Manufacturing Bulk Nanocrystalline Al-3Mg Components Using Cryomilling and Spark Plasma Sintering
Abstract
1. Introduction
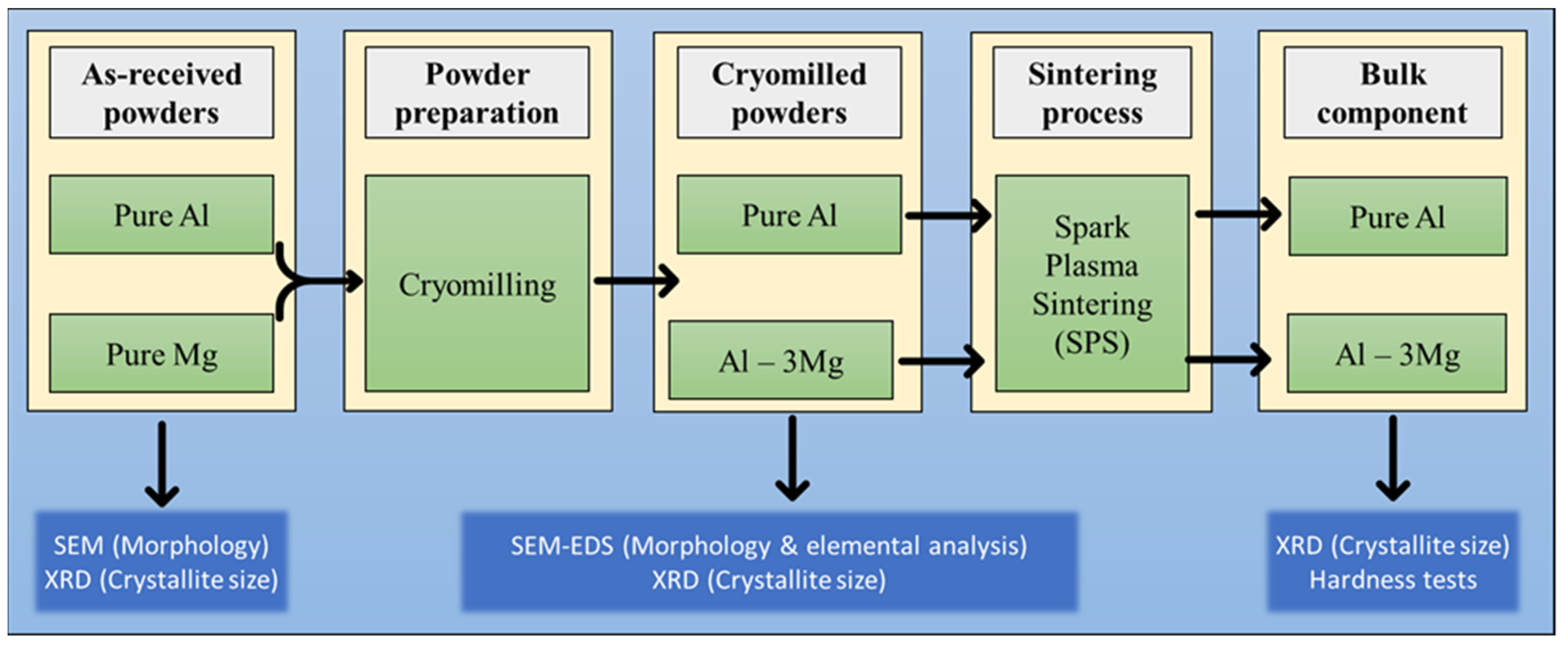
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Methods
3. Results and Discussion
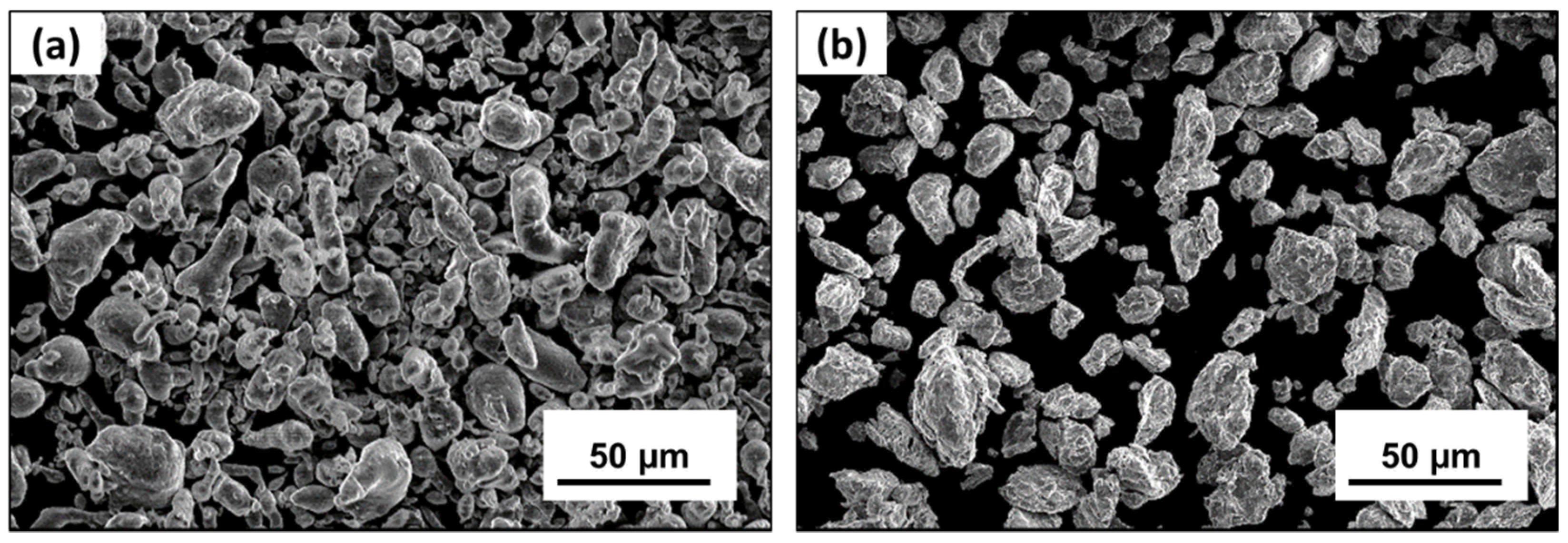
3.1. Cryomilled Powders
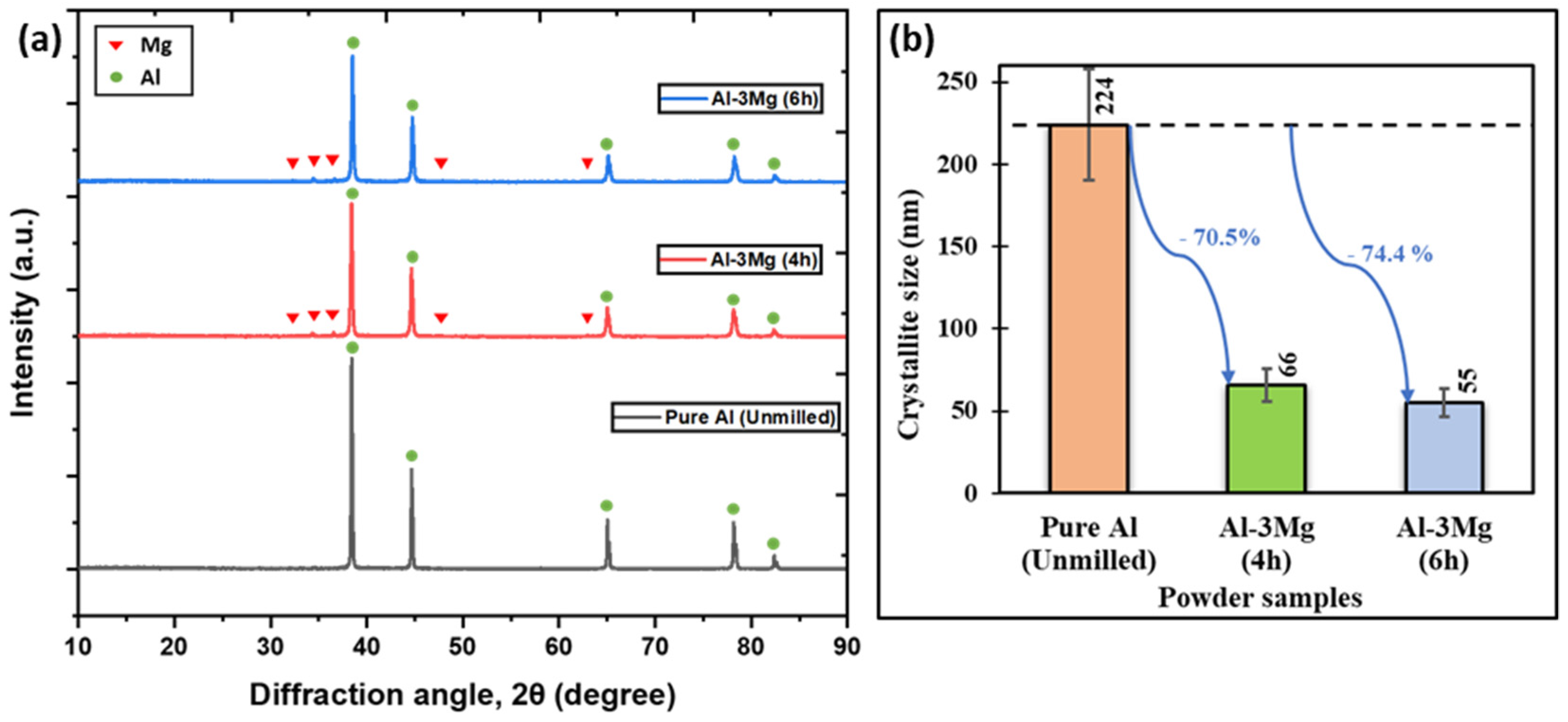
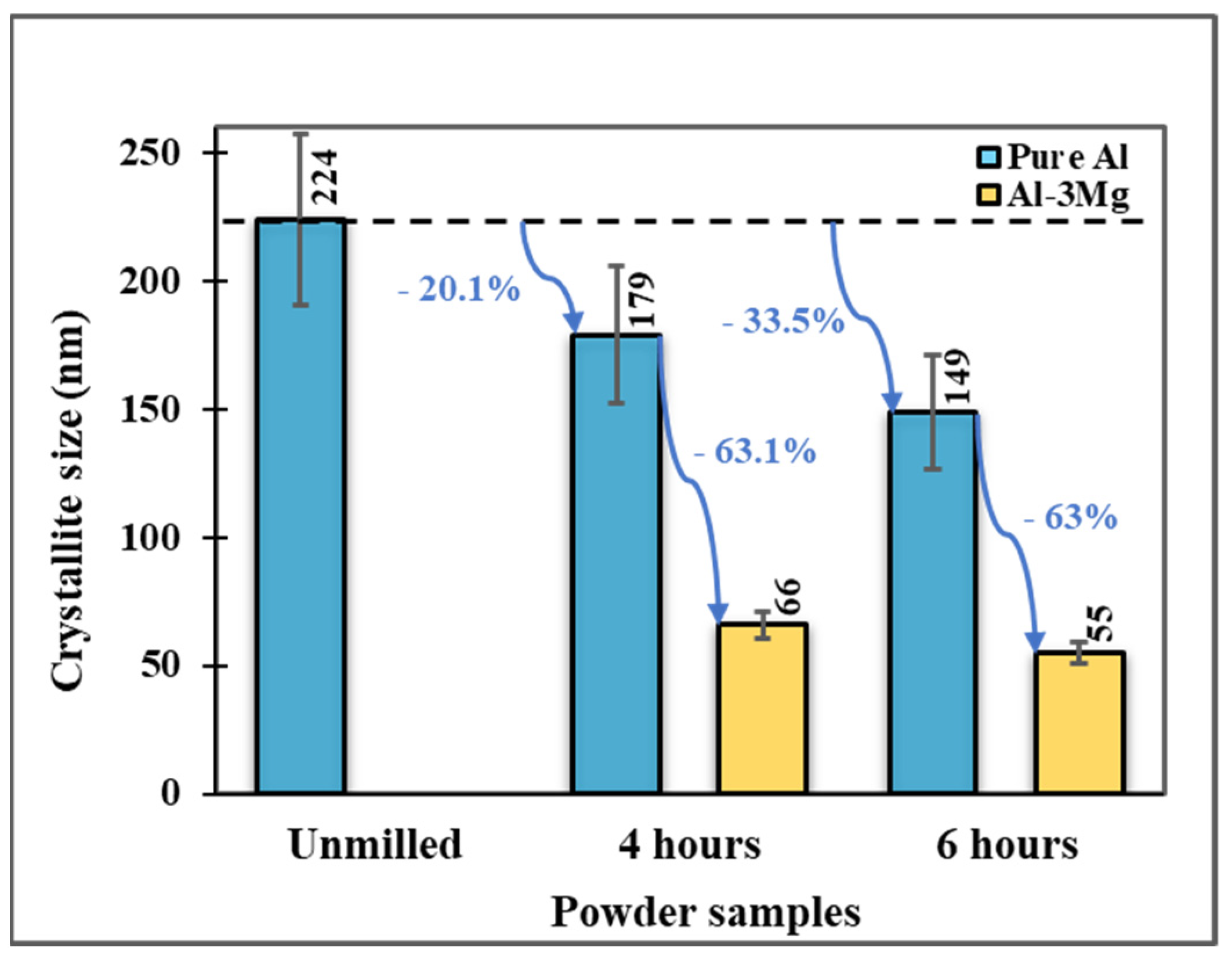
3.1.1. Effect of Cryomilling Duration
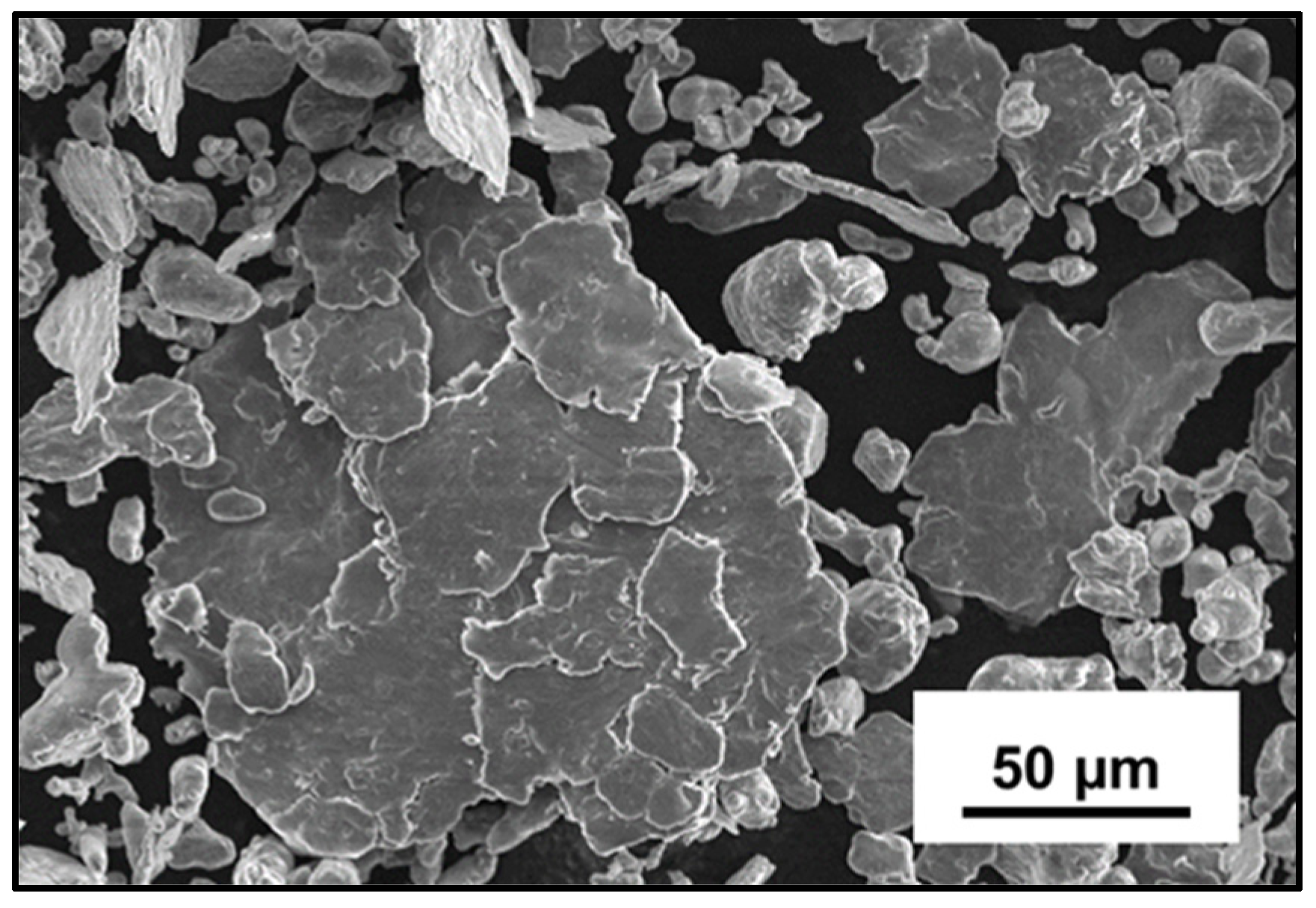
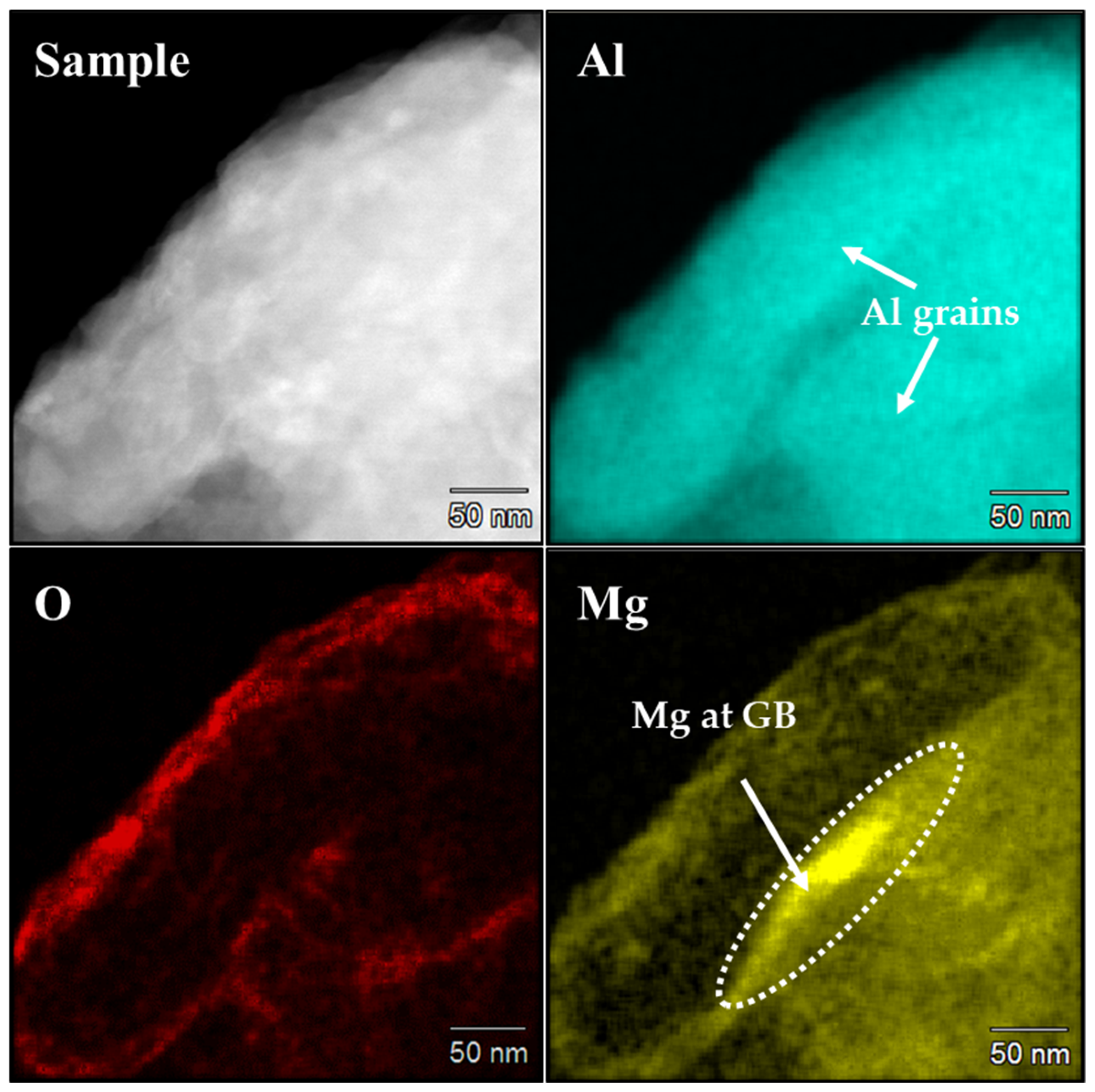
3.1.2. Effect of Mg Doping
3.2. Spark Plasma Sintered Samples
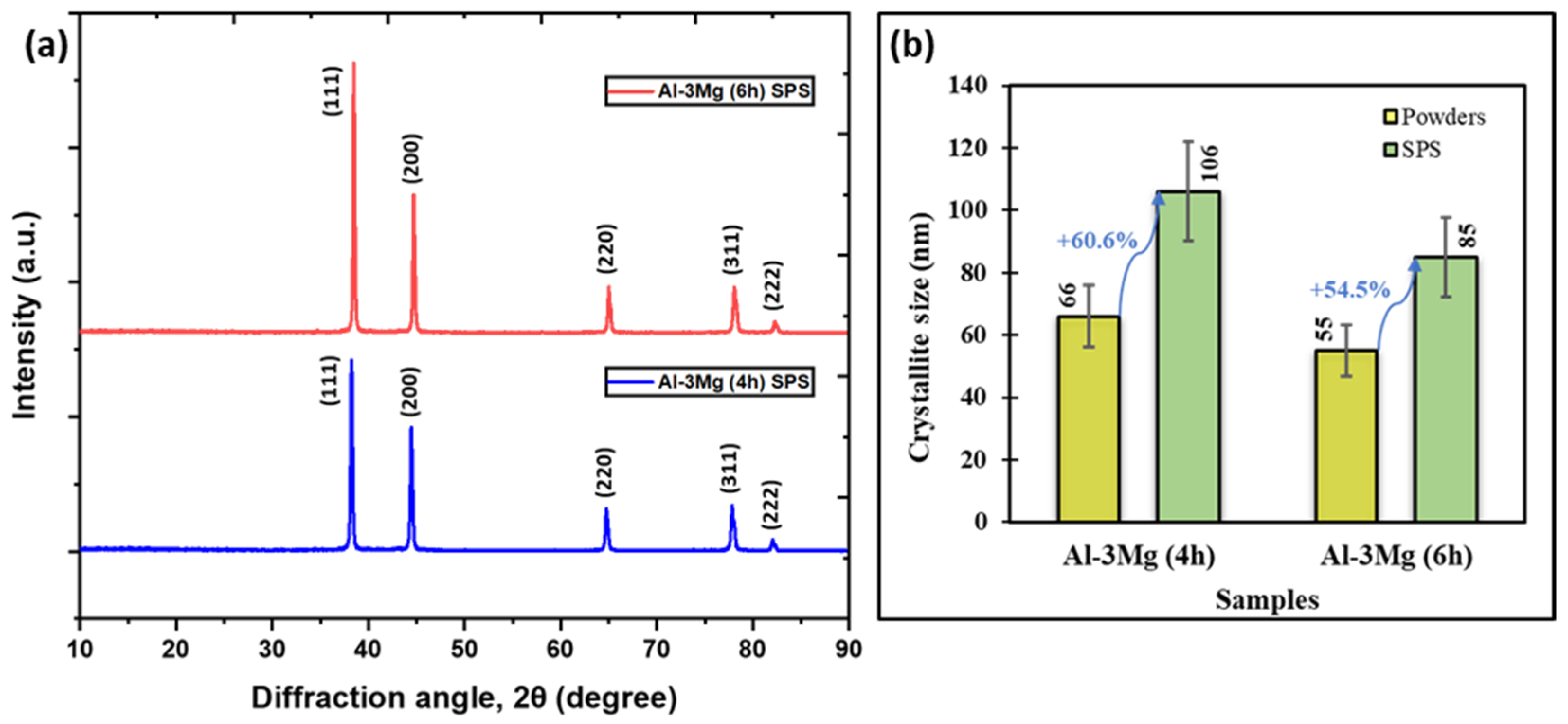
3.2.1. Effect of SPS on Crystallite Size of Sintered Components
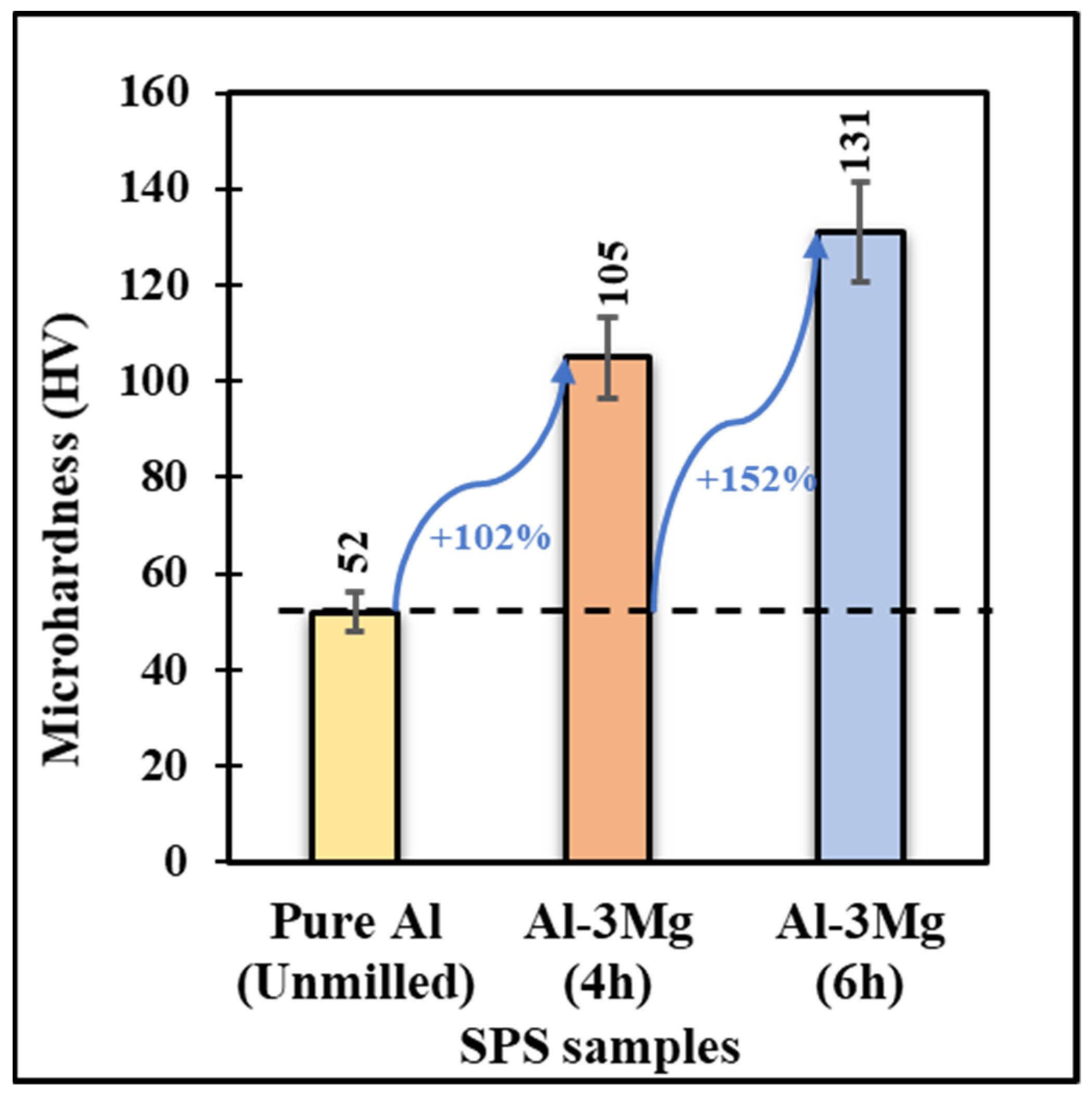
3.2.2. Mechanical Properties
4. Conclusions
- The reduction in the crystallite sizes for Al-3Mg can be attributed to (1) an increase in cryomilling duration and (2) the addition of Mg dopant.
- Al-3Mg powders showed a 74.4% decrement in crystallite size after 6 h of cryomilling which is greater than that achieved with pure Al. This decrease is attributed to the preferential segregation of Mg dopant at the grain boundaries of pure Al, stabilizing its grain boundary energy and preventing grain growth.
- The crystallite size increases during the SPS process due to the elevated temperatures leading to grain coarsening. The Al-3Mg samples showed a lower increase in the crystallite size after SPS indicating that the Mg helped in limiting the effect of grain coarsening by pinning the grain boundaries.
- The Al-3Mg SPS samples showed a 152% increase in microhardness compared to the unmilled pure Al SPS samples. This increase in property can be attributed to the lower crystallite size of the SPS’ed samples.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.T.; Langdon, T.G. Influence of grain size on deformation mechanisms: An extension to nanocrystalline materials. Mater. Sci. Eng. A 2005, 409, 234–242. [Google Scholar] [CrossRef]
- Suryanarayana, C. Nanocrystalline materials. Int. Mater. Rev. 1995, 40, 41–64. [Google Scholar] [CrossRef]
- Whang, S.H. Introduction. In Nanostructured Metals and Alloys; Whang, S.H., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. xxi–xxxv. ISBN 978-1-84569-670-2. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; John, M.; Misra, M.; Menezes, P.L. Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Birringer, R.; Gleiter, H.; Klein, H.-P.; Marquardt, P. Nanocrystalline materials an approach to a novel solid structure with gas-like disorder? Phys. Lett. A 1984, 102, 365–369. [Google Scholar] [CrossRef]
- Ertorer, O.; Topping, T.; Li, Y.; Moss, W.; Lavernia, E.J. Enhanced tensile strength and high ductility in cryomilled commercially pure titanium. Scr. Mater. 2009, 60, 586–589. [Google Scholar] [CrossRef]
- Liu, J.; Khan, A.S.; Takacs, L.; Meredith, C.S. Mechanical behavior of ultrafine-grained/nanocrystalline titanium synthesized by mechanical milling plus consolidation: Experiments, modeling and simulation. Int. J. Plast. 2015, 64, 151–163. [Google Scholar] [CrossRef]
- Deng, H.; Chen, A.; Chen, L.; Wei, Y.; Xia, Z.; Tang, J. Bulk nanostructured Ti-45Al-8Nb alloy fabricated by cryomilling and Spark Plasma Sintering. J. Alloys Compd. 2019, 772, 140–149. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A Novel Low-Density, High-Hardness, High-entropy Alloy with Close-packed Single-phase Nanocrystalline Structures. Mater. Res. Lett. 2015, 3, 95–99. [Google Scholar] [CrossRef]
- Roy, D.; Mahesh, B.V.; Atwater, M.A.; Chan, T.E.; Scattergood, R.O.; Koch, C.C. Grain size stability and hardness in nanocrystalline Cu–Al–Zr and Cu–Al–Y alloys. Mater. Sci. Eng. A 2014, 598, 217–223. [Google Scholar] [CrossRef]
- An, X.; Lin, Q.; Wu, S.; Zhang, Z. Improved Fatigue Strengths of Nanocrystalline Cu and Cu–Al Alloys. Mater. Res. Lett. 2015, 3, 135–141. [Google Scholar] [CrossRef]
- Lu, L.; Pan, Q.; Hattar, K.; Boyce, B.L. Fatigue and fracture of nanostructured metals and alloys. MRS Bull. 2021, 46, 258–264. [Google Scholar] [CrossRef]
- Peng, R.; Fu, L.; Zhou, L. Improved wear resistance by phase transformation of surface nanocrystalline 1090 steel prepared by sandblasting technique. Appl. Surf. Sci. 2016, 388, 406–411. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.; Peng, S. Synthesis and evaluation of TaC nanocrystalline coating with excellent wear resistance, corrosion resistance, and biocompatibility. Ceram. Int. 2021, 47, 20032–20044. [Google Scholar] [CrossRef]
- Renkó, J.B.; Károly, D.; Bonyár, A. Design, manufacture, and characterization of a novel Ti-based nanocrystalline alloy. J. Mater. Res. Technol. 2021, 11, 498–506. [Google Scholar] [CrossRef]
- Baláž, M.; Dobrozhan, O.; Tešinský, M.; Zhang, R.-Z.; Džunda, R.; Dutková, E.; Rajňák, M.; Chen, K.; Reece, M.J.; Baláž, P. Scalable and environmentally friendly mechanochemical synthesis of nanocrystalline rhodostannite (Cu2FeSn3S8). Powder Technol. 2021, 388, 192–200. [Google Scholar] [CrossRef]
- Esteves, L.; Christudasjustus, J.; O’Brien, S.P.; Witharamage, C.S.; Darwish, A.A.; Walunj, G.; Stack, P.; Borkar, T.; Akans, R.E.; Gupta, R.K. Effect of V content on corrosion behavior of high-energy ball milled AA5083. Corros. Sci. 2021, 186, 109465. [Google Scholar] [CrossRef]
- Elangovan, H.; Sengupta, S.; Narayanan, R.; Chattopadhyay, K. Silicon nanoparticles with UV range photoluminescence synthesized through cryomilling induced phase transformation and etching. J. Mater. Sci. 2021, 56, 1515–1526. [Google Scholar] [CrossRef]
- Roy, D.; Chakraborty, S.; Gupta, A.K.; BasuMallick, A.; Scattergood, R.O.; Koch, C.C. Synergistic effect of Nb and Zr additions on the structure-property relationships of nanocrystalline Cu processed by mechanical alloying and hot pressing. J. Alloys Compd. 2021, 854, 157174. [Google Scholar] [CrossRef]
- Youssef, K.M.; Scattergood, R.O.; Murty, K.L.; Koch, C.C. Nanocrystalline Al–Mg alloy with ultrahigh strength and good ductility. Scr. Mater. 2006, 54, 251–256. [Google Scholar] [CrossRef]
- Exploring the Feasibility of Using Cryomilled Aluminum Powder as Feedstock for Laser Powder Bed Fusion Additive Manufacturing. Available online: https://apps.dtic.mil/sti/citations/AD1104814 (accessed on 7 October 2022).
- Ipatov, M.; Zhukova, V.; Dominguez, L.; Alvarez, K.L.; Chizhik, A.; Zhukov, A.; Gonzalez, J. Structural and low-temperature magnetic properties of as-quenched and annealed Ni–Si–B alloys produced by rapid solidification. Intermetallics 2021, 132, 107140. [Google Scholar] [CrossRef]
- Singh, P.K.; Mondal, S.; Das, A.K.; Mishra, S.K.; Singh, D.K.; Singh, S.K. Production of W-based nanoparticles via spark erosion process along with their characterization and optimization for practical application in gas sensor. Appl. Phys. A 2020, 126, 77. [Google Scholar] [CrossRef]
- Priya, S.D.; Nesaraj, A.S.; Selvakumar, A.I. Facile wet-chemical synthesis and evaluation of physico-chemical characteristics of novel nanocrystalline NdCoO3-based perovskite oxide as cathode for LT-SOFC applications. Bull. Mater. Sci. 2021, 44, 115. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Wu, Y.; Zeng, Y.; He, H.; Lin, J.; Ye, Z. Effects of phosphorus doping in ZnO nanocrystals by metal organic chemical vapor deposition. Mater. Lett. 2012, 68, 258–260. [Google Scholar] [CrossRef]
- Ye, J.; Ajdelsztajn, L.; Schoenung, J.M. Bulk nanocrystalline aluminum 5083 alloy fabricated by a novel technique: Cryomilling and spark plasma sintering. Metall. Mater. Trans. A 2006, 37, 2569–2579. [Google Scholar] [CrossRef]
- Rajulapati, K.V.; Scattergood, R.O.; Murty, K.L.; Horita, Z.; Langdon, T.G.; Koch, C.C. Mechanical Properties of Bulk Nanocrystalline Aluminum-Tungsten Alloys. Metall. Mater. Trans. A 2008, 39, 2528–2534. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Rhodin, T.N. Low Temperature Oxidation of Copper. I. Physical Mechanism1a. J. Am. Chem. Soc. 1950, 72, 5102–5106. [Google Scholar] [CrossRef]
- Kumar, N.; Biswas, K. Cryomilling: An environment friendly approach of preparation large quantity ultra refined pure aluminium nanoparticles. J. Mater. Res. Technol. 2019, 8, 63–74. [Google Scholar] [CrossRef]
- Tang, F.; Hagiwara, M.; Schoenung, J.M. Formation of coarse-grained inter-particle regions during hot isostatic pressing of nanocrystalline powder. Scr. Mater. 2005, 53, 619–624. [Google Scholar] [CrossRef]
- Kishimoto, H.; Alinger, M.J.; Odette, G.R.; Yamamoto, T. TEM examination of microstructural evolution during processing of 14CrYWTi nanostructured ferritic alloys. J. Nucl. Mater. 2004, 329–333, 369–371. [Google Scholar] [CrossRef]
- Pandey, V.K.; Shadangi, Y.; Shivam, V.; Basu, J.; Chattopadhyay, K.; Majumdar, B.; Sarma, B.N.; Mukhopadhyay, N.K. Synthesis, Characterization and Thermal Stability of Nanocrystalline MgAlMnFeCu Low-Density High-Entropy Alloy. Trans. Indian Inst. Met. 2021, 74, 33–44. [Google Scholar] [CrossRef]
- Shkodich, N.F.; Kovalev, I.D.; Kuskov, K.V.; Kovalev, D.Y.; Vergunova, Y.S.; Scheck, Y.B.; Vadchenko, S.G.; Politano, O.; Baras, F.; Rogachev, A.S. Fast mechanical synthesis, structure evolution, and thermal stability of nanostructured CoCrFeNiCu high entropy alloy. J. Alloys Compd. 2022, 893, 161839. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Yang, Y.F.; Qian, M. 13—Spark plasma sintering and hot pressing of titanium and titanium alloys. In Titanium Powder Metallurgy; Qian, M., Froes, F.H., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2015; pp. 219–235. ISBN 978-0-12-800054-0. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, F.C.; Lee, S.K.; Liu, Y.; Cheng, J.W.; Liang, Y. Microstructure characteristic, mechanical properties and sintering mechanism of nanocrystalline copper obtained by SPS process. Mater. Sci. Eng. A 2009, 523, 134–138. [Google Scholar] [CrossRef]
- Xie, G.; Ohashi, O.; Chiba, K.; Yamaguchi, N.; Song, M.; Furuya, K.; Noda, T. Frequency effect on pulse electric current sintering process of pure aluminum powder. Mater. Sci. Eng. A 2003, 359, 384–390. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Maccione, R.; John, M.; Lanka, S.; Misra, M.; Menezes, P.L. Influence of Cryomilling on Crystallite Size of Aluminum Powder and Spark Plasma Sintered Component. Nanomaterials 2022, 12, 551. [Google Scholar] [CrossRef]
- Hohl, J.; Kumar, P.; Misra, M.; Menezes, P.; Mushongera, L.T. Thermodynamic stabilization of nanocrystalline aluminum. J. Mater. Sci. 2021, 56, 14611–14623. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Du, K.; Li, X.Y.; Lu, K. Thermally stable nanostructured Al-Mg alloy with relaxed grain boundaries. Acta Mater. 2022, 226, 117640. [Google Scholar] [CrossRef]
- Guan, D.; Gao, J.; Rainforth, W.M. Effect of cryomilling time on microstructure evolution and hardness of cryomilled AZ31 powders. Mater. Charact. 2021, 178, 111311. [Google Scholar] [CrossRef]
- Baig, M.; Rehman, A.U.; Mohammed, J.A.; Seikh, A.H. Effect of Microstructure and Mechanical Properties of Al5083 Alloy Processed by ECAP at Room Temperature and High Temperature. Crystals 2021, 11, 683. [Google Scholar] [CrossRef]
- Bouaziz, O.; Estrin, Y.; Bréchet, Y.; Embury, J.D. Critical grain size for dislocation storage and consequences for strain hardening of nanocrystalline materials. Scr. Mater. 2010, 63, 477–479. [Google Scholar] [CrossRef]



| Process Parameters | Values |
|---|---|
| Speed | 180 rpm |
| Ball to powder Ratio | 30:1 |
| Milling Media diameter | 6.3 mm |
| Milling Time | 4 and 6 h |
| Milling Temperature | −190 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushwaha, A.K.; Misra, M.; Menezes, P.L. Manufacturing Bulk Nanocrystalline Al-3Mg Components Using Cryomilling and Spark Plasma Sintering. Nanomaterials 2022, 12, 3618. https://doi.org/10.3390/nano12203618
Kushwaha AK, Misra M, Menezes PL. Manufacturing Bulk Nanocrystalline Al-3Mg Components Using Cryomilling and Spark Plasma Sintering. Nanomaterials. 2022; 12(20):3618. https://doi.org/10.3390/nano12203618
Chicago/Turabian StyleKushwaha, Amanendra K., Manoranjan Misra, and Pradeep L. Menezes. 2022. "Manufacturing Bulk Nanocrystalline Al-3Mg Components Using Cryomilling and Spark Plasma Sintering" Nanomaterials 12, no. 20: 3618. https://doi.org/10.3390/nano12203618
APA StyleKushwaha, A. K., Misra, M., & Menezes, P. L. (2022). Manufacturing Bulk Nanocrystalline Al-3Mg Components Using Cryomilling and Spark Plasma Sintering. Nanomaterials, 12(20), 3618. https://doi.org/10.3390/nano12203618








