B-Site Fe/Re Cation-Ordering Control and Its Influence on the Magnetic Properties of Sr2FeReO6 Oxide Powders
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of SFRO DP Powders
2.2. Structural and Physical Characterization
3. Results
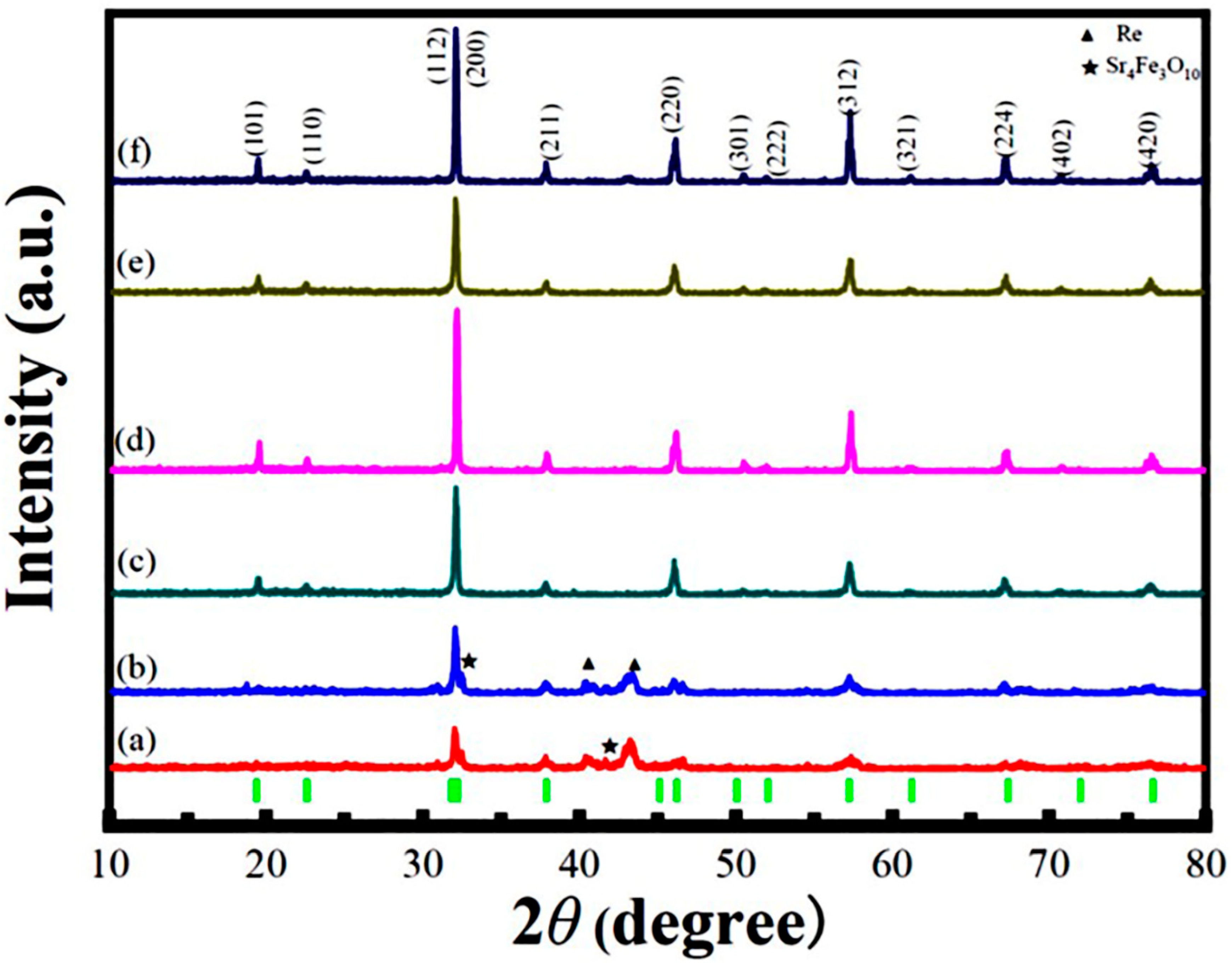
3.1. XRD Analysis and Its Rietveld Refinement
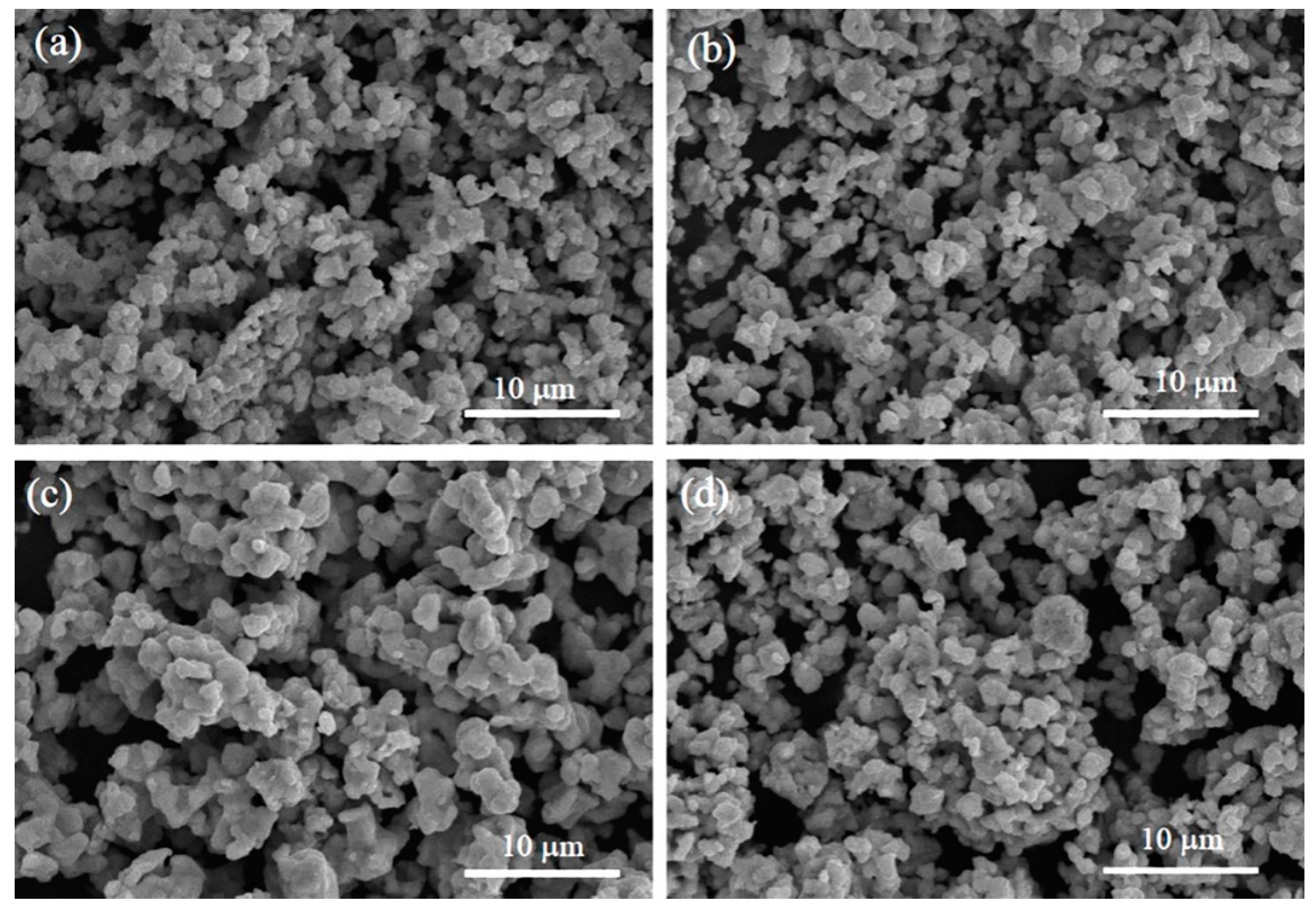
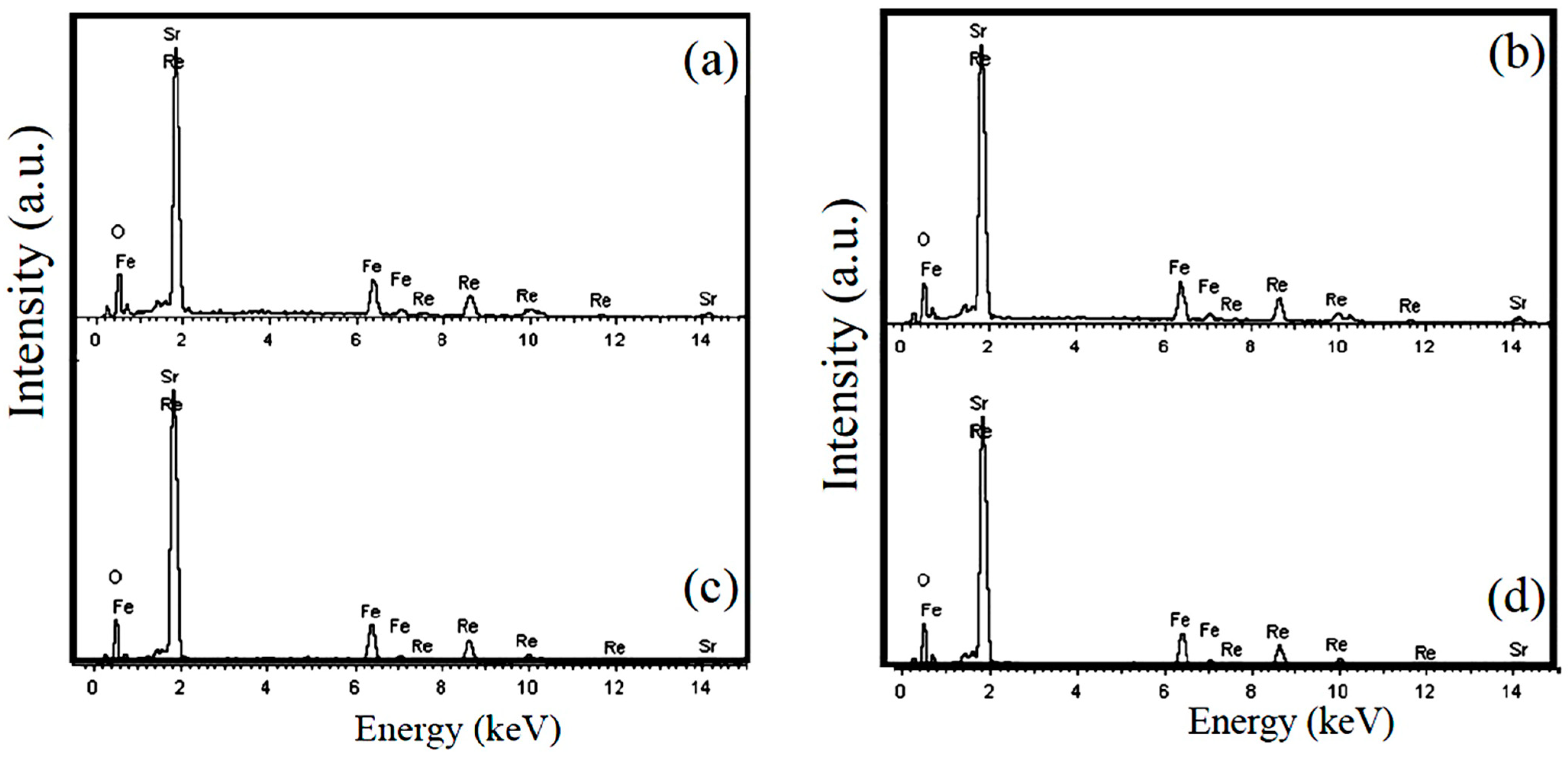
3.2. SEM Observation and EDS Analysis
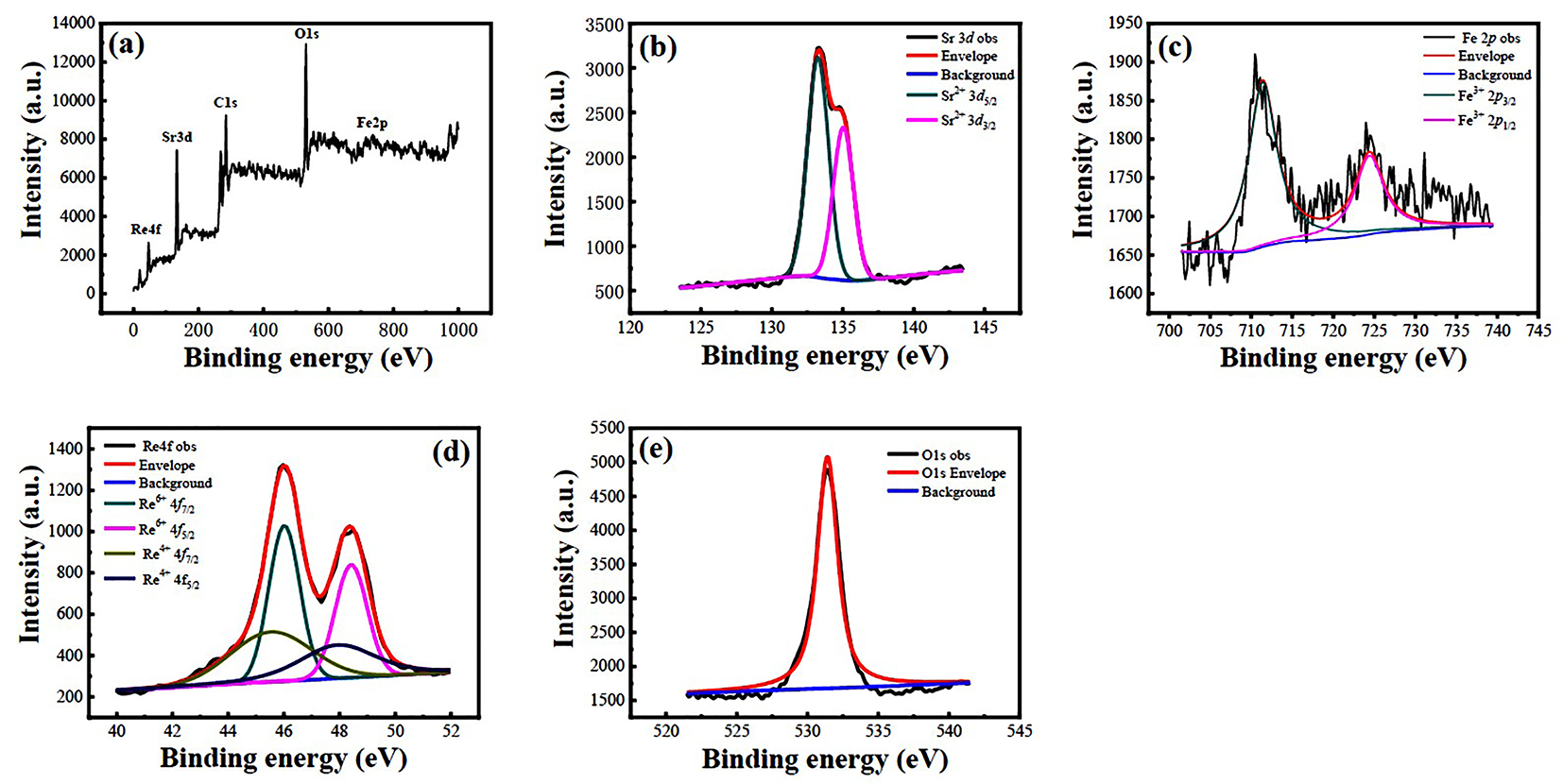
3.3. XPS Spectra Analysis
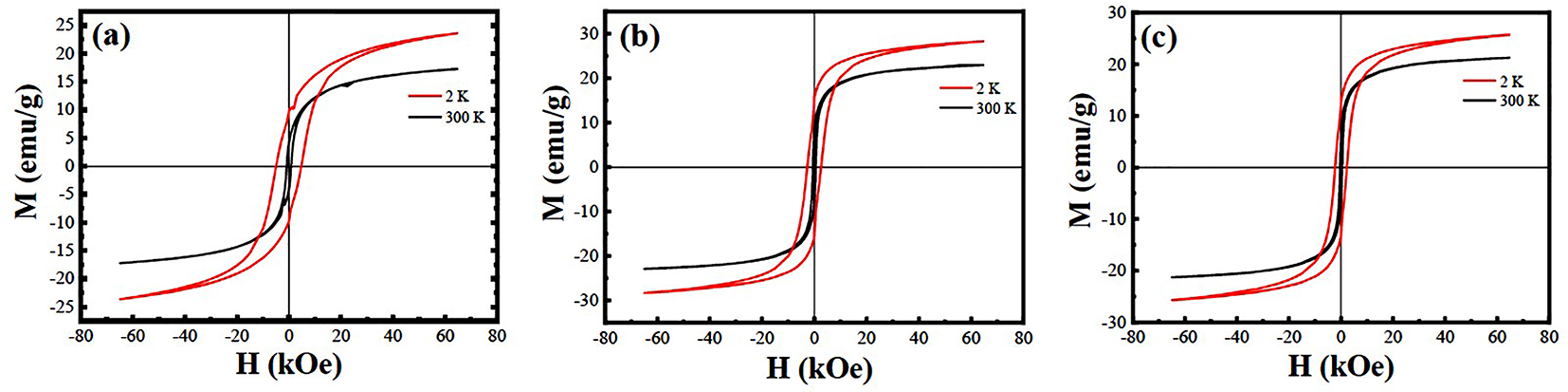
3.4. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolf, S.A.; Awschalom, D.D.; Buhrman, R.A.; Daughton, J.M.; von Molnar, S.; Roukes, M.L.; Chtchelkanova, A.Y.; Treger, D.M. Spintronics: A Spin-based Electronics Vision for the Future. Science 2001, 294, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Makarov, A.; Windbacher, T.; Sverdlov, V.; Selberherr, S. CMOS-compatible spintronic devices: A review. Semicond. Sci. Technol. 2016, 31, 113006. [Google Scholar] [CrossRef]
- Žutić, I.; Fabian, J.; Sarma, S.D. Spintronics: Fundamentals and applications. Rev. Mod. Phys. 2004, 76, 323–410. [Google Scholar] [CrossRef]
- Chrzanowska-Jeske, M.; Goodnick, S.M.; Wybourne, M.N. Nanoelectronics-beyond CMOS computing. IEEE Nanotechnol. Mag. 2021, 15, 6–7. [Google Scholar] [CrossRef]
- Yakout, S.M. Spintronics: Future technology for new data storage and communication devices. J. Supercond. Nov. Magn. 2020, 33, 2557–2580. [Google Scholar] [CrossRef]
- Nikandish, R.; Blokhina, E.; Leipold, D.; Staszewski, R.B. Semiconductor quantum computing: Toward a CMOS quantum computer on chip. IEEE Nanotechnol. Magn. 2021, 15, 8–20. [Google Scholar] [CrossRef]
- Kobayashi, K.I.; Kimura, T.; Sawada, H.; Terakura, K.; Tokura, Y. Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure. Nature 1998, 395, 677–680. [Google Scholar] [CrossRef]
- Serrate, D.; De Teresa, J.M.; Ibarra, M.R. Double perovskites with ferromagnetism above room temperature. J. Phys. Condens. Matter 2007, 19, 023201. [Google Scholar] [CrossRef]
- Blamire, M.G.; MacManus-Driscoll, J.L.; Mathur, N.D.; Barber, Z.H. The materials science of functional oxide thin films. Adv. Mater. 2009, 21, 3827–3839. [Google Scholar] [CrossRef]
- Meetei, O.N.; Erten, O.; Mukherjee, A.; Randeria, M.; Trivedi, N.; Woodward, P. Theory of half-metallic double perovskites. I. Double exchange mechanism. Phys. Rev. B 2013, 87, 165104. [Google Scholar] [CrossRef]
- Mishra, R.; Restrepo, O.D.; Woodward, P.M.; Windl, W. First-principles study of defective and nonstoichiometric Sr2FeMoO6. Chem. Mater. 2010, 22, 6092–6102. [Google Scholar] [CrossRef]
- Kobayashi, K.I.; Kimura, T.; Tomioka, Y.; Sawada, H.; Terakura, K. Intergrain tunneling magnetoresistance in polycrystals of the ordered double perovskite Sr2FeReO6. Phys. Rev. B 1999, 59, 11159–11162. [Google Scholar] [CrossRef]
- Fang, Z.; Terakura, K.; Kanamori, J. Strong ferromagnetism and weak antiferromagnetism in double perovskites: Sr2FeMO6 (M = Mo, W, and Re). Phys. Rev. B 2001, 63, 180407. [Google Scholar] [CrossRef]
- Tomioka, Y.; Okuda, T.; Okimoto, Y.; Kumai, R.; Kobayashi, K.I.; Tokura, Y. Magnetic and electronic properties of a single crystal of ordered double perovskite Sr2FeMoO6. Phys. Rev. B 2000, 61, 422–427. [Google Scholar] [CrossRef]
- Poddar, A.; Das, S. Metal-insulator transition in double perovskite Sr2Fe(Re,W)O6 compound. Phys. B Condens. Matter 2004, 344, 325–333. [Google Scholar] [CrossRef]
- Kim, T.H.; Uehara, M.; Cheong, S.W.; Lee, S. Large room-temperature intergrain magnetoresistance in double perovskite SrFe1−x(Mo or Re)xO3. Appl. Phys. Lett. 1999, 74, 1737–1739. [Google Scholar] [CrossRef]
- Balcells, L.; Navarro, J.; Bibes, M.; Roig, A.; Martinez, B.; Fontcuberta, J. Cationic ordering control of magnetization in Sr2FeMoO6 double perovskite. Appl. Phys. Lett. 2001, 78, 781–783. [Google Scholar] [CrossRef]
- Jacobo, S.E.; Duhalde, S.; Mercader, R.C. Cationic ordering in Sr2FeMoO6 prepared by a new chemical route. Phys. B 2004, 354, 59–62. [Google Scholar] [CrossRef]
- Yuan, C.L.; Wang, S.G.; Song, W.H.; Yu, T.; Dai, J.M.; Ye, S.L.; Sun, Y.P. Enhanced intergrain tunneling magnetoresistance in double perovskite Sr2FeMoO6 polycrystals with nanometer-scale particles. Appl. Phys. Lett. 1999, 75, 3853–3855. [Google Scholar] [CrossRef]
- Woodward, P.; Hoffmann, R.D.; Sleight, A.W. Order-disorder in A2M3+M5+O6 perovskites. J. Mater. Res. 1994, 9, 2118–2127. [Google Scholar] [CrossRef]
- Lim, J.B.; Choi, S.Y.; Kim, M.H.; Suvorov, D.; Jeon, J.H.; Song, T.K.; Kim, W.J. Effect of excess Re on the magnetic properties of Sr2FeReO6 double-perovskite. Mater. Lett. 2012, 7, 143–145. [Google Scholar] [CrossRef]
- Retuerto, M.; Martinez-Lope, M.J.; Garcia-Hernandez, M.; Alonso, J.A. Curie temperature enhancement in partially disordered Sr2FeReO6 double perovskites. Mater. Res. Bull. 2009, 44, 1261–1264. [Google Scholar] [CrossRef]
- Fuoco, L.; Rodriguez, D.; Peppel, T.; Maggard, P.A. Molten-salt-mediated syntheses of Sr2FeReO6, Ba2FeReO6, and Sr2CrReO6: Particle sizes, B/B′ site disorder, and magnetic properties. Chem. Mater. 2011, 23, 5409–5414. [Google Scholar] [CrossRef]
- Yang, L.; Fu, S.; Leng, K.; Tang, Q.K.; Wu, Z.W.; Yi, K.; Zhu, X.H. Crystal structure and magnetic properties of double perovskite Sr2FeReO6 ceramics synthesized by a combined molten-salt synthesis and solid-state reaction method. Open Ceram. 2022, 9, 100238. [Google Scholar] [CrossRef]
- Leng, K.; Tang, Q.K.; Wu, Z.W.; Yi, K.; Zhu, X.H. Double perovskite Sr2FeReO6 oxides: Structural, dielectric, magnetic, electrical, and optical properties. J. Am. Ceram. Soc. 2022, 105, 4097–4107. [Google Scholar] [CrossRef]
- Blanco, J.J.; Insausti, M.; Lezama, L.; Chapman, J.P.; de Gil Muro, I.; Rojo, T. Structural phase transition and magnetic properties of the Sr2FeRe1%−x O6 (B′ = Nb, Ta; x = 0, 0.1) double perovskites. J. Solid State Chem. 2004, 177, 2749–2755. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic-structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Sahu, N.; Panigrahi, S. Mathematical aspects of Rietveld refinement and crystal structure studies on PbTiO3 ceramics. Bull. Mater. Sci. 2011, 34, 1495–1500. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The materials project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Moritomo, Y.; Shimamoto, N.; Xu, S.; Machida, A.; Nishibori, E.; Takata, M.; Sakata, M.; Nakamura, A. Effects of B-site disorder in Sr2FeMoO6 with double perovskite structure. Jpn. J. Appl. Phys. 2001, 40, L672–L674. [Google Scholar] [CrossRef]
- Kato, H.; Okuda, T.; Okimoto, Y.; Tomioka, Y.; Oikawa, K.; Kamiyama, T.; Tokura, Y. Metal-insulator transition of ferromagnetic ordered double perovskites: (Sr1−yCay)2FeReO6. Phys. Rev. B 2002, 65, 144404. [Google Scholar] [CrossRef]
- Kato, H.; Okuda, T.; Okimoto, Y.; Tomioka, Y.; Oikawa, K.; Kamiyama, T.; Tokura, Y. Structural and electronic properties of the ordered double perovskites A2MReO6 (A = Sr, Ca; M = Mg, Sc, Cr, Mn, Fe, Co, Ni, Zn). Phys. Rev. B 2004, 69, 184412. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Chattopadhyay, A.; Ogale, S.B.; Venkatesan, T.; Greene, R.L. Metallic and nonmetallic double perovskites: A case study of A2FeReO6 (A = Ca, Sr, Ba). Phys. Rev. B 2000, 62, 9538–9542. [Google Scholar] [CrossRef]
- De Teresa, J.M.; Serrate, D.; Blasco, J.; Ibarra, M.R.; Morellon, L. Impact of cation size on magnetic properties of (AA’)2FeReO6 double perovskites. Phys. Rev. B 2004, 69, 144401. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation, Physical Electronics Division: Waltham, MA, USA, 1992. [Google Scholar]
- Das, N.; Singh, S.; Joshi, A.G.; Thirumal, M.; Reddy, V.R.; Gupta, L.C.; Ganguli, A.K. Pr2FeCrO6: A type I multiferroic. Inorg. Chem. 2017, 56, 12712–12718. [Google Scholar] [CrossRef]
- Niedrig, T.S.; Weiss, W.; Schlogl, R. Electronic structure of ultrathin ordered iron oxide films grown onto Pt(111). Phys. Rev. B 1995, 52, 17449–17460. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, X.H. Microstructures, magnetic, and dielectric properties of Ba-doped BiFeO3 nanoparticles synthesized via molten salt route. J. Am. Ceram. Soc. 2019, 102, 4698–4709. [Google Scholar] [CrossRef]
- Choi, J.S.; Ahn, C.W.; Bae, J.S.; Kim, T.H. Identifying a perovskite phase in rare-earth nickelates using X-ray photoelectron spectroscopy. Curr. Appl. Phys. 2020, 20, 102–105. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lim, J.B.; Ikuhara, Y.; Suvorov, D.; Jeon, J.H. Direct observation of cationic ordering in double perovskite Sr2FeReO6 crystals. Microsc. Microanal. 2003, 19, 25–28. [Google Scholar] [CrossRef]
- Lim, T.W.; Kim, S.D.; Sung, K.D.; Rhyim, Y.M.; Jeen, H.; Yun, J.; Kim, K.H.; Song, K.M.; Lee, S.S.; Chung, S.Y.; et al. Insights into cationic ordering in Re-based double perovskite oxides. Sci. Rep. 2016, 6, 19746. [Google Scholar] [CrossRef]
- Navarro, J.; Balcells, L.; Sandiumenge, F.; Bibes, M.; Roig, A.; Martínez, B.; Fontcuberta, J. Antisite defects and magnetoresistance in Sr2FeMoO6 double perovskite. J. Phys. Condens. Matter 2001, 13, 8481–8488. [Google Scholar] [CrossRef]
- Yu, X.; Asaka, T.; Tomioka, Y.; Tsuruta, C.; Nagai, T.; Kimoto, K.; Kaneko, Y.; Tokura, Y.; Matsui, Y. TEM study of the influence of anti-site defects on magnetic domain structures in double perovskite Ba2FeMoO6. J. Electron Microsc. 2005, 54, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Asaka, T.; Yu, X.Z.; Tomioka, Y.; Kaneko, Y.; Nagai, T.; Kimoto, K.; Ishizuka, K.; Tokura, Y.; Matsui, Y. Strong pinning effect and magnetic nanodomain formation by coupling between magnetic and crystallographic domains in the ordered double perovskite Ba2FeMoO6. Phys. Rev. B 2007, 75, 184440. [Google Scholar] [CrossRef]
- Esser, B.D.; Hauser, A.J.; Williams, R.E.A.; Allen, L.J.; Woodward, P.M.; Yang, F.Y.; McComb, D.W. Quantitative STEM imaging of order-disorder phenomena in double perovskite thin films. Phys. Rev. Lett. 2016, 117, 176101. [Google Scholar] [CrossRef]
- Yuan, B.; Kim, S.; Chun, S.H.; Jin, W.T.; Nelson, C.S.; Hauser, A.J.; Yang, F.Y.; Kim, Y.J. Robust long-range magnetic correlation across antiphase domain boundaries in Sr2CrReO6. Phys. Rev. B 2021, 103, 064410. [Google Scholar] [CrossRef]
- Singh, V.N.; Majumdar, P. Antisite domains in double perovskite ferromagnets: Impact on magnetotransport and half-metallicity. EPL Europhys. Lett. 2011, 94, 47004. [Google Scholar] [CrossRef][Green Version]
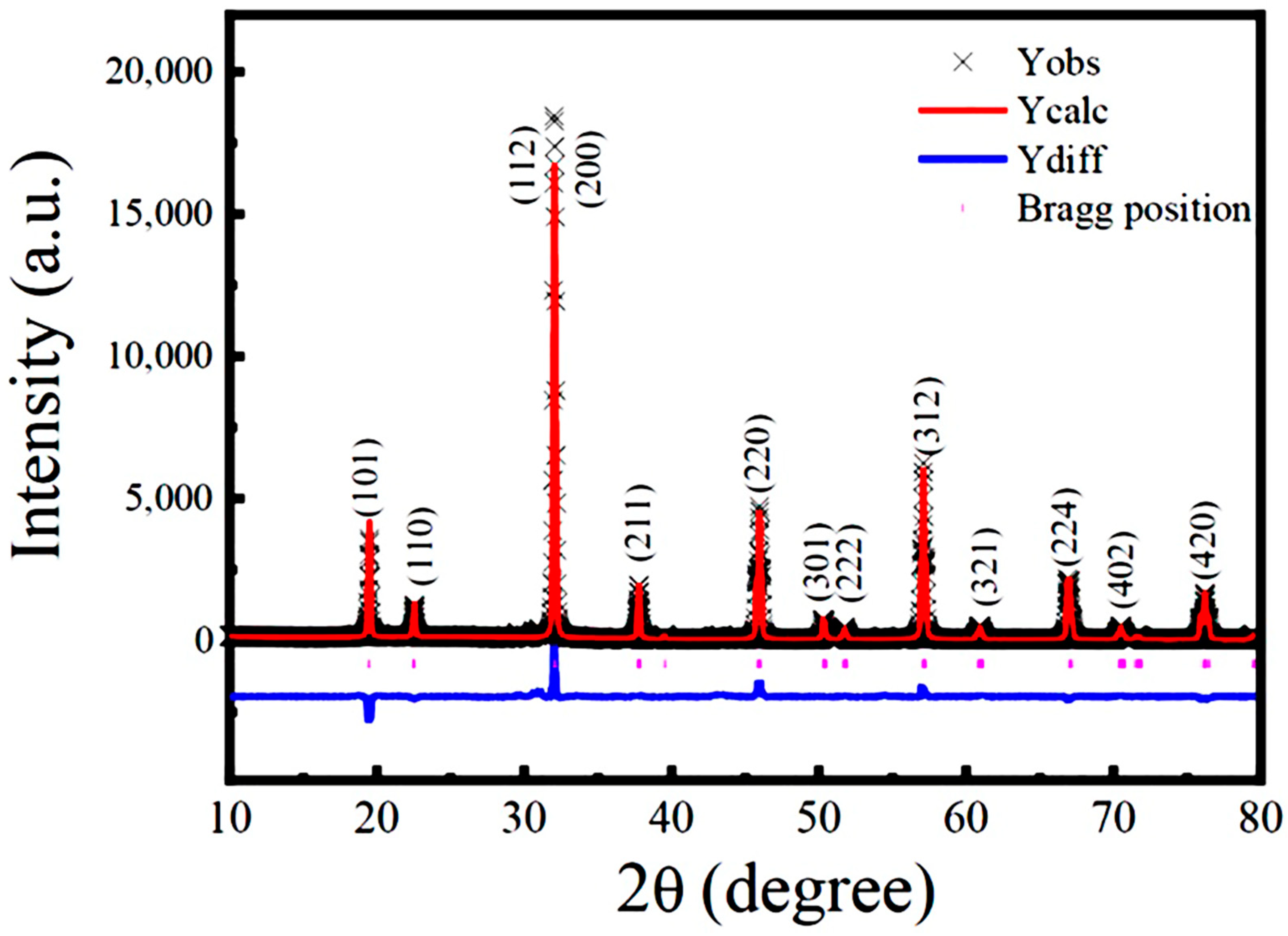
| Sample | Lattice Parameters | Space Group | Average Bond Length (Å) | Average Bond Angles (◦) | Fitting Parameters | ||
|---|---|---|---|---|---|---|---|
| SFRO | Tetragonal structure a = b = 5.57677(1) Å c = 7.91916(1) Å V = 246.29(2) Å3 | I4/m | <Fe-O1> | 1.99196(18) | <Fe1-O1-Re1> | 170.146(0) | χ2 = 0.8665 Rp =8.59% Rwp = 12.66% |
| <Fe-O2> | 1.99642(18) | ||||||
| <Re-O1> | 1.96603(17) | <F1e-O2-Re1> | 180.000(0) | ||||
| <Re-O2> | 1.96316(17) | ||||||
| Sample | 2 K | 300 K | η Value from XRD Data | ASD Content from XRD Data | η Value from Magnetic Data | ASD Content from Magnetic Data | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mr (μB/f.u.) | Hc (Oe) | Mr (μB/f.u.) | Hc (Oe) | |||||||
| A | 2.17 | 0.90 | 9757 | 1.59 | 0.38 | 4107 | 78.6% | 10.70% | 72.3% | 13.85% |
| B | 2.61 | 1.43 | 15,596 | 2.11 | 0.74 | 8001 | 85.1% | 7.45% | 87.0% | 6.50% |
| C | 2.37 | 1.19 | 12,925 | 1.96 | 0.63 | 6531 | 78.1% | 10.95% | 79.0% | 10.50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Tang, Q.; Wu, Z.; Yi, K.; Gu, J.; Zhu, X. B-Site Fe/Re Cation-Ordering Control and Its Influence on the Magnetic Properties of Sr2FeReO6 Oxide Powders. Nanomaterials 2022, 12, 3640. https://doi.org/10.3390/nano12203640
Wang Z, Tang Q, Wu Z, Yi K, Gu J, Zhu X. B-Site Fe/Re Cation-Ordering Control and Its Influence on the Magnetic Properties of Sr2FeReO6 Oxide Powders. Nanomaterials. 2022; 12(20):3640. https://doi.org/10.3390/nano12203640
Chicago/Turabian StyleWang, Zhuowei, Qingkai Tang, Zhiwei Wu, Kang Yi, Jiayuan Gu, and Xinhua Zhu. 2022. "B-Site Fe/Re Cation-Ordering Control and Its Influence on the Magnetic Properties of Sr2FeReO6 Oxide Powders" Nanomaterials 12, no. 20: 3640. https://doi.org/10.3390/nano12203640
APA StyleWang, Z., Tang, Q., Wu, Z., Yi, K., Gu, J., & Zhu, X. (2022). B-Site Fe/Re Cation-Ordering Control and Its Influence on the Magnetic Properties of Sr2FeReO6 Oxide Powders. Nanomaterials, 12(20), 3640. https://doi.org/10.3390/nano12203640






