Advances in Matrix-Supported Palladium Nanocatalysts for Water Treatment
Abstract
1. Introduction and Background
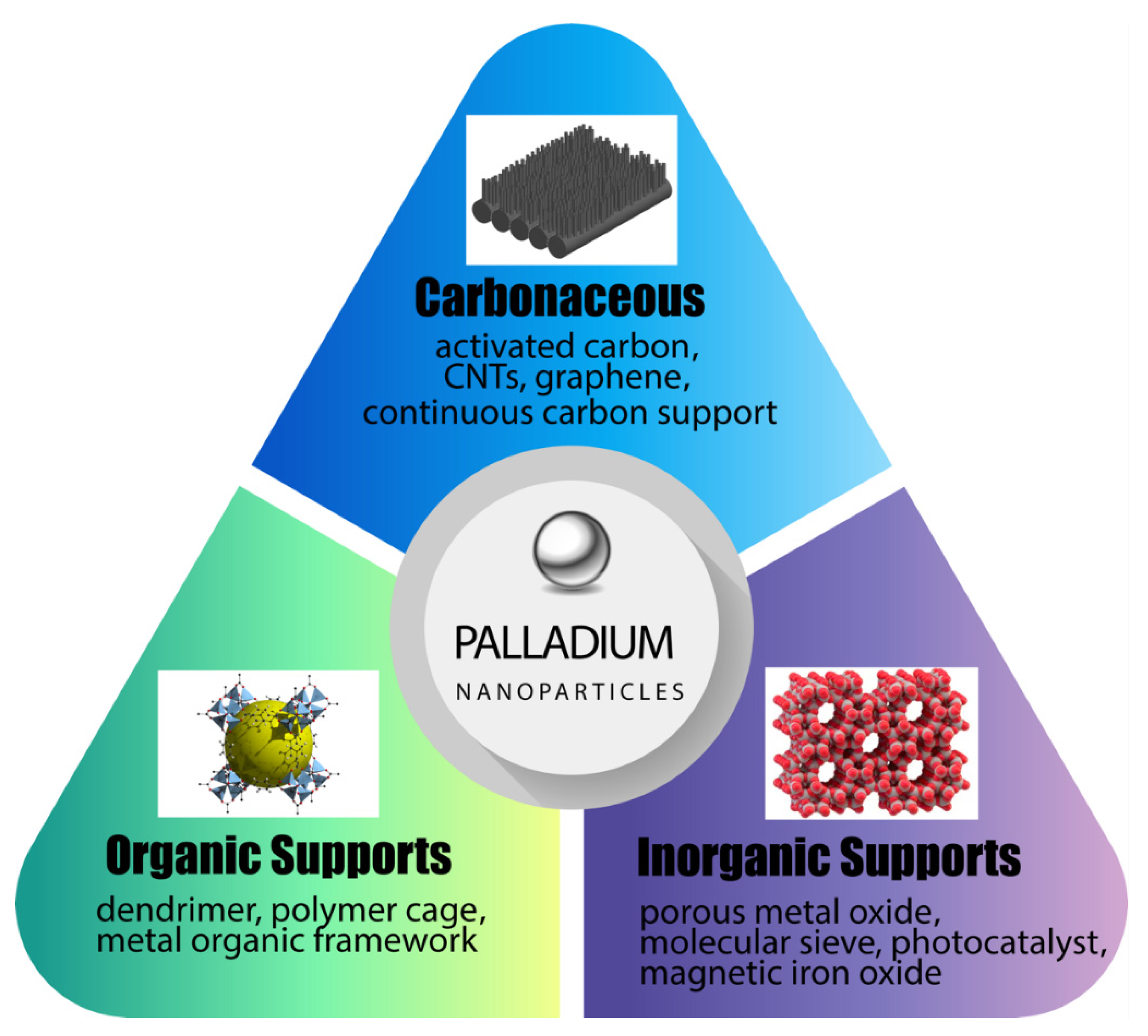
2. Novel Supports for Palladium Nanoparticles and Demonstrated Applications
2.1. Novel Organic and Polymeric Supports for Pd
2.1.1. Dendrimers
2.1.2. Polymer Cage
2.1.3. Porous Metal-Organic Frameworks (MOFs)
2.2. Advances in Inorganic and Ceramic Supports for Palladium Nanocatalysts
2.2.1. Porous Oxide for Supporting Palladium
2.2.2. Photocatalyst-Supported Palladium
2.2.3. Magnetic Supports for Pd Nanoparticles That Can Be Retrieved after Use
2.3. Carbonaceous Supports
2.3.1. Activated Carbon
2.3.2. Graphene
2.3.3. Carbon Nanotubes
3. Supports for Pd Nanocatalysts Developed Specifically for Water Remediation
3.1. Particulate Supports for Pd-Based Catalysts
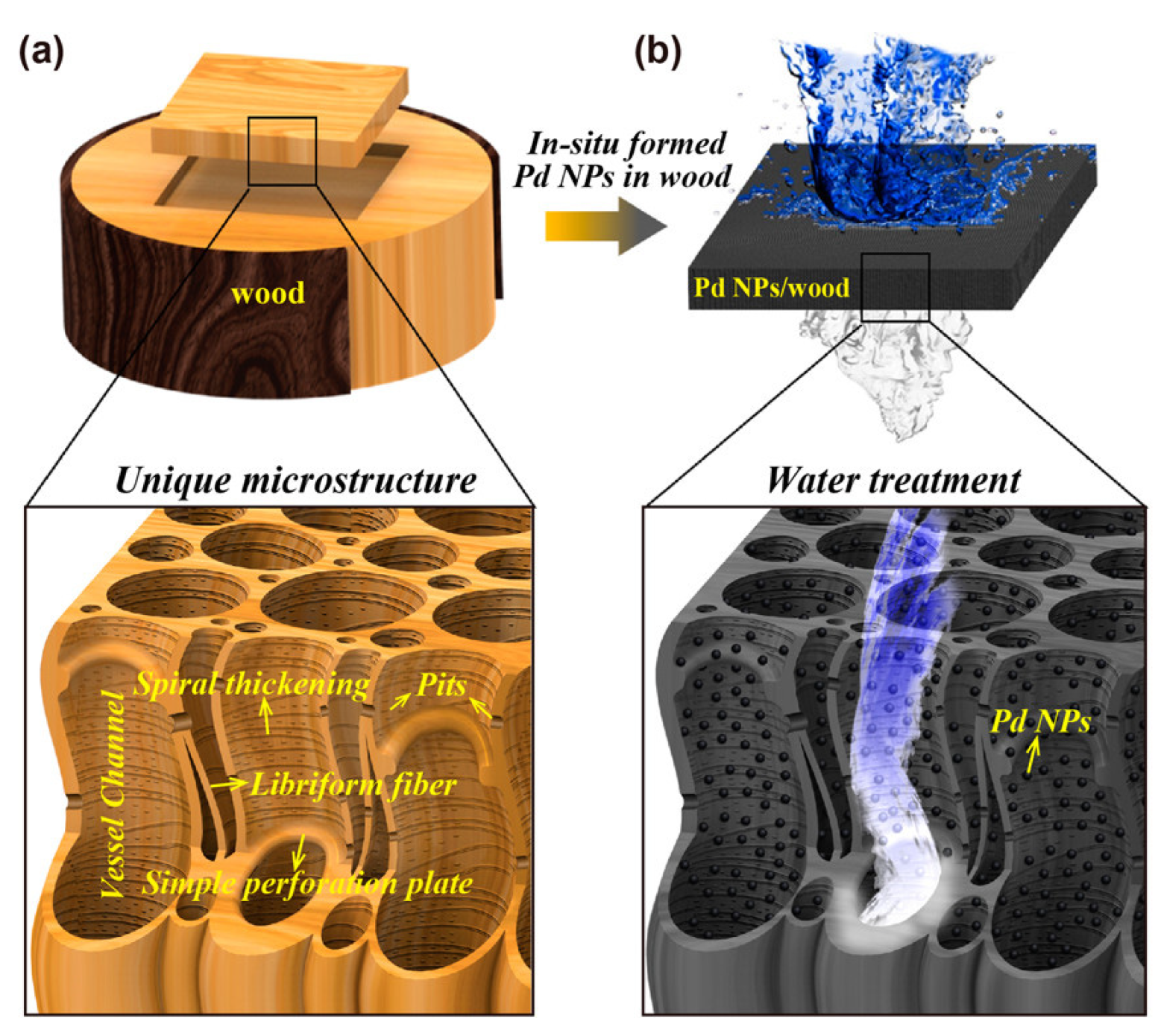
3.2. Pd Nanocatalysts on Continuous Solid Structures Suitable for Water Treatment
3.2.1. Polymeric Membranes
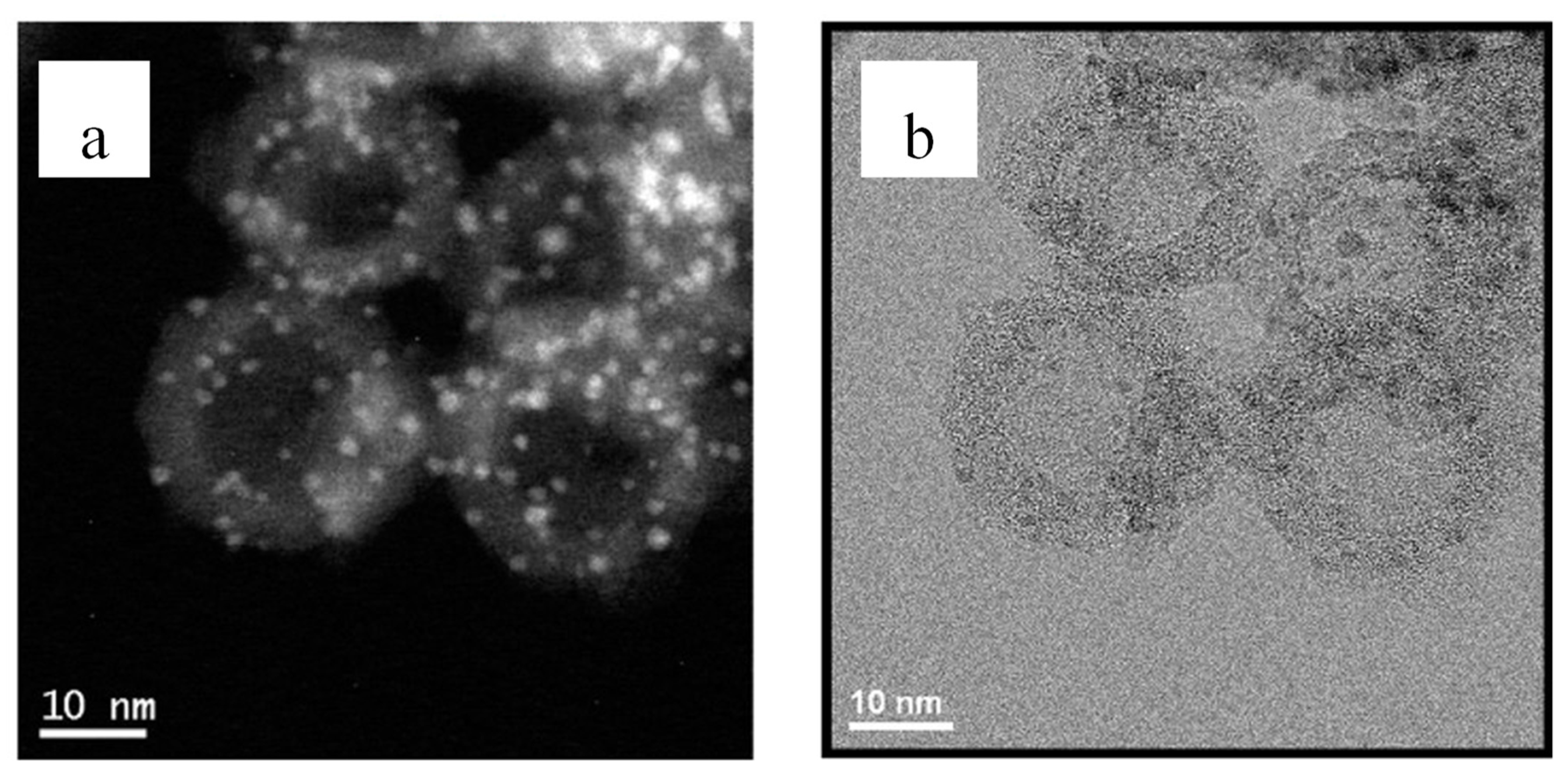
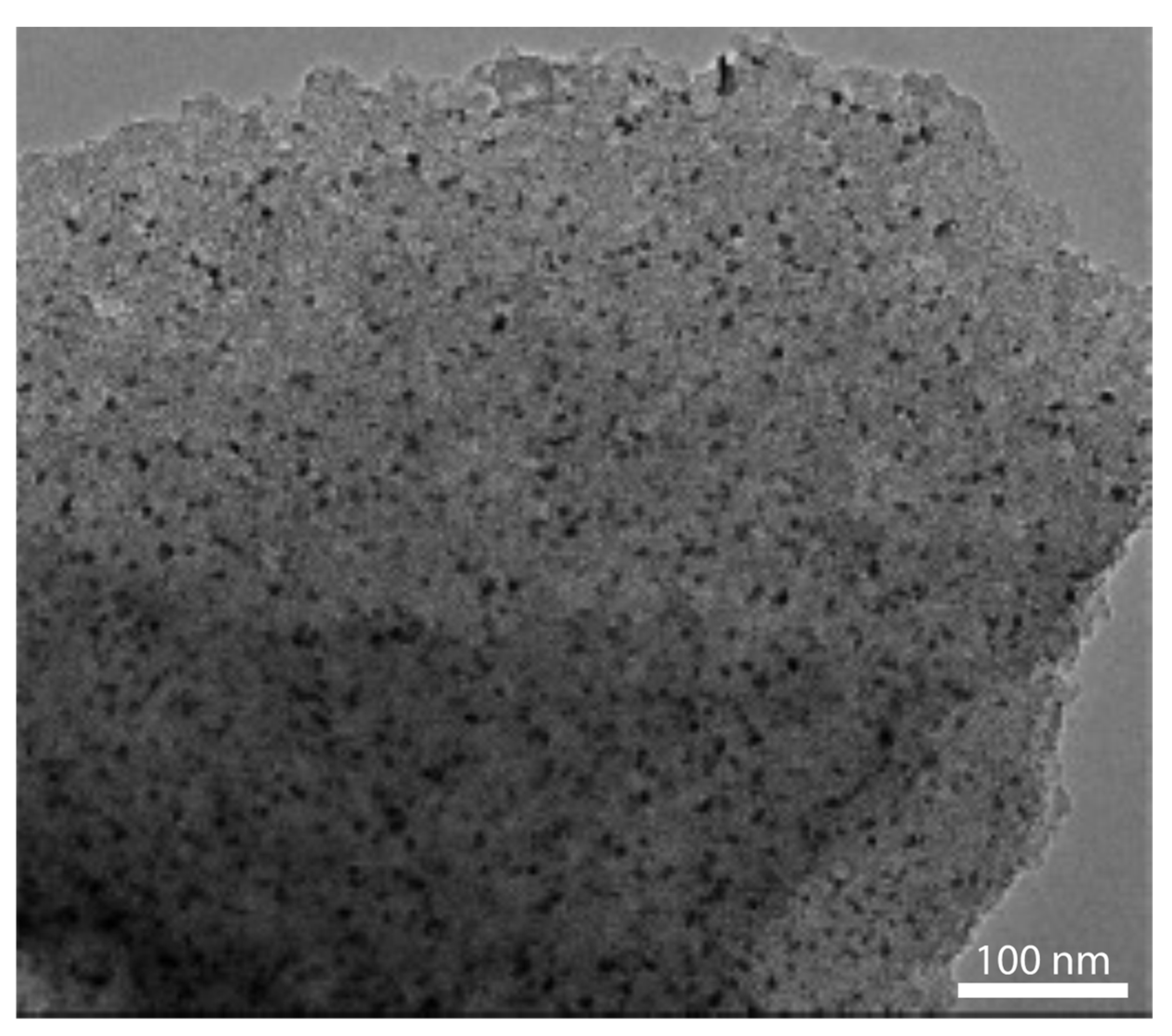
3.2.2. Continuous Carbon Structures
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, Q.; Min, X.; Weng, M. A Review of Polychlorinated Biphenyls (PCBs) pollution in indoor air environment. J. Air Waste Manage. Assoc. 2016, 66, 941–950. [Google Scholar] [CrossRef]
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic Acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 2012, 116, 93–117. [Google Scholar] [CrossRef]
- Steenland, K.; Fletcher, T.; Stein, C.R.; Bartell, S.M.; Darrow, L.; Lopez-Espinosa, M.-J.; Barry Ryan, P.; Savitz, D.A. Review: Evolution of evidence on PFOA and health following the assessments of the C8 science panel. Environ. Int. 2020, 145, 106125. [Google Scholar] [CrossRef]
- Steenland, K.; Fletcher, T.; Savitz, D.A. Epidemiologic evidence on the health effects of Perfluorooctanoic Acid (PFOA). Environ. Health Perspect. 2010, 118, 1100–1108. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the pathways of human exposure to poly- and Perfluoroalkyl Substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Gur-Reznik, S.; Katz, I.; Dosoretz, C.G. Removal of dissolved organic matter by granular-activated carbon adsorption as a pretreatment to reverse osmosis of membrane bioreactor effluents. Water Res. 2008, 42, 1595–1605. [Google Scholar] [CrossRef]
- Aktaş, Ö.; Çeçen, F. Bioregeneration of activated carbon: A review. Int. Biodeterior. Biodegradation 2007, 59, 257–272. [Google Scholar] [CrossRef]
- Barbaro, P.; Liguori, F. Ion exchange resins: Catalyst recovery and recycle. Chem. Rev. 2009, 109, 515–529. [Google Scholar] [CrossRef]
- Huang, H.; Schwab, K.; Jacangelo, J.G. Pretreatment for low pressure membranes in water treatment: A review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, V.; Kim, K.-H.; Rawat, M. Biogenic synthesis of copper oxide nanoparticles using plant extract and its prodigious potential for photocatalytic degradation of dyes. Environ. Res. 2019, 177, 108569. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, S.M.; Lee, H.; Nedrygailov, I.I. Hot-electron-mediated surface chemistry: Toward electronic control of catalytic activity. Acc. Chem. Res. 2015, 48, 2475–2483. [Google Scholar] [CrossRef]
- Khan, M.A.; Nadeem, M.A.; Idriss, H. Ferroelectric polarization effect on surface chemistry and photo-catalytic activity: A review. Surf. Sci. Rep. 2016, 71, 1–31. [Google Scholar] [CrossRef]
- Jian, S.; Cheng, Y.; Ma, X.; Guo, H.; Hu, J.; Zhang, K.; Jiang, S.; Yang, W.; Duan, G. Excellent fluoride removal performance by electrospun La–Mn bimetal oxide nanofibers. New J. Chem. 2022, 46, 490–497. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Munakata, N.; Reinhard, M. Palladium Catalysis for the Treatment of Contaminated Waters: A Review BT—Physicochemical Groundwater Remediation; Smith, J.A., Burns, S.E., Eds.; Springer: Boston, MA, USA, 2002; pp. 45–71. [Google Scholar] [CrossRef]
- Sherbo, R.S.; Kurimoto, A.; Brown, C.M.; Berlinguette, C.P. Efficient electrocatalytic hydrogenation with a palladium membrane reactor. J. Am. Chem. Soc. 2019, 141, 7815–7821. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, C.; Shen, P.K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Salih, K.S.M.; Baqi, Y. Microwave-assisted palladium-catalyzed cross-coupling reactions: Generation of carbon–carbon bond. Catalysts 2019, 10, 4. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Behzadi Pour, G.; Fekri Aval, L.; Nasiri Sarvi, M.; Fekri Aval, S.; Nazarpour Fard, H. Hydrogen sensors: Palladium-based electrode. J. Mater. Sci. Mater. Electron. 2019, 30, 8145–8153. [Google Scholar] [CrossRef]
- Favier, I.; Pla, D.; Gómez, M. Palladium nanoparticles in polyols: Synthesis, catalytic couplings, and hydrogenations. Chem. Rev. 2020, 120, 1146–1183. [Google Scholar] [CrossRef] [PubMed]
- El-Hallag, I.S.; El-Nahass, M.N.; Youssry, S.M.; Kumar, R.; Abdel-Galeil, M.M.; Matsuda, A. Facile in-situ simultaneous electrochemical reduction and deposition of reduced graphene oxide embedded palladium nanoparticles as high performance electrode materials for supercapacitor with excellent rate capability. Electrochim. Acta 2019, 314, 124–134. [Google Scholar] [CrossRef]
- Veisi, H.; Ghorbani, M.; Hemmati, S. Sonochemical in situ immobilization of Pd nanoparticles on green tea extract coated Fe3O4 nanoparticles: An efficient and magnetically recyclable nanocatalyst for synthesis of biphenyl compounds under ultrasound irradiations. Mater. Sci. Eng. C 2019, 98, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, M.H.; Salmani, N.; Heidari, A.; Rezaei, M.R. Bio-synthesis of palladium nanoparticle using spirulina platensis alga extract and its application as adsorbent. Surf. Interfaces 2018, 10, 136–143. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Ricciardi, W.; Hodson, L.L.; Hoover, M.D. Opportunities and challenges of nanotechnology in the green economy. Environ. Health 2014, 13, 78. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the support in gold-containing nanoparticles as heterogeneous catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for environmental remediation: Materials and applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.-X. Sabatier principle of metal-support interaction for design of ultrastable metal nanocatalysts. Science 2021, 374, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Carlsson, P.-A.; Fouladvand, S.; Martin, N.M.; Gustafson, J.; Newton, M.A.; Lundgren, E.; Grönbeck, H.; Skoglundh, M. Chemistry of supported palladium nanoparticles during methane Oxidation. ACS Catal. 2015, 5, 2481–2489. [Google Scholar] [CrossRef]
- Albani, D.; Shahrokhi, M.; Chen, Z.; Mitchell, S.; Hauert, R.; López, N.; Pérez-Ramírez, J. Selective ensembles in supported palladium sulfide nanoparticles for alkyne semi-hydrogenation. Nat. Commun. 2018, 9, 2634. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Schaefer, J.L.; Yang, Z.; Tu, Z.; Archer, L.A. 25th anniversary article: Polymer-particle composites: Phase stability and applications in electrochemical energy storage. Adv. Mater. 2014, 26, 201–234. [Google Scholar] [CrossRef]
- dos Santos, C.A.; Ingle, A.P.; Rai, M. The emerging role of metallic nanoparticles in food. Appl. Microbiol. Biotechnol. 2020, 104, 2373–2383. [Google Scholar] [CrossRef]
- Zare, Y.; Shabani, I. Polymer/metal nanocomposites for biomedical applications. Mater. Sci. Eng. C 2016, 60, 195–203. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, L.; Pan, B.; Zhang, W.; Zhang, S.; Zhang, Q. Polymer-supported nanocomposites for environmental application: A review. Chem. Eng. J. 2011, 170, 381–394. [Google Scholar] [CrossRef]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated metal nanoparticles for catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.C.; Lezutekong, R.; Crooks, R.M. Dendrimer-encapsulated Pd nanoparticles as aqueous, room-temperature catalysts for the Stille reaction. J. Am. Chem. Soc. 2005, 127, 5097–5103. [Google Scholar] [CrossRef]
- Astruc, D.; Liang, L.; Rapakousiou, A.; Ruiz, J. Click dendrimers and triazole-related aspects: Catalysts, mechanism, synthesis, and functions. A bridge between dendritic architectures and nanomaterials. Acc. Chem. Res. 2012, 45, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gregurec, D.; Irigoyen, J.; Martinez, A.; Moya, S.; Ciganda, R.; Hermange, P.; Ruiz, J.; Astruc, D. Precise localization of metal nanoparticles in dendrimer nanosnakes or inner periphery and consequences in catalysis. Nat. Commun. 2016, 7, 13152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fu, F.; Escobar, A.; Moya, S.; Ruiz, J.; Astruc, D. “Click” dendrimer-stabilized nanocatalysts for efficient hydrogen release upon ammonia-borane hydrolysis. ChemCatChem 2018, 10, 2673–2680. [Google Scholar] [CrossRef]
- Kang, N.; Djeda, R.; Wang, Q.; Fu, F.; Ruiz, J.; Pozzo, J.; Astruc, D. Efficient “Click”-dendrimer-supported synergistic bimetallic nanocatalysis for hydrogen evolution by sodium borohydride hydrolysis. ChemCatChem 2019, 11, 2341–2349. [Google Scholar] [CrossRef]
- Kavitha, M.; Parida, M.R.; Prasad, E.; Vijayan, C.; Deshmukh, P.C. Generation of Ag nanoparticles by PAMAM dendrimers and their size dependence on the aggregation behavior of dendrimers. Macromol. Chem. Phys. 2009, 210, 1310–1318. [Google Scholar] [CrossRef]
- Özkar, S. Transition metal nanoparticle catalysts in releasing hydrogen from the methanolysis of ammonia borane. Int. J. Hydrogen Energy 2020, 45, 7881–7891. [Google Scholar] [CrossRef]
- Eghbali, P.; Gürbüz, M.U.; Ertürk, A.S.; Metin, Ö. In situ synthesis of dendrimer-encapsulated palladium(0) nanoparticles as catalysts for hydrogen production from the methanolysis of ammonia borane. Int. J. Hydrogen Energy 2020, 45, 26274–26285. [Google Scholar] [CrossRef]
- Tozawa, T.; Jones, J.T.A.; Swamy, S.I.; Jiang, S.; Adams, D.J.; Shakespeare, S.; Clowes, R.; Bradshaw, D.; Hasell, T.; Chong, S.Y.; et al. Porous organic cages. Nat. Mater. 2009, 8, 973–978. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Zhang, P.; Chai, S.-H.; Chi, M.; Veith, G.M.; Gallego, N.C.; Kidder, M.; Dai, S. Lab-in-a-shell: Encapsulating metal clusters for size sieving catalysis. J. Am. Chem. Soc. 2014, 136, 11260–11263. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.-K.; Kitta, M.; Pang, H.; Xu, Q. Encapsulating highly catalytically active metal nanoclusters inside porous organic cages. Nat. Catal. 2018, 1, 214–220. [Google Scholar] [CrossRef]
- Tao, R.; Kang, K.; Li, X.; Li, R.; Huang, R.; Jin, Y.; Qiu, L.; Zhang, W. Controlled synthesis of palladium nanoparticles with size-dependent catalytic activities enabled by organic molecular cages. Inorg. Chem. 2021, 60, 12517–12525. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yang, R.; Wei, S.; Hu, X.; Xu, D.; Yang, J.; Dong, Z. Ultra-fine Pd nanoparticles confined in a porous organic polymer: A leaching-and-aggregation-resistant catalyst for the efficient reduction of nitroarenes by NaBH4. J. Colloid Interface Sci. 2019, 538, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Aguila, B.; Song, Y.; Ma, S. Tailored porous organic polymers for task-specific water purification. Acc. Chem. Res. 2020, 53, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zeng, Z.; Zeng, G.; Liu, Z.; Xiao, R.; Chen, M.; Tang, L.; Tang, W.; Lai, C.; Cheng, M.; et al. Metal organic frameworks as robust host of palladium nanoparticles in heterogeneous catalysis: Synthesis, application, and prospect. ACS Appl. Mater. Interfaces 2019, 11, 32579–32598. [Google Scholar] [CrossRef]
- Fortea-Pérez, F.R.; Mon, M.; Ferrando-Soria, J.; Boronat, M.; Leyva-Pérez, A.; Corma, A.; Herrera, J.M.; Osadchii, D.; Gascon, J.; Armentano, D.; et al. The MOF-driven synthesis of supported palladium clusters with catalytic activity for carbene-mediated chemistry. Nat. Mater. 2017, 16, 760–766. [Google Scholar] [CrossRef]
- Koo, W.-T.; Qiao, S.; Ogata, A.F.; Jha, G.; Jang, J.-S.; Chen, V.T.; Kim, I.-D.; Penner, R.M. Accelerating palladium nanowire H2 sensors using engineered nanofiltration. ACS Nano 2017, 11, 9276–9285. [Google Scholar] [CrossRef]
- Hyok Ri, S.; Bi, F.; Guan, A.; Zhang, X. Manganese-cerium composite oxide pyrolyzed from metal organic framework supporting palladium nanoparticles for efficient toluene oxidation. J. Colloid Interface Sci. 2021, 586, 836–846. [Google Scholar] [CrossRef]
- Lin, A.; Ibrahim, A.A.; Arab, P.; El-Kaderi, H.M.; El-Shall, M.S. Palladium nanoparticles supported on Ce-metal–organic framework for efficient CO oxidation and low-temperature CO2 capture. ACS Appl. Mater. Interfaces 2017, 9, 17961–17968. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Molnár, Á. Efficient, selective, and recyclable palladium catalysts in carbon−carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar] [CrossRef]
- Fors, B.P.; Davis, N.R.; Buchwald, S.L. An efficient process for Pd-catalyzed C−N cross-coupling reactions of aryl iodides: Insight into controlling factors. J. Am. Chem. Soc. 2009, 131, 5766–5768. [Google Scholar] [CrossRef] [PubMed]
- Carson, F.; Pascanu, V.; Bermejo Gómez, A.; Zhang, Y.; Platero-Prats, A.E.; Zou, X.; Martín-Matute, B. Influence of the base on Pd@MIL-101-NH 2 (Cr) as catalyst for the Suzuki-Miyaura cross-coupling reaction. Chem. A Eur. J. 2015, 21, 10896–10902. [Google Scholar] [CrossRef] [PubMed]
- Losch, P.; Huang, W.; Vozniuk, O.; Goodman, E.D.; Schmidt, W.; Cargnello, M. Modular Pd/zeolite composites demonstrating the key role of support hydrophobic/hydrophilic character in methane catalytic combustion. ACS Catal. 2019, 9, 4742–4753. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Li, J.; Liu, Y.; Zhang, W.; Yang, M.; Jian, Y.; Zuo, P.; Gao, Z. Highly efficient zeolite-supported Pd catalyst activated in C–C cross-coupling reaction. Ind. Eng. Chem. Res. 2020, 59, 11241–11249. [Google Scholar] [CrossRef]
- Friberg, I.; Clark, A.H.; Ho, P.H.; Sadokhina, N.; Smales, G.J.; Woo, J.; Auvray, X.; Ferri, D.; Nachtegaal, M.; Kröcher, O.; et al. Structure and performance of zeolite supported Pd for complete methane oxidation. Catal. Today 2021, 382, 3–12. [Google Scholar] [CrossRef]
- Sreedhar, B.; Yada, D.; Reddy, P.S. Nanocrystalline titania-supported palladium(0) nanoparticles for Suzuki–Miyaura cross-coupling of aryl and heteroaryl halides. Adv. Synth. Catal. 2011, 353, 2823–2836. [Google Scholar] [CrossRef]
- Rossi, L.M.; Costa, N.J.S.; Silva, F.P.; Wojcieszak, R. Magnetic Nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Petrov, A.W.; Ferri, D.; Kröcher, O.; van Bokhoven, J.A. Design of stable palladium-based zeolite catalysts for complete methane oxidation by postsynthesis zeolite modification. ACS Catal. 2019, 9, 2303–2312. [Google Scholar] [CrossRef]
- Petrov, A.W.; Ferri, D.; Krumeich, F.; Nachtegaal, M.; van Bokhoven, J.A.; Kröcher, O. Stable complete methane oxidation over palladium based zeolite catalysts. Nat. Commun. 2018, 9, 2545. [Google Scholar] [CrossRef]
- Mehnert, C.P.; Weaver, D.W.; Ying, J.Y. Heterogeneous heck catalysis with palladium-grafted molecular sieves. J. Am. Chem. Soc. 1998, 120, 12289–12296. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Zhang, J.; Wang, H.; Lewis, J.P.; Xiao, F.-S. Product selectivity controlled by zeolite crystals in biomass hydrogenation over a palladium catalyst. J. Am. Chem. Soc. 2016, 138, 7880–7883. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Q.; Wang, X.; Chen, W.; Leng, L.; Zhang, M.; Horton, J.H.; Liu, B.; Xu, Q.; Wu, W.; et al. Highly active and stable palladium single-atom catalyst achieved by a thermal atomization strategy on an SBA-15 molecular sieve for semi-hydrogenation reactions. ACS Appl. Mater. Interfaces 2021, 13, 2530–2537. [Google Scholar] [CrossRef]
- Shaikh, M.N.; Aziz, M.A.; Helal, A.; Kalanthoden, A.N.; Yamani, Z.H. PdNPs@ZIF-8 Micro-nanostructured catalyst of regioselective Mizoriki-heck olefination. ChemistrySelect 2017, 2, 9052–9057. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, J.; Xie, Z.; Zhang, K.; Wu, Y.; Zuo, P.; Zhang, W.; Li, J.; Gao, Z. Zeolite-enhanced sustainable Pd-catalyzed C–C cross-coupling reaction: Controlled release and capture of palladium. ACS Appl. Mater. Interfaces 2020, 12, 11419–11427. [Google Scholar] [CrossRef]
- Verma, S.; Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Visible light mediated upgrading of biomass to biofuel. Green Chem. 2016, 18, 1327–1331. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Sustainable strategy utilizing biomass: Visible-light-mediated synthesis of γ-valerolactone. ChemCatChem 2016, 8, 690–693. [Google Scholar] [CrossRef]
- Nasir Baig, R.B.; Verma, S.; Nadagouda, M.N.; Varma, R.S. A photoactive bimetallic framework for direct aminoformylation of nitroarenes. Green Chem. 2016, 18, 1019–1022. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Selective oxidation of alcohols using photoactive VO@g-C3N4. ACS Sustain. Chem. Eng. 2016, 4, 1094–1098. [Google Scholar] [CrossRef]
- Verma, S.; Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Aerobic oxidation of alcohols in visible light on Pd-grafted Ti cluster. Tetrahedron 2017, 73, 5577–5580. [Google Scholar] [CrossRef]
- Gao, G.; Jiao, Y.; Waclawik, E.R.; Du, A. Single atom (Pd/Pt) supported on graphitic carbon nitride as an efficient photocatalyst for visible-light reduction of carbon dioxide. J. Am. Chem. Soc. 2016, 138, 6292–6297. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, C.; Puértolas, B.; Ackermann, M.; Chen, Z.; Pérez-Ramírez, J. Enhanced base-free formic acid production from CO 2 on Pd/g-C 3 N 4 by tuning of the carrier defects. ChemSusChem 2018, 11, 2859–2869. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, J.H.; Kim, E.H.; Kim, K.Y.; Choi, Y.H.; Youn, D.H.; Lee, J.S. A Highly active and stable palladium catalyst on a G-C3N4 support for direct formic acid synthesis under neutral conditions. Chem. Commun. 2016, 52, 14302–14305. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Park, S.; Lee, W.; Lee, C. Effects of functionalization of TiO2 nanotube array sensors with Pd nanoparticles on their selectivity. Sensors 2014, 14, 15849–15860. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.K.; Kondamudi, N.; Banerjee, S.; Misra, M. Functionalization of self-organized TiO2 nanotubes with Pd nanoparticles for photocatalytic decomposition of dyes under solar light illumination. Langmuir 2008, 24, 11276–11281. [Google Scholar] [CrossRef]
- Wu, J.; Lu, S.; Ge, D.; Zhang, L.; Chen, W.; Gu, H. Photocatalytic properties of Pd/TiO2 Nanosheets for hydrogen evolution from water splitting. RSC Adv. 2016, 6, 67502–67508. [Google Scholar] [CrossRef]
- Rusinque, B.; Escobedo, S.; de Lasa, H. Hydrogen production via Pd-TiO2 photocatalytic water splitting under near-UV and visible light: Analysis of the reaction mechanism. Catalysts 2021, 11, 405. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 nanotube photocatalysts for solar water splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Carbon-coated magnetic palladium: Applications in partial oxidation of alcohols and coupling reactions. Green Chem. 2014, 16, 4333. [Google Scholar] [CrossRef]
- Veisi, H.; Karmakar, B.; Tamoradi, T.; Tayebee, R.; Sajjadifar, S.; Lotfi, S.; Maleki, B.; Hemmati, S. Bio-inspired synthesis of palladium nanoparticles fabricated magnetic Fe3O4 nanocomposite over Fritillaria imperialis flower extract as an efficient recyclable catalyst for the reduction of nitroarenes. Sci. Rep. 2021, 11, 4515. [Google Scholar] [CrossRef] [PubMed]
- Palliyarayil, A.; Prakash, P.S.; Nandakumar, S.; Kumar, N.S.; Sil, S. Palladium nanoparticles impregnated activated carbon material for catalytic oxidation of carbon monoxide. Diam. Relat. Mater. 2020, 107, 107884. [Google Scholar] [CrossRef]
- Pinilla, J.L.; García, A.B.; Philippot, K.; Lara, P.; García-Suárez, E.J.; Millan, M. Carbon-supported Pd nanoparticles as catalysts for anthracene hydrogenation. Fuel 2014, 116, 729–735. [Google Scholar] [CrossRef]
- Jian, S.; Tian, Z.; Zhang, K.; Duan, G.; Yang, W.; Jiang, S. Hydrothermal synthesis of Ce-doped ZnO heterojunction supported on carbon nanofibers with high visible light photocatalytic activity. Chem. Res. Chinese Univ. 2021, 37, 565–570. [Google Scholar] [CrossRef]
- Zhou, G.; Xiong, T.; Jiang, S.; Jian, S.; Zhou, Z.; Hou, H. Flexible titanium carbide–Carbon nanofibers with high modulus and high conductivity by electrospinning. Mater. Lett. 2016, 165, 91–94. [Google Scholar] [CrossRef]
- Xu, W.; Feng, Y.; Ding, Y.; Jiang, S.; Fang, H.; Hou, H. Short electrospun carbon nanofiber reinforced polyimide composite with high dielectric permittivity. Mater. Lett. 2015, 161, 431–434. [Google Scholar] [CrossRef]
- Peng, X.; Ye, W.; Ding, Y.; Jiang, S.; Hanif, M.; Liao, X.; Hou, H. Facile synthesis, characterization and application of highly active palladium nano-network structures supported on electrospun carbon nanofibers. RSC Adv. 2014, 4, 42732–42736. [Google Scholar] [CrossRef]
- Jia, X.; Hu, G.; Nitze, F.; Barzegar, H.R.; Sharifi, T.; Tai, C.-W.; Wågberg, T. Synthesis of palladium/helical carbon nanofiber hybrid nanostructures and their application for hydrogen peroxide and glucose detection. ACS Appl. Mater. Interfaces 2013, 5, 12017–12022. [Google Scholar] [CrossRef]
- Takasaki, M.; Motoyama, Y.; Higashi, K.; Yoon, S.-H.; Mochida, I.; Nagashima, H. Chemoselective hydrogenation of nitroarenes with carbon nanofiber-supported platinum and palladium nanoparticles. Org. Lett. 2008, 10, 1601–1604. [Google Scholar] [CrossRef]
- Gholinejad, M.; Naghshbandi, Z.; Nájera, C. Carbon-derived supports for palladium nanoparticles as catalysts for carbon-carbon bonds formation. ChemCatChem 2019, 11, 1792–1823. [Google Scholar] [CrossRef]
- Gong, T.; Qin, L.; Zhang, W.; Wan, H.; Lu, J.; Feng, H. Activated carbon supported palladium nanoparticle catalysts synthesized by atomic layer deposition: Genesis and evolution of nanoparticles and tuning the particle size. J. Phys. Chem. C 2015, 119, 11544–11556. [Google Scholar] [CrossRef]
- Veerakumar, P.; Veeramani, V.; Chen, S.-M.; Madhu, R.; Liu, S.-B. Palladium nanoparticle incorporated porous activated carbon: Electrochemical detection of toxic metal ions. ACS Appl. Mater. Interfaces 2016, 8, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kinoshita, H.; Hashimoto, H.; Nishina, Y. Facile preparation of Pd nanoparticles supported on single-layer graphene oxide and application for the Suzuki–Miyaura cross-coupling reaction. Nanoscale 2014, 6, 6501–6505. [Google Scholar] [CrossRef] [PubMed]
- Elazab, H.A.; Siamaki, A.R.; Moussa, S.; Gupton, B.F.; El-Shall, M.S. Highly efficient and magnetically recyclable graphene-supported Pd/Fe3O4 nanoparticle catalysts for Suzuki and heck cross-coupling reactions. Appl. Catal. A Gen. 2015, 491, 58–69. [Google Scholar] [CrossRef]
- Movahed, S.K.; Dabiri, M.; Bazgir, A. Palladium nanoparticle decorated high nitrogen-doped graphene with high catalytic activity for Suzuki–Miyaura and Ullmann-type coupling reactions in aqueous media. Appl. Catal. A Gen. 2014, 488, 265–274. [Google Scholar] [CrossRef]
- Ye, X.R.; Lin, Y.; Wai, C.M. Decorating catalytic palladium nanoparticles on carbon nanotubes in supercritical carbon dioxide. Chem. Commun. 2003, 5, 642–643. [Google Scholar] [CrossRef]
- Ye, X.-R.; Lin, Y.; Wang, C.; Engelhard, M.H.; Wang, Y.; Wai, C.M. Supercritical fluid synthesis and characterization of catalytic metal nanoparticles on carbon nanotubes. J. Mater. Chem. 2004, 14, 908. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Z.; Xiao, J.; Chen, C.; Xiao, F.; Wang, S.; Liu, Y. Facile and green synthesis of palladium nanoparticles-graphene-carbon nanotube material with high catalytic activity. Sci. Rep. 2013, 3, 2527. [Google Scholar] [CrossRef]
- Labulo, A.H.; Martincigh, B.S.; Omondi, B.; Nyamori, V.O. Advances in carbon nanotubes as efficacious supports for palladium-catalysed carbon–carbon cross-coupling reactions. J. Mater. Sci. 2017, 52, 9225–9248. [Google Scholar] [CrossRef]
- Muhulet, A.; Miculescu, F.; Voicu, S.I.; Schütt, F.; Thakur, V.K.; Mishra, Y.K. Fundamentals and scopes of doped carbon nanotubes towards energy and biosensing applications. Mater. Today Energy 2018, 9, 154–186. [Google Scholar] [CrossRef]
- Miners, S.A.; Rance, G.A.; Khlobystov, A.N. Chemical reactions confined within carbon nanotubes. Chem. Soc. Rev. 2016, 45, 4727–4746. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.; Wai, C.M. Microemulsion-templated synthesis of carbon nanotube-supported Pd and Rh nanoparticles for catalytic applications. J. Am. Chem. Soc. 2005, 127, 17174–17175. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, Y.; Jia, X.; Yang, Y. Palladium nanoparticles supported on organosilane-functionalized carbon nanotube for solvent-free aerobic oxidation of benzyl alcohol. Appl. Catal. B Environ. 2014, 156–157, 385–397. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, N.; Pei, X.; Dai, T.; Zhao, Z.; Chen, C.; Ran, M.; Sun, W. Facile one-pot synthesis of superfine palladium nanoparticles on polydopamine-functionalized carbon nanotubes as a nanocatalyst for the heck reaction. J. Mater. Sci. Technol. 2021, 82, 197–206. [Google Scholar] [CrossRef]
- Nie, G.; Zhang, L.; Cui, Y. Preparation of Pd nanoparticles deposited on a polyaniline/multiwall carbon nanotubes nanocomposite and their application in the heck reaction. React. Kinet. Mech. Catal. 2013, 108, 193–204. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, H.; Zhang, B.; Zhang, L.; Wang, H.; Su, D.S. A Pd/CNT-SiC monolith as a robust catalyst for Suzuki coupling reactions. Phys. Chem. Chem. Phys. 2014, 16, 11178–11181. [Google Scholar] [CrossRef]
- Jiao, Z.-F.; Guo, X.-N.; Zhai, Z.-Y.; Jin, G.-Q.; Wang, X.-M.; Guo, X.-Y. The enhanced catalytic performance of Pd/SiC for the hydrogenation of furan derivatives at ambient temperature under visible light irradiation. Catal. Sci. Technol. 2014, 4, 2494–2498. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Reinhard, M.; Schneider, W.F.; Schüth, C.; Shapley, J.R.; Strathmann, T.J.; Werth, C.J. Critical review of Pd-based catalytic treatment of priority contaminants in water. Environ. Sci. Technol. 2012, 46, 3655–3670. [Google Scholar] [CrossRef]
- Antony, A.M.; Kandathil, V.; Kempasiddaiah, M.; Shwetharani, R.; Balakrishna, R.G.; El-Bahy, S.M.; Hessien, M.M.; Mersal, G.A.M.; Ibrahim, M.M.; Patil, S.A. Graphitic carbon nitride supported palladium nanocatalyst as an efficient and sustainable catalyst for treating environmental contaminants and hydrogen evolution reaction. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 647, 129116. [Google Scholar] [CrossRef]
- Wang, Z.; Lü, S.; Yang, F.; Kabir, S.M.F.; Mahmud, S.; Liu, H. Hyaluronate macromolecules reduced-stabilized colloidal palladium nanocatalyst for azo contaminated wastewater treatment. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 628, 127345. [Google Scholar] [CrossRef]
- Huang, B.; Xie, Q.; Yang, Z.; Lei, C.; Chen, W.; Tang, X.; Maran, F. Surfactant-directed Pd-nanoparticle assemblies as efficient nanoreactors for water remediation. EcoMat 2020, 2, e12046. [Google Scholar] [CrossRef]
- Luo, F.; Yang, D.; Chen, Z.; Megharaj, M.; Naidu, R. One-step green synthesis of bimetallic Fe/Pd nanoparticles used to degrade orange II. J. Hazard. Mater. 2016, 303, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Desai, I.; Cruz, C.; Yang, D.J. Novel Pd based catalyst for the removal of organic and emerging contaminants. RSC Adv. 2012, 2, 7540–7548. [Google Scholar] [CrossRef]
- Li, L.; Gong, L.; Wang, Y.-X.; Liu, Q.; Zhang, J.; Mu, Y.; Yu, H.-Q. Removal of halogenated emerging contaminants from water by nitrogen-doped graphene decorated with palladium nanoparticles: Experimental investigation and theoretical analysis. Water Res. 2016, 98, 235–241. [Google Scholar] [CrossRef]
- Darabdhara, G.; Boruah, P.K.; Borthakur, P.; Hussain, N.; Das, M.R.; Ahamad, T.; Alshehri, S.M.; Malgras, V.; Wu, K.C.-W.; Yamauchi, Y. Reduced graphene oxide nanosheets decorated with Au–Pd bimetallic alloy nanoparticles towards efficient photocatalytic degradation of phenolic compounds in water. Nanoscale 2016, 8, 8276–8287. [Google Scholar] [CrossRef]
- Comandella, D.; Woszidlo, S.; Georgi, A.; Kopinke, F.-D.; Mackenzie, K. Efforts for long-term protection of palladium hydrodechlorination catalysts. Appl. Catal. B Environ. 2016, 186, 204–211. [Google Scholar] [CrossRef]
- Xie, W.; Li, T.; Tiraferri, A.; Drioli, E.; Figoli, A.; Crittenden, J.C.; Liu, B. Toward the next generation of sustainable membranes from green chemistry principles. ACS Sustain. Chem. Eng. 2021, 9, 50–75. [Google Scholar] [CrossRef]
- Chappa, S.; Mhatre, A.M.; Adya, V.C.; Pandey, A.K. Egg-shell membrane mimicking synthetic polymer membrane supported palladium nanoparticles for catalyzing reduction of uranyl(VI) ions. Appl. Catal. B Environ. 2017, 203, 53–64. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Gomez-Herrero, E.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Palladium-based catalytic membrane reactor for the continuous flow hydrodechlorination of chlorinated micropollutants. Appl. Catal. B Environ. 2021, 293, 120235. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Watanabe, T.; Ohno, A.; Uozumi, Y. Development of polymeric palladium-nanoparticle membrane-installed microflow devices and their application in hydrodehalogenation. ChemSusChem 2012, 5, 293–299. [Google Scholar] [CrossRef]
- Moloto, B.P.; Vermeeren, P.; Dalla Tiezza, M.; Esterhuysen, C.; Bickelhaupt, F.M.; Hamlin, T.A. Palladium-catalyzed activation of carbon–halogen bonds: Electrostatics-controlled reactivity. European J. Org. Chem. 2022, 2022, e202200722. [Google Scholar] [CrossRef]
- Geise, G.M.; Lee, H.-S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface modification of water purification membranes. Angew. Chemie Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, C.; Zhou, H.; Wang, Y.; Wang, R. Prefunctionalized porous organic polymers: Effective supports of surface palladium nanoparticles for the enhancement of catalytic performances in dehalogenation. Chem. A Eur. J. 2016, 22, 12533–12541. [Google Scholar] [CrossRef]
- Smuleac, V.; Varma, R.; Sikdar, S.; Bhattacharyya, D. Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J. Memb. Sci. 2011, 379, 131–137. [Google Scholar] [CrossRef]
- Chen, F.; Gong, A.S.; Zhu, M.; Chen, G.; Lacey, S.D.; Jiang, F.; Li, Y.; Wang, Y.; Dai, J.; Yao, Y.; et al. Mesoporous, three-dimensional wood membrane decorated with nanoparticles for highly efficient water treatment. ACS Nano 2017, 11, 4275–4282. [Google Scholar] [CrossRef]
- Rashed, M.N. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater; IntechOpen: Rijeka, Croatia, 2013; Chapter 7. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N,Pd Co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Tokazhanov, G.; Ramazanova, E.; Hamid, S.; Bae, S.; Lee, W. Advances in the catalytic reduction of nitrate by metallic catalysts for high efficiency and N2 selectivity: A review. Chem. Eng. J. 2020, 384, 123252. [Google Scholar] [CrossRef]
- Heck, K.N.; Garcia-Segura, S.; Westerhoff, P.; Wong, M.S. Catalytic converters for water treatment. Acc. Chem. Res. 2019, 52, 906–915. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Epron, F.; Garcia, H.; Granger, P. Recent progress and prospects in catalytic water treatment. Chem. Rev. 2022, 122, 2981–3121. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.M.; Karumuri, A.; Barney, I.T. Hierarchical nanostructures by nanotube grafting on porous cellular surfaces. J. Phys. D. Appl. Phys. 2009, 42, 195503. [Google Scholar] [CrossRef]
- He, L.; Karumuri, A.; Mukhopadhyay, S.M. Wettability tailoring of nanotube carpets: Morphology-chemistry synergy for hydrophobic–hydrophilic cycling. RSC Adv. 2017, 7, 25265–25275. [Google Scholar] [CrossRef]
- Karumuri, A.K.; Maleszewski, A.A.; Oswal, D.P.; Hostetler, H.A.; Mukhopadhyay, S.M. Fabrication and characterization of antibacterial nanoparticles supported on hierarchical hybrid substrates. J. Nanoparticle Res. 2014, 16, 2346. [Google Scholar] [CrossRef]
- Pulikollu, R.V.; Mukhopadhyay, S.M. Nanoscale coatings for control of interfacial bonds and nanotube growth. Appl. Surf. Sci. 2007, 253, 7342–7352. [Google Scholar] [CrossRef]
- Wang, W.; Mukhopadhyay, S.M. Hierarchical nanostructured surface design for robust and flexible multifunctional devices. Carbon Trends 2021, 5, 100096. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.M.; Karumuri, A.K. Nanotube attachment for prevention of interfacial delamination. J. Phys. D. Appl. Phys. 2010, 43, 365301. [Google Scholar] [CrossRef]
- Quinton, B.T.; Leedy, K.D.; Lawson, J.W.; Tsao, B.; Scofield, J.D.; Merrett, J.N.; Zhang, Q.; Yost, K.; Mukhopadhyay, S.M. Influence of oxide buffer layers on the growth of carbon nanotube arrays on carbon substrates. Carbon N. Y. 2015, 87, 175–185. [Google Scholar] [CrossRef]
- Quinton, B.T.; Elston, L.; Scofield, J.D.; Mukhopadhyay, S.M. Aligned carbon nanotube arrays bonded to solid graphite substrates: Thermal analysis for future device cooling applications. C J. Carbon Res. 2018, 4, 28. [Google Scholar] [CrossRef]
- Barney, I.T.; Lennaerts, D.S.R.; Higgins, S.R.; Mukhopadhyay, S.M. Specific surface area of hierarchical graphitic substrates suitable for multi-functional applications. Mater. Lett. 2012, 88, 160–163. [Google Scholar] [CrossRef]
- Vijwani, H.; Nadagouda, M.N.; Namboodiri, V.; Mukhopadhyay, S.M. Hierarchical hybrid carbon nano-structures as robust and reusable adsorbents: Kinetic studies with model dye compound. Chem. Eng. J. 2015, 268, 197–207. [Google Scholar] [CrossRef]
- Vijwani, H. Hierarchical Porous Structures with Aligned Carbon Nanotubes as Efficient Adsorbents and Metal-Catalyst Supports. Ph.D. Dissertation, Wright State University, Dayton, OH, USA, 2015. Available online: https://corescholar.libraries.wright.edu/etd_all/1309 (accessed on 13 September 2022).
- Vijwani, H.; Mukhopadhyay, S.M. Palladium nanoparticles on hierarchical carbon surfaces: A new architecture for robust nano-catalysts. Appl. Surf. Sci. 2012, 263, 712–721. [Google Scholar] [CrossRef]
- Vijwani, H.; Nadagouda, M.N.; Mukhopadhyay, S.M. Robust nanocatalyst membranes for degradation of atrazine in water. J. Water Process Eng. 2018, 25, 15–21. [Google Scholar] [CrossRef]
- Jha, K.; Liu, Z.; Vijwani, H.; Nadagouda, M.; Mukhopadhyay, S.; Tsige, M. Carbon nanotube based groundwater remediation: The case of trichloroethylene. Molecules 2016, 21, 953. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nadagouda, M.N.; Mukhopadhyay, S.M. Flexible reusable hierarchical hybrid catalyst for rapid and complete degradation of triclosan in water. Sci. Total Environ. 2021, 766, 144109. [Google Scholar] [CrossRef] [PubMed]
- Bokare, V.; Murugesan, K.; Kim, Y.-M.; Jeon, J.-R.; Kim, E.-J.; Chang, Y.S. Degradation of triclosan by an integrated nano-bio redox process. Bioresour. Technol. 2010, 101, 6354–6360. [Google Scholar] [CrossRef]

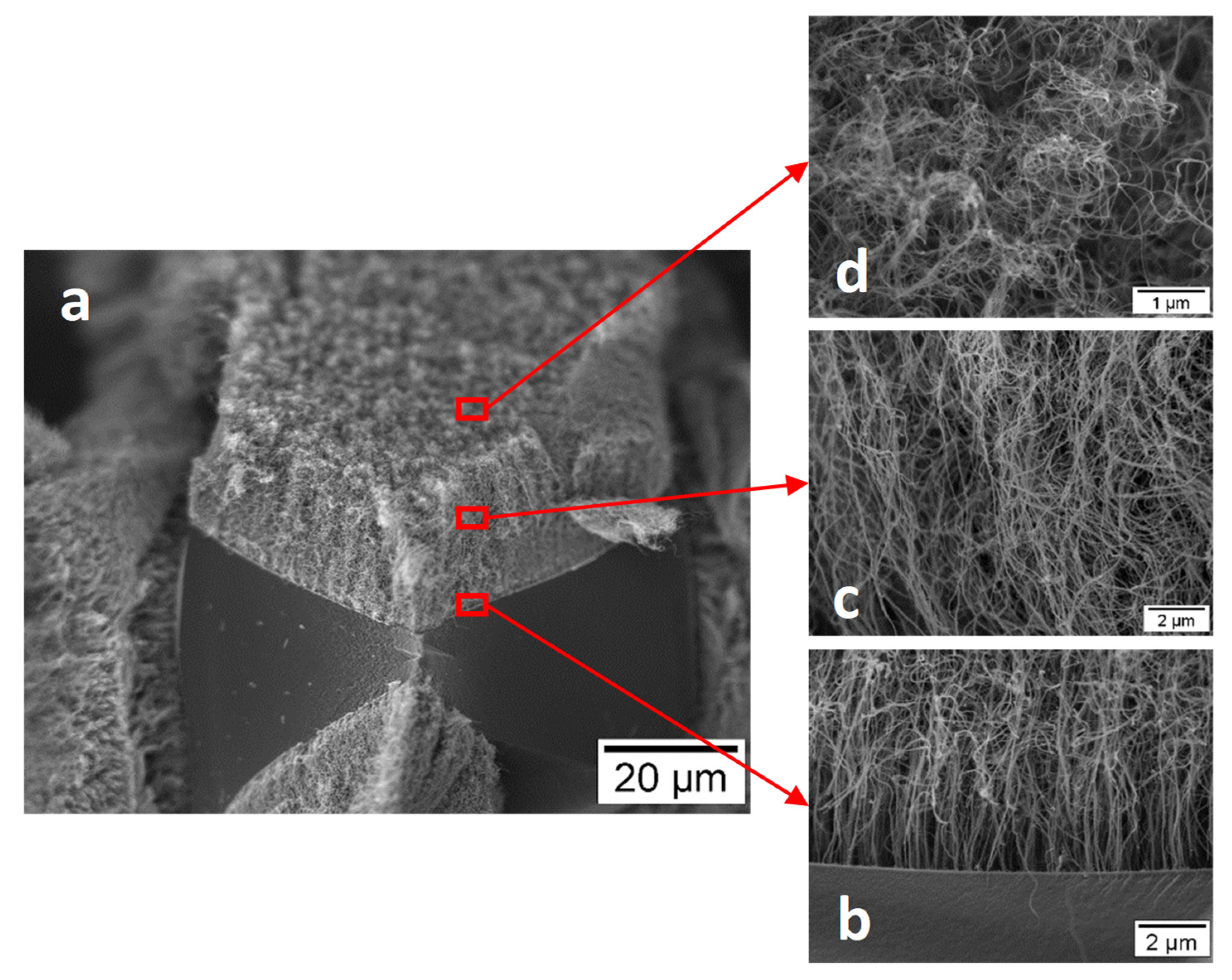

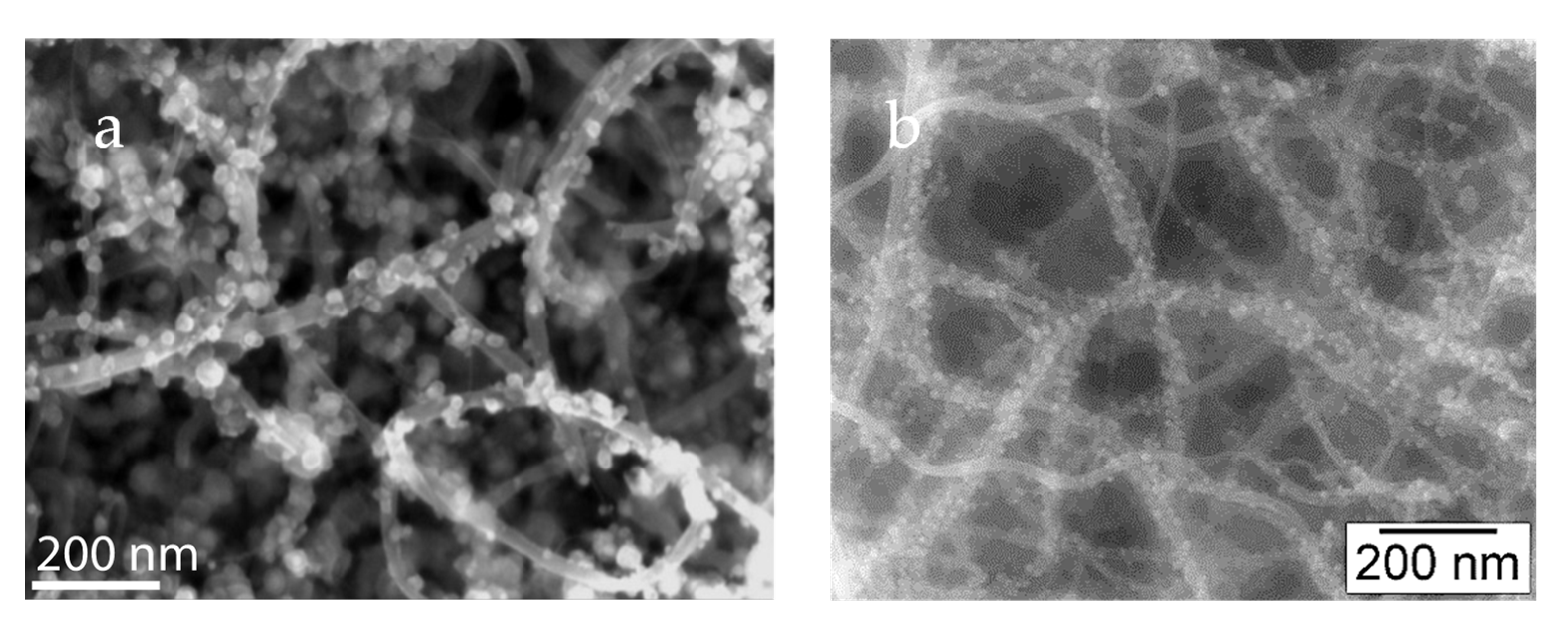


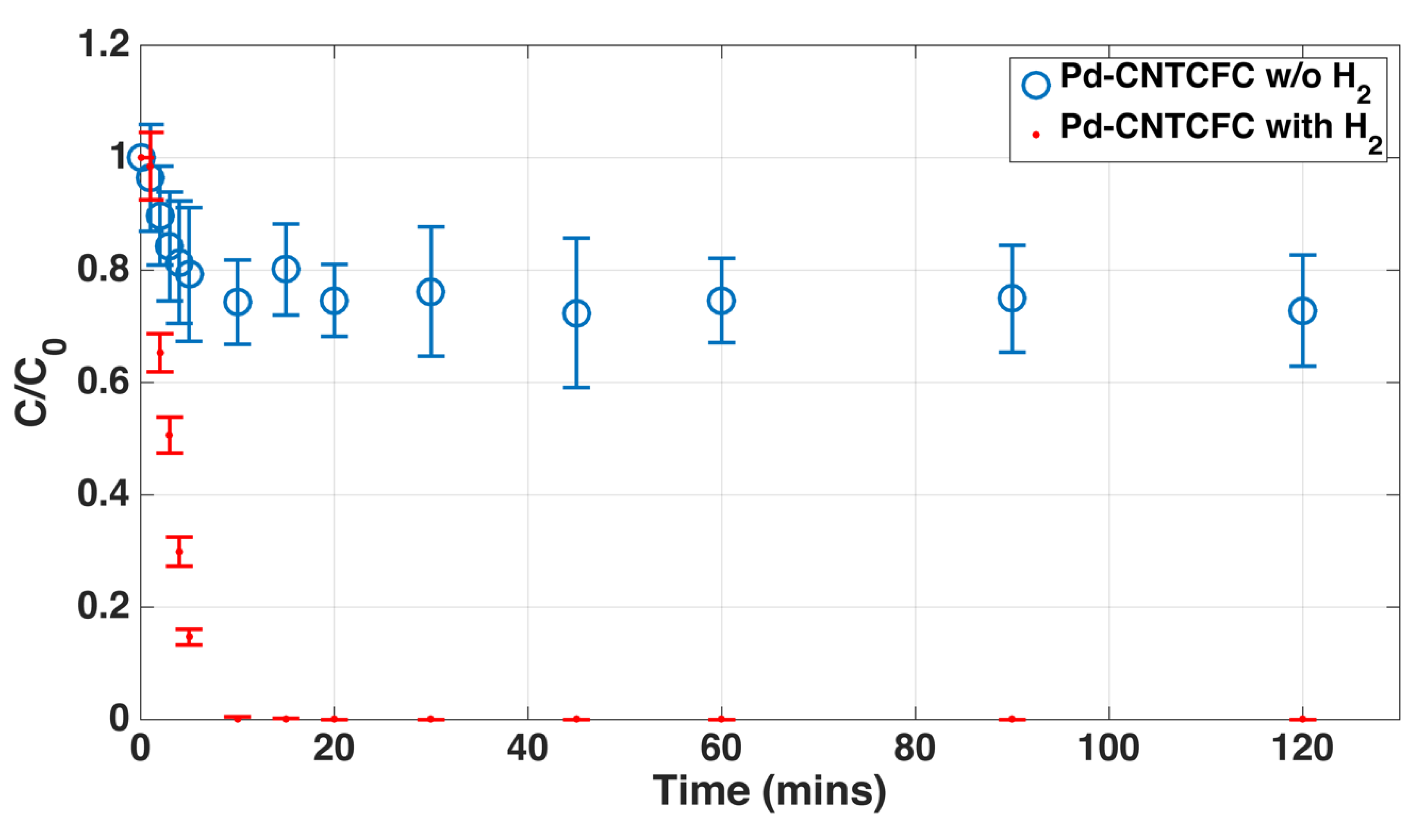
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Nadagouda, M.N.; Mukhopadhyay, S.M. Advances in Matrix-Supported Palladium Nanocatalysts for Water Treatment. Nanomaterials 2022, 12, 3593. https://doi.org/10.3390/nano12203593
Wang W, Nadagouda MN, Mukhopadhyay SM. Advances in Matrix-Supported Palladium Nanocatalysts for Water Treatment. Nanomaterials. 2022; 12(20):3593. https://doi.org/10.3390/nano12203593
Chicago/Turabian StyleWang, Wenhu, Mallikarjuna N. Nadagouda, and Sharmila M. Mukhopadhyay. 2022. "Advances in Matrix-Supported Palladium Nanocatalysts for Water Treatment" Nanomaterials 12, no. 20: 3593. https://doi.org/10.3390/nano12203593
APA StyleWang, W., Nadagouda, M. N., & Mukhopadhyay, S. M. (2022). Advances in Matrix-Supported Palladium Nanocatalysts for Water Treatment. Nanomaterials, 12(20), 3593. https://doi.org/10.3390/nano12203593





