Abstract
Herein, the UV light photocatalytic activity of an Au101NC-AlSrTiO3-rGO nanocomposite comprising 1 wt% rGO, 0.05 wt% Au101(PPh3)21Cl5 (Au101NC), and AlSrTiO3 evaluated for H2 production. The synthesis of Au101NC-AlSrTiO3-rGO nanocomposite followed two distinct routes: (1) Au101NC was first mixed with AlSrTiO3 followed by the addition of rGO (Au101NC-AlSrTiO3:rGO) and (2) Au101NC was first mixed with rGO followed by the addition of AlSrTiO3 (Au101NC-rGO:AlSrTiO3). Both prepared samples were annealed in air at 210 °C for 15 min. Inductively coupled plasma mass spectrometry and high-resolution scanning transmission electron microscopy showed that the Au101NC adhered almost exclusively to the rGO in the nanocomposite and maintained a size less than 2 nm. Under UV light irradiation, the Au101NC-AlSrTiO3:rGO nanocomposite produced H2 at a rate 12 times greater than Au101NC-AlSrTiO3 and 64 times greater than AlSrTiO3. The enhanced photocatalytic activity is attributed to the small particle size and high loading of Au101NC, which is achieved by non-covalent binding to rGO. These results show that significant improvements can be made to AlSrTiO3-based photocatalysts that use cluster co-catalysts by the addition of rGO as an electron mediator to achieve high cluster loading and limited agglomeration of the clusters.
1. Introduction
Green Hydrogen (H2) is an alternative clean and renewable energy source for the global transition toward net-zero carbon emissions [1]. Currently, over 95% of H2 is produced from fossil-fuel feedstocks which is a major contributor to carbon dioxide (CO2) emissions [2]. To comply with net-zero carbon emissions guidelines, a complete transition is required for the production, transportation, and consumption of energy. Therefore, H2 must be generated from renewable sources such as water and energy created from wind, solar, geothermal, tidal, or biomass reforming [3].
Photocatalytic water splitting is one of the most promising approaches for renewable H2 production. The basis of a photocatalyst is a light absorbing semiconductor (i.e., SrTiO3, TiO2, etc.) and co-catalysts (commonly metal nanoparticles) [4,5]. After absorbing light with energy equal or greater than its band gap (Eg), a photoexcited electron and a hole are generated in its conduction band (CB) and valance band (VB), respectively [6]. The photoexcited electron can drive reduction reactions, such as hydrogen evolution reaction (HER), while the hole can drive oxidation reactions, such as oxygen evolution reaction (OER). The co-catalyst can increase charge transfer and minimize electron–hole recombination, maximise reactant molecule activation, reaction selectivity, and catalyst stability [7].
The perovskite oxide SrTiO3 has been extensively used since 1980 for H2 production under UV irradiation due to its outstanding properties [8]. The conduction band minimum (CBM) potential and valence band maximum (VBM) potential make it suitable for H2/O2 evolution [9]. Doping of Al3+ as low-valence cations into the Ti4+ sites (AlSrTiO3) enhances the photocatalytic H2 production up to an apparent quantum yield (AQY) of 96% at 365 nm, which is the highest reported value for metal oxide photocatalysts [10,11,12].
Recently, photocatalytic systems incorporating sub-2 nm sized gold nanoclusters (AuNCs) with a specific number of Au atoms stabilized by ligands have triggered great research interest [13,14,15,16,17]. Typically, AuNCs exhibit non-metallic behaviour (HOMO–LUMO gap), as opposed to localized surface plasmon resonance (LSPR) of nanoparticles (i.e., >2 nm). The presence of HOMO–LUMO gaps in ultra-small AuNCs make them similar to narrow bandgap semiconductors which may be beneficial for photocatalysis [18,19]. AuNCs as co-catalysts can be coupled with semiconductors, resulting in charge transfer which prevents electron–hole recombination [20,21,22]. The catalytic activity of AuNCs is dependent on a number of factors, such as cluster size/structure, ligand type, ligand density, type of support, and metal–support interaction [23]. Use of triphenylphosphine (PPh3) ligands provides a simple approach to synthesize AuNCs with small cluster size, narrow size distribution, and easy ligand derivatization. These features make PPh3-ligated AuNCs unique and different from AuNCs protected by other ligands [23]. As a result, PPh3-ligated AuNCs as the key components of functional materials offer opportunities for both fundamental studies and potential applications, including photocatalysis [16,18,24,25,26].
PPh3-ligated AuNCs do not adhere strongly to metal oxide surfaces. Metal oxides have been shown to fragment PPh3-ligated AuNCs [27]. It has been reported that the deposition of Aun(PPh3)m (n: 1, 8, 9, 101) on acidic supports occurs according to two pathways, where the cluster–support interaction is affected by Brønsted and/or Lewis acid sites on the support. The interaction of PPh3 ligands with Brønsted acid on the supports results in cluster break down by “oxidative fragmentation” [27]. Lewis acid sites on the support results in ligand migration from the Au clusters to the support without fragmentation of the clusters, and their subsequent agglomeration [27].
We have previously reported that the uniform loading of Au101NCs onto reduced graphene oxide (rGO) with no aggregation is facilitated via non-covalent interactions through π–π stacking between the phenyl groups of PPh3 and rGO [28]. rGO with sp2-hybridized 2D structure has extraordinary properties, such as high electrical conductivity, large surface area, charge mobility, and chemical stability [29]. Due to its large surface area, rGO can serve as an “encapsulant” and wrap around semiconductor nanoparticles [30]. Furthermore, rGO as an electron acceptor/mediator facilitates transfer of photo-generated electrons through its π network to the active sites caused by high charge mobility and conductivity, which inhibits electron–hole recombination and promotes photocatalytic H2 production [31]. In addition, the tendency of AuNCs to aggregate (either during deposition or activation on the support [32,33]), or deactivate (following initial reaction [34] or under light illumination [20]) is inhibited due to the strong metal support interaction between rGO and AuNCs [28]. The aforementioned features make rGO a desirable candidate to integrate with a broad range of nanomaterials to form nanocomposites with improved performances in photocatalysis.
Herein, we compare two methods to synthesize a photocatalyst incorporating Au101(PPh3)21Cl5 co-catalyst, rGO electron mediator, and AlSrTiO3 semiconductor. The photocatalytic activity of these nanocomposites was evaluated in photocatalytic HER. Our results highlight the importance of rGO in the synthesis of the nanocomposite to both reduce agglomeration and act as an electron mediator.
2. Materials and Methods
2.1. Reagents and Materials
All chemicals were used as received throughout the study, unless otherwise stated: Natural graphite flakes (Uley, Eyre Peninsula, South Australia), 98% sulfuric acid (H2SO4, RCI Labscan), 85% phosphoric acid (H3PO4, Chem-Supply), 70% nitric acid (HNO3, Chem-Supply), 32% hydrochloric acid (HCl, RCI Labscan), 30% hydrogen peroxide (H2O2, Chem-Supply), potassium permanganate (KMnO4, Merck), Au101(PPh3)21Cl5 was synthesized following the method described by Hutchison and co-workers [35], AlSrTiO3 was provided by K. Domen (University of Tokyo and Shinshu University) and was synthesized following the method described by Ham et al. [10], methanol (CH3OH, Merk, Analysis Grade), high-purity Milli-Q water (18.2 MΩ cm at 25 °C), gold single component standard ICP (TraceCERT, Merck, 999 mg L−1), 68 Component ICP-MS Standard (High Purity Standards, HPS, 10 µg mL−1).
2.2. Au101NC-AlSrTiO3-rGO Photocatalyst Preparation
The procedure for fabrication of AlSrTiO3 with controlled content of 1 wt% rGO and 0.05 wt% Au via an ex situ method is schematically presented in Figure 1.
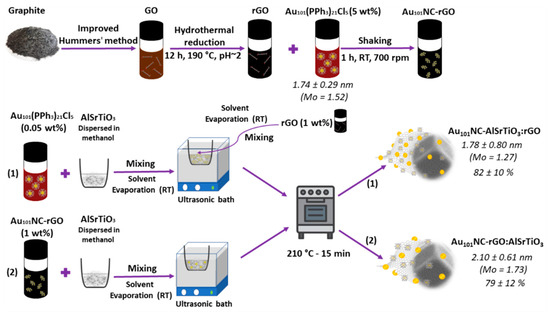
Figure 1.
Schematic illustration of synthesis of Au101NC-rGO nanocomposite and (1) Au101NC-AlSrTiO3:rGO and (2) Au101NC-rGO:AlSrTiO3 with 1 wt% rGO and 0.05 wt% Au. The values on the right-hand side show the Au cluster diameter measured by TEM and final gold loading measured by ICP-MS.
2.2.1. Graphene Oxide, rGO, and Au101NC-rGO Synthesis
Graphene oxide (GO), rGO, and Au101NC-rGO nanocomposite were synthesized following our previously reported method [28]. In brief, GO was synthesized via the improved Hummers’ method and then was reduced by hydrothermal treatment at 190 °C for 12 h in a 500 mL Teflon-lined stainless-steel autoclave. To obtain the 5 mg Au101NC-rGO nanocomposite with 5 wt% Au loading in 1.5 mL methanol, the as-obtained Au101NC was dispersed in methanol (1 mg mL−1). Then, 320 µL Au101NC dispersion (corresponding to 0.25 mg non-ligated Au mass) was added dropwise to 830 µL magnetically stirred as-synthesized rGO dispersion in methanol (5.70 mg mL−1) under ambient temperature and made up to 1.5 mL with methanol. The mixture was wrapped immediately with aluminium foil followed by mixing using an incubator and orbital shaker (THERMOstar), for 1 h at room temperature (RT) and 700 rpm.
2.2.2. Au101NC-AlSrTiO3-rGO Synthesis
The synthesis of Au101NC-AlSrTiO3-rGO with 1 wt% rGO and 0.05 wt% Au (corresponding to 0.0025 mg non-ligated Au mass) was carried out via two different routes, each with 2 steps, as shown in Figure 1 (Hyphen (-) is used to show the two components are first mixed and colon (:) is used to show the component is added last):
- (1)
- Au101NC-AlSrTiO3:rGO: To obtain 25 mg Au101NC-AlSrTiO3:rGO, 16.0 µL Au101NC dispersion (1 mg mL−1 ligated Au mass) was added dropwise to 25 mg of AlSrTiO3 dispersed in 5 mL methanol in a porcelain evaporation dish and homogenized using bath sonication at RT until the solvent was completely evaporated. The as-obtained Au101NC-AlSrTiO3 was dispersed and homogenized in 5 mL methanol via sonication (2 min). Then, 41.7 µL as-synthesized rGO dispersion (5.70 mg mL−1) was added dropwise to the dispersion with bath sonication at RT until the solvent was completely evaporated. The as-obtained Au101NC-AlSrTiO3:rGO was annealed in air in a muffle furnace (S.E.M Pty. Ltd., Adelaide, Australia) at 210 °C for 15 min.
- (2)
- Au101NC-rGO:AlSrTiO3: To obtain 25 mg Au101NC-rGO:AlSrTiO3, 0.25 mg (75 µL) of the as-synthesized Au101NC-rGO nanocomposite was added dropwise to 25 mg of AlSrTiO3 dispersed in 5 mL methanol in a porcelain evaporation dish. The dispersion was homogenized using bath sonication at RT until the solvent had evaporated. The as-obtained Au101NC-rGO: AlSrTiO3 was annealed in air in a muffle furnace at 210 °C for 15 min.
2.3. Characterization
Characterization of obtained materials before and after photocatalysis was performed using: scanning electron microscopy (SEM), high-angle annular diffraction field scanning transmission electron microscopy (HAADF-STEM), bright filed scanning transmission electron microscopy (BF-STEM), inductively coupled plasma mass spectrometry (ICP-MS), and UV–visible diffuse reflectance spectroscopy (UV–Vis DRS).
2.3.1. UV–Visible Diffuse Reflectance Spectroscopy (UV–Vis DRS)
UV–Vis DRS measurements were used to infer the extent of Au101NC agglomeration and to confirm that co-catalysts (rGO and Au101NC) did not affect the bandgap of the photocatalyst (AlSrTiO3). UV–Vis DRS measurements were obtained using a spectrophotometer (Cary 5000 UV–Vis–NIR) fitted with a Praying Mantis Diffuse Reflection accessory (Harrick, DRP-SAP). A PTFE disc was used as reflectance standard. For each measurement, the sample holder was filled with approximately 15 mg of the solid-state sample and the reflectance was measured from 200–800 nm.
2.3.2. Scanning Electron Microscopy (SEM)
The surface morphology, agglomeration state of Au101NC, and interaction of rGO sheets with AlSrTiO3 were measured using a High-Resolution Field Emission Scanning Electron Microscope equipped with EDX Silicon Drift Detectors (FEI-SEM Quanta 450).
2.3.3. High-Angle Annular Dark-Field Scanning TEM (HAADF-STEM)
Images showing the effect of heating and UV irradiation on the size and distribution of Au101NC over AlSrTiO3 and rGO along with interaction of Au101NC, rGO, and AlSrTiO3 with each other were acquired with a FEI Titan Themis STEM operating at 200 keV. The STEM was equipped with a Super-X EDS detector in conjunction with a low-background sample holder to minimize Cu background peaks and maximize X-ray collection efficiency. EDS data were analysed using the Velox™ software from Thermo Fisher Scientific. Samples were prepared by dropping prepared dispersions of as-prepared materials (sonicated for 1 min) onto a 300-mesh copper grid with a lacey carbon support film. The solvent was then allowed to evaporate before placing it into the sample holder for analysis. Image J was employed to measure the size of Au particles (200 particles).
2.3.4. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
ICP-MS (Agilent 8900x QQQ) was employed to determine the total content of Au in Au101NC-rGO, Au101NC-rGO:AlSrTiO3, Au101NC-AlSrTiO3, and Au101NC-AlSrTiO3:rGO by measuring the amount of Au that remained in solution (i.e., not adsorbed onto the solid). The as-synthesized sample dispersions in methanol (1 mL) were centrifuged to precipitate any solid, followed by filtration of the supernatant liquid using a Whatman 13 mm, 0.1 µm disposable nylon syringe filter. Then, 0.05 mL of filtrate was taken, and the solvent was allowed to evaporate. To dissolve the remaining solid, 0.2 mL of fresh aqua regia (analysis grade reagents of 32% hydrochloric acid and 70% nitric acid) was added and left for 30 min, then filled up to 25 mL with water for analysis. Gold and phosphorous single standard solutions in 2% aqua regia with concentrations of 5, 10, 25, 50, 100 and 200 ppb were used for calibration.
2.4. Photocatalysis
Photocatalytic HER was performed in a sealed overhead-irradiation type glass batch reactor (1.7 cm2). For each photocatalytic reaction, 7 mg of sample was immersed in 3 mL of methanol:H2O (1:2) and sonicated for 1 min to form a homogeneous suspension. Before each reaction, air was evacuated from the system and replaced with Ar (1 atm). The suspension was then irradiated with a UV LED (365 nm, 83 mW/cm2, Hongkong UVET Co., UH-82F+L12) for 2 h and stirred during irradiation using a magnetic bar. Starting at 0 h, the evolved gases were sampled hourly and analysed by gas chromatography (Agilent Technologies, 7890B, thermal conductivity detector, Ar carrier gas, molecular sieve 5 Å column).
3. Results and Discussion
The 3 component (Au101NC, AlSrTiO3, and rGO) photocatalysts with 1 wt% rGO and 0.05 wt% Au was prepared by a sequential mixing process via 2 routes. (1) Au101NC was first mixed with AlSrTiO3 followed by the addition of rGO (Au101NC-AlSrTiO3:rGO) and (2) Au101NC was first mixed with rGO followed by the addition of AlSrTiO3 (Au101NC-rGO:AlSrTiO3) (see Figure 1).
Characterization of the Au101NC-AlSrTiO3-rGO nanocomposites was performed to investigate the morphology/structural features, agglomeration state, and interactions between the Au101NCs, AlSrTiO3 and rGO along with their effect on H2 evolution.
3.1. Physical Characterization of Au101NC-AlSrTiO3-rGO
Figure 2a,b shows the SEM images of Au101NC-AlSrTiO3:rGO and Au101NC-rGO:AlSrTiO3. The SEM images of Au101NC-AlSrTiO3:rGO (Figure 2a) and Au101NC-rGO:AlSrTiO3 (Figure 2b) are similar with no obvious change in the morphology/structure and size relative to AlSrTiO3 (Figure S1). Although the rGO loading on AlSrTiO3 is low (1 wt%) in both nanocomposites, it can be seen in both higher resolution images that the rGO is well dispersed and wraps around some of AlSrTiO3 particles.
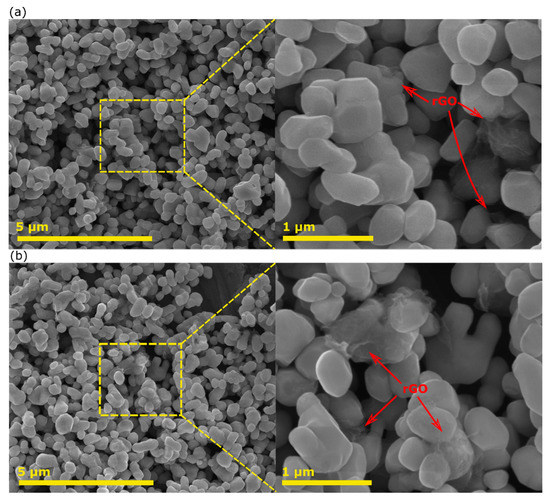
Figure 2.
SEM image of (a) Au101NC-AlSrTiO3:rGO and (b) Au101NC-rGO:AlSrTiO3.
Figure 3a–f shows the BF-STEM images and log-normal size-distribution histograms for Au101NC-AlSrTiO3:rGO (Figure 3a–c) and Au101NC-rGO:AlSrTiO3 (Figure 3d–f). Interestingly, compared with the original cluster (mode (Mo) = 1.52 nm, Figure S2) the cluster size decreases for Au101NC-AlSrTiO3:rGO (Mo = 1.27 nm) but increases for Au101NC-rGO:AlSrTiO3 (Mo = 1.73 nm). This suggests the clusters may fragment upon initial interaction with AlSrTiO3. This will be further addressed when discussing the ICP-MS results (vide infra).
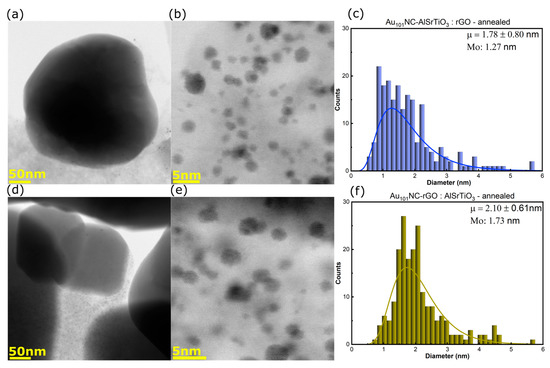
Figure 3.
(a,b) BF-STEM images and (c) size distribution histogram of Au101NC-AlSrTiO3:rGO, (d,e) BF-STEM images and (f) size distribution histogram of Au101NC-rGO:AlSrTiO3. Histogram fit to log-normal distribution with labels indicating mean (μ ± standard deviation) and Mo. Annealing was performed in air at 210 °C for 15 min. AlSrTiO3 and Au101NCs are the particles >50 nm and <5 nm, respectively. The grey thin sheets are rGO.
Beyond determining cluster size, imaging was used to locate where the AuNCs were located, and to determine if this differed for the two synthesis routes (e.g., on rGO or AlSrTiO3). We had expected to observe more Au101NC on the metal oxide in the Au101NC-AlSrTiO3:rGO sample, considering that the first step is the direct mixing of Au101NC with AlSrTiO3 (see Figure S3 for additional images). The STEM images confirm that regardless of the synthetic process, Au101NC was found almost exclusively on the rGO with only very few Au101NC (<5) observed on AlSrTiO3 amongst the thousands of Au101NC observed on rGO within the composite (see Figure S4a for example). This observation shows that the PPh3 ligated Au101NC has a high tendency to interact with rGO, as found in our previous work [28].
For further investigation, ICP-MS was used to determine the amount of Au deposited onto the rGO or AlSrTiO3 at each step of the synthetic process. This was achieved by measuring the Au that had not adsorbed (i.e., remained in solution after centrifugation). Table 1 shows the values obtained for the Au adsorption and the consequent Au loading at each step.

Table 1.
Adsorption (%) of Au on AlSrTiO3 and rGO by ICP-MS.
When following synthesis route (1); after mixing Au101NC and AlSrTiO3 together we find that 69% of the Au has adsorbed onto the metal oxide surface. In the case when rGO (in methanol) is added to the Au101NC-AlSrTiO3, the adsorption increases to 82%. These adsorptions reflect the higher affinity of Au101NC for rGO. When following the alternate synthesis route (2); we find an initial high adsorption of 95% (Au101NC-rGO) which then decreases to 79% after the addition of AlSrTiO3 in methanol. This is likely due to the dissolution of anchored Au101NC in methanol (Figure S10). The ICPMS results are consistent with the STEM observation that Au101NC are decorating the rGO in the composites with minimal loading on the AlSrTiO3 nanoparticles.
3.2. Photocatalytic Hydrogen Evolution Activity of AuNCs-AlSrTiO3-rGO
Figure 4 presents the effect of co-loading of rGO and Au101NC onto AlSrTiO3 via different routes on photocatalytic HER performance under UV light irradiation for 2 h under sacrificial conditions (methanol as hole scavenger). For comparison, the photocatalytic activity of rGO, AlSrTiO3, Au101NC-rGO, Au101NC-AlSrTiO3, and rGO-AlSrTiO3 as control samples under the same experimental conditions were also evaluated. The trend of H2 production rate (Table 2) follows: Au101NC-AlSrTiO3:rGO > Au101NC-rGO:AlSrTiO3 > rGO-AlSrTiO3 ~ Au101NC-AlSrTiO3 > AlSrTiO3 > rGO > Au101NC-rGO. The nanocomposite materials Au101NC-AlSrTiO3:rGO and Au101NC-rGO:AlSrTiO3 demonstrate the highest activity, producing 385 ± 22 and 334 ± 24 nmol h−1 of H2, respectively. This is approximately 10 times that of the activity of the rGO-AlSrTiO3 and Au101NC-AlSrTiO3 where AlSrTiO3 was only decorated with either rGO or Au101NCs. Negative control samples rGO, AlSrTiO3, and Au101NC-rGO produced negligible amounts of H2.
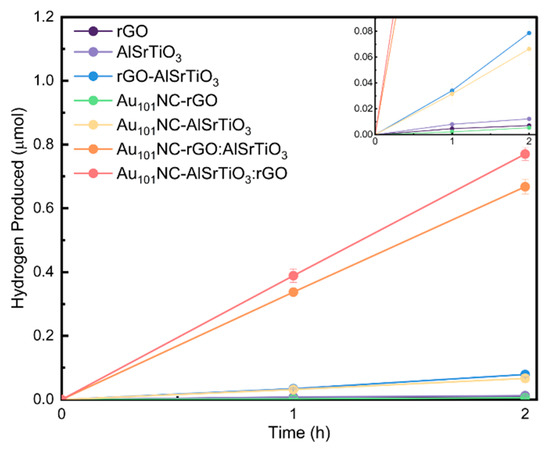
Figure 4.
Liquid-phase sacrificial photocatalytic H2 production of rGO, AlSrTiO3, Au101NC-rGO, Au101NC-AlSrTiO3, rGO-AlSrTiO3, Au101NC-AlSrTiO3:rGO, and Au101NC-rGO:AlSrTiO3. (Conditions: 1:2 methanol:water, LED 365 nm at 83 mW/cm2 for 2 h). Error bars represent standard error. The inset image is scaled up to display the relative activity of rGO, AlSrTiO3, Au101NC-rGO, and Au101NC-AlSrTiO3.

Table 2.
Photocatalytic H2 production rate—with Au mass loading 0.05% (0.0025 mg non-ligated Au): 1:2 methanol:water, 365 nm at 83 mW/cm2, 2 h reaction time. Error is standard deviation.
Extended experiments on the system that we did not completely optimize include the effect of Au101NC loading. Figure S7 indicates that increasing Au mass loading by 20 times (0.05% to 1% wt%) in Au101NC-AlSrTiO3:rGO results in only a small increase in photocatalytic activity (1.6 times). We also altered the annealing condition of the photocatalyst prior to hydrogen evolution. Figure S8 shows that the photocatalytic activity also increases in Au101NC-AlSrTiO3:rGO in order; air annealing > vacuum annealing > not annealed. Further work is required to ascertain the precise reason why annealing in air produced a more active photocatalyst.
STEM was also undertaken after photocatalysis to observe changes in the Au101NC size. Figure 5a–f shows that the average cluster size slightly increased in Au101NC-AlSrTiO3:rGO-HER (Mo from 1.27 to 1.49), and with almost no change for Au101NC-rGO:AlSrTiO3-HER (Mo from 1.73 to 1.72). The changes to the mode reflect changes to the most common cluster size. When comparing the mean average and clusters greater than 2 nm, we see there has been some agglomeration in both samples. The amount of Au particles greater than 2 nm increased from 28.6% to 38.5% (13.5% > 3 nm) and 45.0% to 52.9% (14.8% > 3 nm) for Au101NC-AlSrTiO3:rGO-HER and Au101NC-rGO:AlSrTiO3-HER, respectively (Table 3).
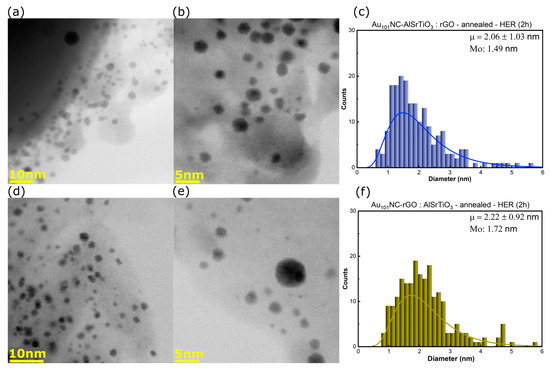
Figure 5.
(a,b) BF-STEM images and (c) size distribution histogram of Au101NC-AlSrTiO3:rGO-HER, (d,e) BF-STEM images and (f) size distribution histogram of Au101NC-rGO:AlSrTiO3-HER. Histogram fit to log-normal distribution with labels indicating mean (μ ± standard deviation) and mode (Mo). Annealing was performed in air at 210 °C for 15 min. AlSrTiO3 and Au101NCs are the particles >50 nm and <5 nm, respectively. The grey thin sheets are rGO.

Table 3.
A comparison of particle size in Au101NC, Au101NC-AlSrTiO3:rGO, and Au101NC-rGO:AlSrTiO3 before and after HER. Annealing was performed in air at 210 °C for 15 min.
4. Discussion
An efficient photocatalyst must possess three critical properties. A semiconductor with a bandgap able to absorb incident light, an electronic structure able to migrate the photo-generated charges to the particle surface, and the redox potential at the surface-active sites must be suitable for the water splitting reactions. Most photocatalysts, AlSrTiO3 included, can only evolve H2 with a co-catalyst [36]. This explains the very low H2 evolved (<7 nmol h−1) in the negative control samples which either do not contain a semiconductor (Au101NC-rGO, rGO) or co-catalyst (AlSrTiO3). When the semiconductor is combined with one of the co-catalyst materials (Au101NC-AlSrTiO3 and rGO-AlSrTiO3) we observe low H2 evolution (30–40 nmol h−1). rGO itself has been previously shown to have moderate activity as a HER co-catalyst [37]. Our previous work on electrocatalytic HER showed Au101NC was the most active of various gold clusters and far more active than rGO alone [38]. When both rGO and Au101NC are combined with AlSrTiO3, regardless of synthetic route, we observe a significantly enhanced HER activity (330–390 nmol h−1). This is attributed to the contributing role of the rGO to facilitate charge transfer from semiconductor to co-catalyst (Figure 6).
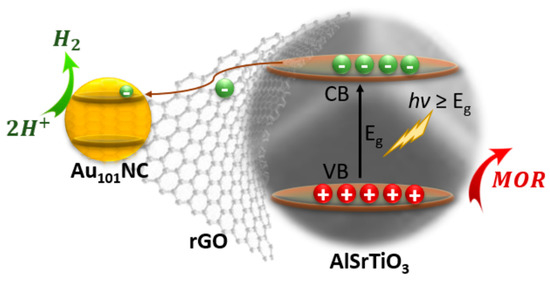
Figure 6.
Possible mechanism of electron transfer during photocatalytic H2 evolution under UV light irradiation. Electrons are transferred from AlSrTiO3 to the CB of Au101NC via rGO. (MOR = methanol oxidation reaction).
The difference in HER activity of the two synthetic routes that were investigated is small but may be due to the difference in Au101NC after deposition with Au101NC-AlSrTiO3:rGO having a smaller size and slightly greater Au loading. Overall, Au101NC-AlSrTiO3:rGO shows less agglomeration than Au101NC-rGO:AlSrTiO3, the differences in clusters size after HER may also be explained by the difference in cluster size before HER, as observed by STEM (Figure 5 and Table 3). The smaller, fragmented, Au101NC in the Au101NC-AlSrTiO3:rGO sample seem to be more prone to agglomeration under UV irradiation. It is difficult to conclude that the agglomeration is caused by the UV light or charge transfer (photoreduction) from rGO and AlSrTiO3 to Au101NCs. Despite small increases in mean average cluster size, both synthetic routes maintain a mode diameter of gold as clusters (<2 nm). This is supported by UV–Vis DRS in which the plasmonic band of the AuNCs was not observe in both of Au101NC-AlSrTiO3:rGO and Au101NC-rGO:AlSrTiO3 (Figure S6). This highlights the strong interaction between Au101NCs and rGO prevents cluster agglomeration, which is in agreement with our previous work [28].
These results demonstrate that the properties of the support determine the agglomeration/fragmentation state of Au101NC and the type of interactions between Au101NCs, AlSrTiO3, and rGO which has a large impact on the photocatalytic activity. The introduction of rGO with a large surface area provides more sites for the adsorption of Au101NCs. The synergistic effect between Au101NCs and rGO and higher tendency of Au101NC to interact with rGO results in agglomeration resistance and migration of Au101NC from AlSrTiO3 to rGO. Such selective loading of Au101NCs on rGO over AlSrTiO3 improves the photogenerated exciton separation. The rGO acts as a charge carrier resulting in improved transfer of photo-generated electrons through its π network to Au101NCs as the active site for H2 production. Our findings suggest that the proposed methodology using Au101NCs with PPh3 ligands to obtain atom-specific metal clusters can be used in photocatalysis with reduced agglomeration without adding a protecting overlayer (e.g., Cr2O3 overlayer for Au25 loaded on BaLa4Ti4O15) [39,40].
The majority of studies on Au co-catalyst systems in photocatalysis focus on nanoparticles where the LSPR is used to extend light absorption into the visible [41,42]. Future work may look at synthesizing size-specific AuNCs which are large enough to exhibit LSPR.
Cluster co-catalysts from metals other than gold have been used in photocatalysis. High density and uniform deposition of Fe, Co, and Ni clusters with sizes less than 1 nm on TiO2 significantly increased the photocatalytic H2 evolution activity caused by efficient carrier separation [43]. The photocatalytic H2 production of Ptn cluster (n: 8, 22, 34, 46, 68) cluster is affected by the size (number of atoms) of cluster [44]. Therefore, our developed methodology can also be applied with other size-specific PPh3-ligated metal clusters such as Pt, Pd, Cu, Ni, Co, Ag, and Ir to design highly efficient clusters/rGO nanocomposites for photocatalytic H2 production.
5. Conclusions
The simple preparation of Au101NC-AlSrTiO3:rGO as a HER photocatalyst is presented for the first time. The incorporation of rGO and Au101NC with AlSrTiO3 increases the catalytic activity of the Au101NC-AlSrTiO3:rGO about 64 times with no change in electronic structure and optical properties of the AlSrTiO3. This is attributed to the small particles size and high loading of Au101NC co-catalysts enabled by addition of rGO to the composite. In addition, the selective loading of Au onto rGO over AlSrTiO3 improves electron–hole separation and facilitates fast charge transfer to the active site and promotes photocatalytic H2 production. This methodology provides a simple and new avenue to design photocatalysts using graphene-PPh3-ligated AuNCs as effective co-catalysts for photocatalytic reactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano12203638/s1, Figure S1. The SEM image of AlSrTiO3; Figure S2. (a,b) HAADF-STEM images and (c) size distribution histogram of unsupported Au101NC drop-cast onto a TEM grid from methanol solution; Figure S3. BF-STEM images of Au101NC-AlSrTiO3:rGO (a) before and (b) after HER and Au101NC-rGO:AlSrTiO3 (c) before and (d) after HER. Au mass loading: 0.05 wt%. Annealing was performed at 210 °C in air for 15 min; Figure S4. EDX spectra of a chosen spot on (a) rGO in Au101NC-AlSrTiO3:rGO and (b) AlSrTiO3 in Au101NC-rGO:AlSrTiO3. Annealing was performed at 210 °C in air for 15 min. The inserts are the HAADF-STEM images of the chosen area; Figure S5. EDX spectra of a chosen spot on rGO in (a) Au101NC-AlSrTiO3:rGO-HER and (b) Au101NC-rGO:AlSrTiO3-HER. Annealing was performed at 210 °C in air for 15 min. The inserts are the HAADF-STEM images of the chosen area; Figure S6. UV Vis DRS of Au101NC-AlSrTiO3:rGO and Au101NC-rGO:AlSrTiO3 compared to AlSrTiO3 (a) before and (b) after HER. Annealing was performed in air at 210 °C for 15 min; Figure S7. Sacrificial liquid-phase H2 photocatalysis of Au101NC-AlSrTiO3:rGO (210 °C in air for 15 min) with Au mass loading of 0.05 and 1 wt%. (Conditions: 1:2 methanol:water, 365 nm at 83 mW/cm2, 2 h reaction time); Figure S8. Sacrificial liquid-phase H2 photocatalysis of unannealed, annealed (air), and annealed (vacuum) Au101NC-AlSrTiO3:rGO. Au mass loading 1 wt%, 1:2 methanol:water, LED 365 nm at 83 mW/cm2 for 2 h. Annealing was performed at 210 °C for 15 min; Figure S9. Photographs showing sample dispersions of (a) Au101NC-AlSrTiO3:rGO and (b) Au101NC-rGO:AlSrTiO3 before (left images) and after (right images) photocatalytic HER. Au mass loading 0.05 wt%, 1:2 methanol:water, LED 365 nm at 83 mW/cm2 for 2 h. Annealing was performed at 210 °C in air for 15 min; Figure S10. Photographs showing dispersions of Au101NCs (1 mg mL−1) in (a) 1:2 methanol:water and (b) methanol. Table S1. Average external quantum efficiencies (EQE)% for synthesized photocatalysts, assuming 2 electrons per H2 molecule produced. References [28,31] were cited in the Supplementary Materials.
Author Contributions
H.M.: Investigation, validation, methodology, visualization, project administration and designed the experiments, provision of study materials/instrumentation, nanomaterial synthesis, writing—original draft preparation, and leading the manuscript writing; T.D.S.: Photocatalysis measurements/DRS and subsequent analysis, writing the relevant experimental text; S.K.S.: Synthesis of Au101NC and manuscript editing; V.B.G.: supervised the synthesis of Au101NC and manuscript editing; C.J.S.: SEM characterisation, supervision, advisory support, and manuscript review and editing; G.F.M.: funding acquisition, supervision, manuscript review and editing, and final approval of the paper for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Australian Solar Thermal Research Initiative (ASTRI) program, which, is supported by the Australian Government, through the Australian Renewable Energy Agency (ARENA). H.M. wishes to acknowledge the University of Adelaide for the awarding of a postgraduate scholarship. SKS acknowledges funding support of the UC Connect PhD scholarship. CJS acknowledges funding support from The Australian Research Council (ARC) for a Future Fellowship (FT190100854).
Data Availability Statement
The majority of data created during this study are available within this manuscript and its Supplemental Material. All other data is available upon reasonable request to the corresponding author.
Acknowledgments
H.M would like to acknowledge Domen Kazunari from the University of Tokyo for providing the AlSrTiO3 sample. The authors acknowledge the instruments and scientific and technical assistance of Microscopy Australia at Adelaide Microscopy, The University of Adelaide, a facility that is funded by the University, and State and Federal Governments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IEA. Net Zero by 2050; International Energy Agency: Paris, France, 2021. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 8 February 2022).
- Taibi, E.; Miranda, R.; Vanhoudt, W.; Winkel, T.; Lanoix, J.-C.; Barth, F. Hydrogen from Renewable Power: Technology Outlook for the Energy Transition; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2018. [Google Scholar]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of photocatalytic performance with the use of noble-metal-decorated TiO2 nanocrystals as highly active catalysts for aerobic oxidation under visible-light irradiation. Appl. Catal. B 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Hou, Q.; Ju, M.; Li, W. Coupling plasmonic and cocatalyst nanoparticles on n–TiO2 for visible-light-driven catalytic organic synthesis. Nanomaterials 2019, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, W.; Dong, F.; Zhang, Z.; Han, L.; Luo, X.; Huang, J.; Feng, Z.; Chen, Z.; Jia, G.; et al. Recent advances in noncontact external-field-assisted photocatalysis: From fundamentals to applications. ACS Catal. 2021, 11, 4739–4769. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.H.; Lyu, H.; Hisatomi, T.; Goto, Y.; Takata, T.; Katayama, M.; Minegishi, T.; Domen, K. Efficient photocatalytic water splitting using Al-doped SrTiO3 coloaded with molybdenum oxide and rhodium–chromium oxide. ACS Catal. 2018, 8, 2782–2788. [Google Scholar] [CrossRef]
- Patial, S.; Hasija, V.; Raizada, P.; Singh, P.; Khan Singh, A.A.P.; Asiri, A.M. Tunable photocatalytic activity of SrTiO3 for water splitting: Strategies and future scenario. J. Environ. Chem. Eng. 2020, 8, 103791. [Google Scholar] [CrossRef]
- Ham, Y.; Hisatomi, T.; Goto, Y.; Moriya, Y.; Sakata, Y.; Yamakata, A.; Kubota, J.; Domen, K. Flux-mediated doping of SrTiO3 photocatalysts for efficient overall water splitting. J. Mater. Chem. A 2016, 4, 3027–3033. [Google Scholar] [CrossRef]
- Goto, Y.; Hisatomi, T.; Wang, Q.; Higashi, T.; Ishikiriyama, K.; Maeda, T.; Sakata, Y.; Okunaka, S.; Tokudome, H.; Katayama, M.; et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen generation. Joule 2018, 2, 509–520. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Munir, A.; Joya, K.S.; Ul Haq, T.; Babar, N.-U.-A.; Hussain, S.Z.; Qurashi, A.; Ullah, N.; Hussain, I. Metal nanoclusters: New paradigm in catalysis for water splitting, solar and chemical energy conversion. ChemSusChem 2019, 12, 1517–1548. [Google Scholar] [CrossRef]
- Attia, Y.; Samer, M. Metal clusters: New era of hydrogen production. Renew. Sustain. Energy Rev. 2017, 79, 878–892. [Google Scholar] [CrossRef]
- Yu, C.; Li, G.; Kumar, S.; Kawasaki, H.; Jin, R. Stable Au25 (Sr) 18/TiO2 composite nanostructure with enhanced visible light photocatalytic activity. J. Phys. Chem. Lett. 2013, 4, 2847–2852. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Wang, M.; Zhang, C.; Luo, N.; Wang, Y.; Abroshan, H.; Li, G.; Wang, F. Visible light gold nanocluster photocatalyst: Selective aerobic oxidation of amines to imines. ACS Catal. 2017, 7, 3632–3638. [Google Scholar] [CrossRef]
- Negishi, Y.; Mizuno, M.; Hirayama, M.; Omatoi, M.; Takayama, T.; Iwase, A.; Kudo, A. Enhanced photocatalytic water splitting by BaLa4Ti4O15 loaded with ∼1 nm gold nanoclusters using glutathione-protected Au25 clusters. Nanoscale 2013, 5, 7188–7192. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Kamat, P.V. Size-dependent excited state behavior of glutathione-capped gold clusters and their light-harvesting capacity. J. Am. Chem. Soc. 2014, 136, 11093–11099. [Google Scholar] [CrossRef] [PubMed]
- Madridejos, J.M.L.; Harada, T.; Falcinella, A.J.; Small, T.D.; Golovko, V.B.; Andersson, G.G.; Metha, G.F.; Kee, T.W. Optical properties of the atomically precise C4 core [Au9(PPh3)8]3+ cluster probed by transient absorption spectroscopy and time-dependent density functional theory. J. Phys. Chem. C 2021, 125, 2033–2044. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Y.-J. Photo-induced transformation process at gold clusters-semiconductor interface: Implications for the complexity of gold clusters-based photocatalysis. Sci. Rep. 2016, 6, 22742. [Google Scholar] [CrossRef]
- Puangpetch, T.; Chavadej, S.; Sreethawong, T. Hydrogen production over Au-loaded mesoporous-assembled SrTiO3 nanocrystal photocatalyst: Effects of molecular structure and chemical properties of hole scavengers. Energy Convers. Manag. 2011, 52, 2256–2261. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Adnan, R.H.; Madridejos, J.M.L.; Alotabi, A.S.; Metha, G.F.; Andersson, G.G. A review of state of the art in phosphine ligated gold clusters and application in catalysis. Adv. Sci. 2022, 9, 2105692. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Peng, L.; Bian, Y.; Shen, X.; Li, J.; Yao, H.-C.; Zang, S.-Q.; Li, Z. Exerting charge transfer to stabilize Au nanoclusters for enhanced photocatalytic performance toward selective oxidation of amines. Appl. Catal. B 2021, 284, 119704. [Google Scholar] [CrossRef]
- Qin, Z.; Zhao, D.; Zhao, L.; Xiao, Q.; Wu, T.; Zhang, J.; Wan, C.; Li, G. Tailoring the stability, photocatalysis and photoluminescence properties of Au11 nanoclusters via doping engineering. Nanoscale Adv. 2019, 1, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Hu, S.; Han, W.; Li, Z.; Xu, W.W.; Zhang, J.; Li, G. Tailoring optical and photocatalytic properties by single-Ag-atom exchange in Au13Ag12(PPh3)10Cl8 nanoclusters. Nano Res. 2022, 15, 2971–2976. [Google Scholar] [CrossRef]
- Longo, A.; de Boed, E.J.J.; Mammen, N.; van der Linden, M.; Honkala, K.; Häkkinen, H.; de Jongh, P.E.; Donoeva, B. Towards atomically precise supported catalysts from monolayer-protected clusters: The critical role of the support. Chem. Eur. J. 2020, 26, 7051–7058. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, H.; Yin, Y.; Howard-Fabretto, L.; Sharma, S.K.; Golovko, V.; Andersson, G.G.; Shearer, C.J.; Metha, G.F. Au101–rGO nanocomposite: Immobilization of phosphine-protected gold nanoclusters on reduced graphene oxide without aggregation. Nanoscale Adv. 2021, 3, 1422–1430. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Wang, X. Graphene-based nanomaterials for catalysis. Ind. Eng. Chem. Res. 2017, 56, 3477–3502. [Google Scholar] [CrossRef]
- Shearer, C.J.; Eder, D. 5. Synthesis strategies of nanocarbon hybrids. In Nanocarbon-Inorganic Hybrids: Next Generation Composites for Sustainable Energy Applications; Dominik, E., Robert, S., Eds.; De Gruyter: Berlin, Germany, 2014; pp. 125–170. [Google Scholar]
- Albero, J.; Mateo, D.; García, H. Graphene-based materials as efficient photocatalysts for water splitting. Molecules 2019, 24, 906. [Google Scholar] [CrossRef]
- Anderson, D.P.; Alvino, J.F.; Gentleman, A.; Qahtani, H.A.; Thomsen, L.; Polson, M.I.J.; Metha, G.F.; Golovko, V.B.; Andersson, G.G. Chemically-synthesised, atomically-precise gold clusters deposited and activated on titania. Phys. Chem. Chem. Phys. 2013, 15, 3917–3929. [Google Scholar] [CrossRef]
- Ruzicka, J.-Y.; Abu Bakar, F.; Hoeck, C.; Adnan, R.; McNicoll, C.; Kemmitt, T.; Cowie, B.C.; Metha, G.F.; Andersson, G.G.; Golovko, V.B. Toward control of gold cluster aggregation on TiO2 via surface treatments. J. Phys. Chem. C 2015, 119, 24465–24474. [Google Scholar] [CrossRef]
- Wang, C.; Li, N.; Wang, Q.; Tang, Z. Hybrid nanomaterials based on graphene and gold nanoclusters for efficient electrocatalytic reduction of oxygen. Nanoscale Res. Lett. 2016, 11, 336. [Google Scholar] [CrossRef]
- Weare, W.W.; Reed, S.M.; Warner, M.G.; Hutchison, J.E. Improved synthesis of small (dcore ≈ 1.5 nm) phosphine-stabilized gold nanoparticles. J. Am. Chem. Soc. 2000, 122, 12890–12891. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Mondal, A.; Prabhakaran, A.; Gupta, S.; Subramanian, V.R. Boosting photocatalytic activity using reduced graphene oxide (rGO)/semiconductor nanocomposites: Issues and future scope. ACS Omega 2021, 6, 8734–8743. [Google Scholar] [CrossRef]
- Mousavi, H.; Yin, Y.; Sharma, S.K.; Gibson, C.T.; Golovko, V.; Andersson, G.G.; Shearer, C.J.; Metha, G.F. Factors influencing catalytic activity of size-specific triphenylphosphine-ligated gold nanoclusters in the electrocatalytic hydrogen evolution reaction. J. Phys. Chem. C 2022, 126, 246–260. [Google Scholar] [CrossRef]
- Kurashige, W.; Kumazawa, R.; Mori, Y.; Negishi, Y. Au25 cluster-loaded SrTiO3 water-splitting photocatalyst; preparation and elucidation of the effect of cocatalyst refinement on photocatalytic activity. J. Mater. Appl. 2018, 7, 1–11. [Google Scholar]
- Kurashige, W.; Kumazawa, R.; Ishii, D.; Hayashi, R.; Niihori, Y.; Hossain, S.; Nair, L.V.; Takayama, T.; Iwase, A.; Yamazoe, S.; et al. Au25-loaded BaLa4Ti4O15 water-splitting photocatalyst with enhanced activity and durability produced using new chromium oxide shell formation method. J. Phys. Chem. C 2018, 122, 13669–13681. [Google Scholar] [CrossRef]
- Luo, J.; Li, D.; Yang, Y.; Liu, H.; Chen, J.; Wang, H. Preparation of Au/reduced graphene oxide/hydrogenated TiO2 nanotube arrays ternary composites for visible-light-driven photoelectrochemical water splitting. J. Alloys Compd. 2016, 661, 380–388. [Google Scholar] [CrossRef]
- Bharad, P.A.; Sivaranjani, K.; Gopinath, C.S. A rational approach towards enhancing solar water splitting: A case study of Au–rGO/n-rGO–TiO2. Nanoscale 2015, 7, 11206–11215. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Yu, L.; Wang, Y.; Huang, Z.; Homewood, K.; Gao, Y. Colour centre controlled formation of stable sub-nanometer transition metal clusters on TiO2 nanosheet for high efficient H2 production. Appl. Surf. Sci. 2020, 511, 145577. [Google Scholar] [CrossRef]
- Schweinberger, F.F.; Berr, M.J.; Döblinger, M.; Wolff, C.; Sanwald, K.E.; Crampton, A.S.; Ridge, C.J.; Jäckel, F.; Feldmann, J.; Tschurl, M.; et al. Cluster size effects in the photocatalytic hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 13262–13265. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).