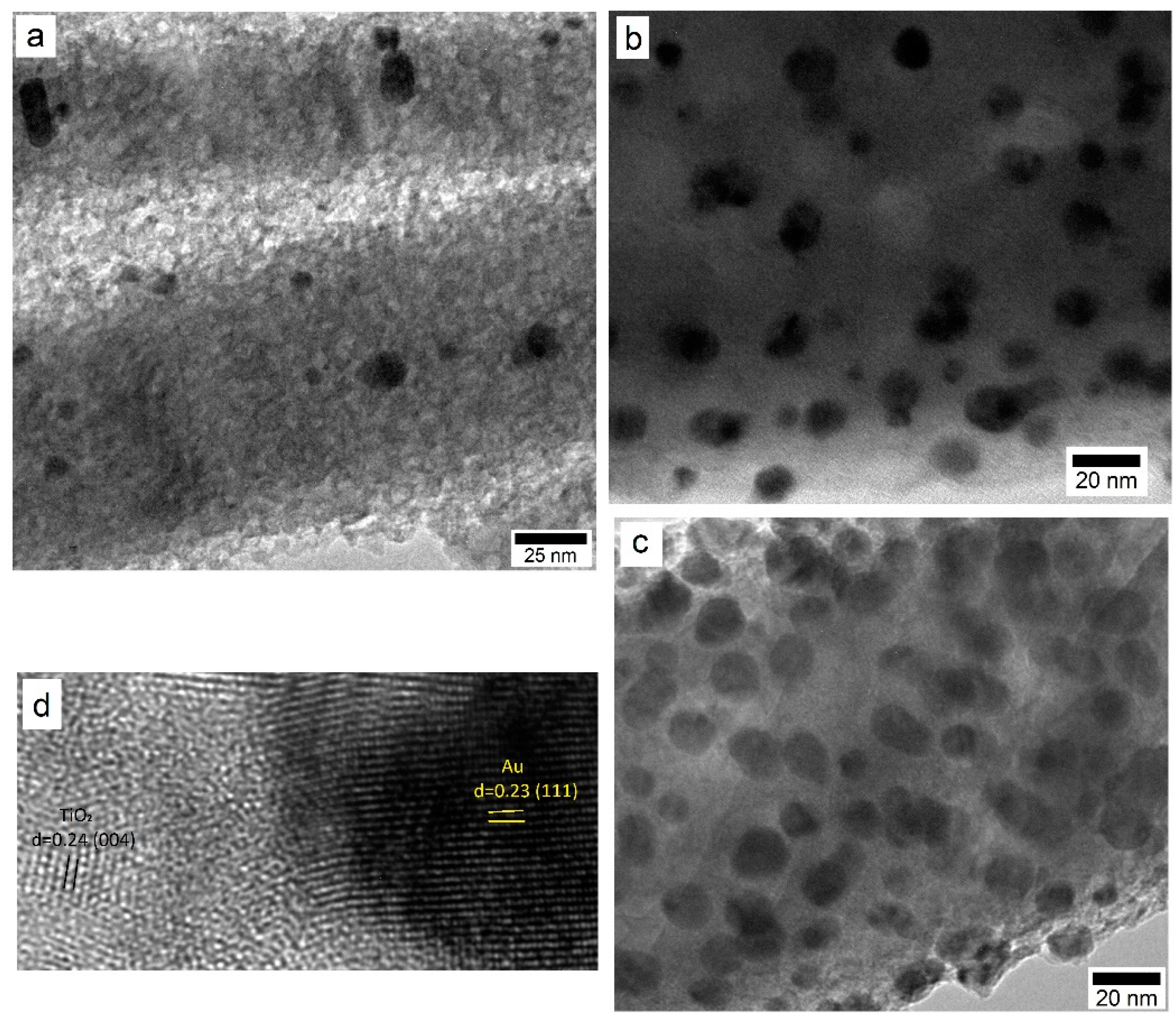
3.1. Characterization of the TiO2 Films and Au NP
3.2. Effect of Au NP Loading
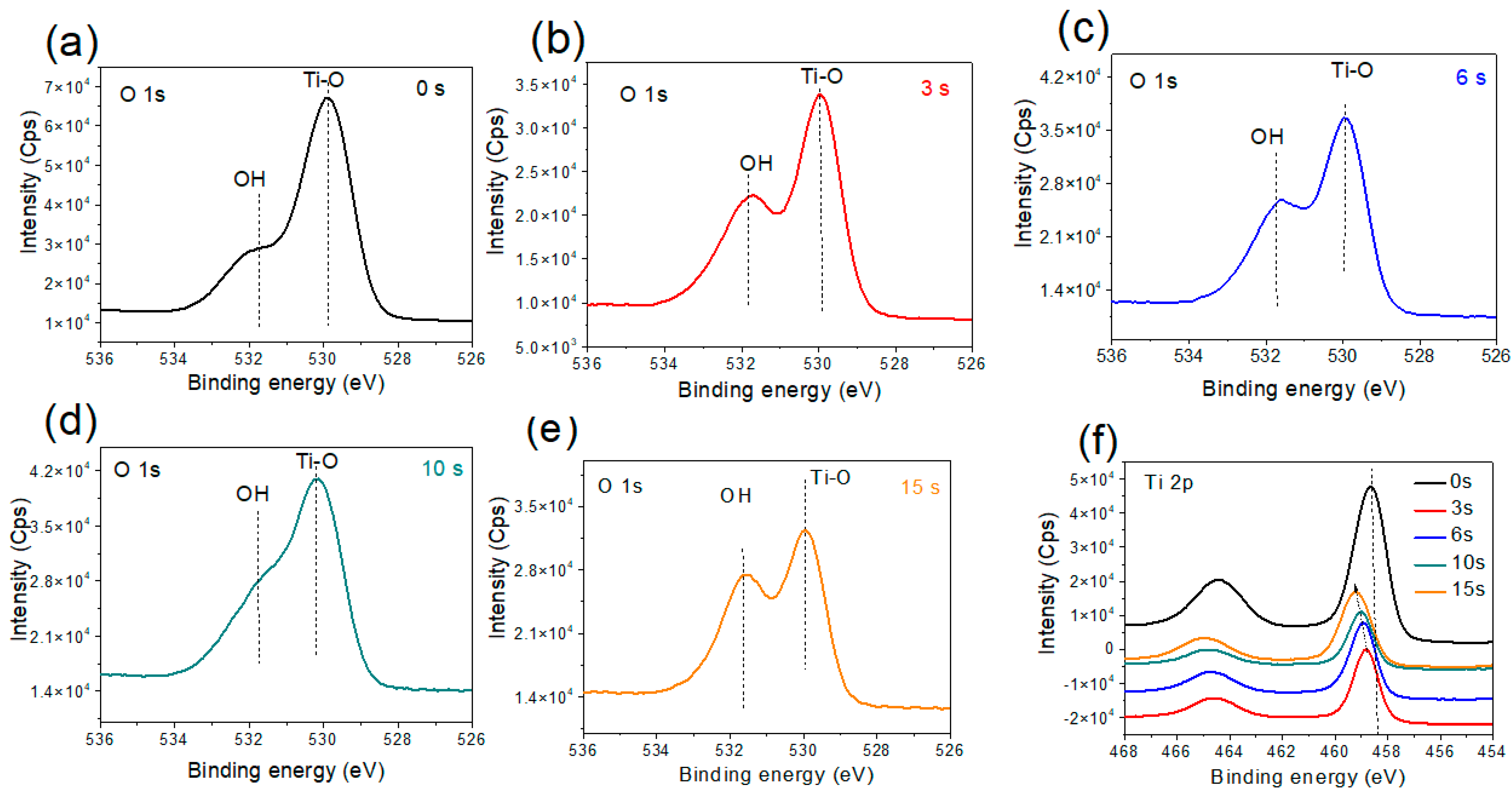
3.2.1. Amorphous TiO2 Support
3.2.2. Annealed Titania Film
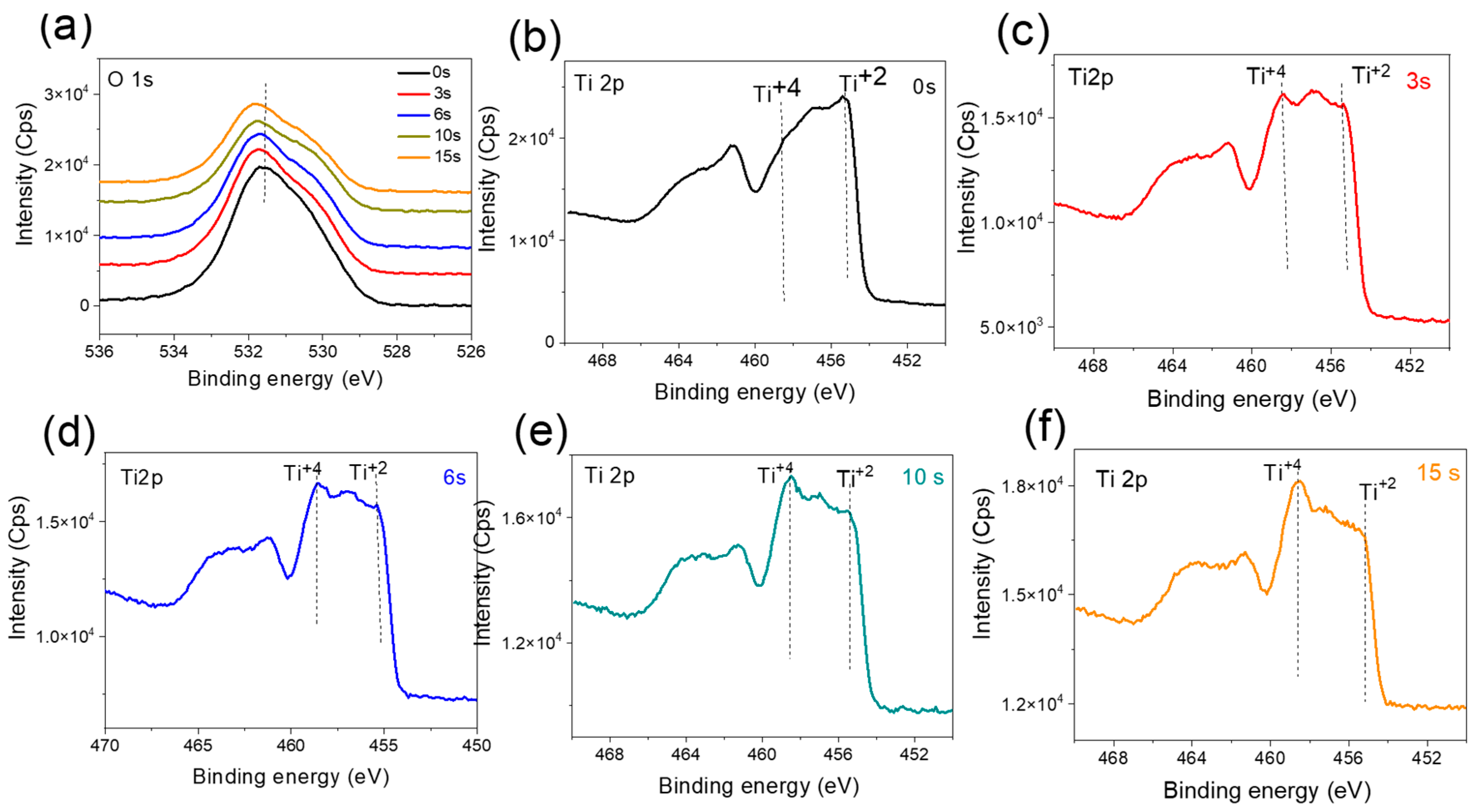
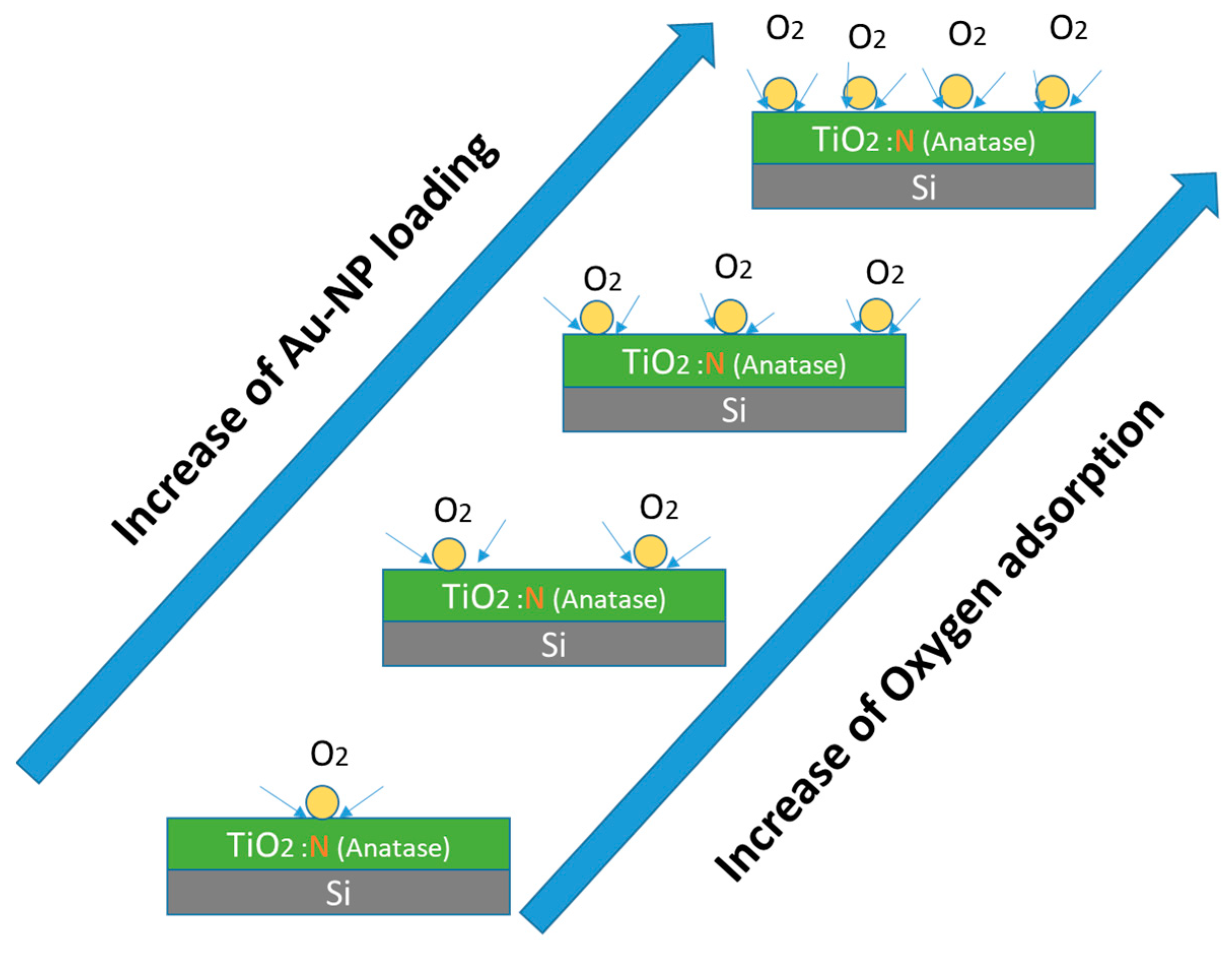
3.2.3. Annealed, Doped Anatase TiO2 Support
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achour, A.; Soussou, M.A.; Ait Aissa, K.; Islam, M.; Barreau, N.; Faulques, E.; Le Brizoual, L.; Djouadi, M.A.; Boujtita, M. Nanostructuration and band gap emission enhancement of ZnO film via electrochemical anodization. Thin Solid Films 2014, 571, 168. [Google Scholar] [CrossRef]
- Marelli, M.; Bossola, F.; Spinetti, G.; Sangalli, E.; Santo, V.D.; Psaro, R.; Polito, L. Microfluidic synthesis of hybrid TiO2-anisotropic gold nanoparticles with visible and near-infrared activity. ACS Appl. Mater. Interfaces 2020, 12, 38522. [Google Scholar] [CrossRef] [PubMed]
- Achour, A.; Islam, M.; Solaymani, S.; Vizireanu, S.; Saeed, K.; Dinescu, G. Influence of plasma functionalization treatment and gold nanoparticles on surface chemistry and wettability of reactive-sputtered TiO2 thin films. Appl. Surf. Sci. 2018, 458, 678. [Google Scholar] [CrossRef]
- Tang, H.; Su, Y.; Zhang, B.; Lee, A.F.; Isaacs, M.A.; Wilson, K.; Li, L.; Ren, Y.; Huang, J.; Haruta, M.; et al. Classical strong metal–support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 2017, 3, e1700231. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ueno, K.; Oshikiri, T.; Sun, Q.; Sasaki, K.; Misawa, H. Enhanced water splitting under modal strong coupling conditions. Nat. Nanotechnol. 2018, 13, 953. [Google Scholar] [CrossRef]
- Islam, M.; Achour, A.; Saeed, K.; Javed, S.; Boujtita, M.; Djouadi, M.A. Metal/Carbon hybrid nanostructures produced from plasma-enhanced chemical vapor deposition over Nafion-supported electrochemically deposited cobalt nanoparticles. Materials 2018, 11, 687. [Google Scholar] [CrossRef]
- Centeno, M.A.; Reina, T.R.; Ivanova, S.; Laguna, O.H.; Odriozola, J.A. Au/CeO2 catalysts: Structure and CO oxidation activity. Catalysts 2016, 6, 158. [Google Scholar] [CrossRef]
- Green, I.X.; Tang, W.; Neurock, M.; Yates, J.T. Spectroscopic Observation of dual catalytic sites during oxidation of CO on a Au/TiO2 catalyst. Science 2011, 333, 736. [Google Scholar] [CrossRef]
- Khan, R.; Javed, S.; Islam, M. Hierarchical nanostructures of titanium dioxide: Synthesis and applications. In Titanium Dioxide—Material for a Sustainable Environment; Yang, D., Ed.; InTechOpen: Basel, Switzerland, 2018; p. 74525. [Google Scholar]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X.; Ma, C.; Wang, X.; Wang, Z. Effect of a ZrO2 support on Cu/Fe2O3-CeO2/ZrO2 catalysts for NO removal by CO using a rotary reactor. Catal. Sci. Technol. 2018, 8, 5623. [Google Scholar] [CrossRef]
- Satitthai, U.; Luengnaruemitchai, A.; Gulari, E. Effect of gold loading on CeO2-Fe2O3 for oxidative steam reforming of methanol. Int. J. Chem. Biol. Eng. 2012, 6, 305. [Google Scholar]
- Jenness, G.R.; Schmidt, J.R. Unraveling the Role of Metal−Support Interactions in Heterogeneous Catalysis: Oxygenate Selectivity in Fischer-Tropsch Synthesis. ACS Catal. 2013, 3, 2881. [Google Scholar] [CrossRef]
- Chen, M.S.; Goodman, D.W. The Structure of Catalytically Active Gold on Titania. Science 2004, 306, 252. [Google Scholar] [CrossRef]
- Saavedra, J.; Doan, H.A.; Pursell, C.J.; Grabow, L.C.; Chandler, B.D. The critical role of water at the gold-titania interface in catalytic CO oxidation. Science 2014, 345, 1599. [Google Scholar] [CrossRef] [PubMed]
- Valdés, H.; Molina, L.M.; Alonso, J.A. Water adsorption and dissociation on gold catalysts supported on anatase-TiO2(101). Appl. Surf. Sci. 2019, 487, 244. [Google Scholar] [CrossRef]
- Kung, M.C.; Davis, R.J.; Kung, H.H. Understanding Au-Catalyzed Low-Temperature CO Oxidation. J. Phys. Chem. C 2007, 111, 11767. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.-C.; Luo, L.; Wang, Y.G.; Zhu, J.; Du, Y.; Li, J.; Mao, S.; Wang, C. Size-dependent dynamic structures of supported gold nanoparticles in CO oxidation reaction condition. Proc. Natl. Acad. Sci. USA 2018, 115, 7700. [Google Scholar] [CrossRef]
- Duan, Z.; Henkelman, G. CO oxidation at the Au/TiO2 boundary: The role of the Au/Ti5C site. ACS Catal. 2015, 5, 1589. [Google Scholar] [CrossRef]
- Widmann, D.; Behm, R.J. Formation and removal of active oxygen species for the non-catalytic CO oxidation on Au/TiO2 catalysts. Chin. J. Catal. 2016, 37, 1684. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Henkelman, G. CO Oxidation Mechanism on CeO2-Supported Au Nanoparticles. J. Am. Chem. Soc. 2012, 134, 1560. [Google Scholar] [CrossRef]
- Tan, S.; Ji, Y.; Zhao, Y.; Zhao, A.; Wang, B.; Yang, J.; Hou, J.G. Molecular Oxygen Adsorption Behaviors on the Rutile TiO2(110)-1 × 1 Surface: An in Situ Study with Low-Temperature Scanning Tunneling Microscopy. J. Am. Chem. Soc. 2011, 133, 2002. [Google Scholar] [CrossRef] [PubMed]
- Setvin, M.; Hulva, J.; Parkinson, G.S.; Schmid, M.; Diebold, U. Electron transfer between anatase TiO2 and an O2 molecule directly observed by atomic force microscopy. Proc. Natl. Acad. Sci. USA 2017, 114, E2556. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Liu, T.; Wang, Z.; Tsukimoto, S.; Saito, M.; Ikuhara, Y. Oxygen Adsorption on Anatase TiO2(101) and (001) Surfaces from First Principles. Mater. Trans. 2010, 51, 171. [Google Scholar] [CrossRef]
- Khorasaninejad, M.; Walia, J.; Saini, S.S. Enhanced first-order Raman scattering from arrays of vertical silicon nanowires. Nanotechnology 2012, 23, 275706. [Google Scholar] [CrossRef] [PubMed]
- Djarri, A.; Achour, A.; Soussou, M.A.; Sobti, N.; Achour, S. Characterization of thin films prepared by co-sputtering iron and titanium precursors and thermal oxidation under air atmosphere. Mater. Charact. 2018, 135, 139. [Google Scholar] [CrossRef]
- Cho, H.W.; Liao, K.L.; Yang, J.S.; Wu, J.J. Revelation of rutile phase by Raman scattering for enhanced photoelectrochemical performance of hydrothermally-grown anatase TiO2 film. Appl. Surf. Sci. 2018, 440, 125. [Google Scholar] [CrossRef]
- Lin, C.-P.; Chen, H.; Nakaruk, A.; Koshy, P.; Sorrell, C.C. Effect of Annealing Temperature on the Photocatalytic Activity of TiO2 Thin Films. Energy Procedia 2013, 34, 627–636. [Google Scholar] [CrossRef]
- Zanatta, A.R. A fast-reliable methodology to estimate the concentration of rutile or anatase phases of TiO2. AIP Adv. 2017, 7, 075201. [Google Scholar] [CrossRef]
- Iliev, M.N.; Hadjiev, V.G.; Litvinchuk, A.P. Raman and Infrared Spectra of Brookite (TiO2): Experiment and Theory. Vib. Spectrosc. 2013, 64, 148. [Google Scholar] [CrossRef]
- Zhou, J.K.; Zhang, Y.X.; Zhao, S.; Ray, A.K. Photodegradation of benzoic acid over metal-doped TiO2. Ind. Eng. Chem. Res. 2006, 45, 3503. [Google Scholar] [CrossRef]
- Batzill, M.; Morales, E.H.; Diebold, U. Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Phys. Rev. Lett. 2006, 96, 026103. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515. [Google Scholar] [CrossRef]
- Rumaiz, A.K.; Woicik, J.C.; Cockayne, E.; Lin, H.Y.; Jaffari, H.G.; Shah, S.I. Oxygen vacancies in N doped anatase TiO2: Experiment and first-principles calculations. Appl. Phys. Lett. 2009, 95, 262111. [Google Scholar] [CrossRef]
- Achour, A.; Islam, M.; Ahmad, I.; LBrizoual Djouadi, A.; Brousse, T. Influence of surface chemistry and point defects in TiN based electrodes on electrochemical capacitive storage activity. Scr. Mater. 2018, 153, 59. [Google Scholar] [CrossRef]
- Javed, F.; Javed, S.; Akram, M.A.; Mujahid, M.; Islam, M.; Bhatti, A.S. Surface Plasmon Mediated Optical Properties of ZnO/Au/TiO2 Nanoheterostructure Rod Arrays. Mater. Sci. Eng. B 2018, 231, 32. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Gan, J.; Tong, Y.; Li, Y. Hydrogenated TiO2 Nanotube Arrays for Supercapacitors. Nano Lett. 2012, 12, 1690–1696. [Google Scholar] [CrossRef]
- Bahri, K.; Eslami, H.; Müller-Plathe, F. Self-assembly of model triblock Janus colloidal particles in two dimensions. J. Chem. Theory Comput. 2022, 18, 1870. [Google Scholar] [CrossRef]
- Eslami, H.; Gharibi, A.; Müller-Plathe, F. Mechanisms of Nucleation and Solid–Solid-Phase Transitions in Triblock Janus Assemblies. J. Chem. Theory Comput. 2021, 17, 1742. [Google Scholar] [CrossRef]
- Wang, X.; Rui, Z.; Ji, H. DFT study of formaldehyde oxidation on silver cluster by active oxygen and hydroxyl groups: Mechanism comparison and synergistic effect. Catal. Today 2020, 347, 124. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Li, K.; Zeng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.Y.; Huang, H. Titanium oxide based photocatalytic materials development and their role in the air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200. [Google Scholar] [CrossRef]
- Javed, S.; Islam, M.; Mujahid, M. Synthesis and characterization of TiO2 quantum dots by sol-gel reflux condensation method. Ceram. Int. 2019, 45, 2676. [Google Scholar] [CrossRef]
- Molina, L.M.; Rasmussen, M.D.; Hammer, B. Adsorption of O2 and oxidation of CO at Au nanoparticles supported by TiO2(110). J. Chem. Phys. 2004, 120, 7673. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Siemer, N.; Todorova, M.; Marx, D.; Neugebauer, J. Deciphering Charge Transfer and Electronic Polarization Effects at Gold Nanocatalysts on Reduced Titania Support. J. Phys. Chem. C 2019, 123, 5495. [Google Scholar] [CrossRef]
- Achour, A.; Porto, R.L.; Soussou, M.-A.; Islam, M.; Boujtita, M.; Aissa, K.A.; le Brizoual, L.; Djouadi, A.; Brousse, T. Titanium nitride films for micro-supercapacitors: Effect of surface chemistry and film morphology on the capacitance. J. Power Sources 2015, 300, 525–532. [Google Scholar] [CrossRef]
- Achour, A.; Chaker, M.; Achour, H.; Arman, A.; Islam, M.; Mardani, M.; Boujtita, M.; le Brizoual, L.; Djouadi, M.A.; Brousse, T. Role of nitrogen doping at the surface of titanium nitride thin films towards capacitive charge storage enhancement. J. Power Sources 2017, 359, 349–354. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matouk, Z.; Islam, M.; Gutiérrez, M.; Pireaux, J.-J.; Achour, A. X-ray Photoelectron Spectroscopy (XPS) Analysis of Ultrafine Au Nanoparticles Supported over Reactively Sputtered TiO2 Films. Nanomaterials 2022, 12, 3692. https://doi.org/10.3390/nano12203692
Matouk Z, Islam M, Gutiérrez M, Pireaux J-J, Achour A. X-ray Photoelectron Spectroscopy (XPS) Analysis of Ultrafine Au Nanoparticles Supported over Reactively Sputtered TiO2 Films. Nanomaterials. 2022; 12(20):3692. https://doi.org/10.3390/nano12203692
Chicago/Turabian StyleMatouk, Zineb, Mohammad Islam, Monserrat Gutiérrez, Jean-Jacques Pireaux, and Amine Achour. 2022. "X-ray Photoelectron Spectroscopy (XPS) Analysis of Ultrafine Au Nanoparticles Supported over Reactively Sputtered TiO2 Films" Nanomaterials 12, no. 20: 3692. https://doi.org/10.3390/nano12203692
APA StyleMatouk, Z., Islam, M., Gutiérrez, M., Pireaux, J.-J., & Achour, A. (2022). X-ray Photoelectron Spectroscopy (XPS) Analysis of Ultrafine Au Nanoparticles Supported over Reactively Sputtered TiO2 Films. Nanomaterials, 12(20), 3692. https://doi.org/10.3390/nano12203692






